Summary
Background
Lyme borreliosis is the most common tick-borne disease in the northern hemisphere. It is a multisystem disease caused by Borrelia burgdorferi sensu lato genospecies and characterised by tissue localisation and low spirochaetaemia. In this study we aimed to describe a novel Borrelia species causing Lyme borreliosis in the USA.
Methods
At the Mayo clinic, from 2003 to 2014, we tested routine clinical diagnostic specimens from patients in the USA with PCR targeting the oppA1 gene of B burgdorferi sensu lato. We identified positive specimens with an atypical PCR result (melting temperature outside of the expected range) by sequencing, microscopy, or culture. We collected Ixodes scapularis ticks from regions of suspected patient tick exposure and tested them by oppA1 PCR.
Findings
100 545 specimens were submitted by physicians for routine PCR from Jan 1, 2003 to Sept 30, 2014. From these samples, six clinical specimens (five blood, one synovial fluid) yielded an atypical oppA1 PCR product, but no atypical results were detected before 2012. Five of the six patients with atypical PCR results had presented with fever, four had diffuse or focal rash, three had symptoms suggestive of neurological inclusion, and two were admitted to hospital. The sixth patient presented with knee pain and swelling. Motile spirochaetes were seen in blood samples from one patient and cultured from blood samples from two patients. Among the five blood specimens, the median oppA1 copy number was 180 times higher than that in 13 specimens that tested positive for B burgdorferi sensu stricto during the same time period. Multigene sequencing identified the spirochaete as a novel B burgdorferi sensu lato genospecies. This same genospecies was detected in ticks collected at a probable patient exposure site.
Interpretation
We describe a new pathogenic Borrelia burgdorferi sensu lato genospecies (candidatus Borrelia mayonii) in the upper midwestern USA, which causes Lyme borreliosis with unusually high spirochaetaemia. Clinicians should be aware of this new B burgdorferi sensu lato genospecies, its distinct clinical features, and the usefulness of oppA1 PCR for diagnosis.
Introduction
Lyme borreliosis is a spirochaetal tick-borne disease caused by some genospecies of the Borrelia burgdorferi sensu lato complex.1–4 With 85 000 cases estimated annually in Europe and 300 000 cases estimated annually in the USA, it is the most common tick-borne disease in the northern hemi sphere.5,6 Nearly all human infections are caused by three B burgdorferi sensu lato genospecies: Borrelia garinii, Borrelia afzelii, and B burgdorferi sensu stricto.1 All three species cause Lyme borreliosis in Europe, whereas only B burgdorferi sensu stricto causes Lyme borreliosis in the USA.7
The clinical features of Lyme borreliosis are broad and seem to be associated with distinct tissue tropisms of specific B burgdorferi sensu lato genospecies.8 Early localised infection typically results in erythema migrans rash, after which spirochaetes can disseminate to the nervous system, joints, and other organs. B burgdorferi sensu stricto is often associated with arthritis, B garinii with neurological effects, and B afzelii with acrodermatitis chronicum atrophicans.8
Lyme borreliosis is characterised by a low level of spirochaetaemia.9 Spirochaetes are detectable by PCR in the peripheral blood of less than 50% of patients with erythema migrans, with average estimation of about 2330 genome copies per mL,9,10 whereas the mean number of spirochaetes detected by culture of peripheral blood is only 0·1 spirochaetes per mL.9 As expected, microscopic detection of B burgdorferi sensu lato spirochaetes has never been reported in peripheral blood, by marked contrast with relapsing fever borreliae, which have loads ranging from 105 spirochaetes per mL to more than 106 spirochaetes per mL, and are readily seen in peripheral blood.11 We describe a new B burgdorferi sensu lato genospecies causing Lyme borreliosis with substantially elevated spirochaetaemia in acutely ill patients.
Methods
Patients
Mayo Medical Laboratories provides diagnostic PCR testing for Lyme borreliosis.12 From Nov 1, 2003, to Sept 30, 2014, physicians throughout the USA submitted 100 545 specimens (synovial fluid, cerebrospinal fluid, EDTA [edetic acid]-anticoagulated whole blood, or fresh tissue) to our laboratory for routine clinical PCR testing. We interviewed patients with specimens yielding a PCR result that differed from that expected for B burgdorferi sensu stricto, B garinii, or B afzelii, to obtain clinical and epidemiological information. We reviewed medical records and requested additional samples. Patient follow-up and DNA sequencing of clinical specimens was approved by the Mayo Clinic institutional review board. Patients were interviewed by state public health officials as part of routine surveillance for a reportable condition.
Real-time PCR and DNA sequencing
We extracted DNA from diagnostic specimens using the MagNA Pure Instrument (Roche) and tested for B burgdorferi sensu stricto, B afzelii, and B garinii with a diagnostic real-time PCR assay that uses hybridisation probes and targets the chromosomal oppA1 gene.12,13 This assay is specific for B burgdorferi sensu lato and does not detect relapsing fever borreliae. We subjected PCR products to melting temperature analysis to differentiate B burgdorferi sensu lato genospecies (appendix). We established the number of oppA1 copies with standard curves that were prepared with genomic DNA from B burgdorferi sensu stricto B31 and the MN14-1420 isolate; oppA1 is present on the chromosome in a single copy in both genospecies. We used the Wilcoxon rank-sum test (two-sided) for oppA1 PCR crossing point comparison.
