Abstract
Mucopolysaccharidosis type I (MPS I) is an inherited lysosomal storage disease. Affected individuals have disease ranging from attenuated to severe with significant disease burden, disability, and premature death. Early treatment with enzyme replacement therapy and/or stem cell transplantation can reduce disease progression and improve outcomes. However, diagnosis is often delayed, particularly for patients with attenuated phenotypes. We conducted a survey of 168 patients and 582 physicians to explore health care seeking patterns and familiarity of physicians with MPS I symptoms. Patients with attenuated MPS I typically first presented with stiff joints or hernia/bulging abdomen, and patients with severe disease with noisy/difficult breathing, or hernia/bulging abdomen. There was a mean delay from time of symptom presentation to diagnosis of 2.7 years for patients with attenuated disease, with a mean of 5 physicians consulted before receiving a correct diagnosis. MPS I was most commonly misidentified by physicians as rheumatoid arthritis (48–72%), with a wide variety of suspected diseases, including lupus. CONCLUSION: Patient and physician real-world surveys show that MPS I is under-recognized and diagnosis of MPS I remains delayed, particularly in patients with attenuated disease. Across regions and specialties, physicians require differential diagnosis education in order to improve early detection and early treatment initiation of MPS I.
Abbreviations: Card, cardiologist; ENT, ear nose and throat; ERT, enzyme replacement therapy; EU, Europe; GAG, glycosaminoglycan; Gen Pract, general practitioner; Gen/Met Dis, geneticist/metabolic disease specialist; HSCT, hematopoietic stem cell transplant; IDUA, α-l-iduronidase; LA, Latin America; MPS I, mucopolysaccharidosis Type I; Neuro, neurologist; Ophth, ophthalmologist; Ortho, orthopedist; Ped or P, pediatrician; Pulm, pulmonologist; Rheum or R, rheumatologist; US, United States
Keywords: MPS I, Diagnosis, Treatment, Education
1. Introduction
Mucopolysaccharidosis I (MPS I) is a life-threatening disease resulting from deficiency of α-l-iduronidase (IDUA), a lysosomal enzyme responsible for glycosaminoglycans (GAGs) dermatan and heparan sulfate metabolism [1]. MPS I is a pan-ethnic, autosomal recessive disease with an estimated incidence of 1/100,000 live births [2]. Disease phenotypes range from severe (Hurler syndrome) to attenuated (Hurler-Scheie and Scheie syndromes) depending on presence or absence of neurocognitive involvement and rate of disease progression [1], [3], [4].
If untreated, MPS I results in significant disease burden, disability, and premature death from respiratory and cardiac disease, and in the most severe phenotype, neurodegeneration due to GAG accumulation [2]. Treatment options include hematopoietic stem cell transplantation (HSCT) for severe disease, and enzyme replacement therapy (ERT) with laronidase (recombinant human IDUA; Aldurazyme®) for attenuated MPS I [5], [6], [7], [8]. Treatment outcomes depend on disease severity and age at treatment initiation [9], [10]. Early treatment considerably improves patient outcomes during long-term therapy and is crucial to reduce disease progression before irreversible damage occurs [10], [11], [12], [13], [14]. However, diagnosis of MPS I is often delayed, particularly for patients with attenuated phenotypes [15], [16], [17].
Early signs and symptoms of MPS I are non-specific and diverse, and suggestive of many other diseases. While pediatricians and primary care physicians are typically consulted first, cardiac symptoms, ocular clouding, recurrent ear infections and hearing loss, hernias, and spinal deformity often result in referrals to specialists [6], [16]. Given the musculoskeletal symptoms associated with MPS I, rheumatologists are often consulted. In order to understand the real-world diagnostic journey for patients with MPS I, we used survey-based data to investigate the patterns of healthcare seeking by patients and the familiarity of pediatricians and rheumatologists with MPS I.
2. Methods
This international, voluntary, quantitative study used purposive, non-random sampling to collect data from surveys administered to patients with MPS I and physicians likely to encounter these patients. No chart review was conducted. Surveys were available in English, French (Canadian and France), Brazilian Portuguese, Mexican Spanish, German, and Italian (Italian for physician surveys only).
2.1. Patient participants
Surveys were distributed to patients/caregivers with confirmed MPS I from 2009 through 2013 by direct mail/email via local MPS patient advocacy/support organizations in Europe, North America, Central America, and Latin America. Participation was not limited by patient age, duration of MPS I diagnosis, or MPS I treatment. All participants were informed of study aims and confidentiality, and gave consent upon survey submission.
