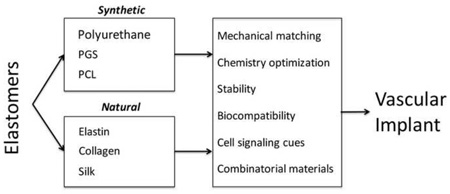
Abstract
Elastomers are popular in vascular engineering applications, as they offer the ability to design implants that match the compliance of native tissue. By mimicking the natural tissue environment, elastic materials are able to integrate within the body to promote repair and avoid the adverse physiological responses seen in rigid alternatives that often disrupt tissue function. The design of elastomers has continued to evolve, moving from a focus on long term implants to temporary resorbable implants that support tissue regeneration. This has been achieved through designing chemistries and processing methodologies that control material behavior and bioactivity, while maintaining biocompatibility in vivo. Here we review the latest developments in synthetic and natural elastomers and their application in cardiovascular treatments.
Graphical Abstract
Introduction
Successful tissue engineering strategies have an inherent requirement to appropriately mimic the natural mechanical properties and signaling cues of host tissues. This is particularly relevant for the vasculature, which presents a dynamic environment that is highly sensitive to compliance mismatch [1]. This requirement for mechanical compliance can be achieved through the use of elastic materials, tailored to match the native tissue to facilitate integration. Two main classes of elastomers exist for cardiovascular engineering: synthetic polymer materials and natural proteins that are often derived from the tissues they are seeking to repair. In selecting an appropriate elastomer for a given application, a number of properties must be considered. The ability to tailor the material mechanically, control degradation to determine implant lifespan, design signaling cues to promote bio-integration and ensure biocompatibility with the local environment are all critical in dictating the ultimate success of a given implant.
Synthetic elastomers
Polyurethanes
Polyurethanes (PUs) are a broad group of copolymer materials composed of aliphatic or aromatic units linked with polar urethane groups. PUs are synthesized through the poly-addition of long chain polyols, di- or triisocyanates combined with a short chain extender to increase mechanical strength [2]. When higher mechanical strength is required, precursors with multiple functional groups are used to produce a 3D cross-linked final structure. Given the range of chemistries that can be employed, formulations of PUs can exhibit a wide range of mechanical and biological properties [3].
In vitro assessments of PU cytocompatibility have demonstrated that a range of cell types such as epithelial, endothelial and fibroblasts attach and proliferate well on PU surfaces [4]. When exposed to blood, PUs present a hemocompatible surface, resisting thrombosis to make them a candidate for vascular applications [5].
The design of PUs has undergone a substantial philosophical change over recent time. Early formulations designed to serve as long term vascular implants suffered from adverse responses, with unforeseen biodegradation due to hydrolysis, micro cracking and enzymatic degradation [6]. Along with weakening the material structure, the degraded products of these chemistries were cytotoxic, resulting in their clinical failure [4]. Long term PU formulations have since seen various improvements, including reduced byproduct cytotoxicity by transitioning away from the use of aromatic diisocyanates. These formulations have also been employed as vascular drug delivery vehicles that show promise in vitro [7]. Clinically, long-term PUs remain a popular choice in the construction of catheters (Vialon), where their smooth surface simplifies insertion and provides a low resistance to flow that resists thrombosis [8].
Modern approaches to PU design have also focused on producing biodegradable PUs designed to operate as temporary healing agents to promote tissue repair [9]. By utilizing high elasticity PUs, thin patterned film cardiac patches have been produced that support cardiomyocyte attachment and growth in linearly aligned organizations. When implanted, these cell-laden films restore contractile tissue of cardiac muscle post infarction, and may be later resorbed, leaving behind only the healthy tissue [10]. PUs have also been applied to the construction of various small diameter vascular conduits, where they display 40% patency in a rat model after 8 weeks. These are further enhanced with the addition of 2-methacryloyloxyethyl phosphorylcholine (MPC), which improves patency to 92% [11], highlighting the benefit of combinatorial drug delivery approaches to further promoting PU biocompatibility.
PCL
Poly(ε-caprolactone) (PCL) is a bioresorbable polymer that is used in vascular medicine on account of its ease of production, mechanical properties and generally favorable biocompatibility [4]. PCL is synthesized by processing ε- caprolactone or 2-methylene-2-3-dioxepane precursors through catalyst or free radical-driven ring-opening polymerization techniques. The resulting PCL is a hydrophobic, semi-crystalline polymer, which is highly miscible and simple to manipulate [12].