We amplified and sequenced portions of the 16S rDNA, ospC, flaB, rrf-rrl, oppA1, uvrA, rplB, recG, pyrG, pepX, clpX, nifS, and clpA genes using previously described primers.12,14–17 We analysed, assembled, and trimmed sequences in Lasergene v9·0 (DNASTAR). Using BLAST, 16S rDNA, ospC, flaB, and rrf-rrl sequences were compared with B burgdorferi sensu lato and relapsing fever borreliae sequences in GenBank. For construction of phylogenetic trees, we obtained homologous B burgdorferi sensu lato and relapsing fever borreliae sequences from GenBank and PubMLST (appendix). We used MEGA 5 (ClustalW) to align sequences and trees constructed by maximum likelihood analysis using the generalised time-reversible nucleotide substitution model with gamma distribution (four categories) followed by bootstrap analysis (1000 replicates).18 Housekeeping genes were concatenated in frame in the order uvrA, rplB, recG, pyrG, pepX, clpX, and clpA, with or without nifS and exported into MEGA 5 to calculate pairwise genetic distances using the Kimura-2 model.
Microscopy and culture
Two clinical blood specimens with an atypical oppA1 PCR melting temperature were available for microscopy and culture after storage at 4°C for either 5 days (one sample) or 39 days (one sample) (appendix). Other specimens were unavailable or previously frozen and not amenable to microscopy and culture. We examined wet mounts from patient samples and cultures with dark-field microscopy at 400 times magnification (appendix).
Serological testing
We tested serum samples and plasma samples for antibodies reacting to B burgdorferi sensu stricto using FDA-cleared commercially available kits, following the recommended two-tiered algorithm (appendix).19
Tick collection and processing
We collected Ixodes scapularis ticks at approximate sites of possible patient exposure in Barron County, WI, USA, during 2013 and 2014 (appendix), and processed the ticks for PCR using a modification of a published protocol.20 We also tested archived DNA from I scapularis collected in Eau Claire County, WI, USA, during 2009–10.
Role of the funding source
The funders of the study, Mayo Clinic and the US Centers for Disease Control and Prevention, had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From Jan 1, 2012, to Sept 30, 2014, 9197 clinical specimens from residents of Minnesota, Wisconsin, and North Dakota were submitted to Mayo Clinic, Rochester, MN, USA, for routine diagnostic B burgdorferi sensu lato oppA1 PCR testing. 3127 (34%) of 9197 specimens tested were blood samples, 1196 (13%) were synovial fluid, 4782 (52%) were cerebrospinal fluid, and 92 (1%) were tissue. 102 specimens were positive for B burgdorferi, including 13 blood, 81 synovial fluid, three cerebrospinal fluid, and five tissue samples. Six specimens (five blood, one synovial fluid) had positive oppA1 PCR results with an atypical melting temperature (60·38–61·24°C), falling between the expected melting temperature for B burgdorferi sensu stricto (61·7–66·7°C) and B afzelii or B garinii (51·7–56·7°C) (table 1, figure 1A, figure 2, appendix).12 No atypical melting temperatures were identified among 24 786 clinical specimens tested from 44 other states during the same time period (Fisher-Exact, p=0·00039), or among more than 66 562 clinical specimens from all states tested by the same method during 2003–11 (0 of 66 562 vs six of 33 983, p=0·00149). The five positive blood specimens with atypical melting temperatures were collected 1–4 days after onset of illness; the synovial fluid specimen was obtained 34 days after onset of illness (table 1). The median oppA1 PCR crossing point was significantly lower (median 29·82, IQR 28·89–30·75) for the five blood specimens with atypical oppA1 melting temperature compared with the 13 blood specimens that tested positive for B burgdorferi sensu stricto (median 34·51, IQR 33·73–35·55; p=0·0016; figure 3).
Table 1.
