Abstract
A lymph node excision was performed on a 45-year-old woman with left cervical swelling. The disorder which developed after the patient had undergone oral surgery for a severe periodontal disease failed to respond to antimicrobial chemotherapy. A mycobacterial strain subsequently identified by high-performance liquid chromatography analysis of cell wall mycolic acids as Mycobacterium lentiflavum grew from the excised specimen. This case and previously published reports highlight the relevance of M. lentiflavum as an emerging causative agent of mycobacterial cervical lymphadenitis.
CASE REPORT
A 45-year-old woman was admitted to hospital with cervical lymphadenitis that lasted for four weeks. Physical examination revealed a firm nodular mass of 3.5 by 4 cm in the left submandibular area that was associated with redness, pain, and swelling of the overlying skin but no systemic illness. Treatment with oral amoxicillin-clavulanate and lincomycin had been unsuccessful. The patient's medical history was unremarkable except for a severe periodontal disease recently treated by oral surgery. Laboratory data revealed a white blood cell count of 14,700/ml and an abnormal erythrocyte sedimentation rate (21 mm/h). Mantoux skin testing with protein purified derivative was positive, while a chest X ray showed no lung abnormalities. A computerized tomogram scan was remarkable for the presence of multiple abscesses within the enlarged lymph node.
The patient was taken to the operating room, where complete surgical excision was performed and samples of the lymph node were collected for pathology investigation and culture. Histologic examination revealed numerous large branching granulomas with ill-defined margins and composed of epithelioid cells and palisading histiocytes. Rare multinucleated giant cells of the Langhans type were highlighted only after meticulous screening. Almost all granulomas exhibited central abscess formation, though no caseating necrosis was observed. Special stains (Gram, Ziehl-Neelsen, Grocott methenamine silver, and Wartin-Starry) failed to demonstrate any microorganism on histologic sections. Microbiological procedures devised to detect acid-fast bacilli by smear microscopy and the Mycobacterium tuberculosis complex genome by a ligase chain reaction commercial amplification assay yielded negative results.
Culture was performed with a couple of BBL Septi-Chek AFB bottles (Becton Dickinson Microbiology Systems, Cockeysville, Md.), which were incubated at 30 and 37°C. After 30 days of incubation at 37°C, the Septi-Chek liquid medium became turbid (smears were positive for acid-fast bacilli), and approximately 10 days later, yellow-pigmented nontuberculous mycobacteria grew on both solid media of the slide. Subsequent subculturing on L-J and Stonebrink media grew a slow-growing, scotochromogenic mycobacterium with some phenotypic resemblance to M. avium complex. The strain was found to be negative for semiquantitative catalase (less than 45 mm of foam production), nitrate reduction, tellurite reduction, Tween hydrolysis, and urease but resistant to carboxylic acid hydrazide (2 μg/ml) and catalase positive after heat inactivation for 20 min at 68°C. Culture confirmation testing with a commercial multiplex line probe assay (Inno-LiPA Mycobacteria; Innogenetics, Brussels, Belgium) specific for M. avium complex and 16 other mycobacterial species (15) was negative, and further high-performance liquid chromatography (HPLC) analysis of mycolic acids revealed a distinctive profile indicative of M. lentiflavum.
For definitive identification of the isolate, sequence analysis of the 16S rRNA gene was performed (Fig. 1). Briefly, a tract of about 500 bases, at the 5′ end of the 16S rRNA gene, was amplified and sequenced with the MicroSeq 500 16S ribosomal DNA bacterial sequencing kit (Applied Biosystems, Foster City, Calif.) and an automated apparatus (ABI Prism 377 DNA sequencer; Applied Biosystems) according to the manufacturer's instructions. The sequence was then compared with those present in the Ridom database (3).
FIG. 1.
Sequence alignment of hypervariable region A within the 16S RNA gene of the isolate studied and related species. M. tuberculosis was used as the reference sequence. Nucleotides different from those of the M. lentiflavum reference strain are indicated; dots indicate identity.