The survey consisted of 17 open- and close-ended questions related to:
-
−
Symptoms prompting physician visits
-
−
History and pattern of referrals to specialists
-
−
Diagnosing physician
-
−
Time to diagnosis
-
−
Alternate diagnoses
-
−
Time to and type of treatment
2.2. Physician participants
Eligible board certified rheumatologists and pediatricians in Europe, North America, Central America, and Latin America identified from WorldOne database (SERMO, Charlotte, NC) were in practice between 3 and 30 years with direct patient care at least 75% of the time. Government employees or paid advisors to pharmaceutical companies were ineligible.
Physicians reviewed an unidentified case of attenuated MPS I and were asked a series of guided open- and close-ended questions in an online survey between 2012 and 2014.
Case Information:
Initial Information: 8 year old female presenting with slow progressive stiffness of joints, particularly of hands and fingers, has impaired fine motor skills, and limited range of motion in shoulders. No clinically apparent signs of inflammation. Past medical history is significant for surgical repair of umbilical hernia and two recent tympanostomy tube placements. Additional information: Patient has not responded to prior courses of steroid therapy, and is negative for rheumatoid factor.
Questions:
-
–
Number of patients similar to the case study seen in the last year
-
–
Possible diagnoses
-
–
Diagnostic tests they would perform
-
–
Specialty of physicians they would refer the patient to
-
–
Experience with seeing and treating patients with MPS I
Physicians assessed the number of currently suspected patients with MPS I and the number of patients they would test for MPS I prior to and after reviewing educational materials.
2.3. Data management and analysis
Descriptive statistics included mean, median, standard deviation, and ranges to tabulate patient and physician characteristics. The percentage of patients or physicians responding to survey questions was stratified by MPS I phenotype, physician specialty, and region.
3. Results
3.1. Patient survey
Seventy percent (168/240) of surveys were completed from Europe (86/168, 51%), Latin America (58/168, 34%), and the US (24/168, 14%). Demographics and disease characteristics are shown in Table 1. Fifty 5 % (93/168) of participants had severe MPS I, 35% (60/168) had attenuated phenotypes, and 8.9% (15/168) were reported as other/unknown. Phenotype distribution was similar in Latin America and Europe, but the majority of US participants were adults and, therefore, few had the severe phenotype (3/24, 13%). Distribution of male and female participants was similar (49% and 51%, respectively) from Latin America and Europe; sex of US participants was not recorded. Most participants (65%) were ≤ 18 years of age (all US participants were > 18 years of age). Data for ERT were available for 110 participants in the US and Europe: 64% (70/110) had received ERT, 60% of whom (42/70) had attenuated phenotypes, and 40% (28/70) of whom had the severe phenotype. Information regarding experience with HSCT was available for European participants (56%, 48/86); 22% (19/86) had received both ERT and HSCT, as ERT is often used to improve overall health prior to HSCT.
Table 1.
Patient characteristics and physician profiles.
| Patient characteristics | All |
Phenotype |
Region |
||||
|---|---|---|---|---|---|---|---|
| N = 168 | Attenuated N = 60 | Severe N = 93 | Other N = 15 | US N = 24 | LA N = 58 | EU N = 86 | |
| Gender (% pts) | n = 142 | n = 53 | n = 89 | n = 56 | n = 86 | ||
| Male, female | 49, 51 | 47, 53 | 53, 47 | 52, 48 | 48, 52 | ||
| Age (% pts) | n = 165 | n = 24 | n = 57 | n = 84 | |||
| 0–2 yr. | 2 | 0 | 2 | 4 | |||
| > 2–11 yr | 44 | 0 | 67 | 40 | |||
| > 11–18 yr | 19 | 0 | 16 | 26 | |||
| > 18 yr | 35 | 100 | 16 | 30 | |||
| Age at presentation, yr | n = 157 | n = 54 | n = 88 | n = 15 | |||
| Mean | 2.9 | 5.5 | 1.2 | 3.9 | |||
| Range | < 1mo–39 yr | < 1mo–39 yr | < mo–8 yr | < 1mo–20 yr | |||
| Age at diagnosis, yr | n = 162 | n = 56 | n = 91 | n = 15 | |||
| Mean | 4.4 | 8.2 | 1.7 | 6.2 | |||
| Range | < 1mo–48 yr | < 1mo–48 yr | < mo–8.5 yr | < 1mo–21 yr | |||
| Treatment history, % pt | n = 110 | n = 51 | n = 59 | n = 24 | n = 86 | ||
| ERT | 64 | 82 | 47 | 71 | 62 | ||
| HSCT | 56 | ||||||
| Both ERT and HSCT | 22 | ||||||
| Physician profile | North America |
Latin America |
Europe |
|||
|---|---|---|---|---|---|---|
| Rheum N = 60 | Ped N = 90 | Rheum N = 60 | Ped N = 81 | Rheum N = 90 | Ped N = 201 | |
| Years in practice | 17.1 | 18.5 | 12.5 | 15.4 | 15.8 | 16.6 |
| % of time in direct patient care | 94 | 94 | 91 | 92 | 91 | 89 |
| % of time in hospital setting | 23 | 28 | 45 | 48 | 60 | 64 |
| % of physicians | ||||||
| > 70% time hospital-based | 8 | 16 | 18 | 26 | 56 | 62 |
| 30–70% time hospital-based | 23 | 22 | 55 | 43 | 11 | 9 |
| < 30% time hospital-based | 68 | 62 | 27 | 31 | 33 | 29 |
| % physicians who have seen a confirmed MPS patient in past 5 years | 18 | 14 | 15 | 26 | 19 | 25 |
| % physicians | ||||||
| Somewhat familiar with MPS I | 30 | 31 | 30 | 30 | 47 | 46 |
| Heard of, but not familiar with MPS I | 52 | 63 | 65 | 63 | 42 | 46 |
| Never heard of MPS I | 15 | 6 | 5 | 4 | 8 | 3 |
| Very familiar with MPS I | 3 | 0 | 0 | 4 | 3 | 5 |
| If at least somewhat familiar with MPS I, % of physicians very comfortable with diagnosing MPS I | 15 | 0 | 6 | 15 | 7 | 7 |
ERT = enzyme replacement therapy; EU = Europe; HSCT = hematopoietic stem cell transplant; LA = Latin America; US = United States.
