Abstract
Protein domains and peptide sequences are a powerful tool for conferring specific functions to engineered biomaterials. Protein sequences with a wide variety of functionalities, including structure, bioactivity, protein-protein interactions, and stimuli responsiveness, have been identified, and advances in molecular biology continue to pinpoint new sequences. Protein domains can be combined to make recombinant proteins with multiple functionalities. The high fidelity of the protein translation machinery results in exquisite control over the sequence of recombinant proteins and the resulting properties of protein-based materials. In this review, we discuss protein domains and peptide sequences in the context of functional protein-based materials, composite materials, and their biological applications.
Graphical Abstract
Introduction
Studies that require biomaterials with well-controlled physical and biological properties have increased dramatically since the concept of tissue engineering was first proposed. Cellular activities are often composite responses to multiple environmental stimuli [1,2]. To study how individual stimuli affect cellular response, precise control over the microenvironment is critical. However, this control is often very difficult to achieve in vivo. On the other hand, a defined in vitro microenvironment could be easily created from biomaterials with controlled properties. Efforts have been made to create such biomaterials with synthetic polymers, including poly(lactic-co-glycolic acid) [3] and poly(ethylene glycol) (PEG) [4], or with natural polymers, such as polysaccharides [5]. In this review, we focus on biomaterials utilizing protein domains or peptide sequences in recombinant proteins or composite materials.
Progress in molecular biology has enabled the facile production of recombinant proteins. Furthermore, maturation in large-scale production techniques makes protein-based materials more economically feasible. New techniques such as incorporation of non-canonical amino acids further expand the possibilities of protein-based biomaterials.
A recombinant protein is designed at the DNA level, and DNA sequences encoding different protein domains can be assembled in the desired order with a specified number of repeats. This modularity in recombinant protein design enables the production of recombinant protein-based materials with diverse properties. The translation machinery also has high fidelity so that the desired recombinant protein will have the specified amino acid sequence. On the other hand, synthetic polymers or proteins harvested from nature often have dispersity in chain length or composition. Thus, the high fidelity in recombinant protein production promises precise control over material properties.
In general, a recombinant protein designed for tissue engineering applications is composed of modular domains that confer specific functions. For example, structural domains provide mechanical properties, and biological domains facilitate the interactions between cells and the materials. In addition, domains can be used that respond to environmental stimuli or enable spatiotemporal control over material properties. In this review, we focus on domains that are being widely used in protein-based materials or are being incorporated into composite materials for added functionality.
Structural domains
Recombinant proteins used as scaffold materials in tissue engineering serve as a temporary matrix before the desired tissue is regenerated. Thus, recombinant proteins should have appropriate structural domains that provide mechanical support and a microenvironment that supports cell proliferation and differentiation. An ideal structural domain should provide appropriate mechanical properties that match those of the surrounding tissues and should not trigger inflammation or adverse immune responses.
Elastin-like polypeptides
Elastin-like polypeptides (ELPs) are based on sequences derived from native elastin and are being actively studied. Elastin is the major component that provides elasticity to the extracellular matrix (ECM). ELPs are able to mimic the mechanical properties of native elastin and are mostly composed of a repeating amino acid sequence (VPGXG)n, where X is a guest residue consisting of any amino acid except proline [6].
The flexibility in choosing the guest residue expands the functionality of ELPs as a structural domain. One example is the use of lysine as a guest residue. This choice allows crosslinking through the primary amine side chain, and a range of mechanical moduli can be achieved with different degrees of crosslinking [7]. Another example is the use of cysteine as a guest residue to facilitate surface immobilization of ELPs or crosslinking of free ELPs [8].
ELPs are also thermo-responsive, and this feature can be modulated using the guest residues. ELPs exhibit lower critical solution temperature (LCST) behavior; they are soluble below the LCST and form a coacervate, or a dense polymer-rich liquid phase, above the LCST. The Rodríguez-Cabello group has utilized the LCST behavior of ELPs to facilitate hydrogel formation of ELP-fusion proteins [9,10]. In addition, spherical structures of ELPs have been triggered by the thermo-responsive behavior [11]. Structures formed by ELPs include hollow spheres or spheres with a dense core, and their size distributions can be controlled by salt concentration [12] or by the number of repeating units [13]. ELP spheres have been used as templates for nanoparticle synthesis [14] and have been applied to other applications, such as drug delivery vehicles [15].