While initially overlooked due to its slow degradation rate (2–3 years), new appreciations for the benefits of PCL have emerged on account of its rheological and viscoelastic properties. This, coupled with its relatively low cost of production and FDA approval have seen it increasingly used in combination with other polymers destined for vascular use [13].
When designing PCL grafts, the method of manufacture is vitally important in establishing correct mechanical behavior for the implantation site [14]. For example, cast PCL undergoes plastic deformation when exposed to long-term cyclic strain, making it unsuitable for vascular graft construction. This issue is averted by producing electrospun PCL grafts that have improved elasticity and better mimic ECM fiber morphology compared to their cast counterparts [15].
Electrospun PCL grafts replacing the abdominal aorta in rats (1.5 – 2 mm ID) outperform similar ePTFE grafts, remaining patent to at least 18 months [16]. However, at 12 months cellular infiltration regresses, leading to the formation of calcified lesions due to insufficient metabolic exchange and signaling factors [17]. Carotid artery replacements in a pig model have also shown benefits over ePTFE in a short-term study [18]. These studies demonstrate the potential for PCL-based grafts if concerns for long term cell viability can be addressed by providing additional cues to support cell survival.
PGS
Poly(glycerol sebacate) (PGS) has widespread application in tissue engineering on account of its thermoset elastomeric properties, biocompatibility and favorable degradation characteristics [19]. PGS is most commonly formulated from glycerol and sebacic acid, which helps underpin its biocompatibility, with both constituents naturally occurring in the body. Both these precursors also have long histories of use in vivo and FDA approval for food use and medical applications respectively [20]. Synthesis typically employs a two-step process of poly-condensation followed by crosslinking. A number of variations to this methodology have been reported, such as varying precursor concentrations and employing photo-crosslinking to allow in vivo polymerization [21].
The elastomeric properties of PGS originate from the three dimensional network of covalently cross-linked random coils within its structure and hydrogen bonding of the backbone hydroxyl groups [4]. Common techniques to vary the mechanical nature of PGS are to modify the temperature, precursor concentration and curing time during synthesis. By altering these parameters, properties such as the Young’s and tangent moduli can be altered [22]. Furthermore, PGS can be processed into a range of forms such as thin films or electrospun constructs that exhibit a wide range of mechanical and cell interactive behaviors.
Degradation of PGS occurs predominantly through surface degradation due to cleavage of ester linkages. This is advantageous in that PGS undergoes a gradual loss of mechanical strength, maintaining predictable mechanics in comparison to materials undergoing bulk degradation [19].
Growth of numerous vascular cell types has been validated on PGS including cardiomyocytes [23], aortic endothelial cells [24], smooth muscle cells [25] and fibroblasts [19]. Casting PGS for cell interactions requires some optimization, as subtle change can have profound impacts on cellular responses. Soft PGS inhibits cardiomyocyte proliferation, while pre-conditioning restores a favorable growth profile [23]. Studies of platelet attachment to assess blood compatibility have demonstrated the PGS outperforms other commonly used polymers such as ePTFE and PLGA in these tests [26]. PGS scaffolds also promote cells to express and deposit elastin and collagen to further enhance the material, increasing the tensile strain 5 fold after 3 weeks [27]. PGS-PCL copolymers designed to mimic the mechanical nature of heart valves have also been shown to promote organized endothelial cell growth [28].
PGS sheets implanted within the left ventricle completely resorb following implantation at a rate of 0.2 mm/month without any discernable immunological response [29]. Other studies support this finding, attributing the low immune response to the surface erosion degradation characteristics that operate to resist lymphocytic infiltration resulting in minimal fibrous capsule formation [30].
Natural Proteins
Collagen
Collagen is found ubiquitously throughout the body, typically organized in triple helix fibers that impart strength and flexibility to their tissue environment. Over 22 varieties of collagen have been described, with the most predominant being I–V [31]. Although it features a very high Young’s modulus (up to ~1 GPa), collagens display high resilience and reversible deformation that confirm its elastic nature [32].