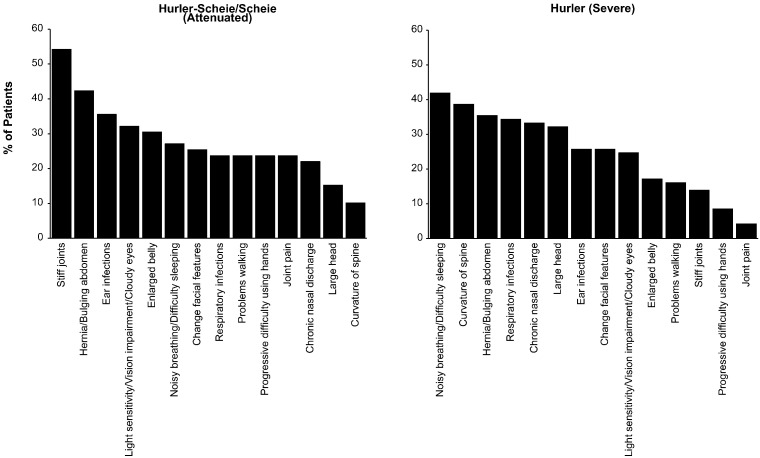
Patients with attenuated MPS I commonly presented with stiff joints or hernia/bulging abdomen, while patients with severe disease reported noisy/difficult breathing, hernia/bulging abdomen, or spine curvature as symptoms triggering initial physician visits (Fig. 1).
Fig. 1.
Symptoms triggering first physician visit percent of patients reporting the symptoms prompting first visits to physicians are shown in descending order by MPS I phenotype.
3.2. Diagnostic history: physician consultations and referrals
As would be predicted, patients with severe MPS I presented and were diagnosed earlier than attenuated patients (Table 1). Nearly 20% of patients with attenuated disease reported the diagnostic process took 5 years or longer.
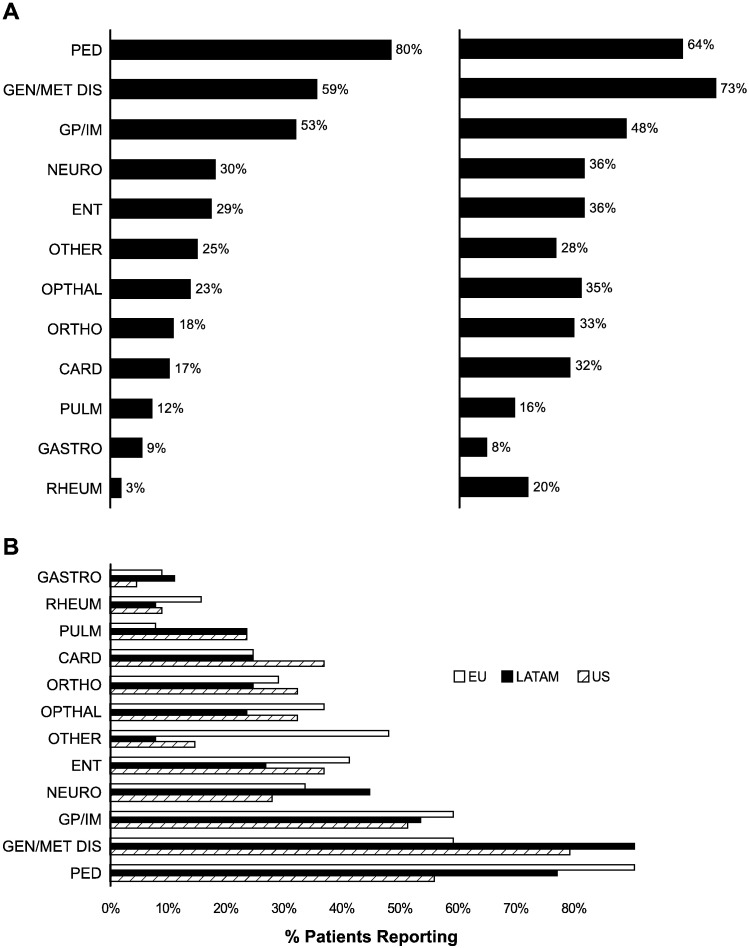
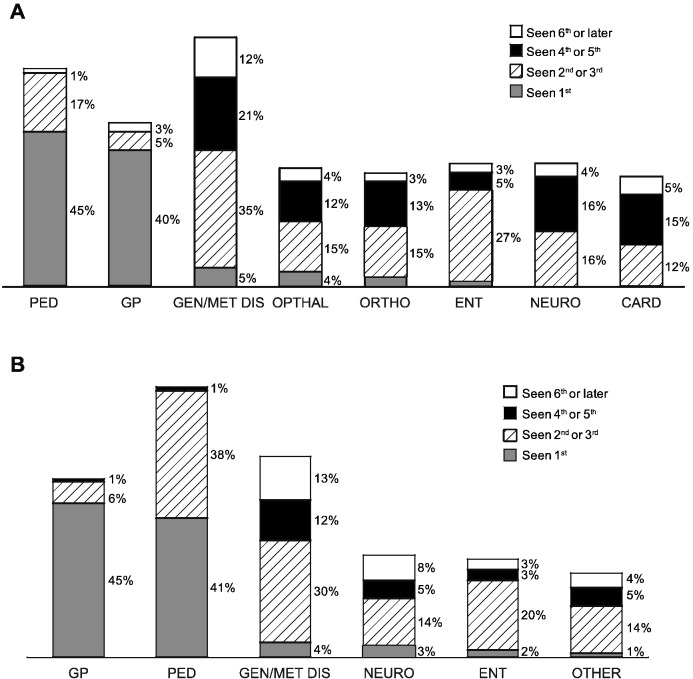
Patients with severe or attenuated disease consulted a mean of 4.7 and 4.5 specialists, respectively, before receiving a correct diagnosis. Twenty-six percent of patients with severe MPS I and 14% with attenuated disease reported seeing > 7 specialists. The specialties consulted are shown stratified by MPS I phenotype in Fig. 2A and by region in Fig. 2B. Despite some variation, the majority of patients reported seeing pediatricians (64–80%), geneticists/metabolic disease specialists (59–73%), and general practitioners (48–53%) regardless of phenotype or region. Patients with attenuated disease were more likely to consult rheumatologists (20% vs. 3%) and orthopedists (33% vs. 18%) than patients with severe MPS I. Patients in Europe were less likely to see geneticists/metabolic disease