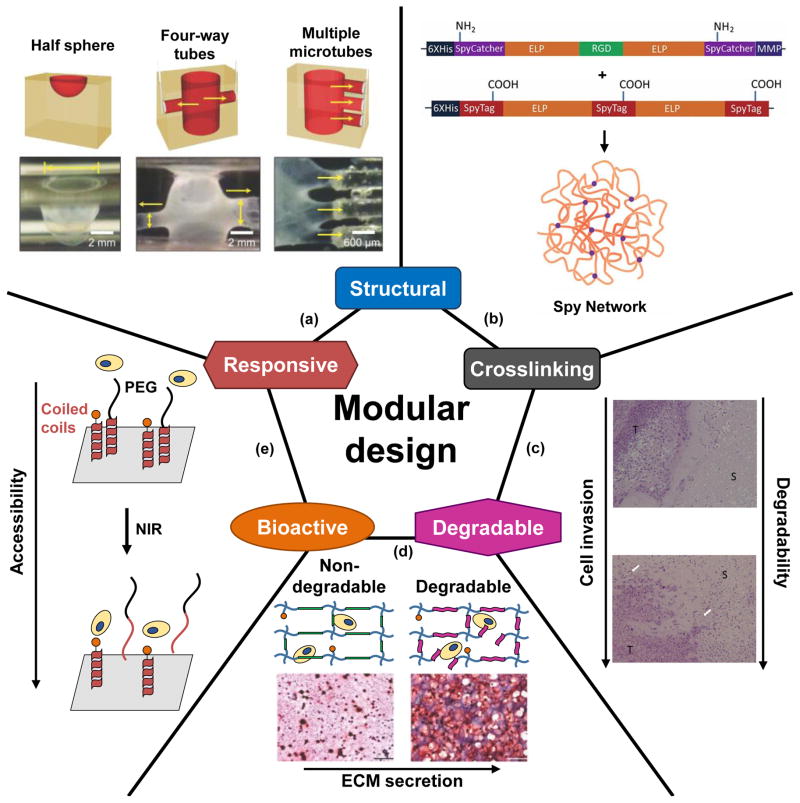
A recent study expanded the versatility of ELP-based materials by mixing them with peptide amphiphiles (PAs) to form self-assembled structures [16]. ELPs mixed with PAs formed multilayer membrane architectures with each layer containing both components. The structure changed when the membrane interface was in contact with other surfaces. The authors demonstrated that a complex multi-way tube structure could be formed from a relatively simple one-way tube (Figure 1a).
Figure 1.
Modular protein domains confer functionality. Five commonly-used functional domains are given as examples. (a) A multilayer structure composed of elastin-like polypeptides (ELPs) and peptide amphiphiles (PAs) can respond to contact with surfaces by changing its geometry. With this responsiveness, complex structures such as interconnected tubes can be fabricated. Reprinted by permission from Macmillan Publishers Ltd: Nature Chemistry (Inostroza-Brito KE, Collin E, Siton-Mendelson O, Smith KH, Monge-Marcet A, Ferreira DS, Rodríguez RP, Alonso M, Rodríguez-Cabello JC, Reis RL et al.: Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system. Nat Chem 2015, 7:897–904.), copyright (2015). (b) The crosslinking domains SpyTag and SpyCatcher were incorporated with ELP domains to form a hydrogel. SpyTag and SpyCatcher can form an isopeptide bond between each other and can therefore form a crosslinked network. Reproduced with permission from Sun F, Zhang WB, Mahdavi A, Arnold FH, Tirrell DA: Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry. Proc Natl Acad Sci U S A 2014, 111:11269–11274. © National Academy of Sciences. (c) Matrix metalloproteinase (MMP)-sensitive sequences were incorporated into recombinant protein-based hydrogels. When the hydrogels were implanted into tumor-bearing mice, a higher cell invasion rate was observed with MMP-sensitive hydrogels compared to MMP-insensitive hydrogels as shown by the histology results. S: protein hydrogel, T: tumor tissue. White arrows indicate vascular infiltration into the hydrogel. Reprinted from J Control Release, 213, Price R, Poursaid A, Cappello J, Ghandehari H, In vivo evaluation of matrix metalloproteinase responsive silk-elastinlike protein polymers for cancer gene therapy, 96–102, copyright (2015), with permission from Elsevier. (d) MMP-sensitive peptides were incorporated into poly(ethylene glycol) (PEG) hydrogels to introduce degradability into the system. When human mesenchymal stem cells (hMSCs) and chondrocytes were co-cultured in these PEG hydrogels, higher levels of extracellular matrix (ECM) secretion were observed with the degradable hydrogels as shown in the histology results where glycosaminoglycans (GAGs) are stained in red and nuclei are stained in black. Adapted with permission from Sridhar BV, Brock JL, Silver JS, Leight JL, Randolph MA, Anseth KS: Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv Healthc Mater 2015, 4:702–713. © John Wiley and Sons. (e) Temperature-sensitive coiled coils served as responsive domains to change the accessibility of the RGD cell-binding domain. The RGD sequence was attached to one of the coils, and a PEG chain was conjugated to the other coil. At low temperatures, the coiled coils remained in the native structure, and the bioactive domains were inaccessible to the cells because of the PEG chains. When the temperature was raised by near infrared (NIR) radiation of gold nanorods, the coiled coils denatured, and the coils with PEG were released from the RGD sequence, which became accessible to cells. Adapted from Yang J, Yao M-H, Du M-S, Jin R-M, Zhao D-H, Ma J, Ma Z-Y, Zhao Y-D, Liu B: A near-infrared light-controlled system for reversible presentation of bioactive ligands using polypeptide-engineered functionalized gold nanorods. Chem. Commun. 2015, 51:2569–2572 with permission of The Royal Society of Chemistry (RSC) on behalf of the Centre National de la Recherche Scientifique (CNRS) and the RSC.
