Abstract
Bioengineering hair follicles using cells isolated from human tissue remains as a difficult task. Dermal papilla (DP) cells are known to guide the growth and cycling activities of hair follicles by interacting with keratinocytes. However, DP cells quickly lose their inductivity during in vitro passaging. Rodent DP cell cultures need external addition of chemical factors, including WNT and BMP molecules, to maintain the hair inductive property. CD133 is expressed by a small subpopulation of DP cells that are capable of inducing hair follicle formation in vivo. We report here that expression of a stabilized form of β-catenin promoted clonal growth of CD133-positive (CD133+) DP cells in in vitro three-dimensional hydrogel culture while maintaining expression of DP markers, including alkaline phosphatase (AP), CD133, and Integrin α8. After a two-week in vitro culture, cultured CD133+ DP cells with up-regulated β-catenin activity led to an accelerated in vivo hair growth in reconstituted skin than control cells. Further analysis showed that matrix cell proliferation and differentiation were significantly promoted in hair follicles when β-catenin signaling was upregulated in CD133+ DP cells. Our data highlight an important role for β-catenin signaling in promoting the inductive capability of CD133+ DP cells for in vitro expansion and in vivo hair follicle regeneration, which could potentially be applied to cultured human DP cells.
Keywords: Hair follicle, Dermal papilla, CD133, β-catenin, regeneration
INTRODUCTION
Interactions between mesenchymal and epithelial cells are at the core of forming and maintaining many tissues [1–3]. In the past decade tissue engineering has greatly benefitted from a better understanding of the role of fibroblast cell populations and their contributions to tissue formation and repair. A highly specialized fibroblast subpopulation is at the heart of hair follicle biology, namely dermal papilla (DP) fibroblasts, and has been intensively studied because of their hair–inducing capabilities [4]. The DP plays a critical role in the activation of hair follicle stem cells (HFSCs) and their progenies required for a new cycle of hair growth at the end of the resting phase (telogen) [5]. Following the onset of growth phase (anagen), the DP continues to provide inducing signals to drive the proliferation and differentiation of hair matrix cells forming the multiple layers of the outgrowing hair shaft and the inner root sheath [5]. Clearly, DP cells are excellent candidates for tissue bioengineering to generate functional skin substitutes for burn victims and patients with alopecia.
Although DP cells from neonatal rodent skin or vibrissae can be used to induce de novo hair follicle development and hair growth [6], the success of using DP cells in hair reconstitution assays has been limited, especially when using human DP cells. Furthermore, DP cells eventually will lose their hair-inducing capacity once cultured and expanded in vitro [7]. To maintain and even prolong these inductive properties in rodent DP cell cultures, external addition of chemical factors, including WNT and BMP molecules, are needed [8, 9]. To fully harness the ability of DP cells in conjunction with keratinocytes and melanocytes to drive the self-assembly of a complex human hair follicle requires improved DP culture methods, better tissue engineering techniques, and most importantly, a better comprehension of the genes and pathways determining “DP-ness” [10].
Several markers specifically demarcate DP cells in the skin. However only a handful of these markers have proven to be useful to study the DP. Alkaline phosphatase has become a widely used marker to identify the DP [11]. Corin is a reliable DP marker and tool to study the DP but only expresses in a short period of time during the hair cycle [12]. Some of the best markers for DP cells that allow purification, targeting, and to distinguish them from other dermal cell populations are Versican and CD133. Versican was the first anagen DP marker to be identified [13]. CD133 has recently been widely considered to be a useful marker for hair-inducing DP cells [14]. While the CD133+ DP cells isolated from embryonic or adult DP had the ability to induce new hair follicles in vivo, CD133- DP cells did not [14]. Further studies using in vitro three-dimensional hydrogel culture system and skin reconstitution assays showed that CD133+ DP cells contributed to the establishment of the DP in both primary and secondary hair follicles [15]. The findings strongly suggest that CD133+ DP cells could be a major cell population in the DP that is capable of promoting hair follicle regeneration and growth. However, it remains unclear how CD133+ DP cells interact with HFSCs in the bulge and matrix keratinocytes in the hair bulb to rebuild the hair follicle structure during anagen phase.
Using the above mentioned markers and tools, it has become clear that several key signaling pathways are crucial for the formation, maintenance, and function of DP cells. One of them is WNT/β-catenin signaling, which is essential for maintaining DP function in vitro and in vivo [8, 16]. To explore the relevance of activating β-catenin signaling in prolonging hair-inducing properties of CD133+ DP cells in in vitro culture and in vivo hair follicle induction, a stabilized ΔN-β-catenin protein was expressed in CD133+ DP cells for in vitro clonal expansion and in vivo skin reconstitution. Here, we show that cultured CD133+ DP cells have enhanced abilities to grow in vitro and induce trichogenesis in vivo when β-catenin signaling is upregulated.
RESULT
β-catenin signaling promotes clonal growth of CD133+ DP cells and preserves their DP characteristics in vitro
To determine whether β-catenin signaling could be targeted to promote DP cell expansion in culture and hair follicle neogenesis in vivo, we generated a triple transgenic mouse line, CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN, which allows for controlled expression of ΔN-β-catenin specifically in CD133+ DP cells. β-catenin protein encoded by Ctnnb1ΔN could no longer be degraded because it lacks 89 amino acids at the N-terminal (ΔN), which are the target site of the ubiquitin-proteasome pathway (Fig. 1A). CD133-CreERT2 mice specifically express a fusion protein (CreER) combining the Cre recombinase and a mutated ligand-binding domain of the human estrogen receptor in CD133+ DP cells [17, 18]. In Rosa-rtTA transgene, a loxP-flanked STOP cassette preventing the transcription of a CAG promoter-driven tet reverse transactivator (rtTA) in the Rosa26 locus [19]. Expression of rtTA will be initiated when Cre recombinase under the control of CD133 is activated to remove STOP cassette when mice are administrated with tamoxifen. The expression of rtTA from CD133-CreERT2; Rosa-rtTA consequently allows the expression of ΔN-β-catenin from tetO-Ctnnb1ΔN if mice are fed with doxycycline diet.