Collagen is usually obtained by harvesting animal tissues, which are purified and treated with stabilizing agents such as glutaraldehyde (GA) to control degradability. Being naturally derived, special care must be taken through this process to control for batch variation, purity and the risk of transmitting infectious agents [33]. Additionally, the use of cross-linkers such as GA to stabilize materials requires optimization to avoid causing tissue calcification when implanted [34].
Collagen-based cardiac patches derived from bovine pericardium (CardioCel) [35] have allowed for the delivery of mesenchymal stem cells (MSC) in human patients for cardiac repair [36]. The Omniflow Vascular Prosthesis (OVP), created by preimplanting a silicone rod within sheep before removing and stabilizing the deposited collagen has been used as a graft for femoral artery repair and peripheral bypass with around 20,000 implants since 1990 [37]. These outperform ePTFE materials by resisting neointimal hyperplasia, however only limited endothelialization is observed in clinical explants [38].
New research has focused on transitioning towards non-animal sourced collagen to address ethical and safety concerns with techniques. Results have been promising, with synthetic collagen matching or outperforming commercial options such as Zyderm in early in vivo immunological evaluations [39].
Silk
Silk fibers are produced naturally by some arthropods and display varying mechanical properties dependent on their species of origin. Of these, the silk worm Bombyx mori is most commonly used for tissue engineering applications [40]. Silk fibers originating from B. mori are made up of a fibroin component encased within an adhesive sericin coat. Studies reporting immunological activation in response to silk sutures containing sericin proteins [41] have driven a large body of work devoted to creating biomaterials derived from the purified fibroin component. However, sericins have since been found not cause a foreign body response, which rather is initiated by the combined fibroin-sericin structure [42].
The primary structure of silk fibroin consists of repeating hydrophobic bulk domains that form beta sheets during isolation. The final properties of silk fibroin materials are therefore heavily dependent on the method of processing used, which alters the extent of beta sheet formation, and has implications on degradability of the final product [43].
In vitro cell attachment and growth on silk fibroin scaffolds is typically low, largely on account of its structure and hydrophobicity. Cultures of endothelial, fibroblast, peripheral blood mononuclear and mesenchymal stem cells on silk scaffolds display slow growth rates, however are able to infiltrate porous scaffolds once established [44].
A range of efforts have been made to improve cellular interactions with silk scaffolds. To reduce the surface hydrophobicity, oxygen plasma treatment has shown to be successful in improving cellular attachment and growth [45]. Another effective strategy has been to combine silk fibroin with extracellular matrix proteins such as collagen [46], fibronectin and elastin [47]. Through this strategy, the cell interactive and mechanical properties are improved, with silk fibroin – elastin films showing dramatically (1.3 MPa vs ~6 GPa) greater elasticity, resembling that of native vascular tissue [48].
Degradation of silk is influenced by the properties of the material, such as beta-sheet content, location of the implant and the method of fabrication. The primary mechanism for silk degradation in vivo is through the actions of proteases (collagenase, protease K, alpha-chymotrypsin), which generally target regions between beta sheets [49]. Silk fibroin structures that allow the infiltration of immune cells such as macrophages will undergo remodeling and degradation [50]. This immunological action is due to activation of the complement cascade by silk fibroin materials, which generally persists through to 14 days post implantation and by 12 weeks no inflammatory cells are present [51,52].
When electrospun into small diameter vessels (1–3 mm diameter), silk fibroin grafts display good biocompatibility, remaining patent after 1 month and 85% patent after 12 months, outperforming PTFE control grafts in a rat abdominal aortic model [53]. Further improvements continue to be investigated to enhance endothelialization, which require 12 weeks to achieve 90% coverage. Silk-PCL-elastin hybrids are mechanically attractive, but are yet to be tested for cell compatibility [54]. Proof-of-concept grafts incorporating heparin into the silk structure to improve hemocompatibiltiy have also shown promise, improving blood contact, while supporting cell growth [55].
Elastin
Elastin is a natural component of the vascular ECM, where it performs vital roles in providing mechanical resilience to tissues, and mediating a range of cellular processes. Within arteries, elastin comprises up to 50% of the dry weight where it persists throughout life due to its high stability, with a half-life of ~70 years [56].