Resilin-like polypeptides
Resilin, which is found in insect cuticle, has drawn great interest with its high elasticity, high resilience, and heat stability [17]. The Elvin group first developed resilin-like polypeptides (RLPs) based on sequences from Drosophila melanogaster (Dros16, (GGRPSDSYGAPGGGN)n) and Anopheles gambiae (An16, (AQTPSSQYGAP)n), and both RLPs possess mechanical properties and heat stability that are similar to those of native resilin [17]. Unlike ELPs, there are no guest residues in RLP sequences; however, RLP amino acid sequences in which lysine residues have been inserted to serve as a crosslinking site still retain resilin-like characteristics [18,19].
Photochemical crosslinking through tyrosine residues in RLPs can be mediated through Ru(II), and crosslinked RLPs have been explored for various applications. For example, Lv et al. reported a recombinant protein based on Dros16 and an RGD-containing domain from tenascin-C [20]. Crosslinked hydrogels of this material had tunable mechanical properties controlled by protein concentration and facilitated spreading of human lung fibroblasts. Another example is the use of photocrosslinked RLPs to modify tissue culture polystyrene surfaces [21]. High coating concentrations prevented fibroblast attachment and spreading, but cell attachment could be restored by incorporating an RGD peptide in the coating.
Outside of the field of tissue engineering, RLPs have been applied to green synthesis of fluorescent gold nanoclusters. Specifically, RLPs served as reducing agents during synthesis and as stabilizers after particle formation [22]. Another example is the development of biocomposite adhesives with enhanced mechanical properties. In particular, RLPs were used to directly introduce nano-crystalline cellulose into epoxy resins [23].
Crosslinking strategies
Chemical crosslinking
A variety of crosslinking strategies have been developed for crosslinking structural domains. A straightforward approach is to include amino acids with reactive side chains in the structural domains. Crosslinking can thus be achieved by reagents that react with those side chains. Examples include crosslinking lysine with N-hydroxysuccinimide (NHS) esters or tris(hydroxymethyl)phosphine (THP) and cysteine with maleimide. Tyrosine is photochemically reactive in the presence of a Ru(II) catalyst. Enzyme-facilitated bond formation has also been utilized for crosslinking. Examples of these enzymes include lysyl oxidase, peroxidase, and transglutaminase [24].
The previous strategies require additional reagents for crosslinking; however, protein domains that form covalent bonds between two protein chains have been developed. The N-terminal and C-terminal domains of a self-splicing intein from Nostoc punctiforme have been utilized to facilitate protein hydrogel formation[25]. Also, the SpyTag and SpyCatcher pair, which is derived from Gram-positive bacterial adhesins, forms an isopeptide bond between specific asparagine and lysine residues. The SpyTag and SpyCatcher domains have been used to form crosslinks in ELP hydrogels (Figure 1b), and the resulting crosslinked network can be controlled by the location of the SpyTag and SpyCatcher domains within ELP backbones [26,27].
Physical crosslinking
Domains with strong protein-protein interactions can serve as physical crosslinking sites. For example, the Heilshorn group has developed a two-component system that forms a gel due to the physical crosslinking of WW and proline-rich peptide domains [28,29]. By using WW domains with varying dissociation constants (Kd), different gelation behaviors and mechanical properties can be achieved. Another example of physical crosslinking is the use of leucine zippers, which are α-helices that form coiled-coil domains. In work by Huang et al., physical crosslinking of leucine zippers was further stabilized by disulfide bond formation through incorporated cysteine residues [30].
Bioactive domains
One advantage of modular recombinant proteins is that bioactive cues can be directly incorporated among the structural domains at the desired location and density. In tissue engineering, the following issues have been addressed by the strategic fusion of bioactive domains: cell-material interactions, cell fate determination, and material response to cellular activities.
Cell-binding domains
Cell attachment to the material is often the first consideration when designing biomaterials. Many cell-adhesive domains are derived from ECM proteins such as fibronectin, collagen, and laminin. The RGD sequence is a classic cell-binding domain that is derived from the 10th domain of fibronectin type III (FNIII). The RGD sequence is often presented with the PHSRN synergy site, which is derived from the 9th domain of FNIII, to increase cell adhesion and target the α5β1 integrin. The CS5 sequence, with the minimum sequence of REDV, is another cell-binding domain derived from FNIII. Hsueh and coworkers reported that ELP proteins containing the CS5 domain supported murine Schwann cell proliferation [31].