Demographic, clinical, and laboratory features in patients infected with suspected novel B burgdorferi sensu lato genospecies
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Demographic features | ||||||
| Age (years) | 10 | 65 | 11 | 21 | 67 | 51 |
| Sex | Male | Male | Male | Female | Female | Male |
| Tick exposure | Probable | Probable | Known bite | Probable | Probable | Known bite |
| Symptoms | ||||||
| Fever | Yes | Yes | Yes | No | Yes | Yes |
| Headache | Yes | Yes | Yes | No | Yes | Yes |
| Neck pain | Yes | Yes | Yes | No | No | No |
| Fatigue | Yes | Yes | Yes | No | Yes | No |
| Myalgia | Yes | Yes | No | No | Yes | Yes |
| Nausea or vomiting | Yes | No | Yes | No | Yes | Yes |
| Arthralgia (site) | No | No | No | Yes (left knee) | No | No |
| Other | Profound somnolence | ‥ | Confused speech | ‥ | Chills, abdominal and lumbar back pain, flashing lights | ‥ |
| Physical findings | ||||||
| Measuredtemperature (°C) | 40 | NA | 39·7 | Afebrile | 38·2 | NA |
| Rash | Many erythematous macules on face, trunk, arms (figure 1A)* | NA | Initial macule, enlarged to erythema migrans; diffuse macular rash after single dose of doxycycline; many erythema migrans 28 days later | NA | Many erythematous macules on trunk and upper extremities | 2 × 2 cm macule on leg at site of possible tick bite |
| Other | ‥ | ‥ | ‥ | Swelling left knee | ‥ | ‥ |
| Laboratory results [normal range for adults and children aged 10–11 years combined] | ||||||
| Days of illness before specimen collection for PCR | 1 | 4 | 2 | 34 | 3 | 1 |
| oppA1 PCR melting temperature (°C) | 61·24 | 60·75 | 60·83 | 61·19 | 60·56 | 60·38 |
| Crossing point | 30·88 | 29·58 | 29·82 | 34·20 | 26·48 | 30·63 |
| Estimated number of oppA1 copies per mL | 4·2×105 | 9·4×105 | 8·1×105 | Not determined | 6·4×106 | 4·9×105 |
| White blood cell count (×10−9/L) [3·4–10·5] | 7·4 | 3·4 | 4·6 | NA | 12·4 | 5·3 |
| Lymphocyte count (×10−9/L) [0·90–6·50] | 0·74 | 0·31 | 0·44 | NA | 0·93 | 0·30 |
| Platelet count (×10−9/L) [150–450] | 184 | 113 | 122 | NA | 215 | 150 |
| Haemoglobin (g/dL) [12·0–17·5] | 14 | NA | 14·7 | NA | 9·6 | 15·5 |
| Aspartate aminotransferase (U/L) [8–60] | 46 | NA | NA | NA | 118 | 23 |
| Alanine aminotransferase (U/L) [7–55] | 33 | NA | NA | NA | 69 | 27 |
| Treatment and outcome | ||||||
| Antimicrobial therapy | Ceftriaxone (1 day), amoxicillin (21 days; dosage NA) | Doxycycline (dosage and duration NA) | Initial treatment: doxycycline (discontinued after 1 × 50 mg dose)† | Initial treatment: doxycycline (100 mg twice per day for 28 days)‡ | Doxycycline (100 mg twice per day for 21 days) | Doxycycline (100 mg twice per day for 14 days) |
| Hospital admission | 4 Days | No | No | No | 1 day | No |
| Outcome | Recovered | Recovered | Recovered | Persistent joint pain | Improved, lingering fatigue (pre-existing anaemia) | Recovered |
Clinical findings and symptoms were recorded by medical staff at time of initial patient presentation. NA=not available.
Rash was reported by patient’s caregiver to involve the palms and soles, but this was not documented in the medical record.
Subsequent treatment for patient 3 was initiated 3 weeks after illness onset, and consisted of cefuroxime, 500 mg twice per day for 21 days.
For patient 4, subsequent treatment consisted of amoxicillin, 500 mg three times per day for 21 days.
Figure 1. Diffuse macular rash in patient 1 and dark-field microscopic visualisation of a spirochaete in patient 6.
(A) Diffuse macular rash seen 4 days after onset of symptoms in patient 1. Rash was reported by patient’s caregiver to involve the palms and soles, but this was not documented in the medical record. (B) Dark-field microscopic visualisation (400× magnification) of a single spirochaete in diluted blood from patient 6.
Figure 2. Representative oppA1 PCR melting temperature peaks of Borrelia genospecies.
Representative melting temperature peaks in °C for B afzelii (A peak; acceptable range 51·7–56·7°C), novel B burgdorferi sensu lato genospecies (B peaks; 60·38–61·24°C), and B burgdorferi sensu stricto (C peaks; 61·7–66·7°C). Y-axis represents the negative derivative of the ratio of the FRET signal (LC-Red640 flouresence) and background fluorescein fluorescence.
Figure 3. Comparison of oppA1 PCR melting temperature, crossing points, and estimated oppA1 copy number in B burgdorferi–positive blood specimens.
Comparison of melting temperature and crossing point for the five atypical oppA1 PCR positive blood specimens (open circles) and 13 B burgdorferi sensu stricto oppA1 PCR positive blood specimens (closed circles).
(B) Comparison of melting temperature and estimated oppA1 copy number (genomes per mL of blood) for five atypical oppA1 PCR positive blood specimens (open circles) and 13 B burgdorferi sensu stricto oppA1 PCR positive blood specimens (closed circles).
Sequence analysis of the atypical oppA1 PCR products that were directly amplified from three patient specimens identified a Borrelia species with 89–95% similarity to B burgdorferi sensu lato genospecies (figure 4). Motile spirochaetes (two per 70 fields of diluted blood) of the B burgdorferi sensu lato genospecies were microscopically recorded in blood from patient 6, obtained 1 day after illness onset and analysed 6 days later (figure 1B). The number of spirochaetes was estimated at around 8·5 × 104/mL (appendix). No spirochaetes were seen in the haemolysed blood specimen from patient 5. Cultures of the B burgdorferi sensu lato genospecies (MN14-1420 and MN14-1539) were established from both available blood specimens (patients 5 and 6) after incubation for about 16 days. Spirochaetes were seen in primary and blind passaged cultures; sustained growth was achieved after cryopreservation and additional passage.
Figure 4. Phylogenetic analyses.