The dominant component of elastic fibers is tropoelastin, a 60 kDa protein that features a well-described alternating hydrophobic-hydrophobilic domain structure[57]. Signaling interactions mediated by tropoelastin occur primarily through adhesion-based mechanisms operating through integrins, glycosaminoglycans and the elastin binding protein [58]. Cell attachment and proliferation on tropoelastin materials has been demonstrated on many cell types, including fibroblasts, endothelial cells, progenitor cells [59] and cardiomyocytes [60]. Tropoelastin materials also exhibit excellent blood compatibility, resisting both platelet adhesion and thrombus formation that make them desirable for cardiovascular engineering [58].
The introduction of recombinant technology has enabled large scale production of tropoelastin and catalyzed its use in the construction of biomedical devices. Tropoelastin is amenable to many production techniques, where its high stability allows it to maintain its structure and cell-interactive properties in harsh conditions such as those seen during electrospinning.
Cardiovascular patches fabricated from tropoelastin mimic cardiac tissue are able to support cardiomyocyte alignment and function, which begin beating on the elastic substrate [61]. Compliance validations in vivo also demonstrate that electrospun tropoelastin – PCL arterial grafts maintain their mechanical properties post implantation, with compliance, elastic modulus and burst pressure remaining unchanged in a rabbit carotid interposition model [62]. Tropoelastin coatings in a sheep carotid model display 79% less neointimal hyperplasia than ePTFE grafts and enhanced endothelialization, addressing the major issues facing current generation grafts [63].
Future Directions
In selecting a graft material for a given location, a main criterion is achieving compliance with the native site. More elastic candidate materials such as PGS, collagen and elastin offer the opportunity for closely matching arterial compliance. For instance, these materials all offer a Young’s moduli between 0.5–1 MPa, placing them at an appropriate range of arterial blood vessels, such as the common carotid artery (750kPa) [65]. Less elastic materials such as PU, PCL and silk have utility when used together with more elastic materials. Here, they can enhance the mechanical strength of the overall material, increasing properties such as burst pressure, while maintaining appropriately matched elasticity to produce in a more resilient final graft [67].
Elastomers represent clear advantages when engineering constructs destined for vascular biological tissues. There continues to be exciting advancements with optimized chemistries and new methods of fabrication allowing for well-tailored biocompatible materials that outperform current vascular treatment standards in pre-clinical tests. Ongoing investigations that employ a combinatorial approach to produce hybrid materials that extract greatest benefits from each will continue to be explored and are likely to shape the next generation of patient treatment.
Highlights.
-
-
Elastic materials can mechanically match the dynamic tissues found in the vasculature
-
-
Elastomers can be processed using different techniques such as casting or electrospinning to tailor their properties
-
-
Synthetic options offer lower costs of production, but natural elastomers often contain important cell signaling cues
-
-
Current trends aim at producing temporary constructs with controlled degradation
-
-
Combinatorial approaches utilizing multiple elastomers or secondary components such as drugs show the greatest promise
Acknowledgments
The authors gratefully acknowledge Dr. Suzanne Mithieux for her insightful comments and assistance in preparing this manuscript. We acknowledge support from the Australian Research Council, National Health & Medical Research Council, National Institutes of Health EB014283 and Wellcome Trust 103328.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kapadia MR, Popowich DA, Kibbe MR. Modified prosthetic vascular conduits. Circulation. 2008;117:1873–1882. doi: 10.1161/CIRCULATIONAHA.107.714170. [DOI] [PubMed] [Google Scholar]
- 2.Krol P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Progress in Materials Science. 2007;52:915–1015. [Google Scholar]
- 3.Kucinska-Lipka J, Gubanska I, Janik H, Sienkiewicz M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater Sci Eng C Mater Biol Appl. 2015;46:166–176. doi: 10.1016/j.msec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Liang S, Thouas GA. Elastomeric biomaterials for tissue engineering. Progress in Polymer Science. 2013;38:584–671. [Google Scholar]
