Abstract
OBJECTIVES:
Dengue cases range from asymptomatic to severe, eventually leading to hospitalization and death. Timely and appropriate management is critical to reduce morbidity. Since 1980, dengue has spread throughout Brazil, affecting an increasing number of individuals. This paper describes age and regional differences in dengue’s clinical presentation and associated risk of hospitalization based on more than 5 million cases reported to the Brazilian Ministry of Health from 2000-2014.
METHODS:
We performed a retrospective analysis of ∼5,450,000 dengue cases, relating clinical manifestations and the risk of hospitalization to age, gender, previous infection by dengue, dengue virus serotype, years of formal education, delay to first attendance and the occurrence of dengue during outbreaks and in different Brazilian regions.
RESULTS:
Complicated forms of dengue occurred more frequently among those younger than 10 years (3.12% vs 1.92%) and those with dengue virus 2 infection (7.65% vs 2.42%), with a delay to first attendance >2 days (3.18% vs 0.82%) and with ≤4 years of formal education (2.02% vs 1.46%). The risk of hospitalization was higher among those aged 6-10 years old (OR 4.57; 95% CI 1.43-29.96) and those who were infected by dengue virus 2 (OR 6.36; 95% CI 2.52-16.06), who lived in the Northeast region (OR 1.38; 95% CI 1.11-2.10) and who delayed first attendance by >5 days (composite OR 3.15; 95% CI 1.33-8.9).
CONCLUSIONS:
In Brazil, the occurrence of severe dengue and related hospitalization is associated with being younger than 10 years old, being infected by dengue virus 2 or 3, living in the Northeast region (the poorest and the second most populated) and delaying first attendance for more than 2 days.
Keywords: Dengue, Morbidity, Epidemiology, Surveillance System, Hospitalization, Brazil
INTRODUCTION
Over the past 50 years, dengue has become one of the top priorities of the World Health Organization (WHO) in terms of global health. This infection occurs in more than 100 countries and is estimated to affect 50-100 million people, with 500,000 severe cases demanding hospitalization and approximately 20,000 deaths every year 1. More than 200 disability-adjusted life years per million population are lost per year 2, with an estimated cost ranging from US$248-571 for ambulatory and hospitalized cases in certain Asian and Latin American countries, implying an annual cost as high as US$500-1000 million per year in countries such as Brazil 3.
Since its reappearance in Brazil in the early 1980s, dengue has progressively spread throughout the country, affecting an increasing number of individuals, states and municipalities 3-7. Initially, it presented as recurrent yearly epidemics with a relatively small number of cases that affected different regions of the country and that occasionally spared the same region one year but not another. However, the increasing circulation of various serotypes of the virus has been associated with increases in the frequency and extent of epidemics, with increasing numbers of complicated dengue cases and hospitalizations 3-6.
Human infection by dengue virus (DENV) leads to a wide spectrum of clinical presentations, often with unpredictable clinical evolution and outcomes 8. Classic dengue fever (DF) is an acute febrile illness, with a sudden onset of fever typically after 5-7 days (full range from 3-12 days) of incubation. The accompanying symptoms are uncharacteristic and the most frequently reported findings are headache and generalized muscular, articular and osseous pain. Occasionally, retro-orbital pain; photophobia; a mild maculopapular skin rash; and minor hemorrhagic manifestations, such as petechiae, ecchymosis, epistaxis and positive tourniquet testing, are also reported 8-10.
However, a small proportion (∼5%) of patients may present or evolve to more severe clinical forms, mostly characterized by plasma leakage with or without hemorrhage, known as dengue hemorrhagic fever (DHF); dengue shock syndrome (DSS); or other complicated clinical forms that have significant morbidity and mortality 8-10. Warning signs of progression to severe dengue usually occur after the first few days of the febrile disease and include severe abdominal pain; persistent vomiting; difficulty breathing; signs of hypovolemic shock; a rapid decline in the platelet count and an increase in hematocrit of at least 10%, with or without mucosal bleeding 8-10.
Early clinical findings are nonspecific and demand a high index of suspicion because recognizing the early signs of potential complications or shock and promptly initiating intensive supportive therapy can reduce the risk of death among patients with severe dengue from 10% to <1% 8,9. This is particularly important during dengue outbreaks, when health services need to cope with the sudden surge in demand 8,9.