Another set of cell-binding domains is derived from laminins, which are heterotrimeric proteins found in the basal lamina. The PPFLMLLKGSTR sequence is derived from the laminin-5 α3 chain globular domain 3 (LG3). Including this domain within an ELP facilitated human keratinocyte attachment [32]. This work also examined keratinocyte attachment with ELPs containing two other binding domains: fibronectin domains containing the RGD and PHSRN sequences and the GEFYFYDLRLKGDK sequence derived from the α1 chain of collagen type IV. Keratinocytes attached more quickly to proteins with the laminin or fibronectin domains compared to the collagen domain. The authors found that keratinocyte attachment to the laminin and fibronectin domains could be reduced with antibodies against the α3 and α5 integrin subunits, respectively, and thus concluded that the keratinocytes were likely utilizing the α3βI and α5βI integrins, respectively, to adhere to those proteins.
Many other cell-binding domains have been reported (e.g., DGEA from collagen type I and IKVAV and YIGSR from laminin) and are described in a review [33]. However, new cell-binding domains are still being identified. Lee and coworkers recently showed that the C-terminal RKRK sequence cannot completely account for cell attachment to human tropoelastin and suggested that there is a new binding domain in domains 17 and 18 of tropoelastin [34].
Domains derived from growth factors
Promoting stem cell differentiation into the desired cell lineage and preventing committed cells from de-differentiating are critical to the success of tissue engineering. Because growth factors play an important role in morphogenesis, it is logical that many of the bioactive domains that are capable of determining cell commitment are derived from the corresponding growth factors.
Bone morphogenetic proteins (BMPs) are a family of growth factors that are involved in the development of different tissues, but they are best known for their roles in bone morphogenesis. Recombinant human BMP-2 and -7 are approved by the Food and Drug Administration (FDA) for clinical applications. The KIPKASSVPTELSAISTLYL peptide is derived from the knuckle epitope of human BMP-2 and has been widely used as a bioactive domain to promote osteogenesis. Kim et al. recently showed that the BMP-2 peptide retained its bioactivity when fused in an RLP backbone and that the fusion protein enhanced osteogenic differentiation [35]. The peptide has also been reported to promote chondrogenesis of human mesenchymal stem cells (hMSCs) in pellet culture [36].
Three peptides have been derived from human BMP-7: SNVILKKYRN, KPSSAPTQLN, and KAISVLYFDDS. In a recent work by Tao and coworkers, these three peptides were incorporated into self-assembling peptides and promoted ECM secretion by human degenerated nucleus pulposus cells [37]. These results demonstrate the potential that these peptides have for intervertebral disc regeneration, which is a major clinical application of recombinant human BMP-7. It is expected that these BMP-7 derived peptides could be easily incorporated into recombinant protein-based materials through the modular design approach.
Growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) play an important role in blood vessel formation in regenerating tissue. The KLTWQELYQLKYKGI sequence (named QK) is based on VEGF. The QK peptide retained its bioactivity and promoted endothelial cell behavior when crosslinked to a protein backbone [38] or presented as a fusion protein [39]. Besides its well-known effects on angiogenesis, VEGF also has protective effects on neuronal cells. Verheyen and coworkers showed that the QK peptide also displayed this function and protected neurons from paclitaxel toxicity and hyperglycemic stress [40].
The previous sections focus on the use of shorter bioactive peptides; however, entire growth factors can also be integrated into fusion proteins without loss of their biological functions. Sun et al. used the SpyTag-SpyCatcher system to fuse chimeric leukemia inhibitory factor (MH35-LIF), which suppresses embryonic stem cell differentiation, to a crosslinked protein hydrogel [27]. Mouse embryonic stem cells cultured in these hydrogels remained pluripotent without any additional LIF supplements. It is noteworthy that, although LIF is expressed as a glycoprotein in mammals, the recombinant version expressed by bacteria remains bioactive. As progress in molecular biology and protein engineering continues, it is anticipated that more growth factor sequences will be identified that can be harnessed as bioactive domains in recombinant protein design.
Degradation domains
Biomaterial degradation is an important factor in tissue engineering. Ideally, degradation should synchronize with cellular regeneration so that there will be room for newly formed tissue. Peptide sequences that are sensitive to proteases can be used as degradation domains in recombinant protein-based materials. For example, ELPs are usually used as structural domains; however, they can also serve as degradation domains due to their sensitivity to elastase [41].
Degradation domains can also be explicitly incorporated into modular designs. Popular choices are matrix metalloproteinase (MMP)-sensitive sequences. MMPs are a family of endopeptidases that can degrade ECM proteins including collagen, elastin, fibronectin, and laminin. MMP-sensitive sequences have been widely used in applications requiring material degradation. For example, Price and coworkers incorporated an MMP-sensitive sequence into a silk-elastin-like protein hydrogel for viral-mediated gene delivery for cancer treatment [42]. When implanted into mice, MMP-sensitive hydrogels had higher cell invasion compared to MMP-insensitive hydrogels (Figure 1c). Tumor-bearing mice treated with MMP-sensitive hydrogels had the highest survival rate.