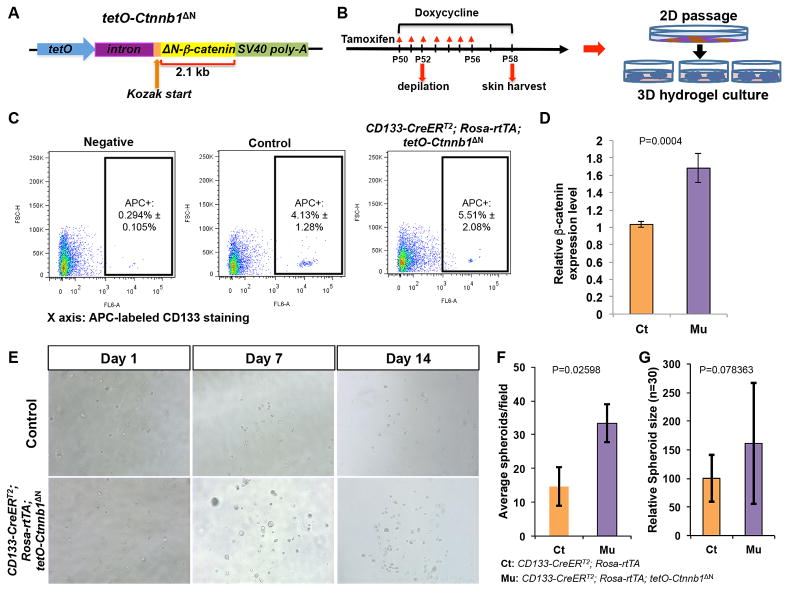
Figure 1. Generation and expansion of ΔN-β-catenin-expressing CD133+ DP cells in in vitro hydrogel culture.
A. Overview of tetO-Ctnnb1ΔN transgenic construct. B. Scheme of mouse induction, skin biology, isolation of CD133+ DP cells and hydrogel culture. C. Representative flow cytometry plots showing a unique CD133+ DP cell population labeled by an APC-conjugate anti-CD133 antibody (n=6). D. Comparison of β-catenin expression between CD133+ DP cells isolated from CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN mice and control littermates of genotype Rosa-rtTA; tetO-Ctnnb1ΔN or CD133-CreERT2; Rosa-rtTA by qPCR (n=6). E. Representative pictures showing spheroids in hydrogel at day 1, 7 and 14. Upper panels: control CD133+ DP cells; lower panels: ΔN-β-catenin-expressing CD133+ DP cells from CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN mice (n=6). F. Comparison of numbers of spheroids formed by ΔN-β-catenin-expressing and control CD133+ DP cells counted in each field under an inverted microscope (10x). At least 5 random fields were picked to count for each well (n=3). G. Sizes of spheroids formed by CD133+ DP cells in hydrogel were measured using ImageJ. At least 30 spheroids formed by control or β-catenin-expressing CD133+ DP cells were measured and averaged. Relative size difference was calculated by setting control spheroid size to 1.
As shown in Fig. 1B, induction of anagen hair growth in CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN mice and control littermates was induced by hair plucking at postnatal day 52 (P52). Six days later, at P58, when hair follicles entered early anagen, CD133+ DP cells were labeled and isolated from the dermis using fluorescence-activated cell sorting (FACS). The percentage of CD133+ DP cell population was always higher in the cell preparations from CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN mice than control littermates (Fig. 1C). As expected, quantitative polymerase chain reaction (qPCR) analysis showed that CD133+ DP cells isolated from CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN mice had higher level of β-catenin expression than control littermates (Fig.1D). FACS-sorted CD133+ DP cells were subsequently cultured using collagen-coated 6-well plates for one passage, and then encapsulated in hydrogel (Extracel) and grown in AmnioMax C-100. Three-dimensional (3D) culture using hydrogel has previously been reported to maintain the hair-inducing property of DP cells in in vitro culture for up to 14 days [7, 15]. After 7 days in culture, spheroids were formed from CD133+ DP cells of both genotypes, and spheroid size and number continued to increase in both cases through day 14. However, while there was no obvious difference at day 1, ΔN-β-catenin-expressing CD133+ DP cells gave rise to significantly more spheroids (ΔN-β-catenin-expressing spheroids) in hydrogel culture than control CD133+ cells at day 7 and 14 (Fig. 1E). As shown in Fig. 1F, there were 35 ± 7 spheroids per field (10X) formed from ΔN-β-catenin-expressing CD133+ DP cells while only 15 ± 6 spheroids formed from control DP cells at day 14. In addition, the average size of ΔN-β-catenin-expressing spheroids was 61% bigger than that of control spheroids (Fig. 1G).
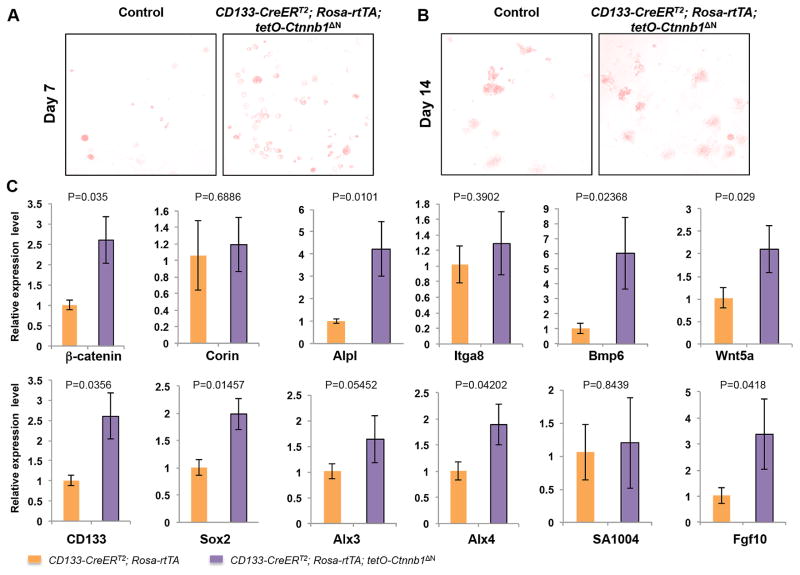
The AP activity has been used as a marker of DP cells and an indicator of their hair inductivity in vivo [11, 20]. At day 7 (Fig. 2A) and 14 (Fig. 2B), AP activity was detected in both ΔN-β-catenin-expressing spheroids and controls. To investigate whether other DP signature genes were expressed during hydrogel culture and whether their expression was altered in ΔN-β-catenin-expressing spheres [21], we isolated RNA from spheroids cultured for 14 days in AmnioMax hydrogel and performed RT-PCR analysis (Fig. 2C). Level of β-catenin in ΔN-β-catenin-expressing spheroids was 2.5 times higher than that in control spheroids. Levels of AP (Alpl), CD133 and Sox2 were significantly increased in ΔN-β-catenin-expressing spheroids. Expression of additional DP signature genes, including Wnt5a, Alx3, Alx4, Fgf10, Bmp6, were also increased in ΔN-β-catenin-expressing spheroids as compared with control spheroids. However, levels of Corin, S100A4, α8 Integrin (Itga8) were only slightly increased in ΔN-β-catenin-expressing spheroids. Collectively, the results suggested that activating β-catenin signaling enhanced the ability of CD133+ DP cells to grow and expand in vitro while maintain hair inductivity.
