Abstract
Background
Rice plant growth is comprised of distinct phases, such as vegetative, reproductive, grain filling and maturity phases. In these phases synthesis and availability of primary and secondary metabolites including volatile organic compounds (VOC’s) is highly variable. In scented rice, aroma volatiles are synthesized in aerial plant parts and deposited in mature grains. There are more than 100 VOCs reported to be responsible for flavor in basmati rice. It will be interesting to keep track of aroma volatiles across the developmental stages in scented rice. Therefore, the aroma volatiles contributing in aroma with special reference to the major compound 2 acetyl-1-pyrroline (2AP) were screened at seven developmental stages in scented rice cultivars Basmati-370 and Ambemohar-157 along with non-scented rice cultivar IR-64 as a control following HS-SPME-GC-MS method. In addition, the expression levels of key genes and precursor levels involved in 2AP biosynthesis were studied.
Results
The study indicated that volatilome of scented rice cultivars is more complex than non-scented rice cultivar. N-heterocyclic class was the major distinguishing class between scented from non-scented rice. A total of 14 compounds including, 2AP were detected specifically in scented rice cultivars. Maximum number of compounds were synthesized at seedling stage and decreased gradually at reproductive and maturity. The seedling stage is an active phase of development where maximum number green leaf volatiles were synthesized which are known to act as defense molecules for protection of young plant parts. Among the 14 odor active compounds (OACs), 10 OACs were accumulated at higher concentrations significantly in scented rice cultivars and contribute in the aroma. 2AP content was highest in mature grains followed by at booting stage. Gene expression analysis revealed that reduced expression of betaine aldehyde dehydrogenase 2 (badh2) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and elevated level of triose phosphate isomerase (TPI) and Δ1-Pyrolline-5-carboxylic acid synthetase (P5CS) transcript enhances 2AP accumulation.
Conclusions
Most diverse compounds were synthesized at seedling stage and OACs were accumulated more at flowering followed by seedling stage. Distinct accumulation pattern exists for 2AP and other aroma volatiles at various developmental stages. The study revealed the mechanism of 2AP accumulation such that 2AP in mature grains might be transported from leaves and stem sheath and accumulation takes place in grains.
Electronic supplementary material
The online version of this article (doi:10.1186/s12284-016-0113-6) contains supplementary material, which is available to authorized users.
Keywords: Rice, Odor active compounds (OAC’s), Volatile organic compounds (VOC’s), Head space solid phase microextraction (HS-SPME), Gas chromatography mass spectrometry (GCMS), Biomarker, Basmati
Background
Rice (Oryza sativa L.) is an annual crop cultivated for grains as a rich source of carbohydrates minerals and vitamins. Based on developmental pattern, rice plant growth is divided into vegetative (germination to panicle initiation), reproductive (panicle initiation to heading), grain filling or ripening and maturity phases. At seedling stage the plant has clearly defined shoot and root parts. Further tillers are formed on main shoot, a gradual increase in plant height and leaf emergence at regular intervals takes place during vegetative development. In reproductive stage plant undergo culm elongation, a decline in tiller number, booting, emergence of the flag leaf, heading or panicle protrude out from sheath and flowering. Grain filling and ripening or maturation stage is characterized by grain growth (Yoshida and Nagota 2011). The developmental phases have direct and indirect influences on the yield and quality characteristics of rice grains. The reproductive growth stage is most sensitive to biotic and abiotic stresses (Fageria 2007). As the plant undergo different developmental phases, simultaneous level of endogenous hormones, primary and secondary metabolites including volatile organic compounds (VOCs) are altered. Photosynthates produced in leaves are transported primarily in the form of sucrose to meristem and developing organs such as flowers. During this phase of development, maximum amino acids and proteins are synthesized in plants and transported to developing organs where they are utilized for flower and embryo development. At grain filling or ripening, grain size and weight increases since starch and sugars are translocated from the culms and leaf sheaths to developing grains. The carbohydrate is stored in the form of starch in grain endosperm. Initially when florets on the main stem show milky accumulation, starch is white and milky in consistency. It loses moisture and changes into bread dough or firmer during dough grain stages. At maturity physiological process of grain filling cease. And when the moisture content of the grain on the main stem is 25 to 30 % the plant reaches to physiological maturity (Paul and Foyer 2001).
A relatively large group of plant natural products consists of VOCs, lipophilic liquids with low molecular weight and high vapor pressure are synthesized during growth developmental stages. Physical properties of these compounds allow them to freely cross cellular membranes and be released into the surrounding environment (Pichersky et al. 2006). Over the years > 1700 VOCs have been identified from 90 different plant families belonging to both angio- and gymnosperms (Knudsen et al. 2006). Biosynthesis of VOCs depends on the availability of carbon, nitrogen and sulfur as well as energy provided by primary metabolism. Biosynthesis of the wide array of different VOCs branches off from only a few primary metabolic pathways. Based on their biosynthetic origin, all VOCs are divided into several classes, including terpenoids, phenylpropanoids (benzenoids), fatty acid derivatives and amino acid derivatives etc. (Dudareva et al. 2013). There are more than 100 volatile compounds were reported responsible for basmati flavor representing 13 hydrocarbons, 14 acids, 13 alcohols, 16 aldehydes, 14 ketones, 8 esters, 5 phenols etc. (Hussain et al. 1987). 2-Acetyl-1-pyrroline (2AP), a principal aroma compound has been detected in all aerial plant parts of scented rice (Yoshihashi et al. 2002a, b; Maraval et al. 2010). Recently, in addition to 2AP, several other major volatiles contributing in the aroma of scented rice cultivars have been identified viz., hexanal, nonanal, octanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, heptanal, pentanal, (E)-2-octenal, 4-vinylphenol, 4-vinylguaicol,1-octen-3-ol, decanal, guaicol, indole and vanillin (Mathure et al. 2011; Mathure et al. 2014). It is interesting to keep track of aroma volatiles synthesized and translocated across various developmental stages in scented rice. Such studies have not yet been reported in any scented rice cultivars. Therefore, in present study 2AP, other aroma volatiles and 2AP precursors (proline and methylglyoxal) were assessed at seven different growth stages in two scented rice cultivars Basmati-370 (BA-370) and Ambemohar-157 (AM-157) and compared with non-scented rice cultivar IR-64. In addition, the expression analysis of genes involved in the biosynthesis of 2AP [betaine aldehyde dehydrogenase 2 (badh2), Δ1-Pyrolline-5-carboxylic acid synthetase (P5CS), triose phosphate isomerase(TPI) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] have been studied.
Results and discussion
Qualitative analysis of aroma volatiles
The volatile compounds identified in three rice cultivars at seven developmental stages (S1 to S7) are depicted in Table 1. Total 88 volatile compounds were identified collectively in three rice cultivars which belongs to 13 chemical classes viz. alkane (7), alkene (6), ketone (12), aromatic hydrocarbon (4), terpenes (11), alcohols (13), aliphatic aldehydes (16), aromatic aldehydes (3), N-heterocyclic (3), ester (7), phenol containing compounds (4), carboxylic acid (1) and furan (1). In scented rice cultivars more number of volatile compounds (72–51) were detected throughout the developmental stages than the non-scented one (58–39). In either one or all stages, 14 compounds were detected specifically in scented rice cultivars. In this 14 compounds eight compounds [2AP (nutty’ or ‘popcorn-like), 2-acetyl-1H-pyrrole (musty and nutty), β-ionone (raspberry, floral), (E,Z)-2,6-nonadienal (green, metallic), p-xylene (sweetish), methyl 2-aminobenzoate (sweet, grape fruity) having characteristic odor and azulene and acetic acid, 1,7,7-trimethyl-bicyclo(2,2,1)hept-2-yl, ester without any contribution in odor] were commonly detected in both scented rice cultivars. Among the scented cultivars, 5 compounds [4-methyldecane (pungent, acrid odor), 4-cyclopentylidene-2-butanone (woody and fruity), toluene (sweet, pungent, benzene), indole (floral, sweet, burnt) and allylcyclohexane (fruity, pineapple)] were detected specifically in AM-157 and camphene (woody, camphoreous) was detected only in BA-370. In present study this five compounds in AM-157 and single compound in BA-370 was observed cultivar specific. In comparative volatile analysis of aromatic and non-aromatic rice cultivars no difference was recorded in presence of volatile compounds but only in the quantity (Widjaja et al. 1996). Other studies showed that in comparative volatiles analysis of mature grains of scented and non-scented rice not a single compound or set of compounds defined a particular aromatic rice cultivar but only the concentration (Jezussek et al. 2002; Zeng et al. 2009). Recently, Bryant and Mcclung (2011) reported presence of 15 compounds in addition to 2AP in mature grains of seven aromatic cultivars over non-aromatic rice cultivars. Similar results were recorded here.
Table 1.
List of volatile compounds identified at various developmental stages in 3 rice cultivars
| Sr.No | Compound | Odor description | Retention Index (RI) | AM-157 | BA-370 | IR-64 | |
|---|---|---|---|---|---|---|---|
| Exp | Ref | Stage of occurrence | |||||
| 1. Alkane | |||||||
| 1 | Nonane | Gasoline-like | 901 | 900 | S4, S5, S6 | S4 to S7 | S5, S6 |
| 2 | 4-Methyldecaneb | Pungent, | 1022 | 1023 | S7 | ND | ND |
| 3 | Dodecane | Gasoline like | 1196 | 1200 | S1, S4 to S7 | S1, S4 to S7 | S1, S4 to S7 |
| 4 | Tetradecanea | Gasoline-like | 1390 | 1400 | S1 to S7 | S1 to S7 | S1 to S7 |
| 5 | Pentadecanea | Mild odor | 1501 | 1500 | S1 to S7 | S1 to S7 | S1 to S7 |
| 6 | Heptadecanea | NA | 1698 | 1700 | S1 to S7 | S1 to S7 | S1 to S7 |
| 7 | Nonadecanea | Sweet, rosy | 1898 | 1900 | S1 to S7 | S1 to S7 | S1 to S7 |
| 2. Alkene | |||||||
| 1 | Allylcyclohexaneb | NA | 965 | 969 | S2, S3 | S1 to S3 | ND |
| 2 | (E)-5-Methyl-4-decene | NA | 1094 | 1100 | S1 to S3, S5 to S7 | S2,S5, S6 | |
| 3 | (Z)-3-Undecene | Hydrocarbon | 1116 | 1123 | S2,S3,S4 - S6 | S1- S3, S7 | S1, S3 |
| 4 | (Z)-3-Dodecene | Pleasant odor | 1194 | 1195 | S1,S2, S4 - S6 | S1,S2,S4-S7 | S1, S4-S7 |
| 5 | 7-Tetradecene | NA | 1362 | 1367 | S1, S2, S7 | S1 to S3 | S1 to S3 |
| 6 | 1-Tetradecenea | Mild pleasant | 1392 | 1385 | S1 to S7 | S1 to S7 | S1 to S7 |
| 3. Ketone | |||||||
| 1 | 2-Heptanonea | Fruit,spicy | 896 | 889 | S1-S7 | S1-S7 | S1-S7 |
| 2 | 6-Methyl-2-heptanonea | Camphoreous | 956 | 957 | S1-S4, S7 | S1-S4, S7 | S1-S4, S7 |
| 3 | 6-Methyl-5-hepten-2-onea | Herby, green | 987 | 988 | S1-S7 | S1-S7 | S1-S7 |
| 4 | (E)-3-Octen-2-onea | Citrus, floral | 1040 | 1036 | S1,S2, S6,S7 | S1,S2,S4,S6,S7 | S1,S2,S6,S7 |
| 5 | 2,2,6-Trimethylcyclohexanone | Citrus, hone | 1043 | 1047 | S1-S4 | S1-S4 | S1-S4 |
| 6 | 2-Nonanone | Green, herbal | 1094 | 1093 | S1, S2, S5 | S1, S2, S7 | S1, S2 |
| 7 | (E)-5-Ethyl-6-methyl-3-hepten-2-one | Citrus type | 1147 | 1144 | S1, S6, S7 | S1, S3-S7 | S6, S7 |
| 8 | 4-Cyclopentylidene-2-butanoneb | Woody, fruity | 1151 | 1158 | S7 | ND | ND |
| 9 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | Musty, citrus | 1156 | 1152 | S1-S6 | S1-S4, S6-S7 | S1-S7 |
| 10 | 2-Undecanone | Floral, pineapple | 1301 | 1302 | S5 | S4 | S4 |