MMPs also play important roles in many physiological events such as morphogenesis and tissue remodeling. For example, Fonseca and coworkers profiled the in vitro gene expression of hMSCs grown in basal or osteogenic medium and found that in osteogenic medium there was an increase in MMP-14 gene expression levels and alkaline phosphatase (ALP) activity at one week [43]. Thus, it is anticipated that by selecting specific MMP-sensitive sequences it is possible to design materials that are selectively degraded by desired cells at specific stages of differentiation. For example, Sridhar et al. used an MMP-sensitive sequence, KCGPQGIWGQCK, as a degradable crosslinker for PEG hydrogels. Cells encapsulated in the degradable gels showed higher glycosaminoglycan (GAG) and collagen deposition compared to those in non-degradable hydrogels (Figure 1d) [44]. In general, MMPs share common features in their cleavage sites. A recent study by Kukreja and coworkers analyzed target sequences of 18 MMPs and identified information for predicting and designing MMP-cleavable sequences [45]. Overall, understanding the specificities of MMPs and identifying their cleavage sequences will expand the possibilities of using degradation domains for targeted applications.
Domains for higher-order control over material properties
In an ideal functional biomaterial, the material properties are precisely controlled, and the material is responsive to dynamic cellular activities and changes in environmental conditions. Therefore, domains that enable material responsiveness and spatial or temporal control over material properties have been explored. The Davies group utilized two MMP sequences with different enzyme specificities (one sequence is recognized by many MMPs whereas the other sequence is recognized by a specific MMP) and successfully modulated the in vitro invasion rates of fibroblasts and vascular smooth muscle cells into PEG hydrogels [46]. They also demonstrated control over in vivo cellular invasion by making hydrogels with a mixture of MMP-sensitive sequences [47].
Leucine zippers have been utilized to control ligand accessibility and density. The stability and oligomerization states of leucine zippers can be easily tuned by changing their amino acid sequences. A heterodimeric leucine zipper pair was used to reversibly enable access to an RGD cell-binding domain. Exposure of gold nanorods to near-infrared (NIR) light resulted in a photothermal effect, which effectively denatured the leucine zippers and enabled access to the RGD sequence (Figure 1e) [48]. Leucine zippers have also been utilized to increase ligand avidity. For example, a trimeric leucine zipper, cartilage matrix protein (CMP), was recently used to present a ligand for epidermal growth factor receptor (EGFR). The EGFR ligand was recombinantly fused to a CMP domain, and the fusion proteins oligomerized to form trimers through the CMP domain. A monomeric control was constructed by fusing the EGFR ligand to a mutated CMP domain, which was unable to oligomerize to form a trimer. When the ligand was presented as a CMP-facilitated trimer, it demonstrated enhanced binding strength compared to the monomeric ligand [49].
Conditional-splicing inteins are an intriguing domain in the recombinant protein toolbox. Unlike normal inteins, conditional-splicing inteins only splice after an environmental stimulus. Informative reviews of recent progress on inteins are available [50,51]. Recently, photoactivatable inteins have been achieved by incorporating non-canonical, photoactive amino acids. For example, both cysteine and serine residues in inteins have been modified with a photocage, and these photoactivatable inteins are promising protein-labeling tools with exquisite spatiotemporal control that can be directly used in live mammalian cells [52,53]. Inteins with photoactive tyrosines have also been used as a tool for making cyclic peptides [54].
Conclusions
Biomaterials with sophisticated control over properties are increasingly needed to address questions regarding cellular behavior for tissue engineering applications. These materials need to meet demanding requirements for specific mechanical properties, macromolecular structure, cell-material interactions, and responsiveness towards environmental changes. Protein domains and peptide sequences provide the desired functionality to recombinant proteins or to composite materials. In particular, recombinant proteins are promising materials because their modularity enables the facile combination of domains that confer the desired structure, bioactivity, and functionality. As we continue to unlock the sequence-structure-function relationship of natural proteins, we can expand the number of domains available as part of our recombinant protein toolbox. Thus, protein domains can be used to design materials that can address a larger variety of questions not only in the field of tissue engineering but also in stem cell and developmental biology, pharmaceutical engineering, and clinical practice.
Highlights.
Protein domains can be designed for specific functions.
Domain functionalities include structure, bioactivity, and stimuli responsiveness.
Recombinant proteins are modular and can be designed by combining functional domains.
Protein domains can be added to composite materials for added functionality.