Figure 2. Upregulated expression of dermal papilla signature genes in ΔN-β-catenin-expressing CD133+ DP cells in hydrogel culture.
A. AP staining of cultured spheroids formed by control (left) and ΔN-β-catenin-expressing (right) CD133+ DP cells in hydrogel at day 7. B. AP staining of cultured spheroids formed by control (left) and ΔN-β-catenin-expressing (right) CD133+ DP cells in hydrogel at day 14. C. Quantitative real-time PCR analysis of expression of DP signature genes, including β-catenin (Ctnnb1), Corin, CD133, AP (Alpl), Integrin α8 (Itga8), Sox2, Bmp6, Wnt5a, Alx3, Alx4, S100A4, Fgf10. The Y-axis represents fold change in expression with the level in control set to 1 (n=6).
Activation of β-catenin signaling in CD133+ DP cells promotes hair follicle neogenesis and accelerates hair growth in reconstituted skin
To determine whether ΔN-β-catenin-expressing CD133+ DP cells cultured in hydrogel retained potency to induce de novo hair follicle formation, we performed skin reconstitution assays by grafting cultured cells to full-thickness wounds in athymic nude mice. As shown in Fig. 3A, ΔN-β-catenin-expressing CD133+ DP cells and control cells were released from spheroids in hydrogel after 14-day culture. Epidermal keratinocytes and dermal cells were freshly isolated from P2 wild type neonates. It was shown before that cultured DP cells need helper fibroblasts in reconstituted skin to make hair follicles [15]. All three types of cells were mixed and inoculated into dry collagen matrix for grafting. We counted the day of grafting as day 0. Grafted nude mice were kept on a doxycycline (Dox) diet to maintain the expression of ΔN-β-catenin in CD133+ DP cells. As reported previously, 21 days after grafting, new hair shafts could be readily observed in nude mice grafted with both ΔN-β-catenin-expressing CD133+ DP cells and control CD133+ DP cells (Fig. 3B). However, ΔN-β-catenin-expressing CD133+ DP cells formed more and longer hair shafts than control CD133+ DP cells did.
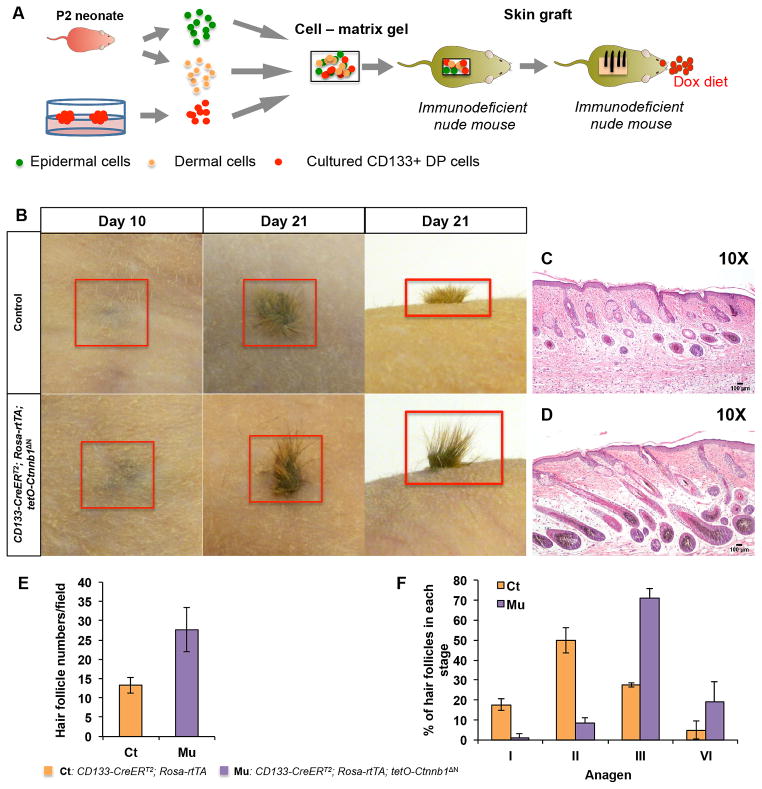
Figure 3. ΔN-β-catenin-expressing CD133+ DP cells induce accelerated hair growth in reconstituted skin.
A. Schematic representation of hair reconstitution assays. Spheroids were released by disaggregating hydrogels and dissociated to release CD133+ DP cells. Same number of control or β-catenin-expressing CD133+ DP cells mixed with P2 dermal cells and epidermal cells was grafted onto nude mice and observed for hair follicle formation, respectively. B. Appearance of newly formed hairs at day 10 and day 21 after grafting. Upper panels: control group containing normal CD133+ DP cells; lower panels: CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN group containing β-catenin-expressing CD133+ DP cells (n=5). Skin biopsies from hair bearing wound area of mice grafted with CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN DP cells (D) or control CD133+ DP cells (C) were stained with H&E and photographed at indicated stages. Scale bars: 100 μm. E. Hair follicle numbers in reconstituted skin formed by either control and β-catenin-expressing CD133+ DP cells were counted in each field after H&E staining. At least 3 random fields were selected and counted for each reconstituted skin sample (n=3). F. Hair follicles at different anagen stages were counted on H&E stained reconstituted skin samples according to the classification system published previously (1). At least 3 random fields were picked and counted for each reconstituted skin sample (n=3).