Drug susceptibility testing was performed by the radiometric system using the macrodilution method developed for M. avium complex (10). MICs were as follows: streptomycin, 6 μg/ml; isoniazid, >0.5 μg/ml; rifampin, 2 μg/ml; ethambutol, 7.5 μg/ml; ciprofloxacin, 1 μg/ml; clarithromycin, 8 μg/ml; and rifabutin, 0.5 μg/ml. Drug susceptibility testing was complete after 6 days. The postoperative course was uneventful, and no relapse occurred within the follow-up period (18 months of observation).
Discussion.
With the decline of tuberculosis, cases of nontuberculous mycobacterial lymphadenitis, which usually involve healthy children between 1 and 5 years of age, have been reported with increasing frequency in industrialized countries (4). Although the causative agent most frequently reported for almost two decades after the first description in 1956 was M. scrofulaceum, currently the major cause of adenitis due to nontuberculous mycobacteria is M. avium complex. In recent years, however, a growing number of mycobacterial organisms, including M. bohemicum, M. celatum, M. genavense, M. haemophilum, M. heidelbergense, M. interjectum, M. lentiflavum, M. malmoense, and M. triplex, have been repeatedly described as emerging and potentially relevant causative agents (14).
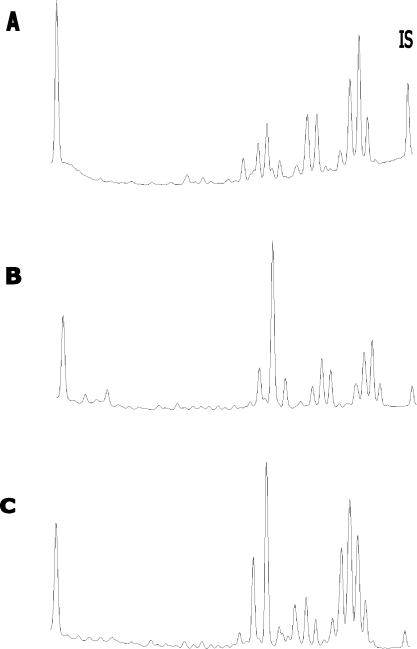
The introduction of comparative 16S rRNA gene sequencing has made recognition of the distinctive characteristics of most of these mycobacteria possible, disclosing the discovery of new species among previously phenotypically indistinguishable organisms. In this context, M. lentiflavum was first described by Springer et al. in 1996 (11). Besides previously described cases of cervical lymphadenitis (Table 1), other clinically significant isolates were reported from a vertebral disk in an elderly patient (11), from blood and synovial fluid in two immunocompromised patients (5, 9), and from sputum in two apparently immunocompetent patients (6, 13). M. lentiflavum is a scotochromogenic slow grower whose results in biochemical tests usually resemble those of M. avium complex. The HPLC profile presents a three-cluster pattern very similar to that of M. simiae (Fig. 2), from which M. lentiflavum is sometimes difficult to distinguish (13). Here, we describe an additional case of lymphadenitis caused by M. lentiflavum and review the literature on this topic.
TABLE 1.
Clinical and histological features of lymphadenitis due to M. lentiflavum
| Patient (reference) | Age (yr) | Sex | Site of infection | Manteaux skin testa | Radiological findings | Isolation specimen | Acid-fast bacilli | Culture medium | Pathology findings | Therapyb | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (3) | 3.5 | M | Cervical | Positive | Abscess formation | Excisional pus | Positive | Bactec 12B | Caseating granulomata | Excision | Healed |
| 2 (3) | 3 | M | Submandibular | Negative | Abscess formation | Lymph node | Negative | Bactec 12B | Caseating granulomata | Excision | Healed |
| 3 (13) | 4 | M | Cervical | NR | NR | Lymph node | NR | NR | NR | Excision | Healed |
| 4 (1) | 1.5 | M | Submandibular | NR | NR | Lymph node | Positive | NR | Noncaseating granulomata, microabscesses | Excision plus chemotherapy (INH, RMP, PZA) | Healed |
| 5 (this study) | 45 | F | Submandibular | Positive | Abscess formation | Lymph node | Negative | Septi-Chek AFB | Noncaseating granulomata, microabscesses | Excision | Healed |
NR, not reported.