Acknowledgments
This work was supported by NIH (NIDCR R03DE021755) and the American Heart Association Scientist Development Grant (12SDG8980014).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyburz KA, Anseth KS. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann Biomed Eng. 2015;43:489–500. doi: 10.1007/s10439-015-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters NJ, Gentleman E. Evolving insights in cell–matrix interactions: Elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 2015;11:3–16. doi: 10.1016/j.actbio.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakaic E, Smeets NM, Hoare T. Injectable hydrogels based on poly (ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015;5:35469–35486. [Google Scholar]
- 5.Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polymer Adv Tech. 2014;25:448–460. [Google Scholar]
- 6.Roberts S, Dzuricky M, Chilkoti A. Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett. 2015;589:2477–2486. doi: 10.1016/j.febslet.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Cai L, Paul A, Enejder A, Heilshorn SC. Hybrid elastin-like polypeptide-polyethylene glycol (ELP-PEG) hydrogels with improved transparency and independent control of matrix mechanics and cell ligand density. Biomacromolecules. 2014;15:3421–3428. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan Y, Koria P. Proliferative activity of elastin-like-peptides depends on charge and phase transition. J Biomed Mater Res A. 2016;104A:697–706. doi: 10.1002/jbm.a.35609. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Colino A, Arias FJ, Alonso M, Rodriguez-Cabello JC. Self-organized ECM-mimetic model based on an amphiphilic multiblock silk-elastin-like corecombinamer with a concomitant dual physical gelation process. Biomacromolecules. 2014;15:3781–3793. doi: 10.1021/bm501051t. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Colino A, Arias FJ, Alonso M, Rodriguez-Cabello JC. Amphiphilic elastin-like block co-recombinamers containing leucine zippers: Cooperative interplay between both domains results in injectable and stable hydrogels. Biomacromolecules. 2015;16:3389–3398. doi: 10.1021/acs.biomac.5b01103. [DOI] [PubMed] [Google Scholar]
- 11.Park WM, Champion JA. Thermally triggered self-assembly of folded proteins into vesicles. J Am Chem Soc. 2014;136:17906–17909. doi: 10.1021/ja5090157. [DOI] [PubMed] [Google Scholar]
- 12.Pinedo-Martín G, Santos M, Testera AM, Alonso M, Rodríguez-Cabello JC. The effect of NaCl on the self-assembly of elastin-like block co-recombinamers: Tuning the size of micelles and vesicles. Polymer. 2014;55:5314–5321. [Google Scholar]
- 13.Hassouneh W, Zhulina EB, Chilkoti A, Rubinstein M. Elastin-like polypeptide diblock copolymers self-assemble into weak micelles. Macromolecules. 2015;48:4183–4195. doi: 10.1021/acs.macromol.5b00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han W, MacEwan SR, Chilkoti A, Lopez GP. Bio-inspired synthesis of hybrid silica nanoparticles templated from elastin-like polypeptide micelles. Nanoscale. 2015;7:12038–12044. doi: 10.1039/c5nr01407g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacEwan SR, Chilkoti A. Applications of elastin-like polypeptides in drug delivery. J Control Release. 2014;190:314–330. doi: 10.1016/j.jconrel.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Inostroza-Brito KE, Collin E, Siton-Mendelson O, Smith KH, Monge-Marcet A, Ferreira DS, Rodríguez RP, Alonso M, Rodríguez-Cabello JC, Reis RL, et al. Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system. Nat Chem. 2015;7:897–904. doi: 10.1038/nchem.2349. A multilayer structure consisting of ELPs and PAs was shown in this work. The structure possessed a dynamic geometry that was capable of elongating, self-healing, and responding to contact with surfaces by changing its geometry. [DOI] [PubMed] [Google Scholar]
- 17.Su RS, Kim Y, Liu JC. Resilin: Protein-based elastomeric biomaterials. Acta Biomater. 2014;10:1601–1611. doi: 10.1016/j.actbio.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Renner JN, Cherry KM, Su RS, Liu JC. Characterization of resilin-based materials for tissue engineering applications. Biomacromolecules. 2012;13:3678–3685. doi: 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Tong Z, Jia X, Kiick KL. Resilin-like polypeptide hydrogels engineered for versatile biological functions. Soft Matter. 2013;9:665–673. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv S, Bu T, Kayser J, Bausch A, Li H. Towards constructing extracellular matrix-mimetic hydrogels: An elastic hydrogel constructed from tandem modular proteins containing tenascin FnIII domains. Acta Biomater. 2013;9:6481–6491. doi: 10.1016/j.actbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Vashi AV, Ramshaw JA, Glattauer V, Elvin CM, Lyons RE, Werkmeister JA. Controlled surface modification of tissue culture polystyrene for selective cell binding using resilin-inspired polypeptides. Biofabrication. 2013;5:035005. doi: 10.1088/1758-5082/5/3/035005. [DOI] [PubMed] [Google Scholar]
