Abstract
Scytalidium dimidiatum is a pigmented dematiaceous coelomycete that typically causes chronic superficial skin diseases and onychomycosis, as well as deeper infections, such as subcutaneous abscesses, mycetoma, and even fungemia in immunocompromised patients. A second species, Scytalidium hyalinum, has hyaline hyphae and arthroconidia and is considered by some authors to be an albino mutant of S. dimidiatum. This study aimed to confirm the presence of melanin or melanin-like compounds (which have been previously implicated in the virulence of other fungal pathogens) in S. dimidiatum from a patient with multiple subcutaneous nodules. Treatment of the hyphae and arthroconidia with proteolytic enzymes, denaturant, and concentrated hot acid yielded dark particles, which were stable free radicals, consistent with their identification as melanins. Extracted melanin particles from S. dimidiatum cultures were labeled by melanin-binding monoclonal antibodies (MAbs) from Sporothrix schenckii, Aspergillus fumigatus, and Cryptococcus neoformans. Lesional skin from the patient infected with S. dimidiatum contained fungal cells that were labeled by melanin-binding MAbs, and digestion of the tissue yielded dark particles that were also reactive. S. hyalinum was also subjected to the melanin extraction protocol, but no dark particles were yielded.
Scytalidium dimidiatum (synanamorph, Nattrassia mangiferae, formerly known as Hendersonula toruloidea) is a thermotolerant fungal plant pathogen that can cause superficial skin and nail infections that mimic those caused by dermatophytes (7). S. dimidiatum can also rarely cause deep subcutaneous infections and disseminated disease, mainly in immunosuppressed patients (2, 17, 31). Subclinical infection is thought to be common in parts of Africa, India, and the Caribbean, where the fungus lives in the soil and on plants (1, 11, 23). S. dimidiatum forms a rapidly growing brown-black colony, which is cycloheximide sensitive (11).
Melanins are enigmatic high-molecular-weight pigments that are produced by a wide variety of microorganisms. They are typically dark brown or black and are insoluble in aqueous and organic solvents and are therefore difficult to study by conventional biochemical techniques (9). Melanin has been isolated from several important human fungal pathogens and is now recognized as an important virulence determinant (21).
In addition, a second species, Scytalidium hyalinum, has also been isolated from human sources (16), although there has been considerable debate in the literature as to whether S. dimidiatum and S. hyalinum are the same or distinct species. Some morphological and genetic characteristics (such as mycelium pigmentation, intronic insertions in the 18S gene, and the A-G polymorphism [16]), apparently do differentiate these species. In contrast, Roeijmans et al. considered S. hyalinum to be a synonym of S. dimidiatum after conducting molecular taxonomy studies using restriction fragment length polymorphisms (26). Indeed, most authors agree that S. hyalinum may be a melanin-deficient cultural mutant of S. dimidiatum (18, 25, 32).
S. dimidiatum was isolated from a renal transplant patient with recalcitrant cutaneous lesions (J. M. Hextall, D. M. MacDonald, J. E. Scoble, and R. J. Hay, abstract from Br. Assoc. Dermatol. Annu. Meet., Br. J. Dermatol. 145:34, 2001). Mycological culture of this isolate grown on Sabouraud dextrose agar revealed a black colony. We were able to extract melanin-like particles for the first time from this human pathogenic fungus, using enzymes, denaturant, and hot acid. Melanin has been implicated in the pathogenesis of fungal infections and may therefore play a role in the recalcitrant nature of Scytalidium infections in humans.