| 11 | 6,10-Dimethyl-2-undecanone | NA | 1404 | 1398 | S3, S5-S7 | S1-S3, S5-S7 | S3, S5-S7 |
| 12 | β-lononeb | Raspberry, floral | 1495 | 1493 | S1, S2 | S2, S7 | ND |
| 4. Aromatic Hydrocarbon | |||||||
| 1 | p-Xyleneb | Strong, Sweetish | 897 | 907 | S1 | S1 | ND |
| 2 | Tolueneb | Sweet, Pungent, | 995 | 1005 | S7 | ND | ND |
| 3 | 1-Isopropyl-2-methylbenzene | Woody, Smoky | 1025 | 1025 | S1-S3 | S1-S3 | S2 |
| 4 | 1-Isopropyl-4-methylbenzene | Woody, Smoky | 1027 | 1023 | S1-S3, S5 | S1-3, S5 | S2 |
| 5. Terpenes | |||||||
| 1 | Pinene | Herbal type odor | 935 | 930 | S2 | S1-S3, S5 | S1, S2 |
| 2 | Campheneb | Woody camphoreous | 950 | 958 | ND | S2, S5 | ND |
| 3 | 3-Carenea | Sweet & pungent | 1014 | 1015 | S1-S6 | S1-S6 | S1-S6 |
| 4 | L-Limonene | Lemon | 1038 | 1040 | S1 | S5 | S5 |
| 5 | Azulenea | NA | 1324 | 1323 | S7 | S3 | ND |
| 6 | β-Elemene | Fruity, Dry | 1405 | 1403 | S1-S3 | S1-S3 | S1, S2 |
| 7 | Isolongifolene | Fresh woody | 1420 | 1416 | S1-S5 | S1-S5 | S1-S5 |
| 8 | Longifolene | vegetal/flowery | 1437 | 1432 | S1-S6 | S1-S7 | S1-S6 |
| 9 | β-Caryophyllene | Spicy type odor | 1443 | 1444 | S1-S6 | S1-S7 | S1-S6 |
| 10 | Aromadendrene a | Woody type odor | 1447 | 1439 | S1 | ND | S1 |
| 11 | Valencen | Sweet, citrus | 1472 | 1477 | S1-S6 | S1-S6 | S5-S6 |
| 6. Alcohol | |||||||
| 1 | 1-Pentanola | Moderately strong | 803 | 792 | S1-S7 | S1-S7 | S1-S7 |
| 2 | (Z)-3-Hexen-1-ola | Green type odor | 880 | 872 | S1-S6 | S1-S7 | S1-S6 |
| 3 | 1-Hexanola | Green | 866 | 860 | S1-S7 | S1-S7 | S1-S7 |
| 4 | 1-Octen-3-ola | Straw, mushroom | 972 | 969 | S1-S7 | S1-S7 | S1-S7 |
| 5 | 1-Hexanol, 2-ethyl- | Heavy, earthy | 1030 | 1033 | S4-S7 | S4, S5, S7 | S3-S5 |
| 6 | 1-Octanola | Fatty, metallic | 1073 | 1073 | S1-S7 | S1-S7 | S1-S7 |
| 7 | Linaloola | Sweet, floral, | 1102 | 1100 | S1-S3,S5,S7 | S1, S2, S4, S7 | S1-S2, S4-S7 |
| 8 | 3, 4-Dimethylcyclohexanol | NA | 1123 | 1126 | S2-S6 | S1-S7 | S1-S7 |
| 9 | 2 Nonen-1-ola | Green type | 1134 | 1135 | S1-S3,S5, S7 | S1-S3, S5-S7 | S1,S2, S4-S7 |
| 10 | Carveol | Spicy type | 1161 | 1188 | S1,S3, S6,S7 | S1, S2, S6, S7 | S1-S3, S5-S7 |
| 11 | 3,7-Dimethyl-1-octanol | Sweet, rosy | 1204 | 1196 | S2, S7 | ND | S5 |
| 12 | 2-Hexyl-1-octanola | Waxy | 1600 | 1591 | S1-S7 | S2, S3, S5-S7 | S1-S3, S5-S7 |
| 13 | 2-Hexadecanol | NA | 1798 | 1774 | S1-S7, | S2-S3, S5-S7 | S1-S3,S5, S6 |
| 7. Aliphatic Aldehyde | |||||||
| 1 | Pentanala | Strong, Acrid | 710 | 707 | S1-S7 | S1-S7 | S1-S7 |
| 2 | Hexanala | Green | 816 | 820 | S1-S7 | S1-S7 | S1-S7 |
| 3 | (E)-2-Hexenal | Sweet, fruity, fresh | 869 | 860 | S2, S3 | S1-S3, S5, S6 | S2 |
| 4 | Heptanala | Grass, fresh | 904 | 905 | S1-S7 | S1-S7 | S1-S7 |
| 5 | (Z)-2-Heptenal | Fatty green | 961 | 960 | S2-S7 | S1-S7 | S3-S7 |
| 6 | Octanala | Citrusy | 1007 | 1005 | S1-S7 | S1-S7 | S1-S7 |
| 7 | (E,E)-2 4-Octadienala | musty, cooked starch | 1017 | 1021 | S1-S7 | S1-S7 | S1-S6 |
| 8 | (E)-2-Octenala | nutty, cooked flour | 1063 | 1068 | S1-S7 | S1-S3, S5-S7 | |
| 9 | Nonanala | Grassy, citrus, floral | 1108 | 1104 | S1-S7 | S1-S7 | S1-S7 |
| 10 | (E,Z)-2,6-Nonadienalb | Green, metallic | 1155 | 1153 | S1, S5, S7 | S2 | ND |
| 11 | (E)-2-Nonenal | Metallic | 1165 | 1162 | S1-S7 | S1-S7 | S1-S7 |
| 12 | Decanala | Fatty, citrusy | 1210 | 1204 | S1-S7 | S1-S7 | S1-S7 |
| 13 | (E,E)-2,4-Nonadienal | Fatty, metallic | 1219 | 1217 | S1-S3, S7 | S1-S3 | S1, S2 |
| 14 | β-Cyclocitral | Minty type odor | 1234 | 1237 | S1-S7 | S1-S7 | S1-S7 |
| 15 | (2,6,6-Trimethyl-1-cyclohexen-1- yl)acetaldehyde | NA | 1269 | 1261 | S1-S6 | S1-S4, S6 | S1-S6 |
| 16 | (E,E)-2,4-Decadienal | Fatty, metallic, citrus | 1325 | 1318 | S1-S5, S7 | S1-S7 | S1-S4 |
| 8. Aromatic aldehyde | |||||||
| 1 | Benzaldehydea | Nutty,sweet | 970 | 965 | S1-S7 | S1-S7 | S1-S7 |
| 2 | Phenylacetaldehydea | Herbal, floral | 1052 | 1048 | S1-S7 | S1-S7 | S1-S7 |
| 3 | Vanillina | Vanilla-like | 1415 | 1403 | S1-S7 | S1-S7 | S1-S7 |
| 9. N-Heterocyclic | |||||||
| 1 | 2-Acetyl-1-pyrrolineab | popcorn-like | 925 | 930 | S1-S7 | S1-S7 | ND |
| 2 | 1-(1H-Pyrrol-2-yl) ethanoneb | Musty and nutty | 1043 | 1035 | S1, S3-S7 | S1-S7 | ND |
| 3 | 1H-Indoleab | Floral, sweet, burnt | 1306 | 1304 | S1-S3 | ND | ND |
| 10. Ester | |||||||
| 1 | Ethyl hexanoate | Fruity, | 989 | 984 | S1 | S1 | S1 |
| 2 | Ethyl heptanoate | Berry like odor | 1088 | 1083 | S1 | S1 | S1 |
| 3 | Ethyl octanoate | Waxy type | 1193 | 1183 | S1, S2 | S1, S2 | S1, S2 |
| 4 | Methylsalicylate | Minty type odor | 1201 | 1206 | S1-S6 | S1-S7 | S1-S7 |
| 5 | Acetic acid, 1,7,7-trimethyl-bicyclo(2,2,1)hept-2-yl, esterb | NA | 1285 | 1277 | S1 | S2, S5 | ND |
| 6 | Methyl 2-aminobenzoateb | sweet, grape fruity | 1365 | 1372 | S2, S4 | S1, S2, S4 | ND |
| 7 | Ethyl laurateb | Waxy type | 1586 | 1581 | S1, S2, S3 | S1,S3 | S1 |
| 11. Phenol-containing | |||||||
| 1 | Phenola | Sweet and tarry | 981 | 980 | S1, S2, S3 | S1-S3, S5 | S1-S3 |
| 2 | 2-Methoxyphenola | Smoky | 1087 | 1090 | S1-S7 | S1-S7 | S1-S7 |
| 3 | 2-Phenoxyethanol | Pleasant odor | 1224 | 1226 | S2-S6 | S1-S6 | S3-S6 |
| 4 | 2-Methoxy-4-vinylphenola | Spicy,fruity | 1320 | 1313 | S1-S7 | S1-S7 | S1-S6 |
| 12. Carboxylic acid | |||||||
| 1 | Benzoic acida | Pleasant odor | 1179 | 1174 | S1-S4 | S1-S4 | S1-S3 |
| 13. Furans | |||||||
| 1 | 2-Pentylfurana | Floral, fruit, nutty | 992 | 996 | S1-S7 | S1-S7 | S1-S7 |
| Total 88 compounds | |||||||
aCompound confirmed using standard
bCompound detected only in scented rice cultivars, Expt experimental, Ref reference, NA not avaliable., ND not detected, S1 seedling, S2 tillering, S3 booting, S4 flowering, S5 milky grains, S6 dough grains, S7 mature grains., AM-157 ambemohar-157., BA-370 Basmati-370
The qualitative analyses of volatile compounds at respective developmental stages are summarized as follows.
Vegetative stages (S1: seedling and S2: tillering)
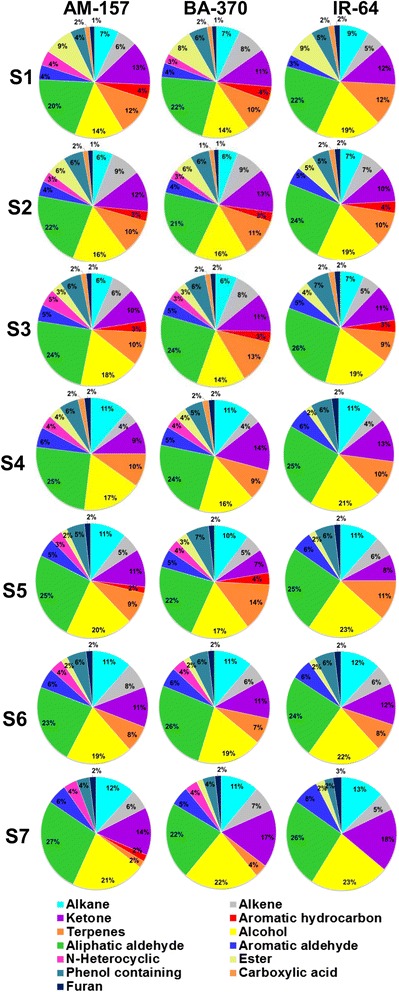
At seedling stage (S1) in scented category, 72 compounds in BA-370 and 70 in AM-157 belonging to all 13 classes were detected. In IR-64, 58 compounds belonging to 11 classes were detected (Fig. 1, Table 1). VOC’s belonging to the chemical classes aliphatic aldehydes (13–16) and alcohols (10–11) were present in maximum number and contributed 20–22 % and 14–19 % share respectively in total volatile components (Fig. 1). N-heterocyclic (3), aromatic hydrocarbons (3) and other 16 compounds belonging to different classes were present only in scented rice cultivars (Table 1). Among these, 8 compounds (2AP, 2-acetyl-1H-pyrrole, (E)-5-methyl-4-decene, (E)-5-ethyl-6-methyl-3-hepten-2-one, p-xylene, 1-isopropyl-2-methylbenzene, 1-isopropyl-4-methylbenzene and valencen) were found common in both the scented cultivars. Seven compounds (β-ionone, 2-hexyl-1-octanol, 2-hexadecanol, (E,Z)-2,6-nonadienal, 1H-indole, L-limonene and acetic acid, 1,7,7-trimethyl-bicyclo(2,2,1)hept-2-yl, ester) were detected specifically in AM-157 and 7 compounds (allylcyclohexane, (Z)-3-undecene, 6,10-dimethyl-2-undecanone, pinene, (Z)-2-heptenal, methyl 2-aminobenzoate and 3,4-dimethylcyclohexanol) were specific to BA-370.
Fig. 1.
Qualitative analysis of aroma volatiles at 7 developmental stages in 3 rice cultivars (S1- seedling, S2: tillering, S3: booting, S4: flowering, S5: miky grains, S6: dough grains, S7: mature grains)
During S2 stage, 70 compounds were present in BA-370, 68 in AM-157 and 58 in IR-64. In scented rice cultivars 13 chemical classes were detected and 12 classes were found in non-scented type. N-heterocyclic (2AP and 2-acetyl-1H-pyrrole) and other 13 compounds from different classes distinguished scented rice cultivars from non-scented at tillering stage (Table 1, Fig. 1). Among these, 11 compounds were commonly detected in both scented type, camphene and acetic acid, 1,7,7-trimethyl-bicyclo(2,2,1)hept-2-yl, ester were specifically present in BA-370 and 1H-indole and 3,7-dimethyl-1-octanol were in AM-157. Percent share of aldehydes (21–24) and alcohols (16–19) was found maximum at this stage (Fig. 1).
Reproductive stages (S3: booting and S4: flowering)
In S3 stage number of compounds were decreased (from 72–68 to 63–62) than S1 and S2 in scented rice cultivars. Compounds belonging to alkenes, alkanes, ketones, aromatic hydrocarbons, terpens and ester were decreased at S3 stage (Fig. 1). In S4 stage, number of compounds decreased further i.e., 55 in BA-370, 51 in AM-157 and 48 in IR-64 than earlier stages. Aromatic hydrocarbon class was found totally absent in all three rice cultivars at this stage. The numbers of compounds belonging to classes’ alkenes (6–4 to 2), esters (6–5 to 2) terpenes (8–7 to 5), alcohols (11–10 to 9) aldehydes (16–14 to 11) and phenols (4 to 3) were decreased in this stage than S1 and S2 stages in all three rice cultivars.
Grain filling stages (S5: milky grain and S6: dough grain)
At S5 stage 58 compounds (BA-370) and 56 compounds (AM-157) belonging to 12 chemical classes and 52 compounds (IR-64) belonging to 10 classes were detected (Fig. 1). N-heterocyclic and aromatic hydrocarbon classes were absent in non-scented rice IR-64. Carboxylic acid class was not detected in any rice cultivar at this stage. Terpenes were present maximum in numbers in BA-370 (8; 14 %) than AM-157 (5; 9 %) and IR-64 (6; 13 %).
At S6 stage, 52 compounds in AM-157, 53 in BA-370 belonging to 11 classes and 50 compounds in IR-64 belonging to 10 classes were detected (Fig. 1). N-heterocyclic class was detected only in scented type and aromatic hydrocarbons and carboxylic acids were absent at S6 stage in all cultivars. The number and percent share of terpenes decreased significantly at S6 stage than S1-S4 stages in all rice cultivars.
Mature grain stage (S7)
At maturity 51 compounds belonging to 12 chemical classes in AM-157 and 54 compounds belonging to 11 chemical classes in BA-370 were detected. At maturity N-heterocyclic (2), terpenes (3), aromatic hydrocarbons (1), alcohols (4), aliphatic aldehydes (4), ketones (2), alkanes (2), alkenes (3) and phenol (1) were specifically present in scented rice cultivars.
The data analysis revealed that N-heterocyclic compounds (2AP, 2-acetyl-1H-pyrrole and 1H-indole) class was the major distinguishing class between scented from non-scented rice cultivars. The N-heterocyclic compounds are characterized for its role in flavor in many food products (Ho and Carlin 1989; Adams et al. 2001). The odor strength and complexity of these compounds makes them desirable as flavoring ingredients (Vernin and Párkányi 1982; Wang and Kays 2000).
In all identified volatile compounds 40 % compounds were belonging to aldehydes, alcohols and phenol classes across developmental stages in all three rice cultivars. Aldehyde was the second most important class of compound contributing into aroma of rice after N-heterocyclic. Among the detected aldehydes, 10 aliphatic aldehydes [pentanal (strong, acrid odor), heptanal (green, fresh), (Z)-2-heptenal (fatty, green), (E,E)-2,4-decadienal (musty, cooked starch aromas), (E)-2-octenal (nutty, cooked flour), nonanal (grassy, citrus, floral), (E,Z)-2,6-nonadienal (green, metallic), decanal (fatty, fruity), (E,E)-2,4-nonadienal (fatty, metallic) and β-cyclocitral (minty, nutty type odor)] and 3 aromatic aldehydes (benzaldehyde with nutty, sweet tone, phenylacetaldehyde contributing for herbal, floral aroma and vanillin imparting vanilla like flavor) were described earlier as positively associated compounds for rice aroma (Buttery et al. 1986; Buttery et al. 1988; Buttery et al. 1999; Mahattanatawee and Rouseff 2014; Calingacion et al. 2015). However, some compounds like octanal (fatty) and hexanal (green) were found to be imparting off flavor to rice (Bergman et al. 2000; Liyanaarachchi et al. 2014). Alcoholic compounds were not significantly differed throughout the developmental stages (9–12). Percent share of phenol containing compounds reduced gradually from vegetative (6–4 %) and grain filling stages (7–5 %) to maturity (4–2 %) stage (Fig. 1). The aldehydes and alcoholic compounds are synthesized through lipoxygenase (LOX), or α/β-oxidation from saturated and unsaturated fatty acids (Schreier and Schwab 2002). These are lipid degradation products and contribute for grassy, fatty and soapy flavors to rice.