In this paper, we present a clinical-epidemiological description of dengue in Brazil based on a reanalysis of nearly 5,450,000 dengue cases reported to the National Epidemiological Surveillance Secretary (SVS) of the Brazilian Ministry of Health (MoH) and assessed by the Brazilian national reportable disease information system, named SINAN (Sistema de Informação de Agravos de Notificação) 11, from 2000-2014. This analysis focuses specifically on age, gender, DENV serotype, the time to first attendance, the number of years of formal education and regional differences in the clinical presentation of dengue and risk factors related to hospitalization.
In 1997, the WHO proposed the grouping of symptomatic DENV infections into three categories: undifferentiated fever, DF and DHF. DHF was further classified into four severity grades, with grades III and IV being defined as DSS 8. However, many have complained that this classification is difficult to use in different clinical settings 12-14, as summarized in a systematic literature review 15. More recently, the WHO conducted a study aimed to “...devise a system that identified patients requiring major intervention with sufficient sensitivity and specificity to be practically useful" 16. However, as mentioned by the authors, only a small fraction of cases evolved to severe disease and “...much larger studies are necessary to fully characterize features associated with disease progression" 16. This issue notwithstanding, the results of the study were incorporated into the 2009-10 WHO simplified classification for dengue, which defined three categories: DF, dengue with warning signs and severe dengue 8,16.
However, the frequency of clinically complicated dengue cases increased, leading to high morbidity and increased hospitalization. The cases did not fulfill the strict WHO criteria for dengue with warning signs or severe dengue, leading the Brazilian MoH to propose a new category of severe dengue, named “dengue with complications” (or CD, an acronym for “complicated dengue”) 10. CD was characterized as all severe dengue cases that did not fulfill the WHO criteria for dengue with warning signs, DHF or DSS and those that presented with any one of the following signs and symptoms: severe alterations in the central nervous system (CNS), cardiorespiratory dysfunction, hepatic or renal insufficiency, a platelet count below 20,000/mm3, gastrointestinal bleeding, pleural effusion or ascites, a global white blood cell count below 1,000/mm3, or any dengue case that led to death without having been characterized as DHF or DSS 10,17. This classification was officially adopted in Brazil and therefore, the cases notified to SINAN are classified accordingly.
Previous studies have qualitatively described the occurrence of dengue and DHF in Brazil since the early 1980s 18, but none has provided a systematic analysis of the clinical-epidemiological-social aspects of dengue occurrence in Brazil.
Brazil is the fifth largest country in the world in terms of both territory and population size. It is divided into 26 states and the Distrito Federal, an independent territory seat for the federal capital, Brasília and certain surrounding municipalities. The 26 states are grouped into five distinct geographical regions that share geographical, physical, ethnic, cultural and economic similarities, known as the North region (with 7 states), the Northeast region (with 9 states), the Southeast region (with 4 states) and the Central-West and South regions (with 3 states each) (see Figure 1).
Figure 1.
Geo-political map of Brazil, with colored representations of its geographic regions (green for North, blue for Northeast, red for Southeast, yellow for Central-West and orange for South).
Brazil has drastically urbanized since the mid-1970s and now, more than 78% of the Brazilian population lives in an urban setting. The North region is nearly entirely in the Amazon and most of it is covered by tropical rain forest. The Northeast region is the poorest in the country and most of it is in a semi-arid area, with sparse rainfalls and poor socio-demographic conditions. Meanwhile, the Southeast region is the most populated and developed region in the country. The South region is characterized by a moderate climate (colder than the rest of the country) and therefore has been spared (with the exception of Paraná state) from dengue epidemics. Finally, the Central-West region is an area with a rich agricultural-based economy on the southern border of the Amazon. Table 1 summarizes several socio-demographic characteristics of Brazil and its regions 19 that are related to dengue transmission and to the risk of hospitalization due to dengue.
Table 1.