CASE REPORT
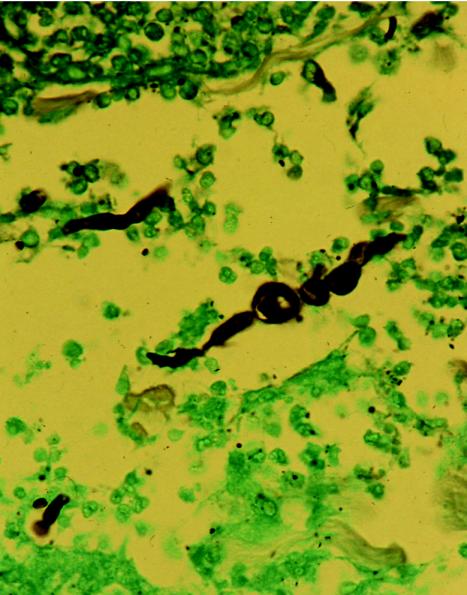
This clinical case was described previously (Hextall et al., Br. J. Dermatol. 145:34, 2001). Briefly, a 66-year-old man with a history of diabetes and renal transplantation presented with violaceous nodular lesions on the left lower leg. The histopathology from a biopsied nodule showed suppurative granulomas in the dermis and numerous fungal elements, which stained positive with methenamine silver (Grocott modification) (Fig. 1). Culture and microscopy grew a grey-brown mould, which had the typical morphology of S. dimidiatum. The patient was treated with intravenous liposomal amphotericin B (Ambisome) for 14 days; the lesions eventually regressed, and the patient was discharged on oral terbinafine to continue long-term therapy.
FIG. 1.
Grocott stain showing S. dimidiatum arthroconidia and hyphae in the dermis. Magnification, ×64.
MATERIALS AND METHODS
Fungal strains and media.
S. dimidiatum (strain 10810) from the index case was obtained from one of the authors (S. D. Morris-Jones). S. hyalinum (strain 2775) was obtained from the National Collection of Pathogenic Fungi in Bristol, United Kingdom.
Isolation and purification of S. dimidiatum hyphal and arthroconidial particles, scanning electron microscopy, transmission electron microscopy, and ESR.
Melanin particles were isolated from pigmented hyphae and arthroconidia grown for 1 week on Sabouraud dextrose agar (SAB) and minimal medium (10, 30). In brief, cells were collected and washed three times with phosphate-buffered saline (0.1 M; pH 7.5) and suspended in 1.0 M sorbitol-0.1 M sodium citrate (pH 5.5). The cells were treated in turn with lysing enzymes (from Trichoderma harzianum; Sigma Poole, Dorset, United Kingdom), 4 M guanidine thiocyanate (denaturant), and proteinase K (Roche Laboratories, Lewes, Sussex, United Kingdom). The resultant material was then boiled in 6 M HCl, washed, and collected. Scanning and transmission electron microscopy and electron spin resonance (ESR) spectroscopy (using a Gunn diode as the microwave source) analyses of melanin particles were then performed, as previously described (4, 30).
Immunofluorescence analysis of melanin expression.
Melanin particles derived from S. dimidiatum were fixed to slides and blocked with Superblock (Roche) overnight at 4°C. Slide cultures of S. dimidiatum and S. hyalinum were prepared as described previously (19) and blocked as described above. All slides were then incubated for 2 h at 37°C with 10 μg of either the anti-Sporothrix schenckii melanin monoclonal antibody (MAb) 8B5 or the melanin-binding MAb 6D2. The anti-S. schenckii melanin MAb 8B5 had been previously generated against S. schenckii yeast cell melanin particles (19), and MAb 6D2 was raised against melanin from Cryptococcus neoformans (29). The slides were washed in phosphate-buffered saline, incubated in a 1:100 dilution of fluorescein isothiocyanate (FITC) GAM-immunoglobulin M (IgM) (Stratech Scientific, West Grove, Pa.) for 2 h at 37°C, and then washed again. Negative controls consisted of either the irrelevant antibody 5C11 (IgM), which binds to lipoarabinomannan of mycobacteria (8), as the primary antibody or FITC-labeled antibody alone. To examine in vivo expression of S. dimidiatum melanin, paraffin-embedded samples from lesional skin of the patient were sectioned. The sections were deparaffinized, rehydrated, treated with 20 μg of proteinase K/ml for 1 h at 21°C, and then heated in citric acid in a microwave for 5 min. The slides were blocked and labeled as for the in vitro work with the same negative controls described above. The paraffin-embedded sections from the patient were also subjected to the melanin extraction protocol. Normal human skin was used as a negative control. S. dimidiatum melanin particles were isolated from the infected tissue and air dried on APES (3-aminopropyltriethoxysilane)-covered slides. The slides were then probed with the various anti-melanin MAbs and FITC GAM-IgM as described above. Wild-type S. schenckii (Mel+) and its albino mutant (Mel−) were used as positive and negative controls (19). The wild-type C. neoformans JEC21 grown in the presence of l-3,4-dihydroxyphenylalanine produces melanin and was also used as a positive control, and the C. neoformans albino mutant MHC6 (melanin deficient) was used as an additional negative control (29).
