Abstract
Clarithromycin-resistant Helicobacter pylori (CRHP) is increasing worldwide, especially in children. We report a family case in which both the mother and child were infected with CRHP. DNA analysis revealed that all of the mother's and daughter's isolates were indistinguishable, suggesting that the same CRHP strain spread between the family members. The spread of CRHP within families may be increasing.
CASE REPORT
The 40-year-old mother in a family living in Shiga, Japan, had experienced epigastralgia since elementary school. She was determined to be Helicobacter pylori infection positive by a 13C-urea breath test in 2002. Endoscopy was performed in 2002, and she was diagnosed with superficial gastritis and was confirmed to be H. pylori positive by culture examinations. Although there was no history of H. pylori eradication treatment, the mother's H. pylori strains were resistant to clarithromycin. Her 41-year-old husband was H. pylori infection negative by a 13C-urea breath test in 2002, and again there was no history of H. pylori eradication. The 10-year-old daughter had experienced epigastralgia since 2000. She was admitted to a hospital because of severe epigastralgia and was determined to be H. pylori infection positive by a 13C-urea breath test in 2001. Treatment with a combination of anti-H. pylori agents (clarithromycin and amoxicillin) and a proton pump inhibitor (lansoprazole) was performed twice in 2001, but the treatment failed, as evidenced by a 13C-urea breath test. Endoscopy was performed in 2002, and she was diagnosed with superficial gastritis. Culture examinations confirmed her H. pylori-positive status. The daughter's H. pylori strains were resistant to clarithromycin.
H. pylori infection is closely associated with gastritis and peptic ulcers and is a bacterial risk factor for gastric cancer (7, 9). For eradication of H. pylori, combination therapy with an anti-acid agent (a proton pump inhibitor or H2 blocker) and one or two anti-H. pylori agents (such as clarithromycin, amoxicillin, or metronidazole) has been recommended (8). Drug resistance has been reported for strains from adults. For clarithromycin, the primary rate of resistance is relatively low, less than 10% in many cases (12, 18), although failure to eradicate the infection with clarithromycin treatment results in extremely high rates of resistance (68%) (2, 16). In our previous study, a strikingly high primary rate (42.9%) of clarithromycin resistance was demonstrated in H. pylori strains from Japanese children, in marked contrast to those from their parents (none of them were resistant to clarithromycin) (14). Therefore, we decided to investigate a family case in which both the mother and child were infected with clarithromycin-resistant H. pylori.
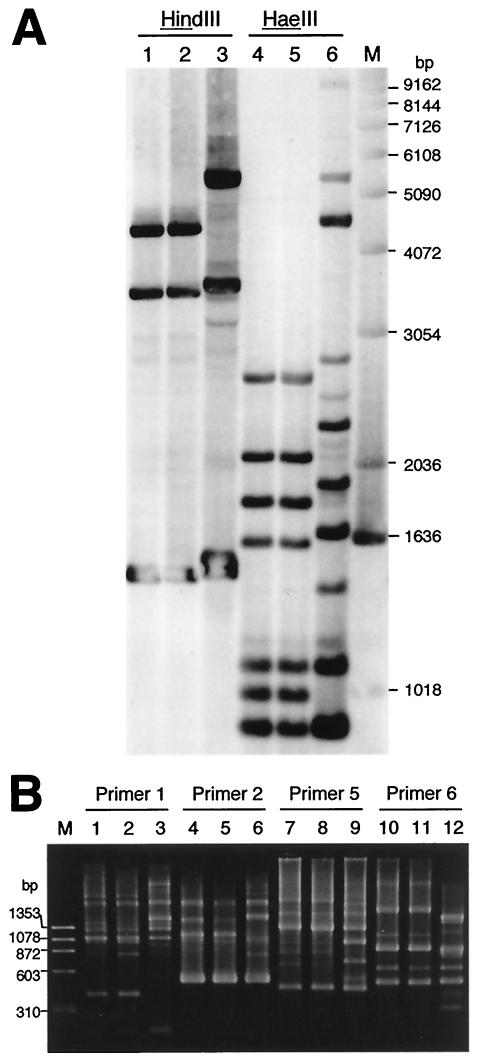
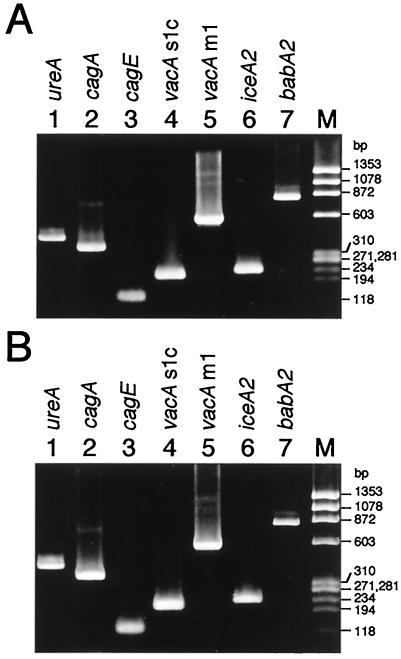