Histological analysis confirmed the macroscopic observation of hair growth. As shown in Fig. 3D, hair follicles in reconstituted skin containing ΔN-β-catenin-expressing CD133+ DP cells exhibited a more advanced hair cycle stage than those in controls at day 21 (Fig. 3C). As shown in Fig. 3E, an average of 28 ± 6 hair follicles per field were counted in reconstituted skin with ΔN-β-catenin-expressing CD133+ DP cells while control reconstituted skin had 13 ± 6 hair follicles. Further analysis of hair follicle stages revealed that on average 71% of the hair follicles reached anagen stage III and 19% reached anagen stage IV in reconstituted skin containing ΔN-β-catenin-expressing CD133+ DP cells. On the contrary, roughly 67.5% of hair follicles in control still remained in anagen I and II (Fig. 3F) and only 27.5% reached anagen III.
Accelerated hair growth in reconstituted skin containing β-catenin-expressing CD133+ DP cells is associated with increased matrix cell proliferation
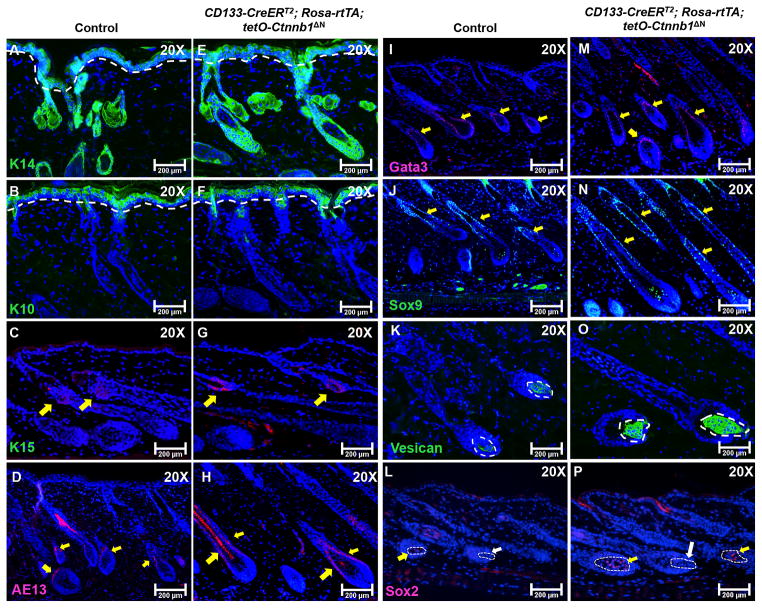
Next, we evaluated the expression of epithelial and hair follicle structural markers, including keratin 14 (basal layer) [22], keratin 10 (spinous layer) [22], keratin 15 (hair follicle stem cells) [23], Sox9 (outer root sheath)[24], Gata3 (inner root sheath) [25], AE13 (hair shaft cortex keratin) [26], and AE15 (medulla) [26], by immunostaining to determine whether their expression levels were consistent with hair follicle morphology. At day 21, expression of keratin 14 and keratin 10 was comparable between β-catenin-expressing grafts (Fig. 4E and 4F) and control grafts (Fig. 4A and 4B), suggesting that ΔN-β-catenin-expressing CD133+ DP cells did not contribute to the wound healing process associated with the grafting process. Immunostaining for the expression of Keratin 15 did not show any abnormality (indicated by yellow arrows in Fig. 4C and 4G), indicating that the stem cell pool for hair follicle morphogenesis and postnatal cycling was not affected. Expression of AE13 was present in both hair follicles, but its expression was much higher in hair follicles containing the ΔN-β-catenin-expressing CD133+ DP cells (Fig. 4H). Levels of Sox9 and Gata3 expression in the outer and inner root sheaths respectively were higher in hair follicles from reconstituted skin containing ΔN-β-catenin-expressing CD133+ DP cells (Fig. 4M and 4N) than in control hair follicles (Fig. 4I and 4J). Versican is a marker for DP cells in anagen hair follicles [27]. As shown in Fig. 4O, the size of the Versican+ DP cell population in ΔN-β-catenin-expressing hair follicles as revealed by immunostaining was larger than that of control DPs (Fig. 4K). Sox2 is considered to be a marker for the DP that specifies particular hair follicle types [28]. No significant difference in Sox2 expression was seen between control (Fig. 4L) and mutant hair follicles (Fig. 4O). Both reconstituted skins contained Sox2+ hair follicles (indicated by yellow arrows) and Sox2- hair follicles (indicated by white arrows).
Figure 4. Activation of β-catenin signaling in CD133+ DP cells accelerates hair follicle growth.
5-μm-thick paraffin slides from reconstituted skin at day 21 were processed for immunofluorescent staining of following markers: K14 for basal epidermis (control: A; mutant: E), K10 for suprabasal epidermis (control: B; mutant F), K15 for hair follicle stem cells and secondary hair germ (control: C; mutant G), AE13 for hair keratins (control: D; mutant: H), Gata3 for inner root sheath (control: I; mutant: M), Sox 9 for outer root sheath (control: J; mutant: N), Versican for anagen DP (control: K; mutant: O) and Sox2 (control: L; mutant: P). Sections were nuclear counterstained with DAPI (blue). Images shown are representative of at least three replicates. Scale bars: 200 μm.
To identify the underlying causes of accelerated hair growth in reconstituted skin generated from cell mixtures that contained ΔN-β-catenin-expressing CD133+ DP cells, we evaluated the proliferation and differentiation states of different cell populations in hair follicles by immunostaining to detect incorporated BrdU in hair matrix cells and to detect Lef1 in hair shaft progenitor cells [29]. As shown in Fig. 5C, there was increased BrdU incorporation in the hair matrix of hair follicles regenerated from ΔN-β-catenin-expressing CD133+ DP cells as compared with control follicles (Fig. 5A). As compared with control hair follicles (Fig. 5B), there were greatly increased numbers of Lef1+ hair shaft precursor cells in hair follicles that contain ΔN-β-catenin-expressing CD133+ DP cells (Fig. 5D). In summary, the data clearly showed that activating β-catenin signaling in CD133+ DP cells enhanced hair follicle regeneration.
Figure 5. ΔN-β-catenin-expressing CD133+ DP cells lead to increased proliferation and differentiation in newly formed hair follicles in reconstituted skin.