Percent share of ketone varied from 7 to 18 % through developmental stages and found minimum at milky grain stage (7–11 %) and maximum in mature grains (14–18 %) (Fig. 1). Bryant and Mcclung (2011) reported maximum ketones (10) in freshly harvested aromatic rice cultivars (Aromatic se2, Dellmati, Dellrose, IAC 600, Jasmine 85, JES and Sierra) which were reduced during storage. Terpene compounds were detected more in number during vegetative stage (S1 and S2) and reduced at maturity (Fig. 1). Terpene compounds are most widely occurring naturally in plants and contribute for plant defenses as well as flavor or fragrances in many spices and fruits (Caputi and Aprea 2011). The presence of terpene compounds in rice may contribute for specific aroma during vegetative (S1 and S2) stage but their number decreased greatly at mature stage (S7) indicating lesser contribution towards grain aroma.
Compounds belonging to some classes like alkane, alkene, aromatic hydrocarbons, esters and carboxylic acids have no contribution in rice aroma and they were found common in all cultivars (Wongpornchai et al. 2003; Ajarayasiri and Chaiseri 2008; Bryant and Mcclung 2011; Goufo et al. 2011; Kong and Zhao 2014a).
Quantitative analysis of aroma volatiles
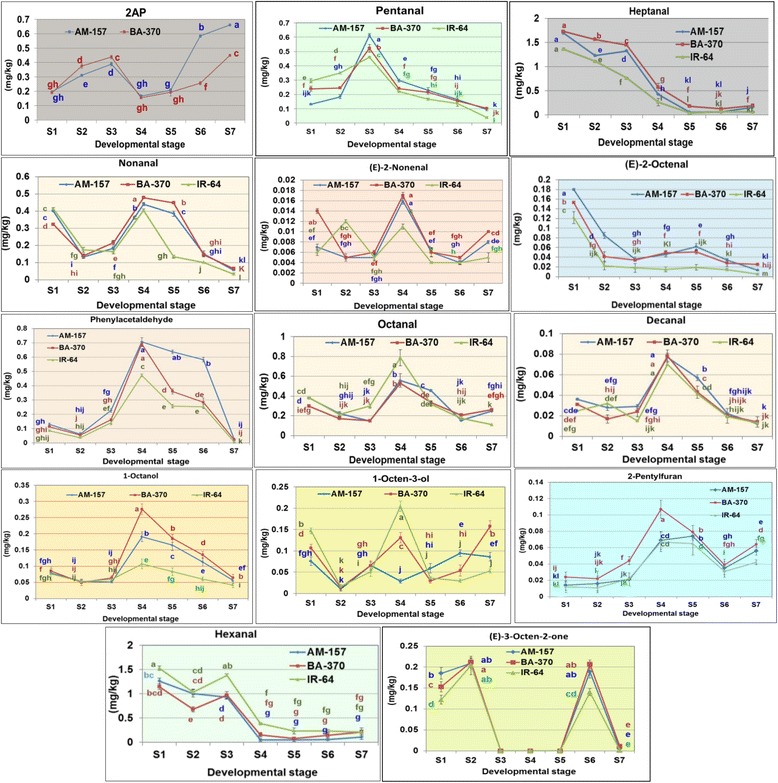
Within the developmental stages, nine OACs (2AP, heptanal, (E)-2-octenal, phenylacetaldehyde, 2-pentylfuran, pentanal, nonanal, (E)-2-nonenal and 1-octanol) were significantly elevated in scented rice cultivars over non-scented either in all stages or from S4 to S7 stages indicating their contribution in the aroma of scented rice grains (Fig. 2). Five OAC (2AP, decanal, pentanal, phenylacetaldehyde and hexanal) were accumulated at higher level in AM-157 than BA-370 during S5 to S7 stages and therefore these five compounds might contribute more towards the characteristic flavor of AM-157 rice. (E)-2-Nonenal, nonanal, heptanal, 1-octanol, 1-octen-3-ol and 2-pentylfuran contents were significantly higher in BA-370 during S5 to S7 stages than AM-157 and thus might be contributing in basmati flavor. Accumulation trend of all 14 OACs across seven developmental stages were observed similar in all three rice cultivars.
Fig. 2.
DNMRT analysis of 14 odor active compounds at 7 developmental stages in 3 rice cultivars (S1: seedling, S2: tillering, S3: booting, S4: flowering, S5: miky grains, S6: dough grains, S7: mature grains, difference in lowercase letter indicates significant difference among the mean value presented at p = 0.05 level, a-most significant)
Quantification of 2AP
2AP content increased from S1 to S3 stage, decreased during S4 stage and again increased at S6 stage and accumulated to its maximum in S7 stage in both the scented rice cultivars (Fig. 2). In Kyeema rice cultivars, 2AP content was increased three times in mature grains (0.295 mg/kg) than in leaves at tillering stage (0.093 mg/kg) (Wilkie and Wootton 2004). In the present study, similar trend was recorded. 2AP analysis through developmental stages revealed that during booting stage higher level of 2AP was accumulated. Sriseadka et al. (2006) reported that secondary metabolites are mostly produced in rice plant during booting stage. Maximum synthesis of free sugar and starch occurs during booting stage in rice (Togari 1954; Yoshida 1968; Perez et al. 1971). Further, during flowering and grain filling stages the stored starch and free sugar content depleted rapidly where it is utilized for panicle and grain development (Perez et al. 1971). As 2AP is highly volatile and lipophilic in nature, it bounds with lipid molecules and get attached to starch granules (Yoshihashi et al. 2005). The decreased 2AP content from the booting stage until the end of grain development stage is probably due to the translocation of starch bounded 2AP from leaves to grains. 2AP contents reported in mature grains of BA-370 and AM-157 cultivars are in agreement with the previous reports (Mathure et al. 2014). Maximum 2AP accumulation in mature grains can be correlated with higher level of 2AP bound starch content in mature grains (Yoshihashi et al. 2005; Hinge et al. 2016). The present result revealed that enhancement of 2AP content at or after booting stage could increase 2AP accumulation in mature grains. Therefore, agronomical and physiological measures could be taken at or after booting stage to enhance 2AP levels in mature grains.
Quantification of Aldehydes
Pentanal content initially increased from S1 to S3 stages and further decreased at S4 to S7 stages in both the scented rice cultivars (Fig. 2). Pentanal content was found significantly higher in the scented rice cultivars at S3 to S7 stages (Fig. 2). High level of pentanal in scented rice cultivars at reproductive and grain filling stages contributes to rice aroma. Previously, pentanal has been reported in cooked, brown rice, basmati and other scented rice cultivars and the contents we found in mature grains are in agreement with previous reports (Mathure et al. 2011; Mathure et al. 2014). Pentanal has fruity, green banana-like odor and it is a lipid derivative volatile compound formed from linoleic acid (Suzuki et al. 1999; Yang et al. 2008; Mathure et al. 2014; Hinge et al. 2016). The synthesis of such C5 aldehydes in plants is catalyzed in part by a 13-lipoxygenase (LOX) (Shen et al. 2014). In the present study, increased pentanal content during early reproductive stages might be due to increased lipid content in rice plant during early reproductive development (Zhang et al. 2010).
Heptanal content was observed significantly more in the scented rice cultivars than non-scented one (Fig. 2). This confirms the contribution of heptanal towards rice aroma. Among the developmental stages, maximum heptanal was recorded at S1 stage and decreased to its minimum at S7 stage in both the scented rice cultivars. In many previous studies heptanal has been identified as a key odorant which gives floral tone to rice scent (Buttery et al. 1988; Yang et al. 2008; Mathure et al. 2014; Nadaf et al. 2016). Heptanal is a fatty acid derived volatile compound associated with green grassy aroma in plants. It is formed by auto-oxidation of palmitoleic acid (Wang et al. 2001). The significantly higher levels of heptanal at S1 stage in both the scented rice cultivars might be due to higher level of fatty acid content during vegetative development. It is also a herbivore induced volatile and more active at early stages of development (Zhou et al. 2003; Aziz et al. 2015; Desurmont et al. 2015). In young leaves of coriander significantly higher level of heptanal was found which decreased further with maturation (Koharai et al. 2006). Moreover, higher level of heptanal was found during vegetative development in tomato (Wang et al. 2001) and early fruit developmental stages of cucumber (Chen et al. 2015).
Nonanal and (E)-2-nonenal contents were significantly increased in scented rice cultivars over non-scented rice at S4 to S7 stages and thus contributed towards the aroma in mature grains (Fig. 2). Higher nonanal level was recorded at S1 and S4 stages and reduced towards S5 to S7 stages in both scented rice cultivars (Fig. 2). Nonanal with fruity, floral aroma is identified as key odorant in various rice cultivars (Yang et al. 2008; Kong and Zhao 2014a; Mahattanatawee and Rouseff 2014; Mathure et al. 2014; Hinge et al. 2016). Nonanal content was elevated in several scented rice cultivars than non-scented one (Yang et al. 2008; Maraval et al. 2008b; Mathure et al. 2011; Mathure et al. 2014). (E)-2-Nonenal content was synthesized to its maximum at S4 stage, reduced at S5 and S6 stages and again increased at S7 stage in both the scented rice cultivars (Fig. 2). (E)-2-Nonenal values in mature grains of scented rice cultivars are in agreement with the previous reports (Widjaja et al. 1996; Mathure et al. 2014). The significantly high levels of (E)-2-nonenal in scented rice cultivars has been reported by Yang et al. (2008); Maraval et al. (2008b). (E)-2-Nonenal having very low odor threshold (0.08 ppb) with fatty, woody, cucumber type aroma was identified as a key odor active compound in AM-157 and BA-370 (Mathure et al. 2014; Hinge et al. 2016). Nonanal, (E)-2-nonenal and other green leaf aldehydes are produced under the lipoxygenase (LOX) pathway through a complex metabolism in leaves. In the lipoxygenase (LOX) pathway lipids are first hydrolyzed in free fatty acids by different types of lipases. Then, LOX catalyses the stereospecific oxidation of unsaturated free fatty acids. (10E,12E,15Z)-9-hydroperoxy-10,12,15-octadecatrienoic acid (9-HPOT) is produced from the linolenic acid which is transformed to C9-oxo-acids and C9-aldehydes like nonanal and (E)-2-nonenal through the action of hydroperoxide lyase (HPL) (Taurino et al. 2013; Chen et al. 2015). In plants, nonanal is also synthesized by autoxidation of oleic acid (Tananuwong and Lertsiri 2010). Lipid content including linolenic acid and oleic acid content was found enhanced in flowering panicles and up to 5 days after flowering and reduced further during seed development (Kim et al. 2015). Therefore, there might be higher level of accumulation of these C9 aldehydes during flowering in rice. Nonanal and (E)-2-nonenal were proved as effective antifungal agent and may plays role in protecting valuable reproductive plant organs in rice (Fernando et al. 2005). They were also observed to be induced upon the attack of armyworm and playing important role in volatile-mediated indirect defense mechanism against insects in rice (Yuan et al. 2008). As among the developmental stages, flowering and seedling stages are most sensitive to abiotic and biotic stresses, synthesis of C9 aldehydes (nonanal and (E)-2-nonenal) during this stages might act as an endogenous defense mechanism (Grattan et al. 2002; Desurmont et al. 2015).
The aldehyde (E)-2-octenal (green, fatty, nutty and cooked rice flavor) was accumulated significantly higher in scented rice cultivars than non-scented rice (Fig. 2). It was found maximum at S1 stage and reduced towards reproductive development and maturity (Fig. 2). The values of (E)-2-octenal reported in mature grains of scented rice cultivars are in agreement with the previous report (Mathure et al. 2014). The preeminent content of (E)-2-octenal in scented rice indicated its contributing role in aroma (Maraval et al. 2008b; Mathure et al. 2014). (E)-2-Octenal in rice plant is synthesized from oleic acid and linoleic acid by the action of LOX enzymes (Schwab et al. 2008). (E)-2-Octenal is involved in triggering plant defense mechanism and also shows antimicrobial activity (Noge et al. 2012). The seedling stage is most susceptible to pathogen and insect attack in rice plant and usually affected by infestation of blast, brown spot and stem borers. Higher expression (E)-2-octenal might offer protection to young seedlings of rice against various pathogens and insects (Desurmont et al. 2015).
Phenylacetaldehyde (intense, green, floral aroma) elevated in scented rice cultivars than non-scented rice cultivar (Fig. 2). Stage wise, it increased from S1 to S4 stages and reduced during S5 to S7 stages in both the scented rice cultivars with maximum accumulation at S4 stage (Fig. 2). Phenylacetaldehyde has been reported previously in O. sativa subsp. japonica cultivar (Lin et al. 2010), Thai fragrant rice (Sunthonvit et al. 2005) and aromatic rice cultivars Yuxiangyouzhan and Nongxiang 18 (Mo et al. 2015). It has been identified as odor active compound in four rice types of O. sativa cultivars (Sungeun 2012) and O. sativa indica spp (Hinge et al. 2016). Synthesis of phenylacetaldehyde occurs through shikimate/phenylalanine biosynthetic pathway in plants. In rice expression of shikimate kinase gene OsSK1 and OsSK3 is up-regulated specifically during the flowering stage (Kasai et al. 2005). Therefore, there might be maximum synthesis of phenylacetaldehyde at flowering in rice plant. Phenylacetaldehyde is a major volatile compound responsible for aroma of petunia and rose (Kaminaga et al. 2006; Farhi and Dupin 2010). In Arabidopsis maximum amount of phenylacetaldehyde is synthesized during flowering (Gutensohn et al. 2011). Phenylacetaldehyde has been known to possess antimicrobial properties (Berrah and Konetzka 1962) and offer protective role for flowers. In flowering panicles, it is involved in attracting pollinating and predatory insects (Raguso et al. 2003; Jhu et al. 2005).