Antrum and corpus biopsy specimens were obtained from the mother and daughter, and one each was examined for H. pylori. H. pylori was cultivated from both sites (antrum and corpus) in both the mother and the daughter. Fifteen colonies each (a total of 30) for the mother and 10 colonies each (a total of 20) for the daughter, that developed after primary cultivation of each biopsy specimen, were stored at −80°C. To determine whether the mother's and daughter's H. pylori isolates were the same, all of the H. pylori isolates were characterized by rRNA gene restriction pattern analysis (ribotyping) with the restriction endonuclease HindIII or HaeIII as described previously (15) and by arbitrarily primed PCR (AP-PCR) analysis with commercially available primers (Amersham Biosciences Corp.). Ribotyping analysis revealed that all of the mother's and daughter's isolates were identical to each other. The representative data are shown in Fig. 1A (lanes 1 and 2 for HindIII digestion and lanes 4 and 5 for HaeIII digestion). All of the mother's and daughter's isolates also had the same amplicon pattern in AP-PCR analysis, as shown in Fig. 1B (lanes 1 and 2 for primer 1, lanes 4 and 5 for primer 2, lanes 7 and 8 for primer 5, and lanes 10 and 11 for primer 6). In addition, when the virulent genotypes of H. pylori were examined by PCR as described previously (3, 6, 19-21), all of the mother's and daughter's isolates were genotypically indistinguishable for ureA, cagA, cagE, vacA s1c and m1, iceA2, and babA2, as shown in Fig. 2. Next, MICs were determined by the agar dilution method in accordance with NCCLS procedures (13). All of the mother's and daughter's isolates were resistant to macrolides and susceptible to amoxicillin, metronidazole, and tetracyclines. The MICs of the antimicrobial agents against the isolates were 8 μg/ml for clarithromycin, 64 μg/ml for roxithromycin, ≥256 μg/ml for azithromycin, 0.008 μg/ml for amoxicillin, 0.5 μg/ml for metronidazole, 0.13 μg/ml for tetracycline, 0.25 μg/ml for doxycycline, and 0.06 μg/ml for minocycline. When the mother's and daughter's isolates were analyzed for the 23S rRNA gene sequence by PCR and sequencing (4, 14), both isolates had the A2143G mutation.
FIG. 1.
Ribotyping (A) and AP-PCR (B) analyses of H. pylori isolates from the members of a family. The results shown are representative of those obtained with 30 H. pylori isolates from the mother and 20 isolates from the daughter. In panel A, H. pylori chromosomal DNA was digested by HindIII or HaeIII. Lanes: 1 and 4, isolate from the daughter; 2 and 5, isolate from the mother; 3 and 6, isolate unrelated to the family; M, molecular size standards (1-kb DNA ladder). Lanes in panel B: 1, 4, 7, and 10, isolate from the daughter; 2, 5, 8, and 11, isolate from the mother; 3, 6, 9, and 12, isolate unrelated to the family; M, molecular size standards (HaeIII fragments of φX174 replicative-form DNA). Primers 1 (5′-d[GGTGCGGGAA]-3′), 2 (5′-d[GTTTCGCTCC]-3′), 5 (5′-d[AACGCGCAAC]-3′), and 6 (5′-d[CCCGTCAGCA]-3′), which gave sharp amplicon bands for H. pylori DNA, were used as the amplification primers.
FIG. 2.