INH, isoniazid; RMP, rifampin; PZA, pirazinamide.
FIG. 2.
Comparison of HPLC phenotypes of M. genavense (A), M. simiae (B), and M. lentiflavum (C). IS, internal standard.
M. lentiflavum has been reported in the literature as the causative agent of cervical lymphadenitis in only four cases (Table 1). The first two cases involved two children (33 and 42 months of age) with unilateral neck and submandibular swelling. Total lymph node excision was performed, and histopathological examination revealed caseating granulomatous inflammation in both cases. The third case occurred in a 4-year-old boy with unilateral cervical lymphadenopathy. The organism was cultured from the excised lymph node sample, but the histopathological diagnosis was not reported. The fourth case involved a 19-month-old boy with acute swelling of a cervical lymph node. In this case also, surgery was required and successful. Histopathological analysis revealed noncaseating granulomatous inflammation with microabscess formation. For our 45-year-old patient, the oral surgical procedures that she underwent were likely to provide the portal of entry for mycobacterial infection. The histopathologic picture which emerged closely resembled that of the previous case and provided strong evidence of nontuberculous mycobacterial lymphadenitis (7).
In recent years, the introduction of HPLC analysis of mycolic acids and 16S rRNA gene sequencing has led to a revolution in the etiology of nontuberculous mycobacterial lymphadenitis, allowing the identification of previously unrecognized or misidentified novel mycobacterial species. In our opinion, this observation is more likely to stem from the availability of more sophisticated diagnostic capabilities and expanded taxonomic tools rather than real changes in the prevalence of different mycobacterial species.
From a practical standpoint, surgical removal of the infected nodes early in the course of the disease is currently considered the treatment of choice (12). It is a well-established fact that this procedure maximizes the ability to recover the causative organism, prevents further cosmetic damage, eliminates extensive spreading which makes later surgery more difficult, and finally brings definitive healing. In this context, incision and drainage of the involved nodes should be avoided, as this technique commonly leads to chronic drainage and/or sinus tract formation. Similarly, fine-needle aspiration should be discouraged. Chemotherapy has been reserved particularly for cases where recurrence occurs after surgery or where the surgeon only succeeds in removing a portion of the abnormal tissue. The optimal chemotherapeutic regimen has yet to be set, but a combination of clarithromycin or azithromycin plus rifampin or rifabutin is commonly used. Some clinicians give ethambutol as the second drug or a combination of clarithromycin, rifampin or rifabutin, and ethambutol (8). The optimal duration of therapy is unknown, and no consensus exists.
The drug susceptibility data presented here and in a previous study (1) indicate that although considerable resistance to the majority of first-line antituberculous drugs is commonly exhibited, the MICs of clarithromycin, rifampin, and rifabutin are low and easily achievable in vivo. Under these conditions, full identification of the agent is warranted not only as a means of defining the role of different species, but also as a therapeutic guide, since in a minority of nontuberculous mycobacterial lymphadenitis cases, the cure was achieved by a combination of surgery and chemotherapy chosen on the basis of in vitro susceptibility testing. In conclusion, M. lentiflavum is a new mycobacterial species described mainly as the cause of cervical lymphadenitis in children.
When properly addressed with adequate control strains, HPLC analysis of mycolic acid is a rapid and reliable method of species identification. Detection of unknown profiles contributes to the characterization of previously undefined mycobacterial species which require 16S rRNA gene sequencing for definitive identification.
Acknowledgments
We thank Enrico Tortoli for HPLC analysis of our strain.