(A) Phylogenetic analysis of a 149 base pair fragment of the oppA1 gene amplified from patient specimens (MN14-1539, MN14-1420, WI133, ND132, and ND121) and tick specimens (CP12150 and EC10N1) compared with seven different species of the B burgdorferi sensu lato complex. There is no homologous sequence in relapsing fever borreliae. Bootstrap support values greater than 50% are shown. The scale bar corresponds to 0·01 substitutions per nucleotide position. Accession numbers are indicated for available Borrelia species oppA1 sequences retrieved from GenBank. The B americana BAA-1877 oppA1 gene sequence was generated in this study. GenBank does not allow deposition of sequences shorter than 200 bp; oppA1 sequences generated in this study are available by request. (B) Phylogenetic analysis of eight concatenated housekeeping genes: uvrA, rplB, recG, pyrG, pepX, clpX, clpA, and nifS, amplified from patient isolates (MN14-1539, MN14-1420) compared with 18 different B burgdorferi sensu lato genospecies and three relapsing fever species. Bootstrap support values greater than 50% are shown. The scale bar corresponds to 0·1 substitutions per nucleotide position. The source of other Borrelia species gene sequences is shown in the supplemental methods. Sequence nomenclature (eg, MN14-1539, WI133) represents the state from which the diagnostic specimens were submitted for testing and does not necessarily show the patient’s state of residence.
Based on the high quantity of spirochaetes that were detected microscopically, we estimated the number of oppA1 copies per mL of blood for all 18 specimens that tested positive for oppA1 PCR identified in Minnesota, Wisconsin, and North Dakota during 2012–14 by comparison of oppA1 crossing point values to standard curves prepared with B burgdorferi sensu stricto B31 or MN14-1420 (appendix). For the five atypical oppA1 positives, the median oppA1 copy number was 180 times higher (median 8·1 × 105, IQR 4·6 × 105–3·6 × 106) when compared with the 13 B burgdorferi sensu stricto positives (median 4·5 × 103, IQR 2·3 × 103–7·5 × 103; figure 3).
Sequence analysis of 16S rRNA (1327 nucleotides), ospC (561 base pairs), flaB (435 base pairs), and rrf-rrl (253 base pairs) amplified from the two blood isolates substantiated that the Borrelia species was not identical to any other Borrelia species in GenBank (appendix). The closest sequence identity was to B burgdorferi sensu lato genospecies at 99% for 16S RNA, 85% for ospC, 97% for flaB, and 95% for rrf-rrl. Multilocus sequence analysis of seven genes, uvrA, rplB, recG, pyrG, pepX, clpX, and clpA genes (3774 nucleotides), showed that the spirochaetes isolated from patients 5 and 6 fell within the B burgdorferi sensu lato genospecies complex and were the same B burgdorferi sensu lato genospecies amplified from the blood of patients 1, 2, and 3. The blood isolates were further compared with 18 B burgdorferi sensu lato genospecies and three relapsing fever borreliae using an eight-gene multilocus sequence analysis (uvrA, rplB, recG, pyrG, pepX, clpX, clpA, and nifS; 4335 nucleotides) previously described for defining B burgdorferi sensu lato genospecies21 (figure 4B). The highest pairwise similarity was to B burgdorferi sensu stricto (94·9–95·2% similarity, genetic distance 0·051–0·048), well above the threshold defined for separating genospecies (98·3% similarity, genetic distance 0·017),21 substantiating that the organism detected in the six patients is a novel B burgdorferi sensu lato genospecies, and not a relapsing fever borrelia (eg, B miyamotoi). For comparison, the genetic distance recorded between the novel B burgdorferi sensu lato genospecies and B burgdorferi sensu stricto B31/Z41293 is greater than that seen between other formally recognised B burgdorferi sensu lato genospecies, including B bissetti and B kurtenbachii (0·035) and B garinii and B bavariensis (0·018).14,21