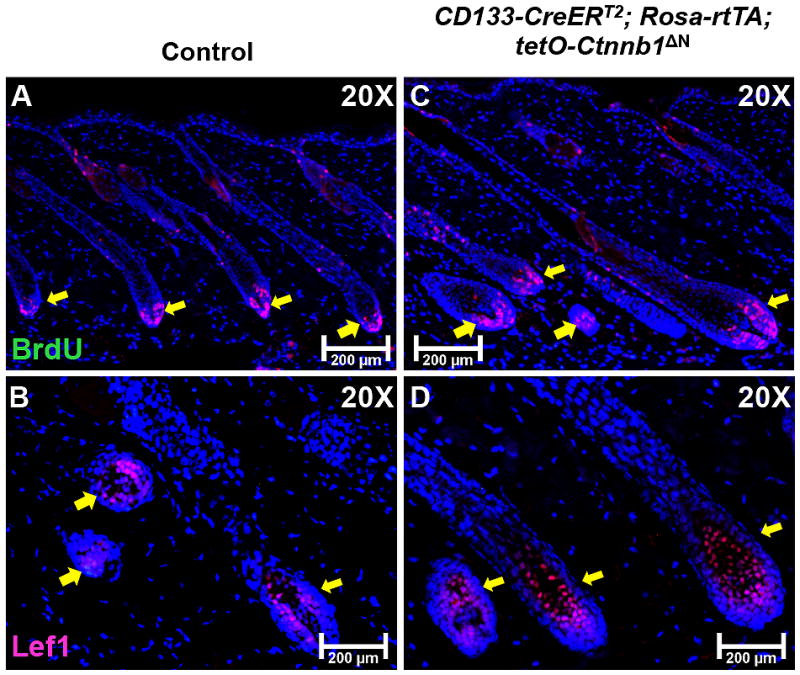
Expression of BrdU (red) and Lef1 (red) was examined on skin samples collected from hair bearing reconstituted skin at day 21 of both the CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN group (C, D) and control group (A, B). At least five mice were analyzed for each group. Scale bars: 200 μm.
DISCUSSION
Life-threatening burn wounds require rapid wound closure. The ultimate goal in clinical approaches to wound healing is to restore all skin functions, including sensitivity, elasticity, normal skin structure, and the function of its adnexa [30]. Currently the gold standard in treating severe burns is the early excision of necrotic tissues followed by wound coverage using autologous split-thickness skin grafts harvested from non-burned parts of the patient’s body. Because of the difficulty of obtaining sufficient donor skin from victims of large burns, alternative techniques for achieving rapid wound closure have been explored. The development of engineered skin replacement to cover burn wounds has provided significant medical benefits [31, 32]. However, because most skin substitutes consist of only one or two cell types, they are not able to provide every function of authentic skin, such as the presence of cycling hair follicles [33]. Such limitation emphasizes the urgent need for a better understanding of molecular mechanisms that could be targeted for bioengineering hair follicles in skin substitutes.
Two critical cell types necessary to produce and maintain hair follicles are fibroblasts in the DP and dermal sheath (DS) and keratinocytes in the epithelial compartment [21]. While keratinocytes are the primary constituents of hair follicles that generate the hair structure [34], it has long been recognized that the growth and cycling activities of hair follicles are largely guided by DP cells. However, after a few passages, cultured DP cells lose their trichogenic properties [35]. Therefore, there remain three major issues that prevent the application of DP cells for hair follicle reconstitution: the isolation of DP cells that possess hair-inducing capability, their expansion in vitro without losing hair inductivity, and the induction of new hair follicle formation in vivo in engineered skin substitutes [10].
Here we report that β-catenin signaling promotes in vitro clonal growth and in vivo hair induction capabilities of CD133+ DP cells. Although the DP is considered to be static and does not proliferate much, the composition of the DP is heterogeneous and dynamic [5]. In murine DP, distinct DP cell subpopulations could be identified based on the expression of different surface markers, including CD133 and Sox2 [36]. CD133+ DP cells have been demonstrated as possessing unique ability to induce hair follicle regeneration [14]. However, CD133+ DP cells have not been tested for their ability to induce hair growth after an extended period of in vitro culture. We have generated a triple transgenic mouse model, CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN, which inducibly expresses a ΔN-β-catenin protein in CD133+ DP cells.
DP cells rarely proliferate in vivo and are difficult to expand in vitro. 3D cultures provides useful model system to mimic and study the complex in vivo environment [37], including the growth factor gradients and cell-cell contacts of the DP microenvironment that is critical for DP cell growth and the maintenance of hair-inducing properties. Different types of 3D spheroids have been shown to be capable of maintaining hair follicle inductivity in both human and rodent DP cells [15, 38]. We adopted a 3D hydrogel culture system that was described previously by the Watt group [15]. Our observation is consistent with prior reports that CD133+ DP cells proliferate and form spheroids in in vitro 3D hydrogels.
It was reported before that specific Wnts, including Wnt3a and Wnt7a, were required in in vitro DP cell culture to maintain their DP-specific gene expression activity to induce hair formation in reconstituted murine skin [8, 35]. Wnt signaling is transmitted through nuclear β-catenin signaling and activates gene expression required for anagen DP cells [39]. Our approach to activate β-catenin signaling in CD133+ DP cells by expressing ΔN-β-catenin achieves the same effects as the external addition of Wnt molecules but may provide additional stimulatory effects. Interestingly, expression of ΔN-β-catenin in CD133+ DP cells led to an increased number of spheroids in 3D hydrogels. Furthermore, DP spheroids formed by ΔN-β-catenin-expressing CD133+ DP cells were larger than those formed from normal CD133+ DP cells. Overall, our data suggest that up-regulating β-catenin signaling not only enhances the survival and proliferation of CD133+ DP cells in in vitro culture, but also effectively promotes their abilities to grow and form spheroids from single cells and maintain hair inductivity.
A series of genes characteristic for the DP has been identified and confirmed to be important for hair inductivity [9, 21]. We confirmed that expression of specific signature genes associated with DP inductivity, including CD133, Sox2, Alpl and igta8, was up-regulated in cultured CD133+ DP cells that expressed ΔN-β-catenin, which was consistent with the hair phenotype observed in the in vivo skin reconstitution assay. In a recent publication from Christiano group [38], they showed that expression of genes in the “Wnt receptor signaling pathways” were disrupted in 2D cultured human DP cells. Furthermore, they demonstrated that the transcriptional profile for DP inductivity was partially restored when human DP cells were cultured in 3D hanging drop culture, which is similar to our 3D hydrogel culture system. Their findings were in agreement with our data as expression of ΔN-β-catenin in CD133+ DP cells using 3D hydrogel culture enhanced the expression of DP signature genes that are essential indicators of in vivo DP inductivity. However, one needs to be cautious when correlating and comparing data from these two different studies since the sources and genetic manipulations of DP cells were different.