RESULTS
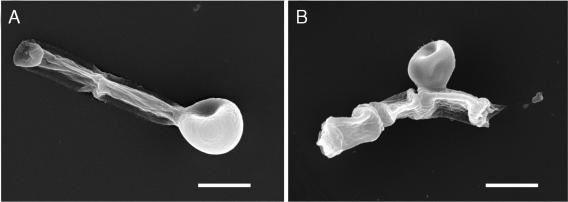
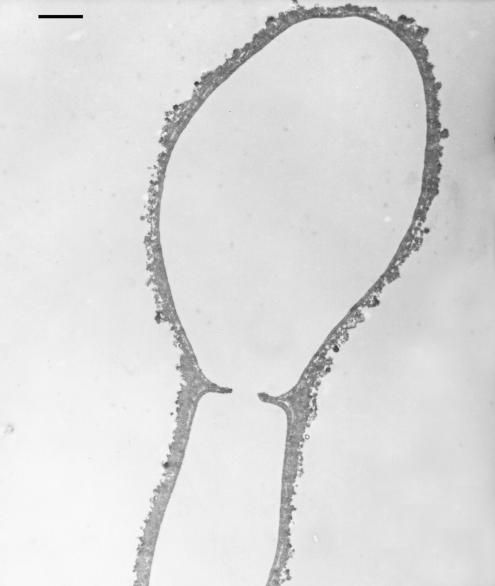
Melanization of S. dimidiatum hyphae and arthroconidia. Mycelia of isolate 10810 were visibly pigmented when grown on minimal medium and Sabouraud dextrose agar for 3 days. Black particles that were of a shape and size similar to the original propagules (hyphae and arthroconidia) were isolated from S. dimidiatum cultures that were subjected to the extraction protocol, as demonstrated by scanning electron microscopy (Fig. 2). Transmission electron microscopy of S. dimidiatum hyphae and arthroconidia extracted with enzymes, denaturant, and hot acid (Fig. 3) showed a layer of electron-dense granules enclosing a void. Mycelia of S. hyalinum (strain 2775) were white when grown on minimal medium or Sabouraud dextrose agar. The hyaline arthroconidia and hyphae of S. hyalinum were also subjected to the melanin extraction protocol, on completion of which no particles were retrieved from the 6 M HCl, as the fungus had been completely solubilized.
FIG. 2.
Scanning electron microscopy of S. dimidiatum hyphae and arthroconidia before (A) and after (B) treatment with enzymes, denaturant, and hot acid. (A) S. dimidiatum culture. (B) Extracted melanin particles. Bars, 5 μm.
FIG. 3.
Transmission electron micrograph of S. dimidiatum ghost showing outer melanin layers. Bar, 0.5 μm.
ESR spectroscopy.
ESR spectroscopy of the melanin-like particles collected from S. dimidiatum produced a stable free-radical population consistent with the pigment being melanin (Fig. 4). The spectrum was virtually identical to the signals generated by melanin previously extracted from C. neoformans (35), Paracoccidioides brasiliensis (10), and Histoplasma capsulatum (22).
FIG. 4.