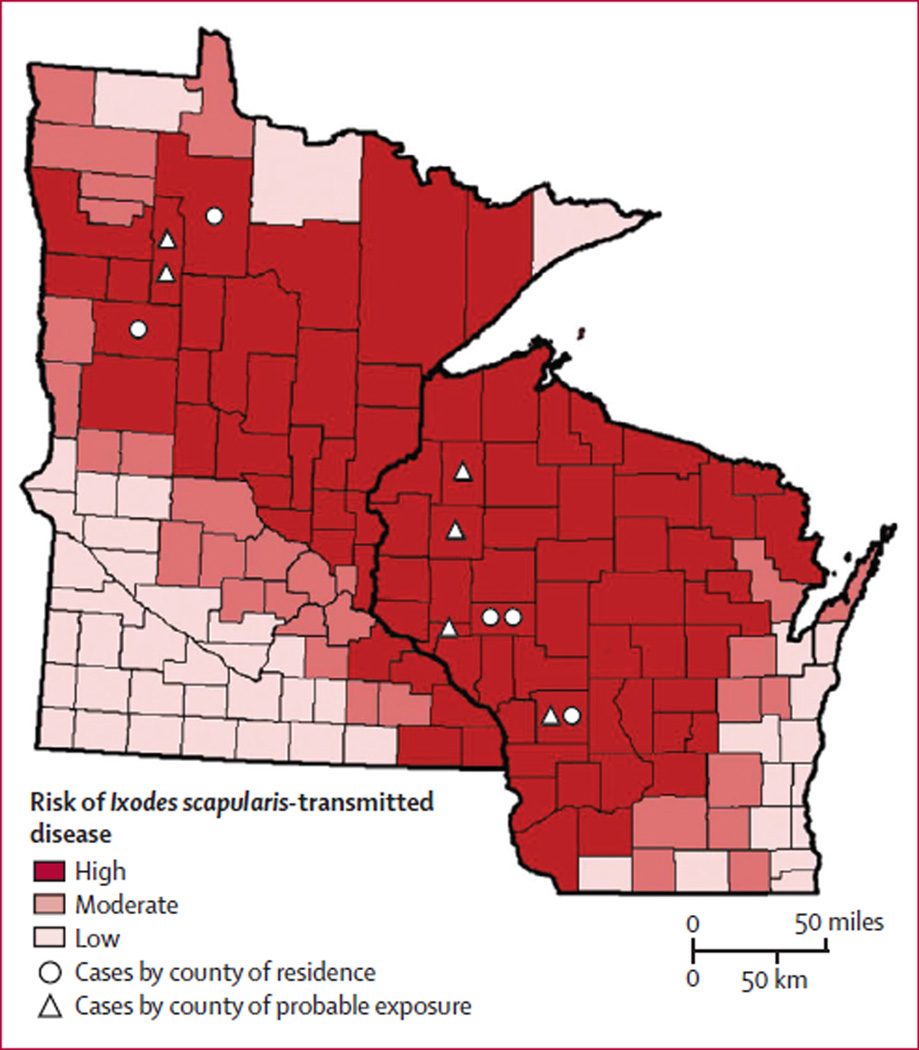
All patients were residents of the upper midwest (Minnesota, North Dakota, or Wisconsin) (figure 5). Median patient age was 36 years (range 10–67 years); four patients were male (table 1). Five presented with acute febrile illnesses, including four with rash. The sixth patient was afebrile but had a 1-month history of unilateral knee pain and swelling. Two patients were admitted to hospital, but none had a known immunocompromising disorder. Testing showed lymphopenia (four of five tested), mild thrombocytopenia (two of five), and high hepatic transaminases (two of three). All patients reported onset of illness between May and July. Exposure to tick habitats in Minnesota or Wisconsin was reported by all patients and two recalled a tick bite less than 30 days before onset of illness. For one patient, the timing between tick bite and PCR sample acquisition was known to be 13 days.
Figure 5. Probable counties of patient exposure to ticks in Minnesota and Wisconsin in relation to risk of diseases transmitted by I scapularis.
I scapularis-transmitted diseases in the figure were Lyme borreliosis, babesiosis, and anaplasmosis. The county of residence for each patient (indicated with a circle) is deemed a county of potential exposure except for the patient from North Dakota, whose county of residence is not shown. Some patients had probable exposures in one or more county in addition to their county of residence (indicated with a triangle). The risk of disease transmitted by I scapularis is based on county-specific mean annual reported incidence of confirmed Lyme borreliosis and confirmed and probable human anaplasmosis and babesiosis in Minnesota and Wisconsin in 2007–13. Counties with 10·0 or fewer cases per 100 000 people were classified as low risk, counties with 10·1–24·9 cases per 100 000 people were classified as moderate risk, and counties with 25·0 or more cases per 100 000 people were classified as high risk.
Descriptions of illness-associated rash varied from diffuse macular rashes involving the face, trunk, and upper extremities (figure 1A, patients 1 and 5) to a single 2 cm diameter erythematous leg lesion at the tick bite location (patient 6; table 1). Patient 3 presented with a single annular erythematous leg lesion with central punctum consistent with erythema migrans, and developed fever, leg and arm pain, and diffuse macular rash on the trunk, upper and lower extremities, and face within 8 h of receiving doxycycline. Differential diagnosis included Jarisch-Herxheimer reaction, drug eruption, and erythema multiforme. Doxycycline was discontinued after one dose. The patient improved without additional treatment, but 3 weeks later developed three erythema migrans lesions on the back and leg, which resolved after treatment with cefuroxime.
All six patients were given antibiotics (table 1). All five patients with febrile illnesses recovered; one with preexisting anaemia reported continuing fatigue. The patient with arthritis improved but reported persistent joint pain 6 months after treatment. Serum or plasma was available for five patients and was tested for reactivity to B burgdorferi sensu stricto antigens using the recommended two–tiered algorithm19 (table 2). Patients 1, 3, and 4 were seropositive to B burgdorferi sensu stricto using this algorithm, including the 30 day cutoff for use of IgM immunoblot. Patient 5 had a positive EIA and IgM immunoblot in a sample obtained 32 days after onset of illness. All four seropositive patients had one or more samples positive using the first-tier C6 EIA; patients 3, 4, and 5 were positive using whole cell EIA. Two seropositive patients (3 and 5) had serial samples and sero converted from a negative to positive IgM immunoblot. The only specimen from patient 6 was obtained 1 day after illness onset and was seronegative.
Table 2.