Socio-demographic characteristics of the population in Brazil and in the Brazilian regions 19.
| Socio-Demographic Characteristic | Brazil | Brazilian Regions | ||||
|---|---|---|---|---|---|---|
| North | Northeast | Southeast | South | Central-West | ||
| Estimated population in 2014 | 203,834,190 | 16,394,272 | 57,454,690 | 85,663,161 | 29,580,665 | 14,741,402 |
| Urbanization rate (%) | 84.8 | 74.6 | 73.3 | 93.2 | 85.5 | 90.1 |
| ≈ Number of dwellings (1000) | 57,557 | 4,010 | 14,994 | 25,310 | 8,993 | 4,250 |
| % without regular water supply | 18.3 | 44.6 | 24.0 | 9.0 | 11.6 | 16.2 |
| % without adequate sanitation | 44.5 | 84.8 | 66.3 | 14.1 | 42.1 | 56.4 |
| % without regular waste collection | 14.1 | 25.8 | 26.7 | 4.4 | 5.8 | 8.6 |
| % not covered by the family health program | 30.1 | 49.0 | 35.2 | 64.1 | 49.7 | 50.9 |
| Per capita GDP in US$ PPP (2014) | 8,619 | 5,725 | 4,203 | 11,478 | 9,748 | 11,845 |
| Gini index | 0.526 | 0.526 | 0.530 | 0.511 | 0.481 | 0.544 |
| Women’s fecundity rate | 1.77 | 2.22 | 1.89 | 1.63 | 1.62 | 1.74 |
| Infant mortality rate (per 1,000 born alive) | 15.0 | 19.2 | 19.4 | 11.6 | 10.4 | 15.6 |
| Life expectancy at birth (years) | 74.8 | 71.5 | 72.2 | 76.6 | 76.9 | 74.4 |
METHODS
This was a population study based on a secondary data analysis of dengue morbidity and hospitalization risk. Unidentified data from dengue cases reported to the Health Surveillance Secretary of the Brazilian MoH, as assessed by the Brazilian national reportable disease information system, named SINAN (Sistema de Informação de Agravos de Notificação) 11 were specifically analyzed. Since its reintroduction in Brazil in the early 1980s, it is mandatory that all dengue cases are notified to SINAN 10,18.
According to the Brazilian MoH classification, DF is defined as an acute febrile illness no more than seven days long, accompanied by at least two of the following symptoms: headache, retro-orbital pain, myalgia, arthralgia, prostration and exanthema, possibly associated with minor hemorrhagic manifestations. In addition, the patient should have been in an area with active dengue transmission in the last 15 days 10.
All reported dengue cases that occurred in municipalities in which dengue epidemics occurred in the period from 2000-2014 were selected for this analysis. A dengue epidemic was defined as the period of time in which the following conditions were simultaneously satisfied:
Having had three consecutive weeks with an increasing number of cases;
The first week of the outbreak was the first in which the number of cases surpassed the 95% upper limit of the confidence interval of the average number of cases in the previous 5 weeks; and
The last week of the epidemic was the first in which the number of cases dropped below the previously mentioned limit after three consecutive weeks with a decreasing number of cases.
The resulting database comprised 5,444,285 dengue cases, corresponding to nearly 80% of the total dengue cases reported to the Brazilian MoH in the study period. The other ∼20% of cases occurred in municipalities without dengue epidemics in the period analyzed (as defined above).
The clinical manifestations of dengue at presentation were related to age, gender, previous infection by DENV, DENV serotype and Brazilian geographic region and were expressed as absolute counts and percentages of patients who presented with each of the symptoms and signs reported. The prevalence of dengue, DHF, DSS and CD, as defined by the Brazilian MoH, were also related to the same variables. Finally, the risk of hospitalization was related to the same variables and to years of formal education, delay to first attendance (in days), a previous dengue episode and whether the dengue case occurred during an outbreak/epidemic.
Associations between variables were assessed by means of chi-square and Bonferroni tests and a multivariate logistic regression analysis was performed to identify the main risk factors related to hospitalization. The analysis was conducted using Statistica 64 (V12)®, StatSoft, INC. and the significance level was set at 5%.
Ethics Statement
This research involved the use of anonymized patient medical data extracted from the Brazilian reportable disease information system and was approved by the Ethics in Research Committee of the Federal University of São Paulo, Brazil (#2059131213).