- 22.Balu R, Bourgeois L, Elvin CM, Hill AJ, Choudhury NR, Dutta NK. A multi-responsive intrinsically disordered protein (IDP)-directed green synthesis of fluorescent gold nanoclusters. J Mater Chem B. 2015;3:6580–6586. doi: 10.1039/c5tb00659g. [DOI] [PubMed] [Google Scholar]
- 23.Verker R, Rivkin A, Zilberman G, Shoseyov O. Insertion of nano-crystalline cellulose into epoxy resin via resilin to construct a novel elastic adhesive. Cellulose. 2014;21:4369–4379. [Google Scholar]
- 24.Heck T, Faccio G, Richter M, Thony-Meyer L. Enzyme-catalyzed protein crosslinking. Appl Microbiol Biotechnol. 2013;97:461–475. doi: 10.1007/s00253-012-4569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez M, Guan D, Ugaz V, Chen Z. Intein-triggered artificial protein hydrogels that support the immobilization of bioactive proteins. J Am Chem Soc. 2013;135:5290–5293. doi: 10.1021/ja401075s. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WB, Sun F, Tirrell DA, Arnold FH. Controlling macromolecular topology with genetically encoded SpyTag-SpyCatcher chemistry. J Am Chem Soc. 2013;135:13988–13997. doi: 10.1021/ja4076452. [DOI] [PubMed] [Google Scholar]
- 27••.Sun F, Zhang WB, Mahdavi A, Arnold FH, Tirrell DA. Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry. Proc Natl Acad Sci U S A. 2014;111:11269–11274. doi: 10.1073/pnas.1401291111. This paper utilized the SpyTag-SpyCatcher system to make protein-based hydrogels. The gelation process was cytocompatible with fibroblasts and mouse stem cells. Leukemia inhibitory factor was also covalently attached to the protein hydrogels and remained bioactive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulyasasmita W, Cai L, Dewi RE, Jha A, Ullmann SD, Luong RH, Huang NF, Heilshorn SC. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Control Release. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Funct Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CC, Ravindran S, Yin Z, George A. 3-D self-assembling leucine zipper hydrogel with tunable properties for tissue engineering. Biomaterials. 2014;35:5316–5326. doi: 10.1016/j.biomaterials.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsueh YS, Savitha S, Sadhasivam S, Lin FH, Shieh MJ. Design and synthesis of elastin-like polypeptides for an ideal nerve conduit in peripheral nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2014;38:119–126. doi: 10.1016/j.msec.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 32•.Tjin MS, Chua AW, Ma DR, Lee ST, Fong E. Human epidermal keratinocyte cell response on integrin-specific artificial extracellular matrix proteins. Macromol Biosci. 2014;14:1125–1134. doi: 10.1002/mabi.201400015. The authors developed ELPs with cell-binding sequences derived from different ECM proteins. Keratinocytes used different integrin receptors to bind these proteins, and the authors studied the effect of these cell-binding domains on cell attachment, proliferation, and colony formation. [DOI] [PubMed] [Google Scholar]
- 33.Rahmany MB, Van Dyke M. Biomimetic approaches to modulate cellular adhesion in biomaterials: A review. Acta Biomater. 2013;9:5431–5437. doi: 10.1016/j.actbio.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Lee P, Bax DV, Bilek MM, Weiss AS. A novel cell adhesion region in tropoelastin mediates attachment to integrin αvβ5. J Biol Chem. 2014;289:1467–1477. doi: 10.1074/jbc.M113.518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Renner JN, Liu JC. Incorporating the BMP-2 peptide in genetically-engineered biomaterials accelerates osteogenic differentiation. Biomater Sci. 2014;2:1110–1119. doi: 10.1039/c3bm60333d. [DOI] [PubMed] [Google Scholar]
- 36.Renner JN, Kim Y, Liu JC. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2012;18:2581–2589. doi: 10.1089/ten.TEA.2011.0400. [DOI] [PubMed] [Google Scholar]
- 37.Tao H, Wu Y, Li H, Wang C, Zhang Y, Li C, Wen T, Wang X, He Q, Wang D, et al. BMP7-based functionalized self-assembling peptides for nucleus pulposus tissue engineering. ACS Appl Mater Interfaces. 2015;7:17076–17087. doi: 10.1021/acsami.5b03605. [DOI] [PubMed] [Google Scholar]
- 38.Cai L, Dinh CB, Heilshorn SC. One-pot synthesis of elastin-like polypeptide hydrogels with grafted VEGF-mimetic peptides. Biomater Sci. 2014;2:757–765. doi: 10.1039/C3BM60293A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulyasasmita W, Cai L, Hori Y, Heilshorn SC. Avidity-controlled delivery of angiogenic peptides from injectable molecular-recognition hydrogels. Tissue Eng Part A. 2014;20:2102–2114. doi: 10.1089/ten.tea.2013.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheyen A, Peeraer E, Lambrechts D, Poesen K, Carmeliet P, Shibuya M, Pintelon I, Timmermans JP, Nuydens R, Meert T. Therapeutic potential of VEGF and VEGF-derived peptide in peripheral neuropathies. Neuroscience. 2013;244:77–89. doi: 10.1016/j.neuroscience.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Ferrero A, Mata A, Mateos-Timoneda MA, Rodriguez-Cabello JC, Alonso M, Planell J, Engel E. Development of tailored and self-mineralizing citric acid-crosslinked hydrogels for in situ bone regeneration. Biomaterials. 2015;68:42–53. doi: 10.1016/j.biomaterials.2015.07.062. [DOI] [PubMed] [Google Scholar]