Serological test results from patients infected with the novel B burgdorferi sensu lato genospecies
| Days from onset of illness to collection of specimen |
B burgdorferi EIA—whole cell |
B burgdorferi EIA-C6 |
B burgdorferi IgM immunoblot (number of bands detected/number of possible bands); specific antigens detected |
B burgdorferi IgG immunoblot (number of bands detected/number of possible bands); specific antigens detected |
|
|---|---|---|---|---|---|
| Patient 1 | 6 | Not available | Positive | Positive (2/3); 23, 41 | Negative (1/10); 41 |
| Patient 3 | 2 | Not available | Equivocal | Negative (0/3) | Negative (0/10) |
| Patient 3 | 29 | Not available | Positive | Positive (3/3); 23, 39, 41 | Negative (2/10); 23, 41 |
| Patient 3 | 104 | Positive | Positive | Negative (0/3) | Negative (4/10); 18, 23, 39, 41 |
| Patient 4 | 266 | Positive | Positive | Negative (1/3); 23 | Positive (5/10); 23, 39, 41, 45, 58 |
| Patient 5 (plasma) | 3 | Negative | Positive | Negative (0/3) | Negative (0/10) |
| Patient 5 | 32 | Positive | Positive | Positive (2/3); 23, 39 | Negative (2/10); 23, 41 |
| Patient 6 (plasma) | 1 | Negative | Negative | Negative (0/3) | Negative (1/10); 41 |
Specimens from patient 2 were not available for testing. IgM immunoblot was deemed second tier positive for B burgdorferi if two or more of a possible three bands (21–25 kDA[OspC], 39 kDA[BmpA], and 41 kDA[Fla]) are detected within 30 days of onset.17 IgG immunoblot was deemed second tier positive for B burgdorferi if five or more of a possible ten bands (18 kDa, 21 kDa[OspC], 28 kDa, 30 kDa, 39 kDa[BmpA], 41 kDa[Fla], 45 kDa, 58 kDa[not GroEL], 66 kDa, and 93 kDa) were detected.
Among archived and prospectively collected ticks I scapularis, 19 (2·9%, range 0–5·2%) of 658 were oppA1 PCR positive for the novel B burgdorferi sensu lato genospecies and 195 (29·6%, range 9·8–33·3%) of 658 were positive for B burgdorferi sensu stricto; two were positive for both. Sequence analysis of oppA1 for two ticks (EC10N1 and CP12150) and seven-gene multilocus sequence analysis for one tick (EC10N1) substantiated that the B burgdorferi sensu lato genospecies detected in I scapularis was the same identified in patients (figure 4A, appendix).
Discussion
We have identified a new B burgdorferi sensu lato genospecies (candidatus Borrelia mayonii) among patients and I scapularis ticks from the upper midwestern USA. A causal role in the patients’ illnesses was suggested by the detection of DNA from this genospecies in patient specimens during acute illness, detection of motile spirochaetes in one blood specimen, culture of the novel B burgdorferi sensu lato genospecies from two patient specimens, development of a patient antibody response after illness onset, and clinical improvement after antimicrobial therapy active against other B burgdorferi sensu lato genospecies. Failure to identify the organism in more than 90 000 clinical samples tested in previous years and from other states might suggest that this new species recently emerged in the upper midwestern USA.
Using an eight-gene multilocus sequence analysis and a published threshold for delineation of B burgdorferi sensu lato genospecies, we showed that the Borrelia species is a member of the B burgdorferi sensu lato group.
Spirochaetes were seen in the diluted blood of a patient who presented with a single erythema migrans lesion, estimated by microscopy at 105 genome copies per mL. The number of genomes in this specimen, based on the single-copy chromosomal gene oppA1, was estimated independently at 5 × 105 per mL. Importantly, the median oppA1 copy number measured for the samples positive for B burgdorferi sensu stricto (4·5 × 102 per mL) agrees with that (2·3 × 102/mL) recorded previously using quantitative flaB PCR, thus supporting the use of the oppA1 gene for B burgdorferi sensu lato quantitation.10 For all five blood specimens from patients infected with the novel B burgdorferi sensu lato genospecies, the number of genomes was estimated to be 105–106 genome copies per mL. This number is similar to what has been reported for patients infected with relapsing fever borreliae and 50–8000 times higher than the blood specimens that tested positive for B burgdorferi sensu stricto during the same time period. The number of spirochaetes as estimated by both microscopy and PCR in blood from patients infected with the novel B burgdorferi sensu lato genospecies is greater than previously estimated for Borrelia miyamotoi (103–104 spirochaetes per mL of blood), a relapsing fever borreliae reported to cause human illness in the USA, Europe, and Russia.22–25 Whether this high spirochaetaemia suggests a different tissue tropism for the new B burgdorferi sensu lato genospecies is an important question that needs to be further addressed; five of six novel B burgdorferi sensu lato genospecies PCR positives were blood specimens, whereas only 13 (13%) of the 102 B burgdorferi sensu stricto PCR positives detected during the same time period were blood, and 81 (79%) of 102 samples were synovial fluids.
Patients infected with the novel B burgdorferi sensu lato genospecies presented with differing clinical presentations when compared with patients infected with B burgdorferi sensu stricto. At least two patients presented with diffuse macular rash not typical of erythema migrans, including one rash that might have involved the palms and soles. Four patients presented with nausea or vomiting and two with fever over 39°C, symptoms not usually reported for Lyme borreliosis7,26–28 but often reported among patients infected with relapsing fever borreliae.29 Similarly, three patients had symptoms potentially consistent with neurological effects (confused speech, profound somnolence, visual difficulties) and two were admitted to hospital.