specialists (53%) than in the US (71%) or Latin America (81%) (Fig. 2B). Approximately one-quarter to one-third of patients in Europe (24% with severe MPS I and 31% with attenuated disease) reported seeing their general practitioner > 10 times before being referred to a specialist. General practitioners and pediatricians were consulted first (Fig. 3A and B), and a variety of specialists were seen second, including geneticists/metabolic disease specialists and ENTs.
Fig. 2.
Physicians consulted by phenotype (A) and region (B) percent of patients reporting which specialists were consulted for MPS I symptoms. CARD = cardiologist; ENT = ear, nose and throat; GASTRO = gastroenterologist; GEN/MET DIS = geneticist/metabolic disease specialist; GP/IM = general practitioner/internal medicine; NEURO = neurologist; ORTHO = orthopedist; PED = pediatrician; PULM = pulmonologist; RHEUM = rheumatologist.
Fig. 3.
Order of specialists seen by patients with attenuated MPS I (A) or severe MPS I (B) percent of patients (within bars) reporting the order of specialists consulted for MPS I symptoms. Card = cardiologist; GP = general practitioner; ENT = ear nose and throat; Geneticist/MDS = geneticist/metabolic disease specialist; Neuro = neurologist; Ophthalm = ophthalmologist; Ortho = orthopedist; Ped = pediatrician.
As shown in Table 2, geneticists/metabolic disease specialists diagnosed most cases (77%), with neurologists a distant second (30%). Rheumatologists and pediatricians were only slightly more likely to refer patients with or without suspicion of a genetic disease (45–50%) as they were to manage patients without referral (32–40%).
Table 2.