PCR analysis of the virulent genotypes of the H. pylori strains isolated from the mother and daughter. The results shown are representative of those obtained with 30 H. pylori isolates from the mother (A) and 20 isolates from the daughter (B). The PCR primers used for ureA, cagA, cagE, vacA s1c and m1, iceA2, and babA2 were those previously described in references 3, 6, 20, and 21. Lane M, molecular size standards (HaeIII fragments of φX174 replicative-form DNA).
In this study, at least 10 colonies from each primary H. pylori culture in the mother and daughter were examined, and all of the colonies selected were determined to have the same characteristics. Therefore, it was strongly suggested that the same clarithromycin-resistant H. pylori strain had spread between the family members. A most likely explanation for such intrafamilial infection is that, since children are at a high risk for H. pylori infection, the daughter was infected with her mother's clarithromycin-resistant H. pylori strain (and thus the treatment failed in the daughter). The reason why the mother carried a clarithromycin-resistant strain is not known (there was no history of H. pylori eradication treatment for the mother). However, the mother was probably infected for more than 20 years, and it is possible that the mother was treated with macrolides for infections other than gastritis, resulting in selection of clarithromycin resistance in H. pylori. Since the mother seemed not to have received macrolides in the last 10 years (since the birth of the daughter), the selection might have occurred around the birth of the daughter or before.
It is also possible that the daughter's clarithromycin-resistant H. pylori strain emerged because of treatment failure with an anti-H. pylori regimen that contained clarithromycin. In that case, the daughter's clarithromycin-resistant H. pylori strain might have been transmitted to her mother. In this household, the grandfather also seemed to be infected with H. pylori and seemed to be the likely source of H. pylori infection (his sample was not available for this study).
High rates of clarithromycin resistance in H. pylori strains from children have been reported in France (21%; reference 10), Spain (28.3%; reference 11), Portugal (44.8%; reference 1), Poland (23.5%; reference 5), and Mexico (21.6%; reference 17). However, since molecular analysis of drug-resistant H. pylori strains has not been reported, the precise reason for such high rates of clarithromycin resistance in children is not known.
In previous studies, we suggested that a history of clarithromycin use by a child (e.g., a children with otitis) caused the development of clarithromycin resistance in previously contracted H. pylori that originated from a parent (14). This seemed to be the major reason for the strikingly high primary rate of clarithromycin resistance in H. pylori strains from Japanese children. In the intrafamilial infection cases in Japan, however, none of the H. pylori strains from the parents examined were resistant to clarithromycin.
This study demonstrated the molecular detection of intrafamilial clustering of clarithromycin-resistant H. pylori infections in a mother and child. We are currently studying another family in which a father and his daughter were infected with the same clarithromycin-resistant H. pylori strain (unpublished data). The spread of clarithromycin-resistant H. pylori within families may be increasing in Japan and may account for some portion of the strikingly high rate of clarithromycin resistance in H. pylori from Japanese children (46.7%, including the data in this study). Further studies on the possible spread of drug-resistant H. pylori among family members are necessary.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, a grant from Research Fellowships of the Japan Society for the Promotion of Science for Young Scientist, and a Grant-in-Aid from the Niigata University Science Foundation, Japan.