Formation of new hair follicles in reconstituted skin is a direct indicator of inductivity of cultured DP cells. Inclusion of P2 dermal cells is a strategic decision for us to make sure skin reconstitution assays will succeed. The critical factor here is the limited number of CD133+ DP cells in our experimental system, even after the in vitro expansion. Considering CD133+ DP cells are required to do multiple jobs in skin reconstitution, including hair follicle formation and the formation of the dermis on the back of nude mice in order to heal the wound, a large-scale in vitro DP cell culture would be needed if we were to test hair follicle induction in reconstituted skin using only CD133+ DP cells. Furthermore, it was reported before that CD133+ DP cells failed to induce hair follicle formation without DP helper cells [15]. Based on this information, we, decided that inclusion of P2 dermal cells is most likely critical and practical for our experiments. Nevertheless, in future experiments, it will be interesting to determine whether CD133+ DP cells by themselves are indeed unable induce hair follicle formation.
The epidermis was restored normally in the wounding area, suggesting CD133+ DP cells did not contribute to the wound healing process. While normal CD133+ DP cells maintained their hair inductivity after 2-weeks in 3D cultures, ΔN-β-catenin-expressing CD133+ DP cells led to increased number of newly formed hair follicles in skin reconstitution assays. We did not observe any indication of abnormal activation of hair follicle stem cells. Therefore, strong proliferation of CD133+ DP cells after grafting induced by ΔN-β-catenin could be the major reason for this increased hair follicle formation. On the other hand, this finding also clearly demonstrated that CD133+ DP cells are involved in early stage hair follicle morphogenesis. It was reported previously that CD133 is expressed in dermal condensate cells during stage 2–5 of murine hair morphogenesis [18, 40]. Our data indicate that involvement of CD133+ DP cells, possibly interacting with epidermal progenitor cells, is a critical step in hair follicle neogenesis. However, further experiments are needed to characterize their exact roles and functions in hair follicle morphogenesis.
A number of studies have shown that Sox2+CD133+ DP cells lead to guard/awl/auchene hair follicles while Sox2-CD133+ DP cells generate zigzag hairs [15, 28]. Since we did not separate sorted CD133+ DP cells based on their Sox2 expression, we, however, did not expect that any specific hair type would preferentially form in reconstituted skin. This expectation was also consistent with reports by Driskell et al. that using CD133+ DP cells led to the formation of different hair types in murine skin reconstitution assay [15]. Furthermore, we evaluated the expression of Sox2 in reconstituted skin and confirmed that not all hair follicles express Sox2 in the DP, indicating that different hair types were formed.
Our observation also showed that growth of newly formed hair follicles was accelerated when ΔN-β-catenin was expressed in CD133+ DP cells. This phenotype was consistent with our in vivo observation that CD133+ DP cells induced increased proliferation in matrix keratinocytes following the expression of ΔN-β-catenin (data not shown). Furthermore, the phenotypes were in agreement with what was reported by Christiano and coworkers that Wnt receptor signaling pathway is crucial for hair-follicle morphogenesis and inductive potential in the papilla cells [38]. Direct or indirect signaling interactions between CD133+ DP cells and hair matrix cells may account for accelerated proliferation and differentiation in both the epidermal and DP compartments.
In conclusion, there was a clear correlation between up-regulation of β-catenin signaling in CD133+ DP cells, maintenance of hair inductivity while culturing in vitro, and enhanced in vivo hair formation. While our studies have been accomplished using genetically engineered mice, the findings could be valuable for future works that aim to induce hair follicle neogenesis and regeneration.
MATERIAL AND METHODS
Mice
CD133-CreERT2 (Prom1C-L) mice were generated as described previously [17]. Rosa-rtTA (Jax 005670) and immunodeficient nude (Jax 002019) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). TetO-Ctnnb1ΔN transgenic mice were generated in Dr. Sarah Millar’s laboratory. For the generation of tetO-Ctnnb1ΔN transgenic mice, a 2.1-kb gene fragment encoding an N-terminal truncated (deleting the first 89 amino acids) mouse β-catenin plus Kozak sequence was amplified by reverse transcription polymerase chain reaction (RT-PCR) from RNA extracted from an embryonic day 14.5 (E14.5) mouse embryo and cloned into the unique BamHI and XbaI sites of a pTLE-Tight vector. The transgene was linearized by digestion with XhoI and injected into fertilized mouse eggs for transgenic founder production. Three mouse lines were generated and all animals were of normal size and did not exhibit any skin phenotypes. All mice were housed in the Laboratory Animal Services Facility of University of Cincinnati under an artificial 12/12 light-dark cycle and were allowed free access to normal mouse feedings and water. The Institutional Animal Care and Use Committee of the University of Cincinnati approved all experimental procedures involving mice.
tetO-Ctnnb1ΔN was crossed with CD133-CreERT2 mice and Rosa-rtTA mice for several generations to generate CD133-CreERT2; Rosa-rtTA; tetO-Ctnnb1ΔN triple transgenic mice. Mice were genotyped by PCR analysis of genomic DNA extracted from tail biopsies. The presence of CreERT2 transgene in CD133 locus was genotyped using forward primer: CAGGCTGTTAGCTTGGGTTC and reverse primer 1: AGGCAAATTTTGGTGTACGG. CD133 wild-type allele was genotyped using forward primer with reverse primer 2: TAGCGTGGTCATGAAGCAAC. Rosa-rtTA was genotyped by PCR using forward primer: AAGTTCATCTGCACCACCG and reverse primer: TCCTTGAAGAAGATGGTGCG. ΔN-β-catenin transgene was genotyped by PCR using forward primer: CCTTGTATCACCATGGACCCTCAT and reverse primer: TAGTGGGATGAGCAGCGTCAAACT. Standard PCR cycle protocol was used.