REFERENCES
- 1.Cabria, F., M. V. Torres, G. I. Cia, M. N. Garrido, J. Esteban, and M. S. Jimenez. 2002. Cervical lymphadenitis caused by Mycobacterium lentiflavum. Pediatr. Infect. Dis. J. 21:574-575. [DOI] [PubMed] [Google Scholar]
- 2.Haase, G., H, Kentrup, H. Skopnik, B. Springer, and E. C. Böttger. 1997. Mycobacterium lentiflavum: an etiologic agent of cervical lymphadenitis. Clin. Infect. Dis. 25:1245-1246. [DOI] [PubMed] [Google Scholar]
- 3.Harmsen, D., J. Rothganger, C. Singer, J. Albert, and M. Frosch. 1999. Intuitive hypertext-based molecular identification of microorganisms. Lancet 353:291. [DOI] [PubMed] [Google Scholar]
- 4.Hazra, R., C. D. Robson, A. R. Atayde, and R. H. Husson. 1999. Lymphadenitis due to nontuberculous mycobacteria in children: presentation and response to therapy. Clin. Infect. Dis. 28:123-129. [DOI] [PubMed] [Google Scholar]
- 5.Ibáñez, R., R. Heranz, M. Palop, C. Román, M. Corteguera, and S. Jiménez. 2002. Disseminated infection caused by slow-growing Mycobacterium lentiflavum. Eur. J. Clin. Microbiol. Infect. Dis. 21:691-692. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto, T., T. Sonobe, K. Hayashi, M. Okazaki, and B. Umeda. 2003. A chronic pulmonary disease caused by Mycobacterium lentiflavum in a patient with a history of pulmonary tuberculosis. Clin. Microbiol. Newsl. 25:79. [Google Scholar]
- 7.Kraus, M., D. Benharroch, D. Kaplan, N. Sion-Vardy, A. Leiberman, H. Dilma, I. Shoham, and D. M. Fliss. 1999. Mycobacterial cervical lymphadenitis: the histological features of non-tuberculous mycobacterial infection. Histopathology 35:534-538. [DOI] [PubMed] [Google Scholar]
- 8.Maltezou, E. C., S. Panagiotis, and D. A. Kafetzis. 1999. Nontuberculous mycobacterial lymphadenitis in children. Pediatr. Infect. Dis. 18:968-970. [DOI] [PubMed] [Google Scholar]
- 9.Ngo Niobe, S., C. M. Bebear, M. Clerc, J.-L. Pellegrin, C. Bebear, and J. Maugein. 2001. Disseminated Mycobacterium lentiflavum infection in a human immunodeficiency virus-infected patient. J. Clin. Microbiol. 39:2030-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui, S. H. 1992. Radiometric (Bactec) tests for slowly growing mycobacteria. 5. Indirect susceptibility tests for slowly growing mycobacteria other than tuberculosis, p. 14-18. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, vol. 1. American Society for Microbiology, Washington, D.C.
- 11.Springer, B., W. K. Wu, T. Bodmer, G. Haase, G. E. Pfyffer, M. Reiner, R. M. Kroppenstedt, K. H. Schröder, S. Emler, J. O. Kilburn, P. Kirschner, A. Telenti, M. B. Coyle, and E. Böttger. 1996. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J. Clin. Microbiol. 34:1100-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starke, J. R. 2000. Management of nontuberculous mycobacterial cervical adenitis. Pediatr. Infect. Dis. 19:674-675. [DOI] [PubMed] [Google Scholar]
- 13.Tortoli, E., A. Bartoloni, M. L. Erba, E, Levrè, N. Lombardi, A. Mantella, and L. Mecocci. 2002. Human infections due to Mycobacterium lentiflavum. J. Clin. Microbiol. 40:728-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortoli, E. 2003. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin. Microbiol. Rev. 16:319-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tortoli, E., A. Mariottini, and G. Mazzarelli. 2003. Evaluation of INNO-LiPA Mycobacteria v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J. Clin. Microbiol. 41:4418-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]