RESULTS
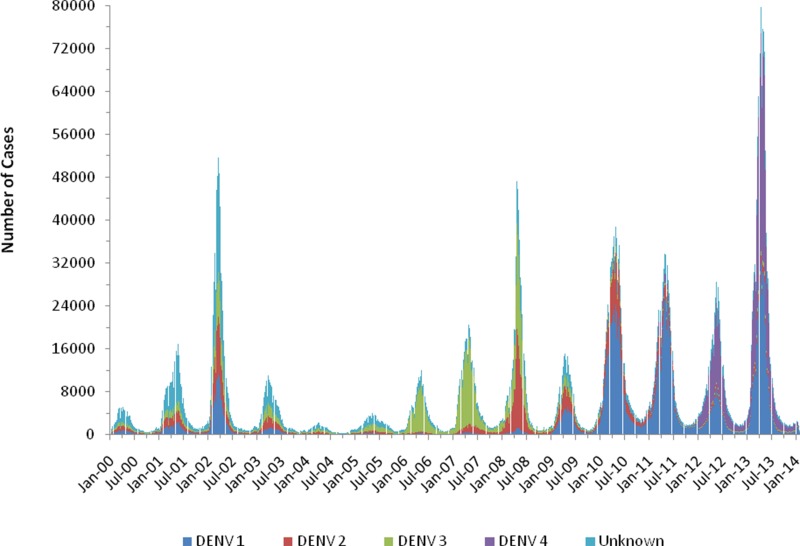
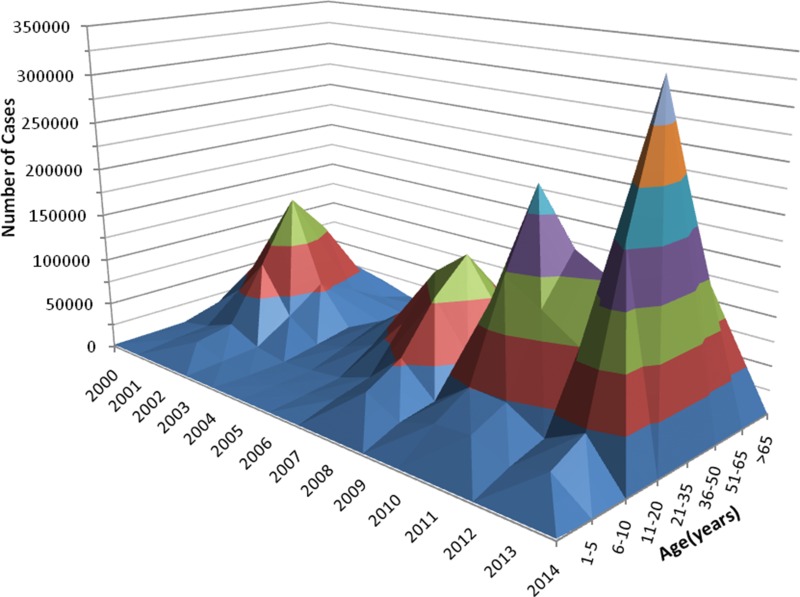
Figure 2 shows the occurrence of dengue as related to the main serotypes involved and the epidemiological week per year between 2000 and early 2014, and Figure 3 shows the successive dengue epidemics as related to the age classes affected during the period from 2000-2014.
Figure 2.
Dengue occurrence per epidemiological week and serotype in Brazil from 2000-2014.
Figure 3.
Successive dengue epidemics in Brazil from 2000-2014, as related to the affected age class and year of occurrence.
Table 2 shows the frequency of dengue symptoms as related to the selected variables. Fever was experienced by approximately 95-97% of the patients. Headache was experienced by approximately 90% but showed some variation, with lower frequency among children below 10 years of age and adults above 65 years. Headaches were also less pronounced in males and in people from the North and Northeast regions. In contrast, diarrhea and rash were the least frequent symptoms and showed marked differences (21-45%) in their frequency among the distinct classes of variables. Rash was more frequent in children younger than 5 years old, those infected by DENV 1 and those living in the North region. Meanwhile, diarrhea was more frequent among adults older than 50 years and those living in the North region. The others symptoms varied in frequency from 38-88%, showing marked variations among the different groups (see Table 2 for details).
Table 2.
