Abstract
Thirty-three isolates of Bartonella spp., including 11 isolates not belonging to previously known species, were isolated from 66 Rattus norvegicus subjects trapped in the city of Marseille, France. Based on seven different gene sequences, the 11 isolates were assigned to Bartonella rattimassiliensis sp. nov. and Bartonella phoceensis sp. nov.
Several cases of human infections due to nonhuman Bartonella species have been reported, including B. henselae, B. elizabethae, B. grahamii, B. vinsonii subsp. arupensis, B. vinsonii subsp. berkhoffii, and possibly B. clarridgeiae (1, 3). Identification of new Bartonella species, especially in animals that have possible contact with humans, can help to identify new human pathogens. In the present study, results of blood cultures taken from rats in the city of Marseille, France, are reported.
Seventy-four rats (8 Rattus rattus and 66 Rattus norvegicus) were trapped in the center of the city and at the limit between city and country. Characteristics of trapped R. norvegicus subjects are summarized in Table 1. Blood and hearts were frozen separately at −80°C. One milliliter of blood was inoculated as described previously (9). A piece of each heart (approximately 8 mm3) was also inoculated. Bartonella spp. were isolated in 30 samples from 20 R. norvegicus subjects. One isolate was obtained from heart, nine were obtained from blood, and 10 were obtained from both heart and blood samples. This finding raises the question of a possible heart involvement in infected rats (myocarditis or endocarditis). For three samples, two different sizes of colonies were observed on the same agar plate; thus, the total number of isolates submitted to molecular identification was 33. For one rat, colonies isolated from blood were different from those isolated from heart. Coinfection by different Bartonella species is not uncommon in animals, as observed in cats infected by both B. henselae and B. clarridgeiae (5).
TABLE 1.
Results of Bartonella sp. culture from 66 R. norvegicus subjects, according to sex, weight, area of capture, and presence of fleas
| Characteristics (n)a | Culture negative (n = 46) | Culture positive (n = 20) | P |
|---|---|---|---|
| Sex | |||
| M (35) | 23 | 12 | |
| F (26) | 19 | 7 | |
| NA (5) | 4 | 1 | |
| Wt (g) | |||
| <100 (11) | 11 | 0 | 0.02 |
| 100-200 (18) | 13 | 5 | 0.04 |
| 200-300 (18) | 10 | 8 | |
| >300 (14) | 9 | 5 | |
| NA (6) | 4 | 2 | |
| Area | |||
| 1 (30) | 29 | 1 | |
| 2 (12) | 11 | 1 | |
| 13 (24) | 6 | 18 | <10−6 |
| Fleas | |||
| No | 35 | 19 | |
| Yes | 9 | 1 |
M, male; F, female; NA, not applicable. Areas 1 and 2 were districts in the inner city; area 13 was a district on the border of the city.
Molecular screening of strains was based on internal transcribed spacer (ITS) sequencing as described previously (8) and allowed for identification of 22 isolates of Bartonella tribocorum (>99% sequence similarity with GenBank sequence no. AF312505), a bacterium already isolated from rats (4, 7). Eleven nonidentified isolates could be classified into three different clusters of sequences corresponding to type strains such as 15908 (three isolates; accession no. AY515121), 16115 (six isolates; accession no. AY515122), and 16120 (two isolates; accession no. 515123). ITS sequences of 15908 and 16115 isolates were very close, with 98.1% homology. The sequence of the 16120 isolate was rather more divergent, with 67.5 and 69.4% homology with the 15908 and 16115 isolate sequences, respectively. Identification of the 11 isolates was performed by sequencing 16S ribosomal DNA (rDNA), gltA, groEL, rpoB, ftsZ, and ribC (10). High sequence similarities were again observed for strains 15908 and 16115 with the other genes tested. They were of 99.8, 99.7, 100, 99.4, 99.6, and 98.3% for 16S rDNA and the gltA, groEL, rpoB, ftsZ, and ribC genes, respectively. Sequence similarities for 16S rDNA and the gltA, groEL, rpoB, ftsZ, and ribC genes of strains 15908, 16115, and 16120 with most close Bartonella species are summarized in Table 2.
TABLE 2.