An important issue raised by identification of the novel B burgdorferi sensu lato genospecies is whether existing Lyme borreliosis diagnostic tests can detect infection with this organism. The six patients described here were fortuitously detected during routine clinical testing, because the diagnostic oppA1 PCR used at Mayo Clinic detects and differentiates B burgdorferi sensu lato genospecies by melting temperature analysis. However, it is unknown if diagnostic PCR assays specific for B burgdorferi sensu stricto will detect the novel genospecies. Regarding serology, the B burgdorferi sensu stricto C6 EIA was positive in all four patients with specimens obtained 3 days or more after onset of illness and B burgdorferi sensu stricto IgM immunoblots of specimens obtained 6 to 32 days after onset were positive for three patients. The B burgdorferi sensu stricto IgG immunoblot, however, was positive only for the patient with more than 30 days of untreated illness. B burgdorferi sensu stricto serology was negative for three specimens drawn 1–3 days after onset of illness.
The patients’ infections were probably acquired by the bite of I scapularis, which transmits B burgdorferi sensu stricto in the USA. Two patients recalled a tick bite before illness and I scapularis that tested positive for the new B burgdorferi sensu lato genospecies were collected at two Wisconsin locations, including one visited by two patients. Prevalence of the novel species in tested I scapularis ranged from 0·6–4·9%. Non-detection of the new B burgdorferi sensu lato genospecies in I scapularis collected in the midwestern USA during 2004–07 further suggests that it might have recently emerged in this region.30,31
The identification of a novel B burgdorferi sensu lato genospecies causing Lyme borreliosis with substantially elevated spirochaetaemia and clinical features distinct from other recognised B burgdorferi sensu lato genospecies has important implications for accurate diagnosis, treatment and disease reporting. In view of the differing clinical manifestations for patients infected with the novel B burgdorferi sensu lato genospecies, it is likely that Lyme borreliosis is not being considered—and therefore not diagnosed—in some patients with this infection. The clinical range of illness must be better defined in additional patients to ensure that physicians can recognise the infection and distinguish it from other tick-borne infections. Many tick-borne pathogens have global distribution, therefore studies are needed to establish the geographic distribution of human beings and ticks infected with the novel B burgdorferi sensu lato genopecies. Finally, clinicians should be aware of the potential role of oppA1 PCR for diagnosing infection with this novel pathogen.
Supplementary Material
Research in context.
Evidence before this study
Lyme borreliosis is a multisystem tick-borne disease of wide public health significance. It is the most frequently reported vector-borne disease in the temperate northern hemisphere and is caused by spirochaetes in the Borrelia burgdorferi sensu lato genospecies complex. There have been no previous descriptions of the pathogenic Borrelia species reported in this study in either ticks or human beings, or reports of Lyme borreliosis with high spirochaetaemia.
Added value of this study
The identification of a new B burgdorferi sensu lato genospecies causing Lyme borreliosis with substantially elevated spirochaetaemia and clinical features distinct from other B burgdorferi sensu lato genospecies has important implications for accurate diagnosis and treatment. In view of the differing clinical manifestations for patients infected with the novel B burgdorferi sensu lato genospecies, it is likely that Lyme borreliosis is not being considered in some patients with this infection. The medical and health-care community need to be aware of this new pathogen to recognise the infection and to treat patients appropriately.
Implications of all the available evidence
The discovery of a novel B burgdorferi sensu lato genospecies was attributable to the use of a diagnostic real-time PCR assay that detects and differentiates B burgdorferi sensu lato genospecies by melting temperature analysis. Those PCR assays designed specifically for detection of a single B burgdorferi sensu lato genospecies do not have the same ability to identify new or different genospecies. Since many tick-borne human pathogens have a global distribution (eg, B burgdorferi, Babesia microti, Anaplasma phagocytophilum, Ehrlichia muris, and Borrelia miyamotoi), the emergence of this pathogen highlights the need for widespread surveillance to look for emergence of this organism or related species in other parts of the world.
Acknowledgments
We would like to thank Marc Dolan, Division of Vector Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO; Deke Haefner, Mayo Medical Laboratories, Rochester, MN; Joni J Franson, BS and Scott A Martin, MD, Mayo Clinic Health System–Eau Claire, WI; Amy L Livermore, Mayo Medical Laboratories New England, Andover, MA; Linda Machmueller, Wisconsin Department of Health Services, Madison, WI; Zachary Muehlenbein, St. John’s University, MN; Kayla Sippl, Jordan Dieckman, Jordan Mandli, Theoren Loo, Nathan Wong, and Ryan Swanson, Tick Surveillance Team at the University of Wisconsin-Madison, Medical Entomology Laboratory, Madison, Wisconsin, USA; and Lindsey Page, North Dakota Department of Health for their contributions to the collection and analysis of data described in this study.