Isolation of CD133+ DP cells
Isolation and sorting of CD133+ DP cells were performed as previously described with modification [14]. Briefly, adult mice were placed on chow containing 6g/kg doxycycline (Bio-Serv, Laurel, MD) at postnatal day 50 (P50). To induce Cre activity, tamoxifen (TAM) (Sigma-Aldrich, St. Louis, MO) in corn oil (10 mg/ml) was simultaneously administered to mice by intraperitoneal injection at 1mg/g body weight once a day until P56. All mice were depilated at P52 to induce a synchronized hair cycle. Skin from plucked areas was harvested for CD133+ DP cell isolation at P58. Collected skin pieces were floated in 0.1% dispase (Thermo Fisher, Waltham, MA) at 37_°C for 2 hours to separate epidermis and dermis. The entire epidermis was then discarded, and the dermis was treated with 0.5% collagenase IV (Thermo Fisher, Waltham, MA). Dissociated dermal cells were collected by centrifugation and followed by resuspension in 100 μl of culture medium and incubation with APC-conjugated-anti-CD133 antibodies (eBioscience, San Diego, CA, 1:50) for 30 min at 4°C. Cell sorting was performed using a MoFlo high-speed sorter (Dako Cytomation, Carpinteria, CA).
DP Cell culture
Sorted CD133+ cells were seeded in AmnioMAX™ C-100 Medium (Thermo Fisher, Waltham, MA) in 6-well culture plates, which were pre-coated with collagen I, for expansion. Cells were incubated at 37_°C with 5% CO2, and the medium was changed every 2 days. After one passage in 2D culture, cells were typsinized, collected and then encapsulated at a density of 106 cells/ml in Extracel hydrogel in wells of 24-well plate (Glycosan Biosystems, Salt Lake City, UT) according to the manufacturer's instructions. Evaluation of AP activity was detected using VECTOR Red Alkaline Phosphatase Substrate Kit (Vector lab, Burlingame, CA). Pictures of spheroids in hydrogel in 24-well plate were taken under a bright-field microscope at 10x magnification. At least 5 random but representative fields from each well were selected, and the number of AP-positive spheroids was manually counted for each field. The measurement and analysis of spheroids size was performed using ImageJ program.
To retrieve cells, hydrogels were incubated for 2 hours at 37 °C in 1× collagenase/hyaluronidase (StemCell Technologies, Vancouver, Canada) in AmnioMax basal medium without adding supplement solution. Cultured spheroids were recovered by centrifuging at 500 × g for 5 minutes. Then, spheroids were treated with 0.25% Trypsin-EDTA at 37 °C and pipetted up and down every 5 min to generate single DP cell suspension. After by adding 10% FBS to stop the trypsin activity, CD133+ DP cells were collected by centrifuging at 1000 × g for 5 minutes and resuspended in AmnioMax medium. Cell number was counted using a hemocytometer.
Quantitative real-time PCR
Total RNA was isolated from cells and spheroids using the RNeasy Micro Kit from Qiagen (Valencia, CA), and reverse-transcribed to complementary DNA (cDNA) using the Superscript III kit (Invitrogen, Carlsbad, CA). qPCR reactions were performed using the Power SYBR green dye in the StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA). qPCR primers for β-catenin (ctnnb1), Sox2, CD133, Corin, Alpl, Itga8, Bmp6, Wnt5a, Alx3, Alx4, SA1004 and Fgf10 were purchased from Real Time Primers, LLC (Elkins Park, PA). All qPCR data were normalized to GAPDH expression.
Skin reconstitution assay
Skin grafting was performed according to published procedures [41] . For each mouse, a mix of 2 × 106 epidermal cells, 5 × 106 freshly isolated wild-type dermal cells, and 5 × 103 cultured CD133+ DP cells released from hydrogels was used for reconstitution assay. Cultured CD133+ DP cells were disaggregated from DP spheroids in hydrogel using trypsin/EDTA. To get freshly isolated epidermal and dermal cells, the trunk skin of P2 neonatal mice was dissected and epidermis and dermis were separated by floating the skin in 0.1% dispase at 37_°C for 2 hours. Subsequently, epidermis was dissociated into a cell suspension by cutting into fine pieces and digested in 0.25% trypsin-EDTA for 15 minutes. Dermis was dissociated in warm 0.25% collagenase IV solution (Thermo Fisher, Waltham, MA) for 30 minutes at 37_°C with manual stirring every 15 minutes using a serological pipette. Collagenase and trypsin activities were stopped by washing cells in medium containing a 10% fetal bovine serum (FBS). Prepared cells were filtered through a 70 mm cell strainer followed with a 40mm cell strainer to ensure single cell suspension and exclude as many tissue clumps as possible.
Freshly isolated epidermal cells, dermal cells and CD133+ DP cells were mixed in 150 μl medium and seeded onto the undersurface of a dry collagen matrix for 30 minutes, which was produced in Dr. Steven Boyce’s laboratory. The collagen matrix with cells were then flipped onto a 1.5 X 1.5 cm full-thickness wound on the back of nude mice and sutured. Sterile dressings were applied to provide constant pressure to the graft so that it would adhere to the wound bed. Dressings were removed for inspection days 5 post-grafting.
Histology and immunohistochemistry
Reconstituted skin biopsies were prepared for histological analysis, BrdU incorporation assays, TUNEL assays and immunostaining according to published protocols [42]. Paraffin sections were deparaffinized, rehydrated and then demasked in citrate buffer (pH6.0) using the microwave heating method. Frozen sections were fixed with −20°C acetone for 10 minutes before immunostaining. After washing with phosphate-buffered saline (PBS), sections were blocked in 10% bovine serum albumin (BSA) in PBS, and subsequently incubated at 4°C overnight with each primary antibody. Next, the slides were washed again with PBS for three times, incubated with the corresponding biotin-conjugated secondary antibodies (Vector lab, Burlingame, CA) at room temperature for 1 h, and followed by incubating with either fluorochrome-labeled Streptavidin for immunofluorescence or VECTASTAIN Elite ABC Reagents (Vector lab) for immunohistochemistry. The slides were examined and images were taken using a Nikon Eclipse 80i fluorescence microscope.
The following primary antibodies were used: anti-CD133 (eBioscience, 1:50), anti-β-catenin (Invitrogen, 15B8, 1:1000), anti-BrdU (Abcam, BU1/75, 1:25), anti-Lef1 (Cell signaling, 1:100), anti-K14 (Covance, 1:1000), anti-K10 (Biolegend, 1:1000), anti-K15 (Vector lab, 1:50), anti-Versican (Millipore, 1:200), anti-AE13 (1:25), anti-AE15 (1:25), anti-Sox9 (Millipore, 1:100), and anti-Gata3 (Santa Cruz Biotechnology, 1:50). AE13 and AE15 antibodies were kind gifts from Dr. Tung-Tien Sun (New York University Medical School, New York, NY).