Octanal (fruity floral) content was elevated in scented rice cultivars during S5 to S7 stages than non-scented rice cultivar (Fig. 2). During development octanal content was increased from S1 to S4 stages and decreased further from S5 to S7 stages. The higher octanal content was previously recorded in basmati than non-aromatic rice cultivars (Yang et al. 2008). Octanal is lipid derivative of oleic acid and imparts off flavor to milled rice (Lam and Proctor 2003; Monsoor and Proctor 2004). The expression of lipoxygenases was found higher during flower and embryo development and moderate in rice leaves (Bañuelos et al. 2008). Thus, there may be higher expression of octanal during flowering in rice. Higher level of octanal was reported as attractants to predatory bugs (Yu et al. 2008). Thus, might be helping rice plant for indirect defense.
Decanal content reduced from S1 to S3 stage and increased significantly at S4 stage. However, in subsequent stages (S5 to S7), it decreased in both scented rice cultivars (Fig. 2). Decanal values reported in mature grains are in agreement with the previous reports (Widjaja et al. 1996; Mathure et al. 2011; Mathure et al. 2014). Though decanal (sweet, citrus, floral type aroma) has been identified as key odorant in many scented rice types like basmati, non-basmati scented, jasmine and tropical aromatic rice cultivars, in present study no significant difference was recorded between scented and non-scented rice cultivars (Yang et al. 2008; Mathure et al. 2014). Similar results were recorded by Maraval et al. (2008a); Maraval et al. (2008b) and Yang et al. (2008). Decanal is a representative of fatty acid-derived volatiles. Similar to other aldehydes, its expression is controlled by LOX enzymes. Abundant availability of free fatty acids and lipid molecules and highest expression of LOX during flowering may lead to its significantly higher accumulation in this stage. Decanal was identified as one of the important volatiles attracting predatory insects in rice (Fujii et al. 2010).
Hexanal with green grass like aroma showed its maximum content at S1 stage and reduced further at S3 and S4 and at S7 stages in both scented rice cultivars (Fig. 2). Hexanal is reported in many fruits (strawberries, blackberries, mandarin peel, pears and walnut etc.), vegetables (tomatoes, carrot, cucumber etc.) imparting characteristic green/grassy aroma and used as marker for green/grassy aromatics (Abegaz et al. 2004; Chambers and Koppel 2013; Baldwin et al. 2004). It is lipid degradation product and formed by the auto-oxidation of linoleic acid resulting in the development of off and stale flavor (Lam and Proctor 2003; Laohakunjit and Kerdchoechuen 2006). 13-HPOT (9Z,11E,15Z)-13-hydroperoxy-9,11,15-octadecatrienoic acid is produced from linolenic acid and is further metabolized by HPL to form 12-oxo-(Z)-9-dodecenoic acid (a precursor of the traumatin) and (Z)-3-hexenal (Grechkin, 1998). Hexanal has been directly related to oxidative off flavor that increases considerably higher after milling and storage (Buttery et al. 1988; Wongpornchai et al. 2003; Laohakunjit and Kerdchoechuen 2006; Wijerathna et al. 2014). It is also used as indicators of rancidity (Yasumatsu et al. 1966; Champagne et al. 1993). In our studies, hexanal associated with green grassy aroma was significantly higher in leaves at vegetative stage than grain filling and maturity stages. The hexanal accumulation was increased in rice leaves upon pathogen infection and wounding specifically through expression of OsHPL3 gene (Chehab et al. 2006). Highest expression of hexanal during seedling stage might be due to induced defense mechanism which protects young seedlings from pathogen and insect infestation.
Quantification of alcohols
Significantly higher level of 1-octanol was recorded at S4 to S7 stages in scented rice cultivars than IR-64 (Fig. 2). Highest content of 1-octanol was recorded at S4 stage and reduced further at S5, S6 and S7 stages in both the scented rice cultivars. Earlier, similar patterns of enhanced expression of 1-octanol during flowering stage was reported (Kong and Zhao 2014b). The presence of 1-octanol has been detected in scented rice (Grimm et al. 2011); Yang et al., 2008; Bryant and Mcclung 2011) and identified as odor active compounds (Mahattanatawee and Rouseff, 2014; Kong and Zhao 2014a, b; Hinge et al., 2016). The role of 1-octanol in flowering panicles may be associated with its antifungal and antibacterial activity (Rodov and Nafussi 2011). 1-Octanol has been observed to induce ROS production in elicited and non-elicited rice cells and showed fungistatic activity against the pathogen Magnaporthe oryzae (Forlani et al. 2011). Thus maximum accumulation of 1-octanol in flowering panicles might protect the flowers and developing embryos from biotic stress.
Significantly higher quantity of 1-octen-3-ol was recorded at S3 and S5 to S7 stages in scented rice cultivars compared to IR-64 (Fig. 2). Highest level of 1-octen-3-ol was recorded in IR-64 at S4 and S1 stages of development. 1-Octen-3-ol has powerful, sweat, earthy, mushroom type odor with strong herbaceous note. It has been reported as odor contributor in many scented rice cultivars (Mo et al. 2015; Mathure et al. 2014; Yang et al. 2008). The role of 1-octen-3-ol at seedling and flowering stages in rice plant may be associated with plant defense mechanisms. 1-Octen-3-ol was reported as attractant for biting insects, contributes to enhanced plant resistance to the necrotropic fungal pathogen Botrytis cinerea by inducing defense signaling cascades and also serves as attractant for fungus-eating beetles (Kanchiswamy et al. 2015).
Ketone (E)-3-octen-2-one was detected only at vegetative and maturity stages and absent at reproductive and milky grain stages in both the scented rice cultivars (Fig. 2). The highest content was recorded at S2 stage followed by S5, S1 and S7 stages. (E)-3-Octen-2-one values recorded in mature grains of scented rice cultivars are in agreement with the previous reports (Maraval et al. 2008b; Luo et al. 2009; Mathure et al. 2014).
Quantification of furans
2-Pentylfuran was found at significantly elevated level in scented rice cultivars than non-scented rice at all developmental stages (Fig. 2). BA-370 showed elevated level of 2-pentylfuran than AM-157 at all developmental stages. 2-Pentylfuran increased from S1 & S2 stages to S4 stage and gradually decreased at S5 & S6 stages and again increased in S7 stage (Fig. 2). 2-Pentylfuran is a furan compound produced by lipid oxidation and also through maillard reaction. It imparts floral, fruity, nutty and caramel-like aroma to rice. Similar elevated contents of 2-pentylfuran in scented rice cultivars than non-scented rice cultivars were reported by several authors (Sunthonvit et al. 2005; Yang et al. 2008; Maraval et al. 2008b; Luo et al. 2009; Grimm et al. 2011). Its higher concentration in scented rice cultivars and odor active property confirms its role in rice aroma. Its enhanced level at reproductive development could be due to abundant availability of lipid and free fatty acids during flowering stage.
The maximum accumulation of OACs at flowering (nonanal, (E)-2-nonenal, phenylacetaldehyde, octanal, decanal, 1-octanol and 2-pentylfuran) followed by seedling (heptanal, hexanal, (E)-2-octenal and (E)-3-octen-2-one) stages might be due to endogenous developmental regulation of volatile emission. The similar developmental regulation of volatile accumulation in many plant species has been observed. Increased volatile accumulation at vegetative and reproductive development and further remained relatively constant or decreasing trend was reported in many plant species (Vassao et al. 2006; Dudareva et al. 2013). In general, rice seedlings are sensitive to abiotic and biotic stress particularly during seedling, flowering stages (anthesis and fertilization) and to a lesser extent at the preceding stage booting (microsporogenesis) stage than other developmental stages (Grattan et al. 2002). Besides aroma and flavor agents volatile compounds have important contribution in direct and indirect defense mechanism of plant. And thus higher level of expression either at flowering or seedlings stages may be result of ontogeny mechanism of plant defense (Desurmont et al. 2015). Similar results were recorded by Fujii et al. (2010) i.e., relative content of green leaf volatiles (GLVs) was higher in seedlings and flowering panicles than in other plant parts of rice.
Correlation analysis between OACs
In the correlation analysis of 14 OACs, 35 significant correlations were observed at seven developmental stages in three rice cultivars (Additional file 1: Table S1). Aldehydes showed significant positive correlations with each other and with alcohols and ketones (except hexanal and heptanal). Hexanal, heptanal and pentanal showed significant positive correlation with each other. Octanal, nonanal, decanal, phenylacetaldehyde, 1-octanol and 2-pentlyfuran were positively correlated with each other. 1-octanol levels correlated positively with octanal, nonanal, decanal and phenylacetaldehyde and negatively with hexanal. 2-Pentlyfuran correlated negatively with hexanal, heptanal and (E)-3-octen-2-one. (E)-3-Octen-2-one was positively correlated with heptanal and (E)-2-octenal. 1-Octen-3-ol level was correlated positively with octanal. There was no significant correlation recorded between 2AP and any OAC in both the scented rice cultivars. This indicated that 2AP expression pattern was unique and specific throughout rice plant development. Mathure et al. (2014) observed positive correlation of 2AP with 1-tetradecene and indole and negative correlation with benzyl alcohol in correlation analysis of 23 headspace volatile compounds in mature grains of 91 rice cultivars. But in the present study among developmental stages in three rice cultivars no any positive or negative correlation was observed for 2AP with other OAC. The correlations among the aliphatic aldehydes (heptanal, octanal, nonanal and decanal) recorded in the present study are in agreement with Mathure et al. (2014). These results revealed that 2AP alone has different accumulation behavior and the remaining OACs have similar accumulation trend through out rice plant development.
PCA analysis between OACs
Principle Component Analysis (PCA) between 14 OACs and seven developmental stages in three rice cultivars are summarized in Fig. 3a and b. Fourteen OACs were separated in two principal components at 55.88 % cumulative variance (Fig. 3a). 2AP was separated at negative side of both the PCs and 2-pentylfuran at negative side of PC2 but positive side of PC1. Aldehydes hexanal, heptanal, pentanal, (E)-2-octenal and (E)-3 octen-2-one were separated at negative side of PC1 with 2AP but positive side of PC2. Majority of the aldehydes exhibited significant loadings on positive side of PC2 indicating positive association between them. Among the 14 OACs, 7 compounds viz. 2AP, pentanal, hexanal, heptanal, 1-octen-3-ol, (E)-2-octenal and (E)-3-octen-2-one displayed significant loadings in more than one principle component (Additional file 2: Table S2). This suggests involvement of multiple factors in accumulation of these compounds at the level of synthesis, developmental accumulation and degradation (Yang et al. 2008; Yang et al. 2010; Mathure et al. 2014). Similar results were recorded by Yang et al. (2008); Griglione et al. (2015).
Fig. 3.
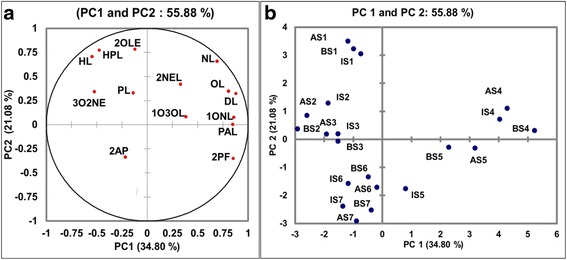
PCA analysis of odor active compounds (a) and developmental stages (b) of 3 rice cultivars (2AP; 2-acetyl-1-pyrroline, PL; pentanal, HL; hexanal, HPL; Heptanal, OL; Octanal, NL; Nonanal, 2NEL; (E)-2-Nonenal, DL; Decanal, PAL; Phenylacetaldehyde, 1ONL; 1-Octanol, 3O2NE; (E)-3-Octen-2-one, 2PF; 2-Pentylfuran, 2OLE; (E)-2-Octenal, 1O3OL; 1-Octen-3-ol, A in AS1 to AS7; AM-157, B in BS1 to BS7; BA-370, I in IS1 to IS7; IR-64, S1; seedlings, S2; tillering, S3; booting, S4; flowering, S5; milky grains, S6; dough grains, S7; mature grains)
Based on 14 OACs, seven developmental stages could be clearly differentiated (Fig. 3b). Vegetative and booting stages of all three rice cultivars were placed at negative side of PC1 and positive side of PC2 respectively. Flowering stages were isolated from all stages at positive side of both the PC and milky grain stages into positive side of PC1 and negative side of PC2. Dough grain and mature grain stages together placed at negative side of both the PCs. This showed that volatile accumulation pattern through developmental stages was specific for each developmental stage. The variation in OACs was more within the developmental stages than between the cultivars. Hence, developmental stages were separated more clearly than cultivars. Maraval et al. (2008b) reported that OACs could differentiate Thai rice cultivar from Aychade and Fidji cultivars. The aromatic cultivars Hyangmibyeo 1, Hyangmibyeo 2, Royal basmati, Jasmine, Black pigmented and non-aromatic rice were separated based on content of 13 OACs (Yang et al. 2008).
OACs as biomarker for defining rice cultivars
The volatile compounds expressing in constant ratios across the developmental stages could be considered as biomarkers for defining specific rice cultivar. The octanal/1-octanol content ratio was served as common marker for AM-157 (3.41), BA-370 (2.66) and IR-64(4.41) (Fig. 4). In AM-157 pentanal/phenylacetaldehyde (1.79) ratio was found constant at all developmental stages and could be used to define this cultivar. Pentanal/heptanal ratio remained constant only in BA-370 (0.54) therefore it could be considered as a good indicator for BA-370. Non-scented rice IR-64 represented constant ratio of pentanal/hexanal (0.47) hence could be taken as biomarker specific for IR-64. Such types of biomarkers were previously reported by Griglione et al. (2015) for defining rice cultivars and as indices of aging. They found that heptanal/1-octen-3-ol and heptanal/octanal ratios as aroma quality indices for six Italian rice cultivars. Aroma volatiles were also used as marker to discriminate the rhizobacterial isolates (Deshmukh et al. 2015). Thus, these OACs can be taken as biomarkers for defining the cultivars under study.
Fig. 4.
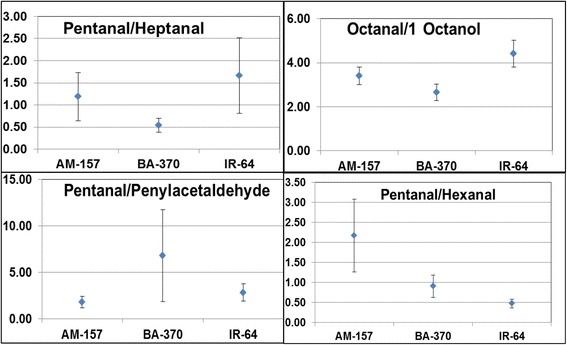
OACs as cultivars specific biomarker identification, average values with standard error for ratio of OACs in respective rice cultivars under study
Proline and methylglyoxal content
Proline content was recorded higher in scented rice cultivars than non-scented rice cultivar IR-64 at all developmental stages (Fig. 5a). Highest proline content was recorded at S4 stage in both the scented rice cultivars (Fig. 5a). Proline content remained constant during vegetative development and increased significantly at flowering and reduced at grain filling and maturity stages in both scented rice cultivars. Proline levels were in agreement with those reported by Kaikavoosi et al. (2015). Proline was identified as a precursor of 2AP (Suprasanna et al. 1998; Suprasanna et al. 2002; Yoshihashi et al. 2002a) and increase in free proline content leads in increased 2AP content in several scented rice cultivars (Poonlaphdecha et al. 2012; Mo et al. 2015). Proline accumulation in rice plant was determined by developmental stage and type of plant organs (Nanjo et al. 1999; Maggio et al. 2002). In flowering stage, the panicles showed highest proline accumulation, which contributes in flower and embryo development. Several studies indicated that proline plays an important role in reproductive development especially at flower development and serving as a readily accessible source of energy in pollen (Lehmann et al. 2010). Analysis of the free amino acids in different tissues revealed that proline content was 60 times higher in pollen than in any other organ (Szabados and Savouré 2010). Thus in reproductive developmental stages proline plays major role. The left over proline through primary metabolism might be utilized for 2AP synthesis; hence, in spite of level of proline, 2AP contents might be low in flowering panicles.