Physician actions and diagnoses for consulting patients (A) and physician responses to survey (B).
| A. Patient survey |
Number of patients consulting specialists |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gen/Met Dis n = 91 |
Rheum n = 15 |
Ped n = 103 |
Neuro n = 44 |
Ophth n = 43 |
Cardio n = 33 |
Ortho n = 35 |
Gen Pract n = 78 |
ENT n = 48 |
Pulm n = 23 |
|
| Physician action | The percent of patients consulting | |||||||||
| MPS suspected/diagnosed | 85 | 47 | 39 | 39 | 16 | 15 | 9 | 5 | 4 | 4 |
| Diagnosed MPS I | 77 | 13 | 17 | 30 | 7 | 3 | 3 | 0 | 0 | 0 |
| Refer; suspicion of genetic disease | 8 | 33 | 22 | 9 | 9 | 12 | 6 | 5 | 4 | 4 |
| Refer without suspicion of MPS | 3 | 12 | 28 | 14 | 21 | 9 | 20 | 45 | 17 | 17 |
| Manage without diagnosis of MPS | 11 | 40 | 32 | 41 | 63 | 73 | 49 | 47 | 58 | 65 |
| No action, told nothing wrong | 4 | 0 | 17 | 14 | 9 | 18 | 11 | 33 | 17 | 17 |
| Monitor, manage symptoms | 5 | 40 | 9 | 16 | 51 | 55 | 23 | 9 | 40 | 40 |
| Incorrect diagnosis | 1 | 0 | 6 | 11 | 2 | 0 | 14 | 5 | 2 | 2 |
| B. Physician survey |
Region |
Region |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA |
LA |
EU |
NA |
LA |
EU |
|||||||
| Suspected diagnosis | R |
P |
R |
P |
R |
P |
R |
P |
R |
P |
R |
P |
| N = 60 |
N = 90 |
N = 60 |
N = 81 |
N = 90 |
N = 201 |
N = 60 |
N = 90 |
N = 60 |
N = 81 |
N = 90 |
N = 201 |
|
| Response following initial information (% of physicians) | Response following full information (% of physicians) | |||||||||||
| Rheumatoid arthritis | 48 | 72 | 25 | 72 | 41 | 65 | 22 | 37 | 8 | 26 | 21 | 39 |
| Lupus | 5 | 9 | 2 | 5 | 0 | 2 | 3 | 18 | 5 | 7 | 2 | 7 |
| Connective tissue disorder | 0 | 8 | 0 | 2 | 4 | 2 | 0 | 2 | 0 | 2 | 9 | 2 |
| Muscular dystrophy/limb girdle | 3 | 2 | 5 | 1 | 2 | 3 | 5 | 7 | 5 | 4 | 2 | 3 |
| Scleroderma | 10 | 0 | 0 | 0 | 3 | 4 | 10 | 4 | 0 | 1 | 4 | 5 |
| Autoimmune disease | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 3 | 0 | 1 | 1 | 2 |
| Dermatomyositis | 2 | 4 | 7 | 2 | 1 | 0 | 2 | 7 | 2 | 5 | 0 | 2 |
| Metabolic disorder | 7 | 1 | 0 | 0 | 0 | 4 | 3 | 1 | 0 | 4 | 2 | 7 |
| Neuromuscular disease | 3 | 1 | 3 | 0 | 1 | 2 | 0 | 1 | 2 | 1 | 3 | 5 |
| Rheumatic fever | 2 | 1 | 3 | 1 | 2 | 2 | 0 | 1 | 8 | 5 | 0 | 1 |
Card = cardiologist; ENT = ear, nose and throat; Gen Pract = general practitioner; Gen/Met Dis = geneticist/metabolic disease specialist; Neuro = neurologist; Ophth = ophthalmologist; Ortho = orthopedist; Ped or P = pediatrician; Pulm = pulmonologist; Rheum or R = rheumatologist.
3.3. Physician survey
Two hundred ten (210) rheumatologists and 372 pediatricians completed the survey. Table 1 shows that approximately 20% of physicians had seen a confirmed case of MPS I in the last 5 years, and overall, approximately 40% were somewhat/very familiar with MPS I. Familiarity varied by region, with a third of North America and Latin America physicians and half of European physicians reporting some familiarity. Among these physicians, fewer than 10% were comfortable diagnosing the disease.
Following case review, pediatricians and rheumatologists suspected MPS I 20% and 33% of the time, respectively. Proposed referrals were to geneticists/metabolic disease specialists 17% of the time, neurologists 20% of the time or other pediatricians/rheumatologists (63% of the time). Possible diagnoses listed by physicians in the suspected order are shown in Table 2 by region, both before and after review of the full case information. MPS I was most commonly misidentified as rheumatoid arthritis following review of the initial information (48–72% of physicians), although there was a wide variety of suspected diseases, including lupus. Fewer rheumatologists made these alternative diagnoses. Following review of full information, 25–50% of pediatricians still suspected rheumatoid arthritis, with the percent of rheumatologists decreasing depending on region.