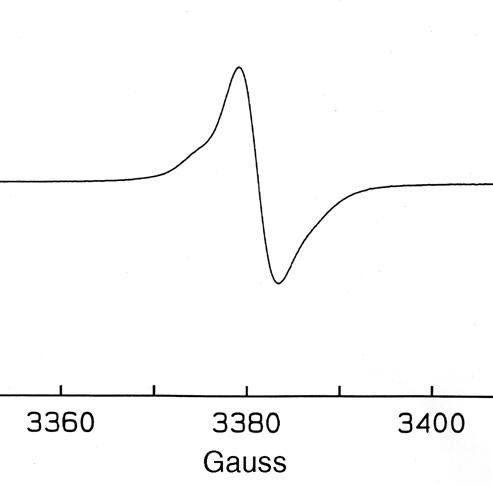
ESR spectroscopy of melanin particles collected from S. dimidiatum grown for 7 days in Sabouraud dextrose agar.
Immunofluorescence analysis.
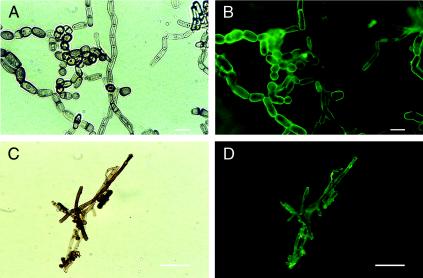
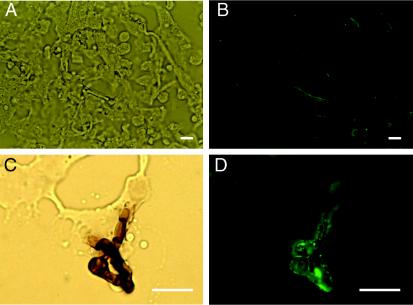
The anti-S. schenckii melanin MAb 8B5 bound to the surfaces of the pigmented hyphae and arthroconidia (Fig. 5A and B), as did MAb 6D2. Melanin-like particles extracted from mycelia were reactive to the anti-melanin MAb 8B5 (Fig. 5C and D). Neither of the anti-melanin MAbs bound to the arthroconidia and hyphae of S. hyalinum.
FIG. 5.
Corresponding bright-field (A) and immunofluorescence (B) microscopic images of S. dimidiatum from slide culture (in vitro) were reacted with anti-S. schenckii melanin MAb 8B5. Corresponding bright-field (C) and immunofluorescence (D) images of melanin particles extracted from S. dimidiatum preparations reacted with anti-S. schenckii melanin MAb 8B5. Bars, 10 μm.
Isolation of melanin particles from infected tissue.
Tissue sections from the skin of the index case patient infected with S. dimidiatum were stained with methenamine silver (Grocott modification) to confirm the presence of fungus (Fig. 1). Anti-melanin MAbs demonstrated reactivity to S. dimidiatum cells in infected tissue (Fig. 6 A and B), which was absent when FITC-labeled GAM-IgM was used alone or when isotype-matched negative control MAb 5C11 (IgM) was used. Treatment of infected human tissue using the melanin extraction protocol resulted in the isolation of dark particles. No particles resembling S. dimidiatum were isolated from normal human skin. The pigmented particles reacted with anti-melanin MAbs in a manner similar to the in vitro-isolated particles (Fig. 6C and D).
FIG. 6.
Corresponding bright-field (A) and immunofluorescence (B) microscopic images of human skin (in vivo) showing labeling of S. dimidiatum by MAb 8B5 (anti-S. schenckii melanin MAb). Corresponding bright-field (C) and immunofluorescence (D) images showing melanin particles recovered from human skin also labeled with MAb 8B5. Bars, 5 μm.
DISCUSSION
S. dimidiatum lives on the roots of certain plants, mainly of the genera Plantus and Pinus (14). It is relatively thermotolerant and can cause significant plant pathology, especially of fruit trees. Since the first report of human disease caused by H. toruloides (S. dimidiatum) (7), there have been numerous case reports from areas of endemicity. In humans, S. dimidiatum is associated with sporadic, as well as epidemic, disease. S. dimidiatum most commonly causes chronic cutaneous infections, but it has been reported to cause mycetoma, subcutaneous abscesses, fungemia, and endophthalmitis following trauma (31). . Two previous cases of S. dimidiatum causing subcutaneous abscesses were reported in patients with underlying diabetes (3; D. A. McGough, C. R. Bodem, K. Fawcett, P. Moody, A. W. Fothergill, and M. G. Rinaldi, Abstr. 92nd Gen. Meet. Am. Soc. Microbiol. 1992, abstr. F-26, p. 503). More recently, cases have been reported in immunosuppressed patients with AIDS (17).