- 5.Fromstein JD, Woodhouse KA. Elastomeric biodegradable polyurethane blends for soft tissue applications. Journal of Biomaterials Science, Polymer Edition. 2002;13:391–406. doi: 10.1163/156856202320253929. [DOI] [PubMed] [Google Scholar]
- 6.Zdrahala RJ, Zdrahala IJ. Biomedical Applications of Polyurethanes: A Review of Past Promises, Present Realities, and a Vibrant Future. Journal of Biomaterials Applications. 1999;14:67–90. doi: 10.1177/088532829901400104. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Farah S, Domb AJ, Lelkes PI. Electrospun rapamycin-eluting polyurethane fibers for vascular grafts. Pharm Res. 2013;30:1735–1748. doi: 10.1007/s11095-013-1016-5. [DOI] [PubMed] [Google Scholar]
- 8.Uslusoy E, Mete S. Predisposing factors to phlebitis in patients with peripheral intravenous catheters: a descriptive study. J Am Acad Nurse Pract. 2008;20:172–180. doi: 10.1111/j.1745-7599.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 9.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 10.Alperin C, Zandstra PW, Woodhouse KA. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials. 2005;26:7377–7386. doi: 10.1016/j.biomaterials.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 11.Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR, Vorp DA. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res A. 2011;96:436–448. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ninago MD, Lopez OV, Lencina MM, Garcia MA, Andreucetti NA, Ciolino AE, Villar MA. Enhancement of thermoplastic starch final properties by blending with poly(epsilon-caprolactone) Carbohydr Polym. 2015;134:205–212. doi: 10.1016/j.carbpol.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Zhao G. Mechanical and thermal properties of conventional and microcellular injection molded poly (lactic acid)/poly (epsilon-caprolactone) blends. J Mech Behav Biomed Mater. 2015;53:59–67. doi: 10.1016/j.jmbbm.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Brugmans M, Soekhradj-Soechit S, van Geemen D, Cox MA, Bouten C, Baaijens F, Driessen-Mol A. Superior tissue evolution in slow degrading scaffolds for valvular tissue engineering. Tissue Eng Part A. 2015 doi: 10.1089/ten.TEA.2015.0203. [DOI] [PubMed] [Google Scholar]
- 15.Nottelet B, Pektok E, Mandracchia D, Tille JC, Walpoth B, Gurny R, Moller M. Factorial design optimization and in vivo feasibility of poly(epsilon-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J Biomed Mater Res A. 2009;89:865–875. doi: 10.1002/jbm.a.32023. [DOI] [PubMed] [Google Scholar]
- 16.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, Walpoth BH. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–2570. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
- 17.de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Moller M, Walpoth BH. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Mrowczynski W, Mugnai D, de Valence S, Tille JC, Khabiri E, Cikirikcioglu M, Moller M, Walpoth BH. Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J Vasc Surg. 2014;59:210–219. doi: 10.1016/j.jvs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2003;66:192–197. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 20.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A. 2010;94:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 21.Nijst CL, Bruggeman JP, Karp JM, Ferreira L, Zumbuehl A, Bettinger CJ, Langer R. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate) Biomacromolecules. 2007;8:3067–3073. doi: 10.1021/bm070423u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsak AG, Dunn AM, Hollister SJ. Mechanical characterization and non-linear elastic modeling of poly(glycerol sebacate) for soft tissue engineering. J Mech Behav Biomed Mater. 2012;11:3–15. doi: 10.1016/j.jmbbm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Chen QZ, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, Chrzanowski W, Boccaccini AR, Ali NN, Knowles JC, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885–3893. doi: 10.1016/j.biomaterials.2010.01.108. [DOI] [PubMed] [Google Scholar]
- 24.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT. Microfabrication of poly (glycerol-sebacate) for contact guidance applications. Biomaterials. 2006;27:2558–2565. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Ensley AE, Nerem RM, Wang Y. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J Biomed Mater Res A. 2007;83:1070–1075. doi: 10.1002/jbm.a.31434. [DOI] [PubMed] [Google Scholar]