Statistical analysis
Data were analyzed using a GraphPad Prism 5.01 software package (GraphPad Software Inc., San Diego, CA, USA) and expressed as the mean ± SEM. Statistical analysis of difference was carried out by Student’s t-test. All graphs were generated using Microsoft Excel. Differences are considered significant at p < 0.05.
Acknowledgments
We thank Dr. Richard J. Gilbertson and the St Jude Children’s Research Hospital f for CD133-CreERT2 (Prom1C-L) mice. This work was supported by NIAMS R03 AR062788-01 (Y.Z.) and Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number UL1RR026314.
Abbreviation
- DP
dermal papilla
- AP (Alpl)
alkaline phosphatase
- HFSCs
hair follicle stem cells
- rtTA
reverse tetracycline-controlled transactivator
- FACS
fluorescence-activated cell sorting
- qPCR
quantitative polymerase chain reaction
- 3D
three-dimensional
- Itga8
α8 Integrin
- Dox
doxycycline
- DS
dermal sheath
Footnotes
The authors have no conflict of interest to declare
AUTHOR CONTRIBUTIONS
| Zhou, Linli: | performed experiments |
| Yang, Kun: | performed experiments |
| Xu, Mingang: | performed experiments |
| Andl, Thomas: | analysed data; wrote the paper |
| Millar, Sarah: | contributed reagents or other essential material; wrote the paper |
| Boyce, Steven: | contributed reagents or other essential material |
| Zhang, Yuhang: | Planned experiments; analysed data; wrote the paper |
References
- 1.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23:917–27. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 3.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 4.Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. The Transplantation of Individual Rat and Guinea-pig Whisker Papillae. Journal of Embryology and Experimental Morphology. 1961;9:117–127. [PubMed] [Google Scholar]
- 7.Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere Formation Increases the Ability of Cultured Human Dermal Papilla Cells to Induce Hair Follicles from Mouse Epidermal Cells in a Reconstitution Assay. J Invest Dermatol. 2012;132:237–239. doi: 10.1038/jid.2011.250. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes & development. 2000;14:1181–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes & development. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenn K, Parimoo S, Zheng Y, Barrows T, Boucher M, Washenik K. Bioengineering the hair follicle. Organogenesis. 2007;3:6–13. doi: 10.4161/org.3.1.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–10. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 12.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol. 1995;105:426–31. doi: 10.1111/1523-1747.ep12321131. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of Murine Hair-Inducing Cells Using the Cell Surface Marker Prominin-1//CD133. J Invest Dermatol. 2006;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 15.Driskell RR, Juneja VR, Connelly JT, Kretzschmar K, Tan DWM, Watt FM. Clonal Growth of Dermal Papilla Cells in Hydrogels Reveals Intrinsic Differences between Sox2-Positive and -Negative Cells In Vitro and In Vivo. J Invest Dermatol. 2012;132:1084–1093. doi: 10.1038/jid.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Developmental Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–608. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushal GS, Rognoni E, Lichtenberger BM, Driskell RR, Kretzschmar K, Hoste E, Watt FM. Fate of Prominin-1 Expressing Dermal Papilla Cells during Homeostasis, Wound Healing and Wnt Activation. J Invest Dermatol. 2015;135:2926–34. doi: 10.1038/jid.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Research. 2005;33:e51–e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Development, Growth & Differentiation. 2007;49:185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 21.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roop DR, Hawley-Nelson P, Cheng CK, Yuspa SH. Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc Natl Acad Sci U S A. 1983;80:716–20. doi: 10.1073/pnas.80.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–8. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 24.Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Current biology : CB. 2005;15:1340–51. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman CK, Zhou P, Amalia Pasolli H, Rendl M, Bolotin D, Lim K-C, Dai X, Alegre M-L, Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes & development. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch MH, O'Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. The Journal of Cell Biology. 1986;103:2593–2606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes & development. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borena BM, Martens A, Broeckx SY, Meyer E, Chiers K, Duchateau L, Spaas JH. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell Physiol Biochem. 2015;36:1–23. doi: 10.1159/000374049. [DOI] [PubMed] [Google Scholar]
- 31.Yannas IV, Orgill DP, Burke JF. Template for Skin Regeneration. Plastic and Reconstructive Surgery. 2011;127:60S–70S. doi: 10.1097/PRS.0b013e318200a44d. [DOI] [PubMed] [Google Scholar]
- 32.Nagase T, Kumagai N. Treatment of burns by grafting of cultured epithelium. Nippon Geka Gakkai Zasshi. 2005;106:750–754. [PubMed] [Google Scholar]
- 33.Wainwright DJ. Burn Reconstruction: the Problems, the Techniques, and the Applications. Clinics in Plastic Surgery. 2009;36:687–700. doi: 10.1016/j.cps.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Morgan BA. Wnt Signaling through the [beta]-Catenin Pathway Is Sufficient to Maintain, but Not Restore, Anagen-Phase Characteristics of Dermal Papilla Cells. J Investig Dermatol. 2004;122:239–245. doi: 10.1046/j.0022-202X.2004.22224.x. [DOI] [PubMed] [Google Scholar]
- 36.Collins CA, Jensen KB, MacRae EJ, Mansfield W, Watt FM. Polyclonal origin and hair induction ability of dermal papillae in neonatal and adult mouse back skin. Dev Biol. 2012;366:290–7. doi: 10.1016/j.ydbio.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gangatirkar P, Paquet-Fifield S, Li A, Rossi R, Kaur P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protocols. 2007;2:178–186. doi: 10.1038/nprot.2006.448. [DOI] [PubMed] [Google Scholar]
- 38.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A. 2013;110:19679–88. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–49. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- 40.Gay DL, Yang CC, Plikus MV, Ito M, Rivera C, Treffeisen E, Doherty L, Spata M, Millar SE, Cotsarelis G. CD133 expression correlates with membrane beta-catenin and E-cadherin loss from human hair follicle placodes during morphogenesis. J Invest Dermatol. 2015;135:45–55. doi: 10.1038/jid.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee LF, Jiang TX, Garner W, Chuong C-M. A Simplified Procedure to Reconstitute Hair-Producing Skin. Tissue Engineering Part C: Methods. 2011;17:391–400. doi: 10.1089/ten.tec.2010.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, Morrisey EE, Overbeek PA, Scheidereit C, Millar SE, Schmidt-Ullrich R. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]