Percentage of similarity of the three closest relatives for strains 16115, 15908, and 16120a
| Strain | Gene | GenBank no. | Closely related sp./similarity (%)
|
||
|---|---|---|---|---|---|
| First | Second | Third | |||
| 16115 | 16S rDNA | AY515118 | BG/99.2 | BK/99 | NG |
| ITS | AY515122 | BG/72.4 | BTr/69.8 | BV/66 | |
| gltA | AY515125 | BG/94.2 | BTr/93.3 | BE/92.9 | |
| groEL | AY515128 | BG/90.2 | BTr/89.9 | BTa/89.4 | |
| rpoB | AY515131 | BG/93.2 | BTr/92.7 | BE/91.5 | |
| ftsZ | AY515134 | BG/94.4 | BE/93.3 | BTr/92.9 | |
| ribC | AY515137 | BE/89.9 | BQ/87 | BA/85.5 | |
| 15908 | 16S rDNA | AY515120 | BG/99.2 | BK/99 | NG |
| ITS | AY515121 | BG/72.5 | BTr/70.3 | BVV/66.3 | |
| gltA | AY515124 | BG/94.5 | BTr/92.9 | BE/92.6 | |
| groEL | AY515127 | BG/90.2 | BTr/89.9 | BTa/89.4 | |
| rpoB | AY515130 | BG/93.1 | BTr/92.5 | BE/91.5 | |
| ftsZ | AY515133 | BG/94 | BE/93.2 | BTr/92.5 | |
| ribC | AY515136 | BE/89.9 | BQ/88 | BA/86.2 | |
| 16120 | 16S rDNA | AY515119 | 16115, 15908/99.9 | BG/98.8 | BK/98.8 |
| ITS | AY515123 | 16115, 15908/73.7 | BQ/70.2 | BQ/70.2 | |
| gltA | AY515126 | BVA/92 | BVV/91.7 | BTa/90.8 | |
| groEL | AY515129 | BVA/90.7 | BTa/90.7 | BH/90.3 | |
| rpoB | AY515132 | BA/92 | BVB/91.8 | BB/91.8 | |
| ftsZ | AY515135 | BA/93.5 | BVV/92.3 | BVA/91.9 | |
| ribC | AY515138 | 16115, 15908/91.7 | BTa/88.2 | BE/87.3 | |
For the 16S rDNA, only the two closest relatives are given when there are several third-closest relatives. NG, not given; BG, B. grahamii; BE, B. elizabethae; BK, B. koehlerae; BTr, B. tribocorum; BQ, B. quintana; BA, B. alsatica; BTa, B. taylorii; BB, B. birtlesii; BVV, B. vinsonii subsp. vinsonii; BVA, B. vinsonii subsp. arupensis; BVB, B. vinsonii subsp. berkhoffii; BH, B. henselae.
Results of culture did not vary significantly with sex, and recovery of fleas was rather associated with negative culture (9 of 46 versus 1 of 20). The presence of fleas was not associated with the weight of the rats (5 of 29 in rats of <200 g versus 4 of 32 in rats of >200 g) but rather with the area of capture (9 of 42 in the inner city versus 1 of 24 at the border of the city; P = 0.05). Positive culture was associated with a weight higher than 200 g (P = 0.04) and capture at the border of the city (P < 10−6) (Table 1). As Bartonella is supposed to be transmitted by ectoparasites, the question of the possibility that B. tribocorum and the new Bartonella species may be transmitted by arthropods other than fleas is raised, but none were detected on rats at the time of trapping. However, we cannot exclude that fleas or other ectoparasites were present when transmission occurred but left their host when infected, e.g., because of fever, other deleterious conditions, or seasonal variation. Bartonella spp. were more frequently isolated from the rats with a weight of >200 g (P = 0.02), which means that they were adults (R. Leblanc, unpublished data). This is different from Bartonella infection in cats, where young cats are mostly culture positive (2, 3, 6), and from a rat model of B. tribocorum infection, where high-concentration bacteremia is observed during the first weeks of the disease (11). This could be explained by a transmission of the disease later in the life of the rat.
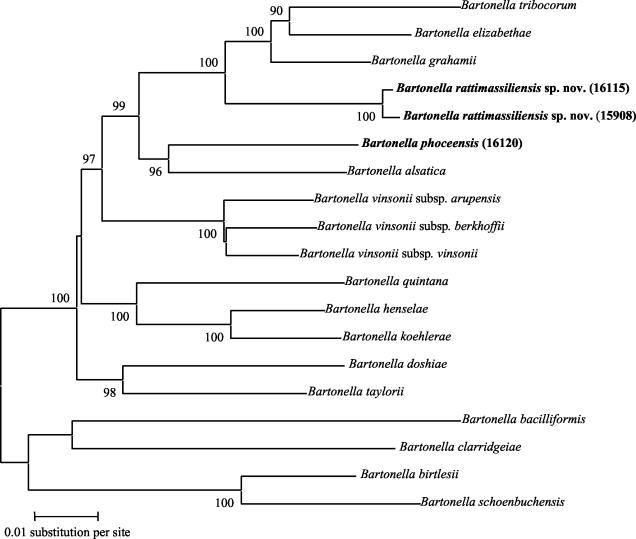
Description of new species is currently based on results of DNA-DNA hybridization and description of phenotypic characteristics (13). Sequencing of housekeeping genes has been proposed to replace DNA-DNA hybridization, with a comparison of at least five genes (12). Based on this proposal, we demonstrated the possibility of delimiting Bartonella species on the basis of the sequences of ITS and the gltA, groEL, rpoB, ftsZ, and ribC genes (10). However, species definition in this genus may be based solely on the results of rpoB and gltA gene sequences, as we demonstrated that a new Bartonella species may be defined on the basis of less than 96 and 95.4% similarities for gltA and rpoB sequences, respectively, provided there is congruency with results of phylogenetic analysis giving high bootstrap values. In the present study, the strains 15908, 16115 and 16120 fulfilled these criteria (Table 2; Fig. 1). We thus propose to name these bacteria as Bartonella rattimassiliensis sp. nov. (ra.tti.mas.si.li.en′sis. L. n. pl. ratti for rattus, rat; L. fem. adj. massiliensis