Fig. 5.
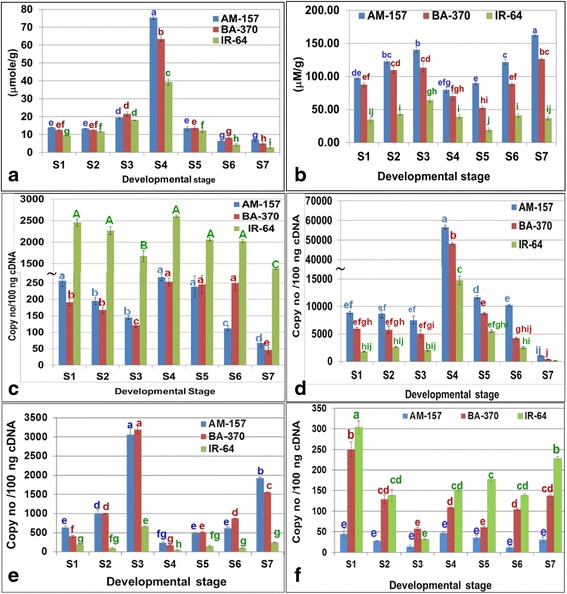
Proline (a), methylglyoxal (b), badh2 (c), P5CS (d), TPI (e), GAPDH (f) transcript at 7 developmental stages in 3 rice cultivars. (S1; seedlings, S2; tillering, S3; booting, S4; flowering, S5; milky grains, S6; dough grains, S7; mature grains. badh2; betaine aldehyde dehydrogenase 2, TPI; triose phosphate isomerase, GAPDH; glyceraldehyde-3-phosphate dehydrogenase, P5CS; Δ1-Pyrolline-5-carboxylic acid synthetase, Difference in lowercase letter indicates significant difference among the mean value, A or a-most significant)
Methylglyoxal was accumulated at significantly higher level in scented rice cultivars than IR-64 (Fig. 5b). At the S7 stage, highest MG content was recorded followed by S3 stage in AM-157. MG contents were positively correlated with 2AP accumulation in both the scented rice cultivars. Increase in MG content leads in increased 2AP content in aromatic rice cultivars Tainung 71 and 72 and in aromatic soybean (Huang et al. 2008; Wu et al. 2009; Szabados and Savouré 2010). The methylglyoxal contents recorded in the present study are comparable with earlier reports (Yadav et al. 2005; Huang et al. 2008; Hossain et al. 2009; Kumar and Yadav 2009). This confirmed the role of methylglyoxal as a precursor in 2AP accumulation.
Gene expression analysis
Expression of badh2 was found to be 9 to 30 fold reduced in both the scented rice cultivars than non-scented IR-64 at all developmental stages (Fig. 5c). At S7 and S3 stages, 1.7 to 5.56 fold lower transcript was recorded than S1, S2, S4 to S6 stage in both the scented rice cultivars. Significantly, higher level of 2AP was accumulated in the S7 and S3 stages of both scented. This indicated that badh2 expression is negatively associated with 2AP accumulation in scented rice. 2AP accumulation is regulated by badh2 transcript (Vanavichit et al. 2008). The down regulation of badh2 gene was also previously reported in aromatic rice cultivars (Chen et al. 2008; Niu et al. 2008; Vanavichit et al. 2008).
P5CS gene expression was 3 to 5 fold higher in both the scented rice cultivars compared to IR-64 at all developmental stages (Fig. 5d). Highest transcript was recorded at S4 stage in both the scented rice followed by S5 stage in AM-157. P5CS expression was low at S7 stage than other stages. P5CS expression was not significantly different among S1, S2 and S3 stages in scented rice cultivars. Positive association of 2AP accumulation with P5CS expression has been reported earlier (Wu et al. 2009; Huang et al. 2008). Up regulation of expression of Δ1-pyrroline-5-carboxylate synthetase (P5CS) in aromatic rice might increase the level of Δ1-pyrroline-5-carboxylic acid (P5C) and thus more 2AP accumulation (Huang et al. 2008). Proline has been also identified as important precursor of 2AP and proline synthesis in plant is mediated largely by P5CS enzyme (Schieberle 1990; Romanczyk et al. 1995; Suprasanna et al. 2002; Yoshihashi et al. 2002b; Thimmaraju et al. 2005). Proline is further metabolized to Δ1-pyrroline that reacts with methylglyoxal to form 2AP (Huang et al. 2008). Kaikavoosi et al. (2015) reported more than 2 fold enhancements in 2AP content after overexpression of P5CS in transgenic calli, vegetative plant parts and seeds of AM-157 and Indrayani rice cultivars over control. Significantly, higher level of P5CS in scented rice cultivars over non-scented in present study confirms the role of P5CS in 2AP accumulation. However across the developmental stages P5CS expression and 2AP accumulation were not correlated positively that might be due to involvement of P5CS and proline in plant development as well as 2AP accumulation. The P5CS dependent proline synthesis from glutamate was associated with flower development (Lehmann et al. 2010). In Arabidopsis P5CS1 was present exclusively in anthers, whereas P5CS2 was found in inflorescence meristems, flower primordia and flower buds (Székely et al. 2008). Both the P5CS genes in Arabidopsis were involved in controlling flowering (Mattioli et al. 2008; Mattioli et al. 2009; Mattioli et al. 2012). Overexpression of P5CS leads to enhance proline accumulation as well as flower development in transgenic plants (Kishor et al. 1995).
Triose phosphate isomerase (TPI) gene expression was found significantly higher (2 to 10 fold) in both the scented rice cultivars than IR-64 at all developmental stages (Fig. 5e). Significantly higher TPI transcript was recorded in S3 stage followed by S7, S2, and S6 stages of scented rice cultivars. 2AP accumulation was higher in S7 and S3 stages of scented rice cultivars. TPI activity controls MG level in plants. MG is produced from non-enzymatic elimination of phosphate from glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP). The inter conversion of DHAP and GAP is catalyzed by a crucial glycolytic enzyme, triose phosphate isomerase (TPI) (Sharma et al. 2012). The TPI expression has been well studied in rice (Sharma et al. 2012) and it was observed that its expression increased during reproductive developmental stages and at grain filling to maturity stages in rice cultivar IR-64. Similar results were observed in the present study. Increased TPI expression led to increase MG concentration; similar results were recorded earlier where increase in MG concentration correlated positively with TPI activity in rice (Sharma et al. 2012).
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was found lower in scented rice cultivars than non-scented rice IR-64 at all developmental stages (Fig. 5f). The expression of GAPDH was lowest in S3 stage than other developmental stages in both the scented rice cultivars. The 2AP accumulation and GAPDH expression were negatively associated and reduced expression of GAPDH may enhance 2AP accumulation in scented rice cultivars. In the present study GAPDH expression varied up to five fold between cultivars and up to 2 fold within cultivars at different developmental stages (Fig. 5f). The range of transcript values of all genes are comparable with previous reports (Whelan et al. 2003; Ohdan et al. 2005; Dhanasekaran et al. 2010). Though glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been used as housekeeping gene, some recent studies showed that GAPDH expression was not stable across different tissue and experimental treatments (Jain et al. 2006; Tong et al. 2009; Li et al. 2010; Ling et al. 2014; Su et al. 2014) in rice. Up to two-fold variation in GAPDH expression were observed between samples in six cultivars of rice (Kim et al. 2003), while among two cultivars of petunia (Petunia hybrida L.), the difference in stability between them was four-fold (Mallona et al. 2010). Thus the present results are in agreement with these reports.
PCA and correlation analysis between 2AP, gene expression and metabolites
The PCA analysis clearly distinguished the parameters under study into 2 PCs with 75.20 % cumulative variance (Fig. 6). 2AP, MG and TPI were placed in positive side of PC1 confirming their close association. Proline and P5CS were placed at positive side of PC1 with 2AP indicating there positive contribution in 2AP accumulation. GAPDH and badh2 were separated opposite to 2AP at negative side of PC1 and PC2. This confirmed there negative correlation in 2AP accumulation. Similar separation of 2AP and proline was reported by Poonlaphdecha et al. (2012) at different developmental stages in Aychade rice cultivar against salt stress.
Fig. 6.
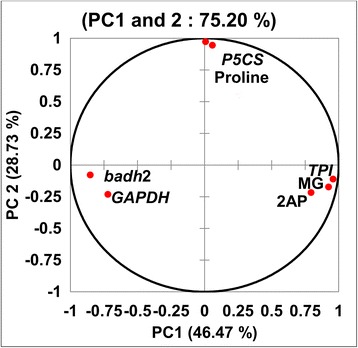
PCA analysis of 2AP, proline, MG, badh2, TPI, GAPDH and P5CS at 7 developmental stages in 3 rice cultivars (badh2: betaine aldehyde dehydrogenase 2; TPI : triose phosphate isomerase;, GAPDH : glyceraldehyde-3-phosphate dehydrogenase and P5CS: Δ1-Pyrolline-5-carboxylic acid synthetase)
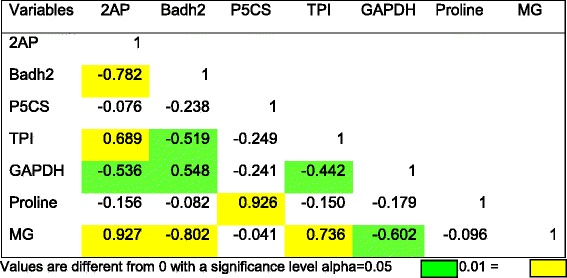
The results of correlation analysis between 2AP, gene expression and metabolites are depicted in Table 2. Significant negative correlation between badh2 and 2AP accumulation confirmed its recessive nature in scented rice cultivars. The negative correlation of 2AP with badh2 expression also reported previously in many scented rice cultivars (Chen et al. 2008; Niu et al. 2008; Vanavichit et al. 2008; Chen et al. 2012). There is no significant positive correlation recorded for 2AP with proline content and P5CS expression across developmental stages. It might be due to developmental regulation of proline synthesis in plants (Lehmann et al. 2010). Significant positive correlation of 2AP with MG confirms that MG is one of the precursors in 2AP biosynthesis. This is in agreement with reports of Huang et al. (2008) and Wu et al. (2009). The positive correlation of TPI with MG and its negative correlation with GAPDH showed TPI and GAPDH as a major gene controlling methylglyoxal level in rice. Sharma et al. (2012) observed increased TPI activity with increase in MG concentration in rice. The role of TPI and GAPDH in biosynthesis of MG and 2AP has been reported earlier in aromatic rice (Huang et al. 2008) and scented soybean (Wu et al. 2009).
Table 2.
Correlation analysis of 2AP, Proline, MG, content with Badh2, P5CS, TPI and GAPDH expression at various developmental stages in three rice cultivars
Mechanism of 2AP accumulation in rice grains
There are two mechanisms proposed for accumulation of 2AP in mature grains. In one mechanism, 2AP is synthesized in leaves and stem sheaths and transported to mature grains. As per the another one, proline translocates from leaves into grains and 2AP synthesis occurs in grains (Poonlaphdecha et al. 2012; Mo et al. 2016; Poonlaphdecha et al. 2016). In the present study, maximum 2AP was recorded in mature grains, with less proline and low P5CS expression as compared to other developmental stages. This suggests the existence of former mechanism in the cultivars under study. This mechanism of 2AP accumulation in mature grains is supported by following reports. Proline level was not significantly affected in grains by salinity or drought stress although these grains contained a significantly higher amount of 2AP (Yoshihashi et al. 2002a; Yoshihashi et al. 2002b; Gay et al. 2010; Summart and Thanonkeo 2010; Poonlaphdecha et al. 2012). Chen et al. (2012) demonstrated that down regulation of BADH2 using amiRNA driven by the maize ubiquitin promoter increased proline and 2AP content in leaf and grains of the transgenic rice. But when the same amiRNA was driven by the endosperm-specific promoter GluC, no effect on BADH2 expression was observed and the 2AP content in grains did not differ from wild-type grains. Among the studied metabolites; enzymes and genes contributing in 2AP biosynthesis are highlighted in Fig. 7.
Fig. 7.
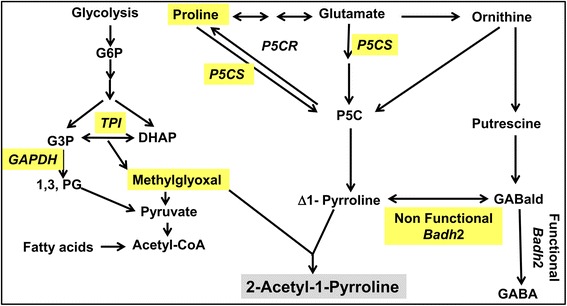
Biosynthesis pathway of 2AP in scented rice cultivars (G6P: Glucose 6 Phosphate; P5CS: Δ1Pyrroline-5 carboxylate synthetase; P5CR: Pyrroline-5 carboxylate reductase; GABald: γ- Amino Butyraldehyde; GABA: γ- Amino Butyric acid. TPI : triose phosphate isomerase; badh2: betaine aldehyde dehydrogenase; GAPDH : glyceraldehyde-3-phosphate dehydrogenase; 1,3, PG : 1,3-bisphosphoglyceric acid; DHAP: Dihydroxyacetone phosphate; Acetyl CoA : Acetyl coenzyme A)
Conclusions
The study indicated that volatilome of scented rice cultivars was more complex than non-scented rice cultivar. N-heterocyclic (2AP, 2-acetyl-1-pyrrole and indole) was the major distinguishing class between scented from non-scented rice cultivars. 2AP and other 13 compounds were detected specifically in scented rice cultivars. Maximum number of compounds were synthesized at seedling stage and decreased gradually at reproductive and maturity. The seedling stage is an active phase of development where maximum number green leaf volatiles were synthesized which could be acting as defense molecules for protection of young plant parts. Among the 14 OACs, 10 OACs were accumulated at higher concentrations significantly in scented rice cultivars and contribute in the aroma. Among the scented rice cultivars, specific set of OACs could distinguish BA-370 and AM-157 and can be taken as Basmati and Ambemohar flavor types. 2AP content was highest in mature grains followed by the booting stage. The maximum accumulation of OACs at flowering stages might be due to endogenous developmental regulation of volatile emission. Gene expression analysis revealed that reduced expression badh2 and GAPDH and elevated level of TPI and P5CS transcript enhances 2AP accumulation.