- 26.Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27:4315–4324. doi: 10.1016/j.biomaterials.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Crapo P, Nerem R, Wang Y. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J Biomed Mater Res A. 2008;85:1120–1128. doi: 10.1002/jbm.a.32028. [DOI] [PubMed] [Google Scholar]
- 28.Gaharwar AK, Nikkhah M, Sant S, Khademhosseini A. Anisotropic poly (glycerol sebacate)-poly (-caprolactone) electrospun fibers promote endothelial cell guidance. Biofabrication. 2015;7:015001. doi: 10.1088/1758-5090/7/1/015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuckey DJ, Ishii H, Chen QZ, Boccaccini AR, Hansen U, Carr CA, Roether JA, Jawad H, Tyler DJ, Ali NN, et al. Magnetic resonance imaging evaluation of remodeling by cardiac elastomeric tissue scaffold biomaterials in a rat model of myocardial infarction. Tissue Eng Part A. 2010;16:3395–3402. doi: 10.1089/ten.TEA.2010.0213. [DOI] [PubMed] [Google Scholar]
- 30.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys. 2011;49:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic proteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramshaw JA. Biomedical applications of collagens. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33541. [DOI] [PubMed] [Google Scholar]
- 34.Neethling WM, Glancy R, Hodge AJ. Mitigation of Calcification and Cytotoxicity of a Glutaraldehyde-Preserved Bovine Pericardial Matrix: Improved Biocompatibility After Extended Implantation in the Subcutaneous Rat Model. J Heart Valve Dis. 2010;19:778–785. [PubMed] [Google Scholar]
- 35.Neethling WM, Strange G, Firth L, Smit FE. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: initial experience with the ADAPT-treated CardioCel(R) patch. Interact Cardiovasc Thorac Surg. 2013;17:698–702. doi: 10.1093/icvts/ivt268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashi AV, White JF, McLean KM, Neethling WM, Rhodes DI, Ramshaw JA, Werkmeister JA. Evaluation of an established pericardium patch for delivery of mesenchymal stem cells to cardiac tissue. J Biomed Mater Res A. 2015;103:1999–2005. doi: 10.1002/jbm.a.35335. [DOI] [PubMed] [Google Scholar]
- 37.Edwards GA, Roberts G. Development of an ovine collagen-based composite biosynthetic vascular prosthesis. Clin Mater. 1992;9:211–223. doi: 10.1016/0267-6605(92)90102-y. [DOI] [PubMed] [Google Scholar]
- 38.Werkmeister JA, White JF, Ramshaw JA. Evaluation of the Omniflow collagen-polymer vascular prosthesis. Med Prog Technol. 1994;203:231–242. [PubMed] [Google Scholar]
- 39.Peng YY, Yoshizumi A, Danon SJ, Glattauer V, Prokopenko O, Mirochnitchenko O, Yu Z, Inouye M, Werkmeister JA, Brodsky B, et al. A Streptococcus pyogenes derived collagen-like protein as a non-cytotoxic and non-immunogenic cross-linkable biomaterial. Biomaterials. 2010;31:2755–2761. doi: 10.1016/j.biomaterials.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leal-Egana A, Scheibel T. Silk-based materials for biomedical applications. Biotechnol Appl Biochem. 2010;55:155–167. doi: 10.1042/BA20090229. [DOI] [PubMed] [Google Scholar]
- 41.Zaoming W, Codina R, Fernández-Caldas E, Lockey RF. Partial characterization of the silk allergens in mulberry silk extract. J Investig Allergol Clin Immunol. 1996;6:237–241. [PubMed] [Google Scholar]
- 42.Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng. 2009;107:556–561. doi: 10.1016/j.jbiosc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Rnjak-Kovacina J, DesRochers TM, Burke KA, Kaplan DL. The effect of sterilization on silk fibroin biomaterial properties. Macromol Biosci. 2015;15:861–874. doi: 10.1002/mabi.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956–2965. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Jeong L, Yeo IS, Kim HN, Yoon YI, Jang da H, Jung SY, Min BM, Park WH. Plasma-treated silk fibroin nanofibers for skin regeneration. Int J Biol Macromol. 2009;44:222–228. doi: 10.1016/j.ijbiomac.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Kim EY, Tripathy N, Cho SA, Joo CK, Lee D, Khang G. Bioengineered neo-corneal endothelium using collagen type-I coated silk fibroin film. Colloids Surf B Biointerfaces. 2015;136:394–401. doi: 10.1016/j.colsurfb.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 47.Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31:8121–8131. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teng W, Cappello J, Wu X. Recombinant Silk-Elastin like Protein Polymer Displays Elasticity Comparable to Elastin. Biomacromolecules. 2009;10:3028–3036. doi: 10.1021/bm900651g. [DOI] [PubMed] [Google Scholar]