referring to Massilia, Latin name of Marseille, where the rats were trapped) and Bartonella phoceensis sp. nov. (pho.ce.en′sis. L. fem. adj. phoceensis referring to Phocea, Greek name of the city which founded Marseille, where the rats were trapped). Both exhibit all of the characteristics of the genus. Good growth is observed on Columbia agar with 5% sheep blood in a 5% CO2 atmosphere, where colonies appear small, white, smooth, and irregular (about 0.5 mm in diameter) after 10 days. Electron microscopic examination shows small bacilli without flagella, approximately 1 to 2 μm long by 0.5 μm wide. Both are gram negative but stain better with Gimenez stain and are nonmotile. The B. rattimassiliensis type strain is 15908 and the B. phoceensis type strain is 16120. They are distinguishable from other Bartonella species by their ITS, 16S rDNA, and gltA, groEL, rpoB, ftsZ, and ribC gene sequences. Both were isolated from the blood of wild rats, R. norvegicus, and are deposited in the Collection des Bacteries de l'Institut Pasteur as CIP 107705T and CIP 107707T, respectively. The strain 16115, which is a genotypic variant of B. rattimassiliensis, is deposited as CIP 107706.
FIG. 1.
Dendrogram representing phylogenetic relationships between Bartonella species. The tree is derived from the concatenated sequences of ITS, 16S rDNA, and the gltA, groEL, rpoB, ftsZ, and ribC genes. The MEGA 2.1 software (http://www.megasoftware.net) was used to infer the tree, by using the neighbor-joining method with Kimura-2 and Jukes-Cantor parameters. The support of each branch, as determined from 1,000 bootstrap samples, is indicated by the value at the node when it is at least 90%. For sequence accession numbers, see Table 2 and reference 10.
REFERENCES
- 1.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomel, B. B., R. C. Abbott, R. W. Kasten, K. A. Floydhawkins, P. H. Kass, C. A. Glaser, N. C. Pedersen, and J. E. Koehler. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33:2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomel, B. B., R. W. Kasten, J. E. Sykes, H. J. Boulouis, and E. B. Breitschwerdt. 2003. Clinical impact of persistent bacteremia in humans and animals. Ann. N. Y. Acad. Sci. 990:267-278. [DOI] [PubMed] [Google Scholar]
- 4.Ellis, B. A., R. L. Regnery, L. Beati, F. Bacellar, M. Rood, G. G. Glass, E. Marston, T. G. Ksiaek, D. Jones, and J. E. Childs. 1999. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a New World disease. J. Infect. Dis. 180:220-224. [DOI] [PubMed] [Google Scholar]
- 5.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. Heller, R. W. Kasten, K. Yamamoto, and Y. Piemont. 1997. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J. Clin. Microbiol. 35:2120-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller, R., M. Artois, V. Xemar, D. de Briel, H. Gehin, B. Jaulhac, H. Monteil, and Y. Piemont. 1997. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J. Clin. Microbiol. 35:1327-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller, R., P. Riegel, Y. Hansmann, G. Delacour, D. Bermond, C. Dehio, F. Lamarque, H. Monteil, B. Chomel, and Y. Piemont. 1998. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int. J. Syst. Bacteriol. 48:1333-1339. [DOI] [PubMed] [Google Scholar]
- 8.Houpikian, P., and D. Raoult. 2001. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol. Lett. 200:1-7. [DOI] [PubMed] [Google Scholar]
- 9.La Scola, B., B. Davoust, M. Boni, and D. Raoult. 2002. Lack of correlation between Bartonella DNA detection within fleas, serological results, and results of blood culture in a Bartonella-infected stray cat population. Clin. Microbiol. Infect. 8:345-351. [DOI] [PubMed] [Google Scholar]
- 10.La Scola, B., Z. Zeaiter, A. Khamis, and D. Raoult. 2003. Gene-sequence based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 11:318-321. [DOI] [PubMed] [Google Scholar]
- 11.Schulein, R., A. Seubert, C. Gille, C. Lanz, Y. Hansmann, Y. Piemont, and C. Dehio. 2001. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J. Exp. Med. 193:1077-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. Grimont, P. Kampfer, M. C. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Trüper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 13.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Trüper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]