Frequency of dengue symptoms at presentation, as related to the selected variables.
| Fever | Headache | Rash | Generalized Pain | Prostration | Myalgia | Arthralgia | Nausea | Diarrhea | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||
| 1-5 | 95.4% | 68.1% | 49.6% | 42.1% | 60.1% | 56.2% | 37.5% | 52.9% | 26.7% |
| 6-10 | 96.2% | 87.5% | 40.3% | 59.5% | 66.3% | 69.6% | 49.5% | 59.5% | 21.7% |
| 11-20 | 96.2% | 92.5% | 36.0% | 73.2% | 70.5% | 81.0% | 62.8% | 59.3% | 22.9% |
| 21-35 | 95.9% | 93.4% | 37.6% | 77.1% | 71.6% | 86.0% | 70.9% | 61.2% | 26.3% |
| 36-50 | 96.0% | 92.9% | 41.8% | 77.3% | 74.2% | 87.6% | 74.2% | 62.2% | 29.2% |
| 51-65 | 95.8% | 91.5% | 40.0% | 73.9% | 75.5% | 87.6% | 74.8% | 63.0% | 31.7% |
| >65 | 94.6% | 88.1% | 33.8% | 67.8% | 74.5% | 85.7% | 72.4% | 60.0% | 30.8% |
| Gender | |||||||||
| Female | 93.5% | 90.3% | 40.1% | 72.7% | 69.3% | 82.6% | 67.5% | 62.1% | 26.3% |
| Male | 94.3% | 88.5% | 34.0% | 68.9% | 67.3% | 80.7% | 64.2% | 52.7% | 25.7% |
| DENV Serotype | |||||||||
| DENV 1 | 97.4% | 92.4% | 45.1% | 76.3% | 76.4% | 83.6% | 73.8% | 53.3% | 22.7% |
| DENV 2 | 95.0% | 92.6% | 33.2% | 76.3% | 76.4% | 85.0% | 72.4% | 57.3% | 24.1% |
| DENV 3 | 98.2% | 92.9% | 33.0% | 75.3% | 74.5% | 86.9% | 72.5% | 59.1% | 23.1% |
| DENV 4 | 96.3% | 92.2% | 36.1% | 73.4% | 72.8% | 83.3% | 72.6% | 64.4% | 22.3% |
| Brazilian Region | |||||||||
| North | 94.4% | 89.6% | 41.9% | 71.4% | 53.5% | 81.6% | 71.1% | 52.9% | 29.7% |
| Northeast | 88.3% | 83.4% | 36.8% | 61.6% | 53.1% | 73.4% | 55.3% | 47.3% | 21.2% |
| Southeast | 96.7% | 93.0% | 38.1% | 76.8% | 80.3% | 86.4% | 70.2% | 65.2% | 27.9% |
| Central-West | 97.3% | 92.1% | 31.9% | 74.0% | 79.9% | 86.0% | 73.4% | 64.1% | 27.3% |
| South | 94.7% | 92.5% | 31.5% | 71.2% | 77.5% | 86.2% | 67.1% | 65.9% | 24.5% |
| Previous Dengue | |||||||||
| Yes | 97.6% | 95.7% | 36.7% | 82.2% | 77.5% | 87.3% | 75.6% | 65.4% | 29.2% |
| No | 97.3% | 93.0% | 40.7% | 76.5% | 74.5% | 85.3% | 71.0% | 61.8% | 28.2% |
Table 3 shows the number of dengue cases and the frequency of its different clinical forms as related to the selected variables. It is noteworthy that the highest frequencies of CD, DHF and DSS were related to younger age classes, DENV types 2 and 3, the North and Northeast regions, a delay to first attendance greater than 3 days and less than 4 years of formal education.
Table 3.
Frequency of the different clinical forms of dengue, as related to the selected variables.