Educational information led to a substantial increase in physicians' suspicions of MPS I and willingness to test at least one current patient for the disease. The percentage of physicians responding that they suspected at least one current patient has MPS I and that they would consider testing at least one current patient following material review increased to 32% (from 5%) and 59% (from 27%), respectively.
4. Discussion
Patients with MPS I display a range of clinical manifestations that vary in severity and age of onset [16], [18], [19] (Table 3). The nonspecific nature of these symptoms results in significant diagnostic delays, particularly in patients with attenuated MPS I [16], [17], [19]. Survey participants confirmed these findings, with on average 3-year delay between first physician visit and diagnosis for attenuated MPS I.
Table 3.
Analysis of symptom frequency by age at symptom onset (adapted from [19]).
| System affected | Problem | % Affected at initial presentation |
||
|---|---|---|---|---|
| Age at symptom onset (years) | ||||
| ≤ 2 | > 2 - ≤ 5 | > 5 | ||
| Respiratory/ENT | Upper airway obstruction → OSA | 82 | 80 | 50 |
| Eustachian tube obstruction → otitis media | 55 | 45 | 20 | |
| Grommet insertion | 56 | 42 | 18 | |
| Reactive airways | 37 | 20 | 18 | |
| Neurological | Cognitive impairment | 60 | 35 | 15 |
| Carpal tunnel | 25 | 50 | 60 | |
| Hearing aid use | 15 | 25 | 25 | |
| General appearance | Coarse facies | 98 | 98 | 58 |
| Enlarged tongue | 60 | 65 | 40 | |
| Ophthalmology | Corneal clouding | 90 | 90 | 90 |
| Glaucoma | 10 | 5 | 20 | |
| Cardiovascular | Cor pumonale | 2 | 5 | 0 |
| Heart failure | 3 | 10 | 3 | |
| Valvular disease | 95 | 75 | 75 | |
| Gastrointestinal | Hepatomegaly | 84 | 88 | 55 |
| Splenomegaly | 60 | 60 | 35 | |
| Hernia | 70 | 65 | 65 | |
| Musculoskeletal | Dysostosis multiplex | 70 | 75 | 60 |
| Kyphosis gibbus | 75 | 60 | 20 | |
| Scoliosis | 35 | 38 | 30 | |
| Hip dysplasia | 42 | 40 | 32 | |
| Joint contractures | 72 | 90 | 90 | |
| Genu valgum | 38 | 56 | 28 | |
| Pes cavus | 18 | 22 | 20 | |
ENT = ear, nose and throat; OSA = obstructive sleep apnea.
Symptoms prompting initial visits included stiff joints, respiratory issues, hernia and spinal curvature. These results are consistent with previous studies where musculoskeletal symptoms and hernia occur in 50–88% of patients with severe or attenuated MPS I [17], [19] and airway-related symptoms are among the first to appear, often before formal diagnosis is made [20]. Regardless of disease severity or initial symptoms, a mean of 5 specialists were consulted before receiving a correct diagnosis. Patients with attenuated disease were more likely to consult with rheumatologists and orthopedists, although this still represented a minority of patients despite the fact that stiff joints and spinal deformity were primary symptoms triggering physician visits.
As might be expected with a rare disease, physician familiarity with MPS I was limited. Fewer than 25% of the pediatricians surveyed had seen a confirmed case of MPS in the past five years, emphasizing the need for consideration of the condition and referral to pediatric specialists to improve MPS I diagnosis and management. Joint stiffness and contractures in the absence of elevated systemic markers of inflammation are early and prominent signs of MPS I, and for patients with attenuated MPS I, are among the earliest symptoms of the disease [21]. While rheumatoid arthritis was the most common misdiagnosis, differential diagnosis can be aided by observations that patients with MPS I usually do not have morning stiffness, have limited response nonsteroidal anti-inflammatory drugs, and very rarely have elevated erythrocyte sedimentation rates/C-reactive protein levels/white blood cell counts, and have negative rheumatoid factor tests [16], [21], [22]. A diagnostic algorithm for rheumatologists to aid in distinguishing attenuated MPS I from inflammatory joint diseases has been developed based on these differences [21].
Educational materials had a positive impact on physician awareness across regions and specialties. Following review there was an increase in the percent of physicians (particularly among rheumatologists) suspecting that at least one current patient had MPS I, and who would test at least one current patient.
This study adhered to recommendations for good practice in the collection and reporting of survey data [23]. Response rate for patient surveys was 70%, which is a level at which reporting bias is reduced [23]. Indeed, real-world observations of patients and physicians and the breadth of coverage of participants from varied countries increased the likelihood of obtaining data based on a representative sample with generalizable results. However, limitations include missing data for some areas of the survey, absence of genotyping for all participants, absence of clear definitions around some physician survey questions (e.g., “being familiar” with the disease could be interpreted in different ways), and differences in regional health care habits/approaches (e.g., in the UK, a patient cannot see a specialist unless referred by a general practitioner).