REFERENCES
- 1.Cabrita, J., M. Oleastro, R. Matos, A. Manhente, J. Cabral, R. Barros, A. I. Lopes, P. Ramalho, B. C. Neves, and A. S. Guerreiro. 2000. Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990-1999). J. Antimicrob. Chemother. 46:1029-1031. [DOI] [PubMed] [Google Scholar]
- 2.Cayla, R., F. Zerbib, P. Talbi, F. Megraud, and H. Lamouliatte. 1995. Pre and post-treatment clarithromycin resistance of Helicobacter pylori strains. Gut 37:A55. [Google Scholar]
- 3.Clayton, C. L., H. Kleanthous, P. J. Coates, D. D. Morgan, and S. Tabaqchali. 1992. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J. Clin. Microbiol. 30:192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debets-Ossenkopp, Y. J., M. Sparrius, J. G. Kusters, J. J. Kolkman, and C. M. Vandenbroucke-Grauls. 1996. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol. Lett. 142:37-42. [DOI] [PubMed] [Google Scholar]
- 5.Dzierzanowska-Fangrat, K., E. Rozynek, P. Jowiak, D. Celinska-Cedro, K. Madalinski, and D. Dzierzanowska. 2001. Primary resistance to clarithromycin in clinical strains of Helicobacter pylori isolated from children in Poland. Int. J. Antimicrob. Agents 18:387-390. [DOI] [PubMed] [Google Scholar]
- 6.Gerhard, M., N. Lehn, N. Neumayer, T. Boren, R. Rad, W. Schepp, S. Miehlke, M. Classen, and C. Prinz. 1999. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. USA 96:12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin, C. S. 1997. Helicobacter pylori gastritis, peptic ulcer, and gastric cancer: clinical and molecular aspects. Clin. Infect. Dis. 25:1017-1019. [DOI] [PubMed] [Google Scholar]
- 8.Graham, D. Y. 2000. Therapy of Helicobacter pylori: current status and issues. Gastroenterology 118:S2-S8. [DOI] [PubMed] [Google Scholar]
- 9.IARC Working Group. 1994. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 61:177-241. [PMC free article] [PubMed] [Google Scholar]
- 10.Kalach, N., M. Bergeret, P. H. Benhamou, C. Dupont, and J. Raymond. 2001. High levels of resistance to metronidazole and clarithromycin in Helicobacter pylori strains in children. J. Clin. Microbiol. 39:394-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Brea, M., M. J. Martínez, D. Domingo, and T. Alarcón. 2001. A 9 year study of clarithromycin and metronidazole resistance in Helicobacter pylori from Spanish children. J. Antimicrob. Chemother. 48:295-297. [DOI] [PubMed] [Google Scholar]
- 12.Megraud, F., N. Lehn, T. Lind, E. Bayerdorffer, C. O'Morain, R. Spiller, P. Unge, S. V. van Zanten, M. Wrangstadh, and C. F. Burman. 1999. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob. Agents Chemother. 43:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2001. MIC interpretive standards (μg/ml) for Helicobacter pylori, p. 107. In Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. M7-A5, vol. 21, no. 1 (Performance standards for antimicrobial susceptibility testing; 11th informational supplement. M100-S11). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Taneike, I., S. Goshi, Y. Tamura, N. Wakisaka-Saito, N. Matsumori, A. Yanase, T. Shimizu, Y. Yamashiro, S. Toyoda, and T. Yamamoto. 2002. Emergence of clarithromycin-resistant Helicobacter pylori (CRHP) with a high prevalence in children compared with their parents. Helicobacter 7:297-305. [DOI] [PubMed] [Google Scholar]
- 15.Taneike, I., Y. Tamura, T. Shimizu, Y. Yamashiro, and T. Yamamoto. 2001. Helicobacter pylori intrafamilial infections: change in source of infection of a child from father to mother after eradication therapy. Clin. Diagn. Lab. Immunol. 8:731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tompkins, D. S., J. Perkin, and C. Smith. 1997. Failed treatment of Helicobacter pylori infection associated with resistance to clarithromycin. Helicobacter 2:185-187. [DOI] [PubMed] [Google Scholar]
- 17.Torres, J., M. Camorlinga-Ponce, G. Pérez-Pérez, A. Madrazo-De la Garza, M. Dehesa, G. González-Valencia, and O. Muñoz. 2001. Increasing multidrug resistance in Helicobacter pylori strains isolated from children and adults in Mexico. J. Clin. Microbiol. 39:2677-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakil, N., B. Hahn, and D. McSorley. 1998. Clarithromycin-resistant Helicobacter pylori in patients with duodenal ulcer in the United States. Am. J. Gastroenterol. 93:1432-1435. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka, Y., E. Orito, M. Mizokami, O. Gutierrez, N. Saitou, T. Kodama, M. S. Osato, J. G. Kim, F. C. Ramirez, V. Mahachai, and D. Y. Graham. 2002. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 517:180-184. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka, Y., T. Kodama, M. Kita, J. Imanishi, K. Kashima, and D. Y. Graham. 1999. Relation between clinical presentation, Helicobacter pylori density, interleukin 1β and 8 production, and cagA status. Gut 45:804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka, Y., T. Kodama, O. Gutierrez, J. G. Kim, K. Kashima, and D. Y. Graham. 1999. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J. Clin. Microbiol. 37:2274-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]