| Dengue Cases | Classic Dengue | Complicated Dengue (CD) | Dengue Hemorrhagic Fever (DHF) | Dengue Shock Syndrome (DSS) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 1-5 | 158616 | 96.87% | 2.29% | 0.83% | 0.02% |
| 6-10 | 258372 | 94.75% | 3.94% | 1.28% | 0.02% |
| 11-20 | 801997 | 97.65% | 1.85% | 0.49% | 0.01% |
| 21-35 | 1270950 | 98.37% | 1.32% | 0.30% | 0.01% |
| 36-50 | 879684 | 98.02% | 1.59% | 0.38% | 0.01% |
| 51-65 | 462687 | 97.57% | 2.00% | 0.41% | 0.02% |
| >65 | 163873 | 96.54% | 2.86% | 0.56% | 0.04% |
| Gender | |||||
| Female | 2408847 | 97.77% | 1.76% | 0.46% | 0.01% |
| Male | 1913157 | 97.47% | 2.01% | 0.50% | 0.02% |
| DENV Serotype | |||||
| DENV 1 | 11546 | 95.96% | 2.41% | 1.51% | 0.13% |
| DENV 2 | 2711 | 85.57% | 7.65% | 5.53% | 1.25% |
| DENV 3 | 4416 | 91.04% | 5.59% | 3.15% | 0.22% |
| DENV 4 | 3922 | 95.73% | 2.45% | 1.20% | 0.61% |
| Brazilian Region | |||||
| North | 446450 | 98.38% | 1.03% | 0.57% | 0.02% |
| Northeast | 999774 | 97.60% | 1.65% | 0.73% | 0.02% |
| Southeast | 2239451 | 97.54% | 2.09% | 0.35% | 0.01% |
| Central-West | 547436 | 97.25% | 2.17% | 0.57% | 0.01% |
| South | 90775 | 99.38% | 0.36% | 0.25% | 0.01% |
| Previous Dengue | |||||
| Yes | 105965 | 90.7% | 1.3% | 0.6% | 0.0% |
| No | 786398 | 93.1% | 1.0% | 0.4% | 0.0% |
| Delay to First Attendance (days) | |||||
| 0-2 | 1312749 | 99.01% | 0.82% | 0.16% | 0.01% |
| 3-5 | 964121 | 97.33% | 2.10% | 0.55% | 0.02% |
| 6-7 | 345171 | 95.21% | 3.71% | 1.05% | 0.03% |
| >7 | 328240 | 95.86% | 3.20% | 0.91% | 0.03% |
| Years of Formal Education | |||||
| ≤4 years | 336604 | 97.17% | 2.02% | 0.79% | 0.01% |
| >4 years | 1801282 | 98.06% | 1.46% | 0.46% | 0.01% |
Table 4 shows the univariate analysis of the risk of hospitalization as related to the selected variables. During the period analyzed, 113,726 patients with age information were hospitalized, with ∼60% of them aged from 11-50 years. This finding notwithstanding, the highest proportion of hospitalizations was observed among the youngest patients, with more than 15% of children younger than 10 years old being hospitalized. Additionally, a relatively high proportion of elders (>65 years old) were hospitalized (12.4%) due to dengue. Regarding the DENV serotypes, the highest proportion of hospitalizations (25.5%) was related to DENV 2, followed by DENV 3 (16.8%). The Northeast region was the region with the highest (13.5%) hospitalization rate in Brazil. Delaying the first attendance by more than two days was related to an increase in the risk of hospitalization (the risk nearly tripled for delays between 3-5 days and nearly quintupled for delays greater than 5 days). Having had less than four years of formal education increased the risk of hospitalization by 1.5 times. Finally, having had dengue during a period not defined as a dengue epidemic nearly doubled the risk of hospitalization.
Table 4.
Results of the univariate analysis of the risk of hospitalization due to dengue, as related to the selected variables.
| # Hospitalized | % Hospitalized | p-value | |
|---|---|---|---|
| Age (years) | |||
| 1-5 | 7485 | 13.87% | <0.0001 |
| 6-10 | 16573 | 17.47% | |
| 11-20 | 23897 | 8.11% | |
| 21-35 | 25142 | 5.64% | |
| 36-50 | 19571 | 6.38% | |
| 51-65 | 13276 | 7.61% | |
| >65 | 7782 | 12.37% | |
| Gender | |||
| Female | 61541 | 7.68% | <0.0001 |
| Male | 55389 | 8.50% | |
| DENV Serotype | |||
| DENV 1 | 708 | 11.55% | <0.001 |
| DENV 2 | 309 | 25.52% | |
| DENV 3 | 313 | 16.84% | |
| DENV 4 | 267 | 8.55% | |
| Brazilian Region | |||
| North | 10506 | 9.22% | <0.0001 |
| Northeast | 30000 | 13.47% | |
| Southeast | 55275 | 4.91% | |
| Central-West | 18707 | 6.32% | |
| South | 2483 | 9.70% | |
| Previous Dengue | |||
| Yes | 50404 | 10.69% | <0.0001 |
| No | 369147 | 10.42% | |
| Delay to First Attendance (days) | |||
| 0-2 | 22761 | 3.80% | <0.0001 |
| 3-5 | 41214 | 9.06% | |
| 6-7 | 25656 | 15.24% | |
| >7 | 21494 | 13.33% | |
| Years of Formal Education | |||
| ≤4 years | 11712 | 11.41% | <0.0001 |
| >4 years | 31812 | 7.67% | |
| During Outbreak/Epidemic | |||