5. Conclusions
Early diagnosis is crucial for the best therapeutic outcomes with both ERT and HSCT [10], [13]. [12], [14], [24]. The present study demonstrates that diagnosis of MPS I is often delayed, particularly in patients with attenuated disease, and that MPS I is under-recognized. These results are similar to those of smaller surveys of MPS patients/caregivers conducted previously in the US and the Netherlands [25], [26]. Collectively, these data illustrate that across regions and specialties, improvement in MPS I awareness is still needed. It is hoped that education initiatives in combination with evolving newborn screening programs in several countries, including the US and Taiwan [27], [28] will aid in earlier diagnosis, which is key to improving outcomes and lives of patients with MPS I.
Contributions
All authors provided strategic input for manuscript development, critically revised all manuscript drafts, and approved the final version of the manuscript.
Potential conflict of interest and disclosures
Stefano Bruni is an employee of Sanofi Genzyme.
Christine Lavery is supported by the MPS Society, which has received unrestricted educational grants from: Synageva, BioMarin, Sanofi Genzyme, Shire, Amicus, and Ultragenyx.
Alexander Broomfield has received consultancy fees from Sanofi Genzyme, Shire, and BioMarin Pharmaceutical.
Acknowledgments
The authors thank the patients, families and physicians for their participation, and the following patient advocacy groups: Vaincre les Maladies Lysosomales, France - www.vml-asso.org; Society for Mucopolysaccharide Diseases, United Kingdom - www.mpssociety.org.uk Asociación MPS España, Spain - www.mpsesp.org; Gesellschaft für Mukopolysaccharidosen e.V., Germany - www.mps-ev.de/index.php; National MPS Society, United States - www.mpssociety.org; Allianca Brasil de Mucopolissacaridoses, Brazil - www.aliancabrasilmps.org.br. The study was sponsored by Sanofi Genzyme, Cambridge, MA, USA. Fulcrum Research provided data analysis and was funded by the sponsor. Patrice C. Ferriola, PhD provided medical writing and editing assistance in preparation of the manuscript and was funded by the sponsor.
Contributor Information
Stefano Bruni, Email: stefano.bruni@genzyme.com.
Christine Lavery, Email: C.Lavery@mpssociety.org.uk.
Alexander Broomfield, Email: Alexander.Broomfield@cmft.nhs.uk.
References
- 1.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2012;50(Suppl. 5) doi: 10.1093/rheumatology/ker394. v4-12. ker394 [pii] 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 2.Moore D., Connock M.J., Wraith E., Lavery C. The prevalence of and survival in mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK. Orphanet J. Rare Dis. 2008;3:24. doi: 10.1186/1750-1172-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neufeld E., Muenzer J. The Metabolic and Molecular Basis of Inherited Disease. McGraw Hill; New York: 2001. The mucopolysaccharidoses. [Google Scholar]
- 4.Thomas J.A., Beck M., Clarke J.T., Cox G.F. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J. Inherit. Metab. Dis. 2010;33(4):421–427. doi: 10.1007/s10545-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolar J., Orchard P.J. alpha-l-iduronidase therapy for mucopolysaccharidosis type I. Biologics. 2008;2(4):743–751. doi: 10.2147/btt.s3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muenzer J., Wraith J.E. Clarke LA. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123(1):19–29. doi: 10.1542/peds.2008-0416. 123/1/19 [pii] 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 7.de Ru M.H., Boelens J.J., Das A.M., Jones S.A., van der Lee J.H., Mahlaoui N. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J. Rare Dis. 2011;6:55–62. doi: 10.1186/1750-1172-6-55. 1750-1172-6-55 [pii] 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wraith J.E. The first 5 years of clinical experience with laronidase enzyme replacement therapy for mucopolysaccharidosis I. Expert. Opin. Pharmacother. 2005;6(3):489–506. doi: 10.1517/14656566.6.3.489. EOP060313 [pii] 10.1517/14656566.6.3.489. [DOI] [PubMed] [Google Scholar]