Most diverse compounds were synthesized at seedling stage and OACs were accumulated more at flowering followed by seedling stage. Distinct accumulation pattern exists for 2AP and other aroma volatiles at various developmental stages. OACs were identified as biomarkers for defining rice cultivars and selective OACs could be enhanced for inducing basmati or ambemohar type flavor. By designing proper management practices, 2AP contents at booting stage could be maintained to further enhance aroma in mature grains.
Methods
Plant material
The seeds of scented rice cultivars Ambemohar-157 (AM-157) and Basmati-370 (BA-370) were procured from Rice Research Station, Vadgaon Maval, Maharashtra, India. The seeds of non-scented rice cultivar IR-64 were procured from Balashaeb Sawant Kokan Krishi Vidyapeeth (BSKKV), Dapoli, Maharashtra, India. The seeds of three rice cultivars were sown in pots under greenhouse following completely randomized block design in three replications at Vilasrao Deshmukh College of Agricultural Biotechnology (VDCOAB), Latur, Maharashtra, India. The four seedlings were planted into each pot and kept 30 pots per cultivar. Routine cultivation practices were adapted thought growing season of rice (Hukkeri 1981; Kediyal and Dimri 2009). Seven developmental stages selected for study are seedling (S1), tillering (S2), booting (S3), flowering (S4), milky grains (S5), dough grains (S6) and mature grains (S7). Leaf samples were collected during S1 to S3 stages, panicles during S4 to S6 and mature grains at S7 stages. Every sampling was done at morning 10.00 am and samples of nine randomly selected plants (3 from each replication) of each cultivars were pulled together. Further samples were powdered using liquid nitrogen and distributed into 3 aliquots and stored at −80 °C, one used for volatile analysis, 2nd for RNA extraction and 3rd for other metabolite analysis. Three biological and two technical replicates were used for biochemical and molecular analysis of each sample.
Extraction, identification and quantitation of volatiles
Volatile compounds were extracted using head-space solid phase micro extraction (HS-SPME) method and 1 cm long fibers coated with Carboxen/Divinyl-benzene/Poly-dimethyl-siloxane (CAR/DVB/PDMS) with a manual holder (Wongpornchai et al. 2003; Laguerre et al. 2007; Grimm et al. 2011). 4 ml screw top vials (15 × 45 mm) with PTFE (Polytetrafluoroethylene) silicon septa (Chromatography research supplies, Louiseville, KY, USA) were used for analysis. The vials were heated in oven at 150 °C for 1 h prior to use for elimination of unintended volatile compounds. The optimized HS-SPME conditions were used for volatile analysis of leaf (Hinge et al. 2016) and mature grain (Mathure et al. 2011) samples.
Separation and analysis of volatile compounds was done using HS-SPME coupled with GC-MS (Varian 430-GC and 210-MS, Japan) with a factor four capillary column VF5-MS (30 m × 0.25 mm × 0.25 μm) (Varian, Inc., Palo Alto, CA) of 5 % diphenyl, 95 % dimethyl polysilosane. The research grade helium (99.999 %) was used as the carrier gas under a constant flow of 28.6 cm/s (1 ml/min). Volatiles were extracted and concentrated by using preconditioned SPME fiber at 250 °C for 30 min attached to the SPME manual holder (57330-U) (Supelco, Bellefonte, PA, USA). The SPME fiber was desorbed for 5 min in GC injector having temperature at 260 °C. Optimum performance of SPME fiber was monitored after every ten extractions with the standard fiber maintained separately. The fiber showing comparable performance was continued in further analysis. Blanks were run following every fourth sample as a control. The GC oven program was optimized for the separation of 2AP from seed and leaf samples. Initially, oven temperature was kept at 45 °C for 1 min and ramped to 55 °C at the rate 5 °C/min, then at the rate of 9 °C/min up to 120 °C, further ramped to 240 °C at the rate 15 °C/min with final hold of 1 min. The total GC cycles consisted of 19.22 min runs and 1 min re-stabilization time. Blank run of GC-MS was also performed after every sixth samples to remove traces (if any) from earlier runsin GC column. The injector temperature was 260 °C and the transfer line was held at 230 °C. The detection was performed by a Saturn III mass spectrometer in the EI mode (ionization energy, 70 eV; source temperature, 180 °C). The MS was operated in the scan mode from m/z 35 to 275. Identification and quantification of 2AP and other volatiles were done at selected seven developmental stages as described previously (Hinge et al. 2016). The comparative GC-chromatograms for three rice cultivars at seven developmental stages are shown in Additional file 3: Figure S1. During the analysis, 14 volatile compounds were identified as odor active compounds (OACs) that were analyzed quantitatively in the seven developmental stages in all three rice cultivars. This includes 2AP, 8 aliphatic aldehydes (pentanal, hexanal, heptanal, octanal, (2E)-2-nonenal, nonanal, decanal and (E)-2-octenal), 1 aromatic aldehyde (phenylacetaldehyde), 2 alcohols (1-octanol and 1-octen-3-ol), 1 furan (2-pentylfuran), and 1 ketone (E)-3-octen-2-one).
RNA extraction and cDNA synthesis
RNA was extracted from the leaves, panicles of rice cultivars under study using TRIzol reagent following the manufacturer’s protocol (Invitrogen, Carlsbad, CA) and treated with DNase (Fermentas, Germany). RNA was extracted from ~100 mg mature grains using extraction buffer (100 mMTris-HCl, 150 mM LiCl2, 50 mM EDTA, 1.5 % SDS, 1.5 % β-mercaptoethanol), and phenol/chloroform extraction followed by TRIzol reagent. RNA concentration in samples was determined using a Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE, USA). First-strand cDNA synthesis was done using ~1 μg total RNA from each sample and RevertAid first strand cDNA synthesis kit (Thermo Scientific). cDNA was then aliquoted and stored at −80 °C.
Quantitative Real-Time PCR (qRT-PCR)
For performing qRT-PCR selected genes (badh2, P5CS, TPI, GAPDH and EF1α as housekeeping gene) were amplified from cDNA of leaves of BA-370 using gene specific primers and cloned into pJET cloning vector (Life Technologies, Carlsbad, CA, USA). For badh2 gene, primers (forward: TGTGCTAAACATAGTGACTGGA, reverse: CTTAACCATAGGAGCAGCT) were designed to target exon 6 and 7 region of badh2 gene [Genebank: FJ70385]. For GAPDH primers (forward: ATGGCGAAGATTAAGATCGGGAT, reverse: CACAGTGTCATACTTGAACA) were designed based reference gene sequence [Genebank:GQ848049]. Primers for P5CS, TPI and EF1α were used as reported earlier by Yooyongwech et al. (2012), Sharma et al. (2012) and Jain et al. (2006). The purified linear plasmid containing gene of interest was used as standard DNA for developing standard curves (Taverniers et al. 2004; Ohdan et al. 2005) for each gene under study (Additional file 4: Figure S2). The real-time quantitative PCR was carried out in a total volume of 25 μl containing12.5 μl VeriQuest™ Fast SYBR® Green qPCR Master Mix with ROX (2X), 1 μl of each primer (10 pmol/μl), 1.0 μl of cDNA, and 9.5 μl DDH2O. Thermal cycling consisted of a hold at 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30s. The PCR reactions were performed on the Mastercycler® eprealplex PCR system (Eppendorf, Hamburg, Germany) in triplicate. Primer specificity and gene specific amplification were confirmed with melt curve analysis. Each primer pair has specific TM and only single peak detected in melt curve analysis (Additional file 5: Figure S3). Three biological replicates were used for each sample and reaction for each test sample was repeated three times. Mean ct values were used quantification. Expression analysis in terms of copy number for badh2 (R2 = 0.996, e = 1.16); P5CS (R2 = 0.983, e = 1.14); TPI (R2 = 0.992, e = 1.12); GAPDH (R2 = 0.990, e = 1.09) and EF1aplha (R2 = 0.995, e = 1.11) as a control gene were calculated at selected developmental stages from three rice cultivars. The transcript abundance was expressed as copy number/100 ng of cDNA.
Proline and methylglyoxal estimation
Free proline and methylglyoxal content were estimated following the method of (Bates et al. 1973) and (Yadav et al. 2005), respectively. Standard curve for proline (Y = 0.011X + 0.0064; R2 = 0.988) and methylglyoxal (Y = 0.011X + 0.0064; R2 = 0.988) were developed using standard proline and methylglyoxal (Himedia, India). The concentration of extracted proline and methylglyoxal were extrapolated using these standard curves. The estimation of both was performed in triplicates using three biological replicates.
Statistical analysis
Descriptive analysis of volatiles was performed to determine the mean, standard deviation, standard error and % coefficient of variation (CV %). Duncan’s multiple range tests were performed on the mean values of each OAC, gene transcripts and other metabolites at various developmental stages in three rice cultivars to identify significant variation between means. Principle component analysis (PCA) was performed to study variations in the composition of 14 OACs, gene transcripts and other metabolites among seven developmental stages in three rice cultivars using XLSTAT software (Version 2014, Addinsoft™).
Abbreviations
2AP, 2-acetyl-1-pyrroline; AM-157, Ambemohar-157; amiRNA, artificial micro RNA; BA-370, Basmati-370; badh2, betaine aldehyde dehydrogenase 2; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde-3-phosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPL, hydroperoxide lyase; LOX, lipoxygenase; MG, methylglyoxal; OACs, odor active compounds; P5CS, Δ1-Pyrolline-5-carboxylic acid synthetase; PCA, principle component analysis; qRT-PCR, Quantitative Real-Time PCR; TPI, triose phosphate isomerase; VOC’s, volatile organic compounds
Acknowledgments
This study was supported by Department of Science and Technology New Delhi (India) under WOS-A program grant number SR/WOS-A/LS433/2011(G) for the period 2012–2015. The authors are thankful of Rice Research Station, Vadgaon Maval and BSKKV, Dapoli for providing seed material. The authors are thankful to Dr. R. L. Chavhan, for supporting research work at Department of Plant Biotechnology, VDCOAB, Latur.