The first description of S. dimidiatum (32) documented its tendency to form colonies with dark pigmentation. Subsequently, there have been numerous reports of a white, pigment-deficient variant, namely, S. hyalinum, being isolated from human infections (19). Recent work has suggested that members of the genus Scytalidium may synthesize melanin—thus, Scytalidium species produce scytalols A to D, which are modulators of melanin biosynthesis (5, 33). In addition, the enzyme laccase (which is involved in melanization [30]) has been isolated from Scytalidium thermophilum (39). However, no comprehensive study has been done to confirm the presence of melanin, despite the potentially important role that the pigment may play in pathogenesis. There is an increasing body of evidence that melanization prolongs the survival of fungi in the environment (9). Thus, C. neoformans is protected from environmental insults, such as UV radiation, extremes of temperature, and oxygen and nitrogen free radicals, by melanin in its cell wall (27, 37, 38). Interestingly, S. dimidiatum, which is pigmented, is found in the environment, whereas the nonpigmented S. hyalinum has been found only in humans and never in the environment.
In the present study, we have confirmed experimentally that S. dimidiatum does indeed produce melanin-like pigment. Our evidence for this is as follows: (i) treatment of S. dimidiatum hyphae and arthroconidia with enzymes and chemicals resulted in the isolation of black particles that were of a shape and size similar to the original propagules; (ii) ESR spectroscopy analysis of pigmented particles showed the presence of a stable free-radical compound consistent with melanin; (iii) melanin-binding MAbs reacted with the cell surface of S. dimidiatum grown in vitro and with the pigmented particles derived from these cells, and hyphal structures were melanized, which is in contrast to H. capsulatum (22) and P. brasiliensis (10); (iv) melanin-binding MAbs reacted with the cell wall of S. dimidiatum in infected human skin; and (v) we recovered melanin-like particles that were similar in shape and size to S. dimidiatum from infected human tissue after enzymatic and chemical treatment and which were also reactive with the melanin-binding MAbs. Together these observations provide good evidence that S. dimidiatum can produce melanin in vitro and in vivo. We have also confirmed for the first time that S. hyalinum is unable to produce melanin in vitro.
There is evidence from studies of C. neoformans that melanin synthesis is associated with increased virulence (20, 28). This finding might lead us to expect only S. dimidiatum to be isolated from patients; however, the melanin-deficient S. hyalinum has also been found in vivo. This observation may initially seem contradictory to the view that fungal melanization plays a role in virulence. However, the published data pertaining to the Scytalidium species is somewhat fragmentary. In fact, S. dimidiatum and S. hyalinum can be singularly and concurrently isolated by mycological culture from individual patients (1, 12, 23). Given that S. hyalinum has not been isolated from the external environment, this strongly suggests that the latter must arise in vivo from the black S. dimidiatum, possibly through phenotypic switching, for which there is evidence from other pathogenic fungi (6, 15). In keeping with this is evidence from ribosomal gene studies that has confirmed that S. hyalinum is genetically indistinguishable from S. dimidiatum (26). Further investigations are needed to explore the enzymes and genetic components of S. hyalinum to see if it has the necessary machinery to synthesize melanin. From this information, we may be able to identify possible triggers for the production and degradation of melanin in the environment and in vivo.
S. dimidiatum infections remain highly resistant to the available antifungal drugs, despite adequate drug levels in serum and tissue (determined from MICs) (11). The reasons for this are not yet known; however, melanin has been shown to be important in resistance to antifungal drugs in C. neoformans (13, 36) and H. capsulatum (34). It is thus possible that melanization in S. dimidiatum may also play a role in drug resistance. Clinically, however, it appears that the white and the black isolates of Scytalidium are equally resistant to antifungals. This observation is in keeping with studies of Wangiella dermatitidis, in which loss of melanin did not increase susceptibility to antifungal agents (24). Our patient did not respond to treatment with itraconazole, and clinical improvement was evident only after a prolonged course of liposomal amphotericin B. His toenail clippings remained culture positive for Scytalidium up to 1 year after this treatment. The patient continues on the immunosuppressant drugs needed to protect his renal allograft, and therefore long-term secondary prophylaxis with terbinafine may be needed to prevent relapse of the cutaneous Scytalidium infection.