- 9.Giugliani R. Mucopolysacccharidoses: from understanding to treatment, a century of discoveries. Genet. Mol. Biol. 2012;35(4 (suppl)):924–931. doi: 10.1590/s1415-47572012000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laraway S., Breen C., Mercer J., Jones S., Wraith J.E. Does early use of enzyme replacement therapy alter the natural history of mucopolysaccharidosis I? Experience in three siblings. Mol. Genet. Metab. 2013;109(3):315–316. doi: 10.1016/j.ymgme.2013.04.023. S1096-7192(13)00153-4 [pii] 10.1016/j.ymgme.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014;111(2):63–72. doi: 10.1016/j.ymgme.2013.11.015. S1096-7192(13)00418-6 [pii] 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Poe M.D., Chagnon S.L., Escolar M.L. Early treatment is associated with improved cognition in hurler syndrome. Ann. Neurol. 2014;76(5):747–753. doi: 10.1002/ana.24246. [DOI] [PubMed] [Google Scholar]
- 13.Gabrielli O., Clarke L.A., Bruni S., Coppa G.V. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2009;125(1):e183–e187. doi: 10.1542/peds.2009-1728. peds.2009-1728 [pii] 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- 14.Aldenhoven M., Wynn R.F., Orchard P.J., O'Meara A., Veys P., Fischer A. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood. 2015;125(13):2164–2172. doi: 10.1182/blood-2014-11-608075. [DOI] [PubMed] [Google Scholar]
- 15.D'Aco K., Underhill L., Rangachari L., Arn P., Cox G.F., Giugliani R. Diagnosis and treatment trends in mucopolysaccharidosis I: findings from the MPS I Registry. Europ JPed. 2012;171(6):911–919. doi: 10.1007/s00431-011-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijay S., Wraith J.E. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94(7):872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. Q28MP8422662W725 [pii] 10.1080/08035250510031584. [DOI] [PubMed] [Google Scholar]
- 17.Beck M., Arn P., Giugliani R., Muenzer J., Okuyama T., Taylor J. The natural history of MPS I: global perspectives from the MPS I Registry. Genet Med. 2014;16(10):759–765. doi: 10.1038/gim.2014.25. gim201425 [pii] 10.1038/gim.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary M.A., Wraith J.E. The presenting features of mucopolysaccharidosis type IH (Hurler syndrome) Acta Paediatr. 1995;84(3):337–339. doi: 10.1111/j.1651-2227.1995.tb13640.x. [DOI] [PubMed] [Google Scholar]
- 19.Pastores G.M., Arn P., Beck M., Clarke J.T., Guffon N., Kaplan P. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol. Genet. Metab. 2007;91(1):37–47. doi: 10.1016/j.ymgme.2007.01.011. S1096-7192(07)00034-0 [pii] 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Arn P., Bruce I.A., Wraith J.E., Travers H., Fallet S. Airway-Related Symptoms and Surgeries in Patients With Mucopolysaccharidosis I. Ann. Otol. Rhinol. Laryngol. 2015;124(3):198–205. doi: 10.1177/0003489414550154. 0003489414550154 [pii] 10.1177/0003489414550154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cimaz R., Coppa G.V., Kone-Paut I., Link B., Pastores G.M., Elorduy M.R. Joint contractures in the absence of inflammation may indicate mucopolysaccharidosis. Pediatr. Rheumatol. Online J. 2009;7:18. doi: 10.1186/1546-0096-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimaz R., Vijay S., Haase C., Coppa G.V., Bruni S., Wraith E. Attenuated type I mucopolysaccharidosis in the differential diagnosis of juvenile idiopathic arthritis: a series of 13 patients with Scheie syndrome. Clin. Exp. Rheumatol. 2006;24(2):196–202. [PubMed] [Google Scholar]
- 23.Kelly K., CLark B., Brown V., Sitzia J. Good practice in the conduct and reporting of survey research. Int. J. Qual. Health Care. 2003;15(3):261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 24.Aldenhoven M., Jones S.A., Bonney D., Borrill R.E., Coussons M., Mercer J. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol. Blood Marrow Transplant. 2015;21(6):1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 25.de Ru M.H., Bouwman M.G., Wijburg F.A., van Zwieten M.C. Experiences of parents and patients with the timing of Mucopolysaccharidosis type I (MPS I) diagnoses and its relevance to the ethical debate on newborn screening. Mol. Genet. Metab. 2012;107(3):501–507. doi: 10.1016/j.ymgme.2012.08.008. S1096-7192(12)00308-3 [pii] 10.1016/j.ymgme.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 26.National MPS I Organization http://mpssociety.org/education/mps-i-survey-results/2004, 2007.
- 27.Lin S.P., Lin H.Y., Wang T.J., Chang C.Y., Lin C.H., Huang S.F. A pilot newborn screening program for Mucopolysaccharidosis type I in Taiwan. Orphanet J. Rare Dis. 2013;8:147. doi: 10.1186/1750-1172-8-147. 1750-1172-8-147 [pii] 10.1186/1750-1172-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins P.V., Campbell C., Klug T., Rogers S., Raburn-Miller J., Kiesling J. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J. Pediatr. 2015;166(1):172–177. doi: 10.1016/j.jpeds.2014.09.023. [DOI] [PubMed] [Google Scholar]