Declaration of interests
BSP, LMS, CLI, EST and RP are employed by Mayo Clinic, which provides commercial PCR and serologic laboratory testing for Borrelia burgdorferi and related species through its reference laboratory, Mayo Medical Laboratories. BSP received partial funding for this project from the Mayo Clinic Department of Laboratory Medicine and Pathology Small Grant Program, and all authors from the Minnesota Department of Health (DFN, ES, JAR, JB), Wisconsin Department of Health Services (DKHJ, JPD, CRS, AD), and North Dakota Department of Health (MAF and TKM) received funding from the Centers for Disease Control and Prevention through the Epidemiology and Laboratory Capacity Cooperative Agreement. SMP received funding from the State of Wisconsin through the CDC Epidemiology and Laboratory Capacity Cooperative Agreement. RP reports grant funding from Pfizer, Pradama, Tornier, Astellas, Procared, nanoMR, BioFire, Curetis, 3M, Cubist, Hutchinson Biofilm Medical Solutions, and Accelerate Diagnostics. She receives royalties from Up-To-Date and an editor’s stipend and travel reimbursement from the American Society of Microbiology. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding US Centers for Disease Control and Prevention Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) Cooperative Agreement and Mayo Clinic Small Grant programme.
Footnotes
Contributors
BSP and JMP did the literature search, created tables and figures, participated in study design, data collection, analysis and interpretation, co-drafted the manuscript, and edited and approved the final report. PSM, LMS, MES, LBR-K, and AJR contributed to the literature search, helped to create figures, participated in study design, data collection, analysis and interpretation, and culture edited and approved the final manuscript. DKHJ, JPD, DFN, and ES contributed to literature search, helped to create figures, participated in data collection, analysis and interpretation, and edited and approved the final manuscript. SMP, JAR, JB, CRS, AD, XL, TKM, MAF, EST, RP, LCK, and CLI participated in data collection, analysis and interpretation, and edited and approved the final manuscript.
References
- 1.Baranton G, Postic D, Saint Girons I, et al. Delineation of Borrelia burgdorferi sensu stricto Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Bartenhagen NH, Craft JE, et al. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RC, Schmid GP, Hyde FW, Steigerwalt AG, Brenner DJ. Borrelia burgdorferi sp. nov.: etiologic agent of lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 5.Lindgren E, Jaenson TGT. Influences of climate and climate change, epidemiology, ecology and adaptation measures. Copenhagen, Denmark: World Health Organization; 2006. Lyme borreliosis in Europe. [Google Scholar]
- 6.Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 8.van Dam AP, Kuiper H, Vos K, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 9.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liveris D, Schwartz I, McKenna D, et al. Quantitation of cell-associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur J Clin Microbiol Infect Dis. 2012;31:791–795. doi: 10.1007/s10096-011-1376-x. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin MS, Schwan TG, Anderson DE, Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–468. viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babady NE, Sloan LM, Vetter EA, Patel R, Binnicker MJ. Percent positive rate of Lyme real-time polymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagn Microbiol Infect Dis. 2008;62:464–466. doi: 10.1016/j.diagmicrobio.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Hu LT, Pratt SD, Perides G, Katz L, Rogers RA, Klempner MS. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect Immun. 1997;65:4989–4995. doi: 10.1128/iai.65.12.4989-4995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margos G, Hojgaard A, Lane RS, et al. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010;1:151–158. doi: 10.1016/j.ttbdis.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kugeler KJ, Gurfield N, Creek JG, Mahoney KS, Versage JL, Petersen JM. Discrimination between Francisella tularensis and Francisella-like endosymbionts when screening ticks by PCR. Appl Environ Microbiol. 2005;71:7594–7597. doi: 10.1128/AEM.71.11.7594-7597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudenko N, Golovchenko M, Lin T, Gao L, Grubhoffer L, Oliver JH., Jr Delineation of a new species of the Borrelia burgdorferi sensu lato complex Borrelia americana sp. nov. J Clin Microbiol. 2009;47:3875–3880. doi: 10.1128/JCM.01050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Liveris D, Mukherjee P, Jungnick S, Margos G, Schwartz I. Molecular typing of Borrelia burgdorferi. Curr Protoc Microbiol. 2014;34:12C.5.1–12C.5.31. doi: 10.1002/9780471729259.mc12c05s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Mor B Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 20.Cao WC, Gao YM, Zhang PH, et al. Identification of Ehrlichia chaffeensis by nested PCR in ticks from southern China. J Clin Microbiol. 2000;38:2778–2780. doi: 10.1128/jcm.38.7.2778-2780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margos G, Vollmer SA, Cornet M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molloy PJ, Telford SR, III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 23.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis. 2006;43:704–710. doi: 10.1086/506936. [DOI] [PubMed] [Google Scholar]
- 27.Strle F, Ruzic-Sabljic E, Logar M, et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis. 2011;11:1253–1258. doi: 10.1089/vbz.2010.0230. [DOI] [PubMed] [Google Scholar]
- 28.Logar M, Ruzic-Sabljic E, Maraspin V, et al. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–19. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 29.Dworkin MS, Anderson DE, Jr, Schwan TG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 30.Hamer SA, Hickling GJ, Walker ED, Tsao JI. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the midwestern United States. Infect Genet Evol. 2014;27:531–542. doi: 10.1016/j.meegid.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Hoen AG, Margos G, Bent SJ, et al. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci USA. 2009;106:15013–15038. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.