Acknowledgments
We thank the Wellcome Trust (United Kingdom) for supporting R. Morris-Jones. J. D. Nosanchuk and A. Casadevall were supported by National Institutes of Health grant NIH AI52733.
We thank P. Aisen from the Department of Physiology and Biophysics, Albert Einstein College of Medicine, for ESR spectroscopy of the melanin samples and A. Robson (St John's Institute of Dermatology) for dermatopathological support.
REFERENCES
- 1.Allison, V. Y., R. J. Hay, and C. K. Campbell. 1984. Hendersonula toruloidea and Scytalidium hyalinum infections in Tobago. Br. J. Dermatol. 111:371-372. [DOI] [PubMed] [Google Scholar]
- 2.Dhindsa, M. K., J. Naidu, and S. M. Singh. 1998. A case of subcutaneous infection in a patient with discoid lupus erythematosus caused by a Scytalidium synanamorph of Nattrassia mangiferae, and its treatment. Med. Mycol. 36:425-427. [PubMed] [Google Scholar]
- 3.Dickinson, G. M., T. J. Cleary, T. Sanderson, and M. R. McGinnis. 1983. First case of subcutaneous phaeophyphomycosis caused by Scytalidium lignicola in a human. J. Clin. Microbiol. 17:155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enochs, W. S., M. J. Nilges, and H. M. Swartz. 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPM) spectroscopy. Pigment Cell Res. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 5.Findlay, J. A., and D. Kwan. 1973. Scytalone (3,6,8-trihydroxytetralone), a metabolite from Scytalidium species. Can. J. Chem. 51:1617-1619. [Google Scholar]
- 6.Fries, B. C., D. L. Goldman, and A. Casadevall. 2002. Phenotypic switching in Cryptococcus neoformans. Microbes Infect. 4:1345-1352. [DOI] [PubMed] [Google Scholar]
- 7.Gentles, J. C., and E. G. Evans. 1970. Infection of the feet and nails with Hendersonula toruloidea. Sabouraudia 8:72-75. [PubMed] [Google Scholar]
- 8.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez, B. L., and J. D. Nosanchuk. 2003. Melanin and fungi. Curr. Opin. Infect. Dis. 16:91-96. [DOI] [PubMed] [Google Scholar]
- 10.Gomez, B. L., J. D. Nosanchuk, S. Diez, S. Youngchim, P. Aisen, L. E. Cano, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 69:5760-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay, R. J. 2002. Scytalidium infections. Curr. Opin. Infect. Dis. 15:99-100. [DOI] [PubMed]
- 12.Hay, R. J., and M. K. Moore. 1984. Clinical features of superficial fungal infections caused by Hendersonula toruloidea and Scytalidium hyalinum. Br. J. Dermatol. 110:677-683. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, R., T. Sugita, E. S. Jacobson and T. Shinoda. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 47:271-277. [DOI] [PubMed] [Google Scholar]
- 14.Lacaz, C. S., A. D. Pereira, E. M. Heins-Vaccari, L. C. Cuce, R. C. Benatti, and S. Nunes. 1999. Onychomycosis caused by Scytalidium dimidiatum. Report of two cases. Review of taxonomy of the synanamorph and anamorph forms of the coelomycete. Rev. Inst. Med. Trop. Sao Paulo 41:319-323. [PubMed] [Google Scholar]
- 15.Liu, H. 2002. Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int. J. Med. Microbiol. 292:299-311. [DOI] [PubMed] [Google Scholar]
- 16.Machouart-Dubach, M., C. Lacroix, C. Vaury, M. Feuillhade de Chauvin, C. Bellanné, F. Derouin, and F. Lorenzo. 2001. Nucleotide structure of the Scytalidium hyalinum and Scytalidium dimidiatum 18S subunit ribosomal RNA gene: evidence for the insertion of a group IE intron in the rDNA gene of S. dimidiatum. FEMS Microbiol. Lett. 208:187-196. [DOI] [PubMed] [Google Scholar]
- 17.Marriott, D. J., K. H. Wong, E. Aznar, J. L. Harkness, D. Cooper, and D. Muir. 1997. Scytalidium dimidiatum and Lecythophora hoffmani: unusual causes of fungal infections in a patient with AIDS. J. Clin. Microbiol. 35:2949-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, M. K., and R. J. Hay. 1986. Circulating antibodies and antigenic cross-reactivity in Hendersonula toruloidea and Scytalidium hyalinum infection. Br. J. Dermatol. 115:435-445. [DOI] [PubMed] [Google Scholar]