- 42•.Price R, Poursaid A, Cappello J, Ghandehari H. In vivo evaluation of matrix metalloproteinase responsive silk-elastinlike protein polymers for cancer gene therapy. J Control Release. 2015;213:96–102. doi: 10.1016/j.jconrel.2015.06.022. In this work, silk-elastinlike recombinant protein hydrogels were used as vehicles for gene therapy. MMP-sensitive sequences were incorporated into the protein backbone. Higher survival rates were observed in tumor-bearing mice implanted with hydrogels with MMP-sensitive sequences compared to hydrogels that did not contain the degradation sequence. The authors also found that the location of the MMP-sensitive sequence in the protein backbone had an effect on cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonseca KB, Maia FR, Cruz FA, Andrade D, Juliano MA, Granja PL, Barrias CC. Enzymatic, physicochemical and biological properties of MMP-sensitive alginate hydrogels. Soft Matter. 2013;9:3283–3292. [Google Scholar]
- 44•.Sridhar BV, Brock JL, Silver JS, Leight JL, Randolph MA, Anseth KS. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv Healthc Mater. 2015;4:702–713. doi: 10.1002/adhm.201400695. This work showed that the extent of ECM secretion of cultured cells can be influenced by the degradability of the culture scaffold. An MMP-degradable sequence was incorporated into PEG hydrogels. Higher secretion of GAG and collagen was observed with a co-culture of hMSCs and chondrocytes in MMP-degradable hydrogels compared to those in non-degradable hydrogels. Constructs were cultured with cells, and the degradable gels had a higher compression modulus than non-degradable gels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kukreja M, Shiryaev SA, Cieplak P, Muranaka N, Routenberg DA, Chernov AV, Kumar S, Remacle AG, Smith JW, Kozlov IA, et al. High-throughput multiplexed peptide-centric profiling illustrates both substrate cleavage redundancy and specificity in the MMP family. Chem Biol. 2015;22:1122–1133. doi: 10.1016/j.chembiol.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bracher M, Bezuidenhout D, Lutolf MP, Franz T, Sun M, Zilla P, Davies NH. Cell specific ingrowth hydrogels. Biomaterials. 2013;34:6797–6803. doi: 10.1016/j.biomaterials.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 47.Goetsch KP, Bracher M, Bezuidenhout D, Zilla P, Davies NH. Regulation of tissue ingrowth into proteolytically degradable hydrogels. Acta Biomater. 2015;24:44–52. doi: 10.1016/j.actbio.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 48•.Yang J, Yao M-H, Du M-S, Jin R-M, Zhao D-H, Ma J, Ma Z-Y, Zhao Y-D, Liu B. A near-infrared light-controlled system for reversible presentation of bioactive ligands using polypeptide-engineered functionalized gold nanorods. Chem Commun. 2015;51:2569–2572. doi: 10.1039/c4cc09516b. Recombinant leucine zippers were used to functionalize gold nanorods. In particular, one half of the leucine zipper, which was fused to the RGD sequence, was attached to gold nanorods. The second half of the leucine zipper was attached to a PEG polymer chain. When the leucine zippers associated, the RGD sequence was inaccessible to cells because of the PEG chain. However, when exposed to NIR radiation, gold nanorods generated heat. The elevated temperature denatured the leucine zipper, and as a result, the RGD sequence became accessible to cells. [DOI] [PubMed] [Google Scholar]
- 49.Kim D, Kim SK, Valencia CA, Liu R. Tribody: Robust self-assembled trimeric targeting ligands with high stability and significantly improved target-binding strength. Biochemistry. 2013;52:7283–7294. doi: 10.1021/bi400716w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah NH, Muir TW. Inteins: Nature’s gift to protein chemists. Chem Sci. 2014;5:446–461. doi: 10.1039/C3SC52951G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood DW, Camarero JA. Intein applications: From protein purification and labeling to metabolic control methods. J Biol Chem. 2014;289:14512–14519. doi: 10.1074/jbc.R114.552653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Ren W, Ji A, Ai HW. Light activation of protein splicing with a photocaged fast intein. J Am Chem Soc. 2015;137:2155–2158. doi: 10.1021/ja508597d. A photoactivatable intein was developed by introducing a photocaged cysteine residue. The intein was inserted in the middle of the sequences for mCherry fluorescent protein or a Src tyrosine kinase. These chimeric proteins were expressed by mammalian cells, the intein activity was triggered with light, and mCherry or the kinase was reconstituted. [DOI] [PubMed] [Google Scholar]
- 53.Jung D, Sato K, Min K, Shigenaga A, Jung J, Otaka A, Kwon Y. Photo-triggered fluorescent labelling of recombinant proteins in live cells. Chem Commun. 2015;51:9670–9673. doi: 10.1039/c5cc01067e. [DOI] [PubMed] [Google Scholar]
- 54.Bocker JK, Friedel K, Matern JC, Bachmann AL, Mootz HD. Generation of a genetically encoded, photoactivatable intein for the controlled production of cyclic peptides. Angew Chem Int Ed Engl. 2015;54:2116–2120. doi: 10.1002/anie.201409848. [DOI] [PubMed] [Google Scholar]