Authors’ contributions
ABN defined the research theme, designed experiments, guided during experiments, manuscript preparation and finalization. VRH carried out the experiments, analyzed data and prepared manuscript. HBP supervised and guided part of research work at VDCOAB Latur. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional files
Correlation analysis of 14 odor active compounds at various developmental stages in three rice cultivars (2AP; 2-acetyl-1-pyrroline, PL;pentanal, HL-hexanal, HPL; Heptanal, OL; Octanal, NL; Nonanal, 2NEL; (E)-2-Nonenal, DL; Decanal, PAL; Phenylacetaldehyde, 1ONL; 1-Octanol, 3O2NE; (E)-3-Octen-2-one, 2PF; 2-Pentylfuran, 2OLE; (E)-2-Octenal, 1O3OL; 1-Octen-3-ol). (DOCX 25 kb)
PCA analysis of 14 odor active compounds at various developmental stages in three rice cultivars (2AP; 2-acetyl-1-pyrroline, PL;pentanal, HL-hexanal, HPL;Heptanal, OL;Octanal, NL;Nonanal, 2NEL; (E)-2-Nonenal, DL; Decanal, PAL; Phenylacetaldehyde, 1ONL; 1-Octanol, 3O2NE; (E)-3-Octen-2-one, 2PF; 2-Pentylfuran, 2OLE; (E)-2-Octenal, 1O3OL; 1-Octen-3-ol). (DOCX 15 kb)
Comparative GC chromatograms at 7 developmental stages in 3 rice cultivars, S1; seedlings, S2; tillering, S3; booting, S4; flowering, S5; milky grains, S6; dough grains, S7; mature grains. (TIFF 9631 kb)
Standard curves developed for badh2, P5CS, TPI, GAPDH and EF1α genes. (TIFF 1042 kb)
Melt curves of badh2, P5CS, TPI, GAPDH and EF 1α genes. (TIFF 2645 kb)
Contributor Information
Vidya R. Hinge, Email: vidyahinge17@gmail.com
Hemant B. Patil, Email: hbpatil2003@yahoo.co.in
Altafhusain B. Nadaf, Email: abnadaf@unipune.ac.in
References
- Abegaz EG, Tandon KS, Scott JW, Baldwin EA, Shewfelt RL. Partitioning taste from aromatic flavor notes of fresh tomato (Lycopersicon esculentum, Mill) to develop predictive models as a function of volatile and nonvolatile components. Postharvest Biol Technol. 2004;34:227–235. doi: 10.1016/j.postharvbio.2004.05.023. [DOI] [Google Scholar]
- Adams RL, Mottram DS, Parker JK, Brown HM. Flavor − Protein Binding: Disulfide Interchange Reactions between Ovalbumin and Volatile Disulfides. J Agric Food Chem. 2001;49:4333–4336. doi: 10.1021/jf0100797. [DOI] [PubMed] [Google Scholar]
- Ajarayasiri J, Chaiseri S. Comparative study on aroma-active compounds in Thai, black and white glutinous rice varieties. Kasetsart J Nat Sci. 2008;42:715–722. [Google Scholar]
- Aziz A, Siti-Fairuz M, Abdullah MA, Ma NL, Marziah M. Fatty Acid Profile of Salinity Tolerant Rice Genotypes Grown on Saline Soil. Malays Appl Biol. 2015;44:119–124. [Google Scholar]
- Baldwin EA, Goodner K, Plotto A, Pritchett K, Einstein M. Effect of Volatiles and their Concentration on Perception of Tomato Descriptors. J Food Sci. 2004;69:S310–S318. doi: 10.1111/j.1750-3841.2004.tb18023.x. [DOI] [Google Scholar]
- Bañuelos GR, Argumedo R, Patel K, Ng V, Zhou F, Vellanoweth RL. The developmental transition to flowering in Arabidopsis is associated with an increase in leaf chloroplastic lipoxygenase activity. Plant Sci. 2008;174:366–373. doi: 10.1016/j.plantsci.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldron RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bergman CJ, Delgado JT, Bryant R, Grimm C, Cadwallader KR, Webb BD. Rapid Gas Chromatographic Technique for Quantifying 2-Acetyl-1-Pyrroline and Hexanal in Rice (Oryza sativa, L.) Cereal Chem J. 2000;77:454–458. doi: 10.1094/CCHEM.2000.77.4.454. [DOI] [Google Scholar]
- Berrah G, Konetzka WA. Selective And Reversible Inhibition of The Synthesis of Bacterial Deoxyribonucleic Acid By Phenethyl Alcohol. J Bacteriol. 1962;83:738–744. doi: 10.1128/jb.83.4.738-744.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RJ, Mcclung AM. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC –MS. Food Chem. 2011;124:501–513. doi: 10.1016/j.foodchem.2010.06.061. [DOI] [Google Scholar]
- Buttery RG, Ling LC, Mon TR. Quantitative analysis of 2-acetyl-1-pyrroline in rice. J Agric Food Chem. 1986;34(1):112–114. doi: 10.1021/jf00067a031. [DOI] [Google Scholar]
- Buttery RG, Turnbaugh JG, Ling LC. Contribution of volatiles to rice aroma. J Agric Food Chem. 1988;36(5):1006–1009. doi: 10.1021/jf00083a025. [DOI] [Google Scholar]
- Buttery RG, Orts WJ, Takeoka GR, Nam Y. Volatile Flavor Components of Rice Cakes. J Agric Food Chem. 1999;47(10):4353–4356. doi: 10.1021/jf990140w. [DOI] [PubMed] [Google Scholar]
- Calingacion M, Fang L, Quiatchon-baeza L, Mumm R, Riedel A, Hall RD, Fitzgerald M. Delving deeper into technological innovations to understand differences in rice quality. Rice. 2015;8(6):2–10. doi: 10.1186/s12284-015-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi L, Aprea E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric. 2011;3:9–16. doi: 10.2174/2212798411103010009. [DOI] [PubMed] [Google Scholar]
- Chambers E, IV, Koppel K. Associations of volatile compounds with sensory aroma and flavor: the complex nature of flavor. Molecules. 2013;18:4887–4905. doi: 10.3390/molecules18054887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne ET, Hron RJ Sr, Abraham G (1993) Stabilizing unmilled brown rice by ethanol vapors. U.S. Patent No. 5,209,940. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1992001391
- Chehab EW, Raman G, Walley JW, Perea JV, Banu G, Theg S, Dehesh K. Rice HYDROPEROXIDE LYASES with unique expression patterns generate distinct aldehyde signatures in Arabidopsis. Plant Physiol. 2006;141:121–134. doi: 10.1104/pp.106.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, Cheng Z, Liu X, Xu M. Badh2, Encoding Betaine Aldehyde Dehydrogenase, Inhibits the Biosynthesis of 2-Acetyl-1-Pyrroline, a Major Component in Rice Fragrance. Plant Cell Online. 2008;20:1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wei X, Shao G, Tang S, Luo J, Hu P. Fragrance of the rice grain achieved via artificial microRNA-induced down-regulation of OsBADH2. Plant Breed. 2012;131:584–590. doi: 10.1111/j.1439-0523.2012.01989.x. [DOI] [Google Scholar]
- Chen S, Zhang R, Hao L, Chen W, Cheng S. Profiling of volatile compounds and associated gene expression and enzyme activity during fruit development in two cucumber cultivars. PLoS One. 2015;10:1–22. doi: 10.1371/journal.pone.0119444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh Y, Khare P, Nadaf AB, Patra D. Discrimination between 2AP producing and non‐producing rice rhizobacterial isolates using volatile profiling: a chemometric approach. J Chemom. 2015;29:648–658. doi: 10.1002/cem.2760. [DOI] [Google Scholar]
- Desurmont GA, Laplanche D, Schiestl FP, Turlings TCJ. Floral volatiles interfere with plant attraction of parasitoids: ontogeny-dependent infochemical dynamics in Brassica rapa. BMC Ecol. 2015;15:17. doi: 10.1186/s12898-015-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran S, Doherty TM, Kenneth J. Comparison of different standards for real-time PCR-based absolute quantification. J Immunol Methods. 2010;354:34–39. doi: 10.1016/j.jim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Klempien A, Muhlemann JK, Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- Fageria NK. Yield Physiology of Rice. J Plant Nutr. 2007;30:843–879. doi: 10.1080/15226510701374831. [DOI] [Google Scholar]
- Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100–108. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol and Biochem. 2005;37:955–964. doi: 10.1016/j.soilbio.2004.10.021. [DOI] [Google Scholar]
- Forlani G, Occhipinti A, Bossi S, Bertea CM, Varese C, Maffei ME. Magnaporthe oryzae cell wall hydrolysate induces ROS and fungistatic VOCs in rice cell cultures. J Plant Physiol. 2011;168:2041–2047. doi: 10.1016/j.jplph.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Fujii T, Hori M, Matsuda K. Attractants for Rice Leaf Bug, Trigonotylus caelestialium (Kirkaldy), are Emitted from Flowering Rice Panicles. J Chem Ecol. 2010;36:999–1005. doi: 10.1007/s10886-010-9839-6. [DOI] [PubMed] [Google Scholar]
- Gay F, Maraval I, Roques S, Gunata Z, Boulanger R, Audebert A, Mestres C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France) F Crop Res. 2010;117:154–160. doi: 10.1016/j.fcr.2010.02.008. [DOI] [Google Scholar]
- Goufo P, Wongpornchai S, Tang X. Decrease in rice aroma after application of growth regulators. Agron Sustain Dev. 2011;31(349):359. [Google Scholar]
- Grattan SR, Shannon MC, Roberts SR. Rice is more sensitive to salinity than previously thought. Calif Agric. 2002;56(6):189–198. doi: 10.3733/ca.v056n06p189. [DOI] [Google Scholar]
- Grechkin A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog Lipid Res. 1998;37(5):317–352. doi: 10.1016/S0163-7827(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Griglione A, Liberto E, Cordero C, Bressanello D, Cagliero C, Rubiolo P, Bicchi C, Sgorbini B. High-quality Italian rice cultivars: Chemical indices of ageing and aroma quality. Food Chem. 2015;172:305–313. doi: 10.1016/j.foodchem.2014.09.082. [DOI] [PubMed] [Google Scholar]
- Grimm CC, Champagne ET, Lloyd ISW, Easson IM. Analysis of 2-Acetyl-l-Pyrroline in Rice by HSSE/GC/MS. Cereal Chem J. 2011;88:271–277. doi: 10.1094/CCHEM-09-10-0136. [DOI] [Google Scholar]
- Gutensohn M, Klempien A, Kaminaga Y, Nagegowda DA, Negre‐Zakharov F, Huh JH, Luo H, Weizbauer R, Mengiste T, Tholl D, Dudareva N. Role of aromatic aldehyde synthase in wounding/herbivory response and flower scent production in different Arabidopsis ecotypes. Plant J. 2011;66:591–602. doi: 10.1111/j.1365-313X.2011.04515.x. [DOI] [PubMed] [Google Scholar]
- Hinge V, Patil H, Nadaf A. Comparative Characterization of Aroma Volatiles and Related Gene Expression Analysis at Vegetative and Mature Stages in Basmati and Non-Basmati Rice (Oryza sativa L.) Cultivars. Appl Biochem Biotechnol. 2016;178(4):619–639. doi: 10.1007/s12010-015-1898-2. [DOI] [PubMed] [Google Scholar]
- Ho CT, Carlin JT (1989) Formation and Aroma Characteristics of Heterocyclic Compounds in Foods Flavor Chemistry. pp 92–104. doi:10.1021/bk-1989-0388.ch008
- Hossain MA, Hossain MZ, Fujita M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci. 2009;3:53. [Google Scholar]
- Huang TC, Teng CS, Chang JL, Chuang HS, Ho CT, Wu ML. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J Agric Food Chem. 2008;56:7399–7404. doi: 10.1021/jf8011739. [DOI] [PubMed] [Google Scholar]
- Hukkeri SB. Cultural Practices in the Cultivation of Rice. Water Int. 1981;6:37–41. doi: 10.1080/02508068108685893. [DOI] [Google Scholar]
- Hussain A, Naqvi SHM, Hammerschimdt FJ. Isolation and identification of volatile compo- nents from Basmati rice (Oryza sativa L) In: Martens M, Dalen GA, Russwurm H, editors. Flavor Sci Tech Wiley. 1987. pp. 95–100. [Google Scholar]
- Jain M, Tyagi AK, Khurana JP. Genome-wide analysis, evolutionary expansion, and expression of early auxin-responsive SAUR gene family in rice (Oryza sativa) Genomics. 2006;88:360–371. doi: 10.1016/j.ygeno.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Jezussek M, Juliano BO, Schieberle P. Comparison of key aroma compounds in cooked brown rice varieties based on aroma extract dilution analyses. J Agric Food Chem. 2002;50(5):1101–1105. doi: 10.1021/jf0108720. [DOI] [PubMed] [Google Scholar]
- Kaikavoosi K, Kad TD, Zanan RL, Nadaf AB. 2-Acetyl-1-Pyrroline Augmentation in Scented indica Rice (Oryza sativa L.) Varieties Through Δ(1)-Pyrroline-5-Carboxylate Synthetase (P5CS) Gene Transformation. Appl Biochem Biotechnol. 2015;177:1466–1479. doi: 10.1007/s12010-015-1827-4. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y, Schnepp J, Peel G, Kish CM, Ben-Nissan G, Weiss D, Orlova I, Lavie O, Rhodes D, Wood K, Porterfield DM. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J Biol Chem. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- Kanchiswamy CN, Malnoy M, Maffei ME. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015;20:206–211. doi: 10.1016/j.tplants.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Kasai K, Kanno T, Akita M, et al. Identification of three shikimate kinase genes in rice: characterization of their differential expression during panicle development and of the enzymatic activities of the encoded proteins. Planta. 2005;222:438–447. doi: 10.1007/s00425-005-1559-8. [DOI] [PubMed] [Google Scholar]
- Kediyal VK, Dimri S. Traditional methods of rice cultivation and SRI in Uttarakhand hills. Asian Agrihist. 2009;13:293–306. [Google Scholar]
- Kim B-R, Nam H-Y, Kim S-U, et al. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett. 2003;25:1869–1872. doi: 10.1023/A:1026298032009. [DOI] [PubMed] [Google Scholar]
- Kim N, Kwak J, Yeon J, et al. Changes in lipid substances in rice during grain development. Phytochemistry. 2015;116:170–179. doi: 10.1016/j.phytochem.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Kishor PBK, Hong Z, Miao G-H, et al. Overexpression of Δ-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Bot Rev. 2006;72:1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- Koharai K, Sakamotoi Y, Hasegawa H. Fluctutations in Volatile Compounds in Leaves. Stems and Fruits of Growing Coriander (Coriandrum sativum L.) Plants. J Japan Soc HortSci. 2006;75:267–269. doi: 10.2503/jjshs.75.267. [DOI] [Google Scholar]
- Kong Z, Zhao D (2014a) The Enhancing Effect of Jasmonic Acid on Fragrance of Kam Sweet Rice. J Food Nutr Res 2:395–400. doi:10.12691/jfnr-2-7-11
- Kong Z, Zhao D (2014b) The Inhibiting Effect of Abscisic Acid on Fragrance of Kam Sweet Rice. J Food Nutr Res 2:148–154. doi:10.12691/jfnr-2-7-11
- Kumar V, Yadav SK. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant. 2009;31:261–269. doi: 10.1007/s11738-008-0227-6. [DOI] [Google Scholar]
- Laguerre M, Mestres C, Davrieux F, et al. Rapid discrimination of scented rice by solid-phase microextraction, mass spectrometry, and multivariate analysis used as a mass sensor. J Agric Food Chem. 2007;55:1077–1083. doi: 10.1021/jf0623992. [DOI] [PubMed] [Google Scholar]
- Lam HS, Proctor A. Milled Rice Oxidation Volatiles and Odor Development. J Food Sci. 2003;68:2676–2681. doi: 10.1111/j.1365-2621.2003.tb05788.x. [DOI] [Google Scholar]
- Laohakunjit N, Kerdchoechuen O. Aroma enrichment and the change during storage of non-aromatic milled rice coated with extracted natural flavor. Food Chem. 2006;101:339–344. doi: 10.1016/j.foodchem.2005.12.055. [DOI] [Google Scholar]
- Lehmann S, Funck D, Szabados L, Rentsch D. Proline metabolism and transport in plant development. Amino Acids. 2010;39:949–962. doi: 10.1007/s00726-010-0525-3. [DOI] [PubMed] [Google Scholar]
- Li Q-F, Sun SSM, Yuan D-Y, et al. Validation of candidate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol Biol Report. 2010;28:49–57. doi: 10.1007/s11105-009-0124-1. [DOI] [Google Scholar]
- Lin J-Y, Fan W, Gao Y-N et al (2010) Study on volatile compounds in rice by HS-SPME and GC-MS. 10th Int Work Conf Stored Prod Prot, pp 125–134. doi:10.5073/jka.2010.425.217
- Ling H, Wu Q, Guo J, et al. Comprehensive selection of reference genes for gene expression normalization in sugarcane by real time quantitative RT-PCR. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanaarachchi GD, Kottearachchi NS, Samarasekera R. Volatile profiles of traditional aromatic rice varieties in Sri Lanka. J Natl Sci Found Sri Lanka. 2014;42:1. [Google Scholar]
- Luo T, Fan W, Xu Y. Characterization of volatile and semi-volatile compounds in Chinese rice wines by headspace solid phase microextraction followed by gas chromatography–mass spectrometry. J InstBrew. 2009;1114:172–179. [Google Scholar]
- Maggio A, Miyazaki S, Veronese P, et al. Does proline accumulation play an active role in stress‐induced growth reduction. Plant J. 2002;31:699–712. doi: 10.1046/j.1365-313X.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- Mahattanatawee K, Rouseff RL. Comparison of aroma active and sulfur volatiles in three fragrant rice cultivars using GC-Olfactometry and GC-PFPD. Food Chem. 2014;154:1–6. doi: 10.1016/j.foodchem.2013.12.105. [DOI] [PubMed] [Google Scholar]
- Mallona I, Lischewski S, Weiss J, et al. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol. 2010;10:1. doi: 10.1186/1471-2229-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraval I, Mestres C, Pernin K et al (2008a) Impact flavour compounds in cooked rice cultivars from the Camargue area (France). In Expression of Multidisciplinary Flavour Science:479–482, Proceedings of the 12th Weurman Symposium Interlaken, Switzerland, 2008. Institute of Chemistry and Biological Chemistry Zürich University of Applied Sciences Wädenswil, Switzerland.