- 19.Morris-Jones, R., S. Youngchim, B. L. Gomez, P. Aisen, R. J. Hay, J. D. Nosanchuk, A. Casadevall, and A. J. Hamilton. 2003. Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect. Immun. 71:4026-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosanchuk, J. D., R. Ovalle, and A. Casadevall. 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 183:1093-1099. [DOI] [PubMed] [Google Scholar]
- 21.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203-223. [DOI] [PubMed] [Google Scholar]
- 22.Nosanchuk, J. D., B. L. Gomez, S. Youngchim, S. Diez, P. Aisen, R. M. Zancope-Oliveira, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2002. Histoplasma capsulatum synthesizes melanin-like pigment in vitro and during mammalian infection. Infect. Immun. 70:5124-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyeka, C. A., and I. Okoli. 2003. Isolation of dermatophytes and non-dermatophytic fungi from soil in Nigeria. Mycoses 46:318-320. [DOI] [PubMed] [Google Scholar]
- 24.Polak, A., and D. M. Dixon. 1989. Loss of melanin in Wangiella dermatitidis does not result in greater susceptibility to antifungal agents. Antimicrob. Agents Chemother. 33:1639-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rippon, J. W. 1988. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. W. B. Saunders Co., Philadelphia, Pa.
- 26.Roeijmans, H. J., G. S. De Hoog, C. S. Tan, and M. J. Figge. 1997. Molecular taxonomy and GC/MS of metabolites of Scytalidium hyalinum and Nattrassia mangiferae (Hendersonula toruloides). J. Med. Vet. Mycol. 35:181-188. [PubMed] [Google Scholar]
- 27.Rosas, A. L., and A. Casadevall. 1997. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol. Lett. 153:265-272. [DOI] [PubMed] [Google Scholar]
- 28.Rosas, A. L., J. D. Nosanchuk, and A. Casadevall. 2001. Passive immunisation with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 69:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosas, A. L., J. D. Nosanchuk, M. Feldmesser, G. M. Cox, H. C. McDade, and A. Casadevall. 2000. Synthesis of polymerized melanin by Cryptococcus neoformans in rodents. Infect. Immun. 68:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salas, S. D., E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigler, L., R. C. Summerbell, and L. Poole. 1997. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J. Clin. Microbiol. 35:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton, B. C., and B. J. Dyko. 1989. Revision of Hendersonula. Mycol. Res. 4:466-488. [Google Scholar]
- 33.Thines, E., H. Anke, and O. Sterner. 1998. Scytalols A, B, C and D and other modulators of melanin biosynthesis from Scytalidium sp. 26-93. J. Antibiot. 51:387-393. [DOI] [PubMed] [Google Scholar]
- 34.Van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibility to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, Y., and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 64:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Y., and A. Casadevall. 1997. Growth of Cryptococcus neoformans in the presence of l-DOPA decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 38:2646-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Y., and A. Casadevall. 1994. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 60:3864-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, Y., and A. Casadevall. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen and oxygen derived oxidants. Infect. Immun. 62:3004-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, F. 2001. Dioxygen reactivity of laccase: dependence on laccase source, pH, and anion inhibition. Appl. Biochem. Biotechnol. 95:125-133. [DOI] [PubMed] [Google Scholar]