- 49.Brown J, Lu CL, Coburn J, Kaplan DL. Impact of silk biomaterial structure on proteolysis. Acta Biomater. 2015;11:212–221. doi: 10.1016/j.actbio.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta S, Park SH, Seok GE, Patel A, Numata K, Lu CL, Kaplan DL. Quantifying osteogenic cell degradation of silk biomaterials. Biomacromolecules. 2010;11:3592–3599. doi: 10.1021/bm101054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Etienne O, Schneider A, Kluge JA, Bellemin-Laponnaz C, Polidori C, Leisk GG, Kaplan DL, Garlick JA, Egles C. Soft tissue augmentation using silk gels: an in vitro and in vivo study. J Periodontol. 2009;80:1852–1858. doi: 10.1902/jop.2009.090231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–155. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 53.Aytemiz D, Sakiyama W, Suzuki Y, Nakaizumi N, Tanaka R, Ogawa Y, Takagi Y, Nakazawa Y, Asakura T. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv Healthc Mater. 2013;2:361–368. doi: 10.1002/adhm.201200227. [DOI] [PubMed] [Google Scholar]
- 54.McClure MJ, Simpson DG, Bowlin GL. Tri-layered vascular grafts composed of polycaprolactone, elastin, collagen, and silk: Optimization of graft properties. J Mech Behav Biomed Mater. 2012;10:48–61. doi: 10.1016/j.jmbbm.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Seib FP, Herklotz M, Burke KA, Maitz MF, Werner C, Kaplan DL. Multifunctional silk-heparin biomaterials for vascular tissue engineering applications. Biomaterials. 2014;35:83–91. doi: 10.1016/j.biomaterials.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersen E, Wågberg F, Ängquist KA. Serum Concentrations of Elastin-derived Peptides in Patients with Specific Manifestations of Atherosclerotic Disease. European Journal of Vascular and Endovascular Surgery. 2002;24:440–444. doi: 10.1053/ejvs.2002.1750. [DOI] [PubMed] [Google Scholar]
- 57.Wise SG, Yeo GC, Hiob MA, Rnjak-Kovacina J, Kaplan DL, Ng MK, Weiss AS. Tropoelastin: a versatile, bioactive assembly module. Acta Biomater. 2014;10:1532–1541. doi: 10.1016/j.actbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiob MA, Wise SG, Kondyurin A, Waterhouse A, Bilek MM, Ng MK, Weiss AS. The use of plasma-activated covalent attachment of early domains of tropoelastin to enhance vascular compatibility of surfaces. Biomaterials. 2013;34:7584–7591. doi: 10.1016/j.biomaterials.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Y, Wise SG, Michael PL, Bax DV, Yuen GS, Hiob MA, Yeo GC, Filipe EC, Dunn LL, Chan KH, et al. Characterization of Endothelial Progenitor Cell Interactions with Human Tropoelastin. PLoS One. 2015;10:e0131101. doi: 10.1371/journal.pone.0131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Annabi N, Selimovic S, Acevedo Cox JP, Ribas J, Afshar Bakooshli M, Heintze D, Weiss AS, Cropek D, Khademhosseini A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip. 2013;13:3569–3577. doi: 10.1039/c3lc50252j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater. 2013;23:4950–4959. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Weiss AS, Ng MK. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Byrom M, Wise S, Liu H, Bao B, Bilek M, Weiss T, Bannon P, Ng M. Enhancement of Biocompatibility of Synthetic Vascular Grafts by Covalent Immobilisation of Recombinant Human Tropoelastin. Heart, Lung and Circulation. 2012;21:1. [Google Scholar]
- 64.Gamble G, Zorn J, Sanders G, MacMahon S, Sharpe N. Estimation of Arterial Stiffness, Compliance, and Distensibility From M-Mode Ultrasound Measurements of the Common Carotid Artery. Stroke. 1994;25:11–16. doi: 10.1161/01.str.25.1.11. [DOI] [PubMed] [Google Scholar]
- 65.Khamdaeng T, Luo J, Vappou J, Terdtoon P, Konofagou EE. Arterial stiffness identification of the human carotid artery using the stress-strain relationship in vivo. Ultrasonics. 2012;52:402–411. doi: 10.1016/j.ultras.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed M, Ramos TA, Damanik F, Quang Le B, Wieringa P, Bennink M, van Blitterswijk C, de Boer J, Moroni L. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci Rep. 2015;5:14804. doi: 10.1038/srep14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melchiorri AJ, Hibino N, Fisher JP. Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Eng Part B Rev. 2013;19:292–307. doi: 10.1089/ten.teb.2012.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]