- Maraval I, Mestres C, Pernin K et al (2008b) Odor-active compounds in cooked rice cultivars from Camargue (France) analyzed by GC-O and GC-MS. J Agric Food Chem 56:5291–5298. doi:10.1021/jf7037373 [DOI] [PubMed]
- Maraval I, Sen K, Agrebi A, et al. Quantification of 2-acetyl-1-pyrroline in rice by stable isotope dilution assay through headspace solid-phase microextraction coupled to gas chromatography–tandem mass spectrometry. Anal Chim Acta. 2010;675:148–155. doi: 10.1016/j.aca.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Mathure SV, Wakte KV, Jawali N, Nadaf AB. Quantification of 2-Acetyl-1-pyrroline and Other Rice Aroma Volatiles Among Indian Scented Rice Cultivars by HS-SPME/GC-FID. Food Anal Methods. 2011;4:326–333. doi: 10.1007/s12161-010-9171-3. [DOI] [Google Scholar]
- Mathure SV, Jawali N, Thengane RJ, Nadaf AB. Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food Chem. 2014;142:383–391. doi: 10.1016/j.foodchem.2013.07.066. [DOI] [PubMed] [Google Scholar]
- Mattioli R, Marchese D, D’Angeli S, et al. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol Biol. 2008;66:277–288. doi: 10.1007/s11103-007-9269-1. [DOI] [PubMed] [Google Scholar]
- Mattioli R, Falasca G, Sabatini S, et al. The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plant. 2009;137:72–85. doi: 10.1111/j.1399-3054.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- Mattioli R, Biancucci M, Lonoce C, et al. Proline is required for male gametophyte development in Arabidopsis. BMC Plant Biol. 2012;12:236. doi: 10.1186/1471-2229-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Li W, Pan S, et al. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice. 2015;8:1–10. doi: 10.1186/s12284-015-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Huang J, Xiao D et al (2016) Supplementation of 2-Ap, Zn and La Improves 2-Acetyl-1-Pyrroline Concentrations in Detached Aromatic Rice Panicles In Vitro. pp 1–15. doi:10.1371/journal.pone.0149523 [DOI] [PMC free article] [PubMed]
- Monsoor MA, Proctor A. Volatile component analysis of commercially milled head and broken rice. J Food Sci. 2004;69:C632–C636. doi: 10.1111/j.1365-2621.2004.tb09911.x. [DOI] [Google Scholar]
- Nadaf A, Mathure S, Jawali N (2016) Scented Rice (Oryza Sativa L.) Cultivars of India: A Perspective on Quality and Diversity. Springer India. doi:10.1007/978-81-322-2665-9
- Nanjo T, Kobayashi M, Yoshiba Y, et al. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis Thaliana. Plant J. 1999;18:185–193. doi: 10.1046/j.1365-313X.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- Niu X, Tang W, Huang W, et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.) BMC Plant Biol. 2008;8:100. doi: 10.1186/1471-2229-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noge K, Kimura H, Abe M et al (2012) Antibacterial Activity of 4-Oxo-(E)-2-hexenal from Adults and Nymphs of the Heteropteran, Dolycoris baccarum (Heteroptera: Pentatomidae). Biosci Biotechnol Biochem 76:1975–1978. doi:10.1271/bbb.120321 [DOI] [PubMed]
- Ohdan T, Francisco PB, Sawada T, et al. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot. 2005;56:3229–3244. doi: 10.1093/jxb/eri292. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. Sink regulation of photosynthesis. J Exp Bot. 2001;52(360):1383–1400. doi: 10.1093/jexbot/52.360.1383. [DOI] [PubMed] [Google Scholar]
- Perez CM, Palmiano EP, Baun LC, Juliano BO. Starch metabolism in the leaf sheaths and culm of rice. Plant Physiol. 1971;47:404–408. doi: 10.1104/pp.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Noel J, Dudareva N. Biosynthesis of plant volatiles: nature‟s diversity and ingenuity. Science. 2006;311(5762):808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonlaphdecha J, Maraval I, Roques S, et al. Effect of timing and duration of salt treatment during growth of a fragrant rice variety on yield and 2-Acetyl-1-pyrroline, proline, and GABA levels. J Agric Food Chem. 2012;60(15):3824–3830. doi: 10.1021/jf205130y. [DOI] [PubMed] [Google Scholar]
- Poonlaphdecha J, Gantet P, Maraval I, et al. Biosynthesis of 2-acetyl-1-pyrroline in rice calli cultures: Demonstration of 1-pyrroline as a limiting substrate. Food Chem. 2016;197:965–971. doi: 10.1016/j.foodchem.2015.11.060. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Levin RA, Foose SE, et al. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/S0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Rodov V, Nafussi B. Essential Oil Components as Potential Means to Control Penicillium digitatum Pers. (Sacc.) and Other Postharvest Pathogens of Citrus Fruit. Fresh Produce. 2011;5(1):43–50. [Google Scholar]
- Romanczyk LJ, Jr, McClelland CA, Post LS, Aitken WM. Formation of 2-acetyl-1-pyrroline by several Bacillus cereus strains isolated from cocoa fermentation boxes. J Agric Food Chem. 1995;43:469–475. doi: 10.1021/jf00050a040. [DOI] [Google Scholar]
- Schieberle P. The role of free amino acids present in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2-acetyl tetrahydropyridine in wheat bread crust. Zeitschrift für Leb und Forsch. 1990;191(3):206–209. doi: 10.1007/BF01197621. [DOI] [Google Scholar]
- Schreier P, Schwab W (2002) Enzymic Formation of Flavor Volatiles from Lipids. In: Lipid Biotechnology (Kuo, T.M.and Gardner, H.W., eds). New York: Marcel Dekker, pp 293–318
- Schwab W, Davidovich-Rikanati R, Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Mustafiz A, Singla-Pareek S, et al. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal Behav. 2012;7:1337–1345. doi: 10.4161/psb.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tieman D, Jones JB, et al. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J Exp Bot. 2014;65:419–428. doi: 10.1093/jxb/ert382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriseadka T, Wongpornchai S, Kitsawatpaiboon P. Rapid Method for Quantitative Analysis of the Aroma Impact Compound, 2-Acetyl-1-pyrroline, in Fragrant Rice Using Automated Headspace Gas Chromatography. J Agric Food Chem. 2006;54:8183–8189. doi: 10.1021/jf0614490. [DOI] [PubMed] [Google Scholar]
- Su J, Wang CY, Chen S, Zhu XY (2014) Expression of cytosolic glyceraldehyde-3-phosphate dehydrogenase in response to rice blast infection. In Advanced Materials Research. Vol 884 : pp 615–618, Trans Tech Publications
- Summart J, Thanonkeo P. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr J Biotechnol. 2010;9:145–152. [Google Scholar]
- Sungeun C. Aroma Chemistry of Wild Rice (Zizania palustris) and African Rice Species (Oryaza sativa, Oryza glaberrima, and Interspecific Hybrids) 2012. [Google Scholar]
- Sunthonvit N, Srzednicki G, Craske J. Effects of high-temperature drying on the flavor components in Thai fragrant rice. Dry Technol. 2005;23:1407–1418. doi: 10.1081/DRT-200063491. [DOI] [Google Scholar]
- Suprasanna P, Ganapathi TR, Ramaswamy NK, et al. Aroma synthesis in cell and callus cultures of rice. Rice Genet News. 1998;15:123–125. [Google Scholar]
- Suprasanna P, Bharati G, Ganapathi TR, Bapat VA. Aroma in rice: effects of proline supplementation and immobilization of callus cultures. Rice Genet Newsl. 2002;19:9–11. [Google Scholar]
- Suzuki Y, Ise K, Li C, et al. Volatile Components in Stored Rice [Oryza sativa (L.)] of Varieties with and without Lipoxygenase-3 in Seeds. J Agric Food Chem. 1999;47:1119–1124. doi: 10.1021/jf980967a. [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Székely G, Ábrahám E, Cséplő Á, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- Tananuwong K, Lertsiri S. Changes in volatile aroma compounds of organic fragrant rice during storage under different conditions. J Sci Food Agric. 2010;90(2010):1590–1596. doi: 10.1002/jsfa.3976. [DOI] [PubMed] [Google Scholar]
- Taurino M, De Domenico S, Bonsegna S, Santino A. The Hydroperoxide Lyase Branch of the Oxylipin Pathway and Green Leaf Volatiles in Plant/Insect Interaction. J Plant Biochem Physiol. 2013;1:102. doi: 10.4172/2329-9029.1000102. [DOI] [Google Scholar]
- Taverniers I, Van Bockstaele E, De Loose M. Cloned plasmid DNA fragments as calibrators for controlling GMOs: different real-time duplex quantitative PCR methods. Anal Bioanal Chem. 2004;378:1198–1207. doi: 10.1007/s00216-003-2372-5. [DOI] [PubMed] [Google Scholar]
- Thimmaraju R, Bhagyalakshmi N, Narayan MS, et al. In vitro culture of Pandanus amaryllifolius and enhancement of 2-acetyl-1-pyrroline, the major flavouring compound of aromatic rice, by precursor feeding of L-proline. J Sci Food Agric. 2005;85:2527–2534. doi: 10.1002/jsfa.2286. [DOI] [Google Scholar]
- Togari Y. Studies on the production and behavior of carbohydrates in rice plant. II. On the accumulation and distribution of starches in the organs of rice plant with its development of growth. Nippon Sakumotsu Gakkai Kiji. 1954;22(3):98–99. [Google Scholar]
- Tong Z, Gao Z, Wang F, et al. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol. 2009;10(1):1. doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanavichit A, Tragoonrung S, Toojinda T et al (2008) Transgenic Rice Plants with Reduced Expression of OS2AP and Elevated Levels of 2-acetyl-1-pyrroline. U.S. Patent No. 7,319,181. Washington, DC: U.S. Patent and Trademark Office
- Vassao DG, Gang DR, Koeduka T, et al. Chavicol formation in sweet basil (Ocimum basilicum): cleavage of an esterified C9 hydroxyl group with NAD(P)H-dependent reduction. Org Biomol Chem. 2006;4:2733–2744. doi: 10.1039/B605407B. [DOI] [PubMed] [Google Scholar]
- Vernin G, Párkányi C (1982) Mechanisms of formation of heterocyclic compounds in Maillard and pyrolysis reactions. In The Chemistry of Heterocyclic Flavoring and Aroma Compounds. Vernin, G. Ed; Ellis Horwood Publishers, Chichester, 1982, pp 151–207
- Wang Y, Kays SJ. Contribution of Volatile Compounds to the Characteristic Aroma of BakedJewel’Sweetpotatoes. J Am Soc Hortic Sci. 2000;125:638–643. [Google Scholar]
- Wang C, Xing J, Chin CK, et al. Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry. 2001;58:227–232. doi: 10.1016/S0031-9422(01)00233-3. [DOI] [PubMed] [Google Scholar]
- Whelan JA, Russell NB, Whelan MA (2003) A method for the absolute quantification of cDNA using real-time PCR. J Immunol Methods 278:261–269. doi:10.1016/S0022-1759(03)00223-0 [DOI] [PubMed]
- Widjaja R, Craske JD, Wootton M. Comparative studies on volatile components of non-fragrant and fragrant rices. J Sci Food Agric. 1996;70(2):151–161. doi: 10.1002/(SICI)1097-0010(199602)70:2<151::AID-JSFA478>3.0.CO;2-U. [DOI] [Google Scholar]
- Wijerathna YMAM, Kottearachchi NS, Gimhani DR, Sirisena DN. Exploration of relationship between fragrant gene and growth performances of fragrant rice (Oryza sativa L.) seedlings under salinity stress. J Expt Biol Agril Sciences. 2014;2(1):7–12. [Google Scholar]
- Wilkie K, Wootton APM (2004) Flavour qualities of new Australian fragrant rice cultivars. Australian Government-Rural Industries Research and Development Corporation (RIRDC). https://rirdc.infoservices.com.au/downloads/04-160
- Wongpornchai S, Sriseadka T, Choonvisase S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze) J Agric Food Chem. 2003;51(2):457–462. doi: 10.1021/jf025856x. [DOI] [PubMed] [Google Scholar]
- Wu ML, Chou KL, Wu CR, et al. Characterization and the possible formation fechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L.) J Food Sci. 2009;74:192–197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, et al. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun. 2005;337:61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Yang DS, Shewfelt RL, Lee KS, Kays SJ. Comparison of odor-active compounds from six distinctly different rice flavor types. J Agric Food Chem. 2008;56(8):2780–2787. doi: 10.1021/jf072685t. [DOI] [PubMed] [Google Scholar]
- Yang DS, Lee KS, Kays SJ. Characterization and discrimination of premium-quality, waxy, and black-pigmented rice based on odor-active compounds. J Sci Food Agric. 2010;90:2595–2601. doi: 10.1002/jsfa.4126. [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Moritaka S, Wada S. Studies on Cereals: Part IV Volatile Carbonyl Compounds of Cooked Rice Part V Stale Flavor of Stored Rice. Agric Biol Chem. 1966;30:478–486. [Google Scholar]
- Yoshida H, Nagato Y (2011) Flower development in rice. J of Expt Bot. 1;62(14):4719–30 [DOI] [PubMed]
- Yoshida S. The accumulation process of carbohydrates in rice varieties in relation to their response to nitrogen in the tropics. Soil Sci Plant Nutr. 1968;14:153–161. doi: 10.1080/00380768.1968.10432759. [DOI] [Google Scholar]
- Yoshihashi T, Huong NTT, Inatomi H (2002a) Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J Agric Food Chem 50:2001–2004. doi:10.1021/jf011268s [DOI] [PubMed]
- Yoshihashi T, Kabaki N, Nguyen TTH, Inatomi H (2002b) Formation of flavor compound in aromatic rice and its fluctuations with drought stress. Research Highlights JIRCAS 2003(2004):32–33
- Yoshihashi T, Huong NTT, Surojanametakul V, et al. Effect of storage conditions on 2-acetyl-1 pyrroline content in aromatic rice variety, Khao Dawk Mali 105. J Food Sci. 2005;70:S34–S37. doi: 10.1111/j.1365-2621.2005.tb09061.x. [DOI] [Google Scholar]
- Yooyongwech S, Cha-um S, Supaibulwatana K (2012). Proline related genes expression and physiological changes in indica rice response to water-deficit stress. Plant Omics. Nov 1;5(6):597
- Yu H, Zhang Y, Wu K, et al. Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. Environ Entomol. 2008;37:1410–1415. doi: 10.1603/0046-225X-37.6.1410. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Ko TG, Wiggins G, Grant J. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008;55:491–503. doi: 10.1111/j.1365-313X.2008.03524.x. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Zhang H, Zhang T, et al. Analysis of flavor volatiles of glutinous rice during cooking by combined gas chromatography–mass spectrometry with modified headspace solid-phase microextraction method. J Food Compos Anal. 2009;22:347–353. doi: 10.1016/j.jfca.2008.11.020. [DOI] [Google Scholar]
- Zhang D, Liang W, Yin C, et al. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice.". Plant Physiol. 2010;154(1):149–162. doi: 10.1104/pp.110.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Blanchard C, Helliwell S, Robards K. Fatty Acid Composition of Three Rice Varieties Following Storage. J Cereal Sci. 2003;37:327–335. doi: 10.1006/jcrs.2002.0502. [DOI] [Google Scholar]