| Yes | 90576 | 6.96% | <0.0001 |
| No | 77101 | 12.21% | |
Table 5 shows a summary of the multivariate analysis of the risk of hospitalization due to dengue. All variables described in Table 4 were included in the logistic model, as they were all significantly associated with the risk of hospitalization. It is noteworthy that those aged less than 5 years had nearly triple the risk and that those aged between 6-10 years had nearly quintuple the risk. Moreover, people older than 65 years had nearly double the risk of hospitalization, although this result was not significant in the multivariate analysis. With respect to dengue serotypes, DENV 2 was associated with a six times higher risk of hospitalization than for DENV 4, while DENV 3 was associated with a nearly two times higher risk than for DENV 4. In contrast, DENV 1 was associated with only one third of the risk of hospitalization for DENV 4 (this result was not significant in the multivariate analysis). With respect to the different Brazilian regions, the Northeast region was associated with a 38% higher risk of hospitalization relative to the South region. In contrast, both the Southeast and the Central-West regions had lower risks (88% and 41%, respectively) of hospitalization compared with the South region. Finally, delaying the first attendance by more than 7 days was associated with a nearly four times higher risk of hospitalization compared with a delay of less than 2 days. In addition, compared with a delay of less than 2 days, a delay between 5 and 7 days increased the risk by 2.5 times.
Table 5.
Summary of the multivariate analysis of the risk of hospitalization due to dengue, as related to the selected variables.
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-value | |
|---|---|---|---|---|
| Intercept 1 | 14.05 | 7.74 | 25.51 | 0.0001 |
| Age (years) | ||||
| 1-5 | 2.87 | 1.40 | 5.86 | 0.0385 |
| 6-10 | 4.57 | 1.43 | 29.96 | 0.0039 |
| 21-35 | 1.00 | --- | --- | --- |
| 36-50 | 1.31 | 0.76 | 2.84 | 0.1035 |
| >65 | 1.81 | 0.90 | 3.64 | 0.0958 |
| DENV Serotype | ||||
| DENV 1 | 0.32 | 0.22 | 2.14 | 0.0747 |
| DENV 2 | 6.36 | 2.52 | 16.06 | 0.0001 |
| DENV 3 | 1.94 | 1.17 | 3.20 | 0.0100 |
| DENV 4 | 1.00 | --- | --- | --- |
| Brazilian Region | ||||
| South | 1.00 | --- | --- | --- |
| Southeast | 0.12 | 0.08 | 0.18 | 0.0001 |
| Central-West | 0.59 | 0.43 | 0.82 | 0.0018 |
| Northeast | 1.38 | 1.11 | 2.10 | 0.0138 |
| Delay to First Attendance (days) | ||||
| 0-2 | 1.00 | --- | --- | --- |
| 3-5 | 0.40 | 0.28 | 1.72 | 0.0871 |
| 6-7 | 2.49 | 1.33 | 4.66 | 0.0044 |
| >7 | 3.62 | 1.62 | 8.09 | 0.0017 |
Obs: Only the variable categories that remained in the final model are shown.
DISCUSSION
Infection by any serotype of DENV results in a large spectrum of nonspecific clinical manifestations with an unpredictable clinical course and outcome, ranging from asymptomatic cases to severe clinical forms leading to hospitalization, a need for intensive care treatment and death. Timely and appropriate monitoring and clinical management of dengue patients, mainly entailing early fluid replacement interventions, is critical to reduce morbidity and mortality 8,10.
DENV and its vectors are now widely distributed throughout tropical and subtropical regions, spreading particularly over the last half-century and threatening even temperate regions, such as North America and Europe 20. Throughout the world, the significant geographic expansion of dengue has been coupled with rapid increases in the numbers of cases and epidemics, leading to an increasing number of more severe forms of dengue, hospitalizations and deaths 20. In the Southeast Asia (SEA) and Western Pacific (WP) regions, the expansion of dengue has occurred over the past few decades; epidemics are occurring persistently in regular 3- to 5-year cycles, with an increasing number of reported cases in many countries that are now classified as hyperendemic and all four DENV serotypes being reported as present 20. Severe dengue is endemic in most SEA countries and is a leading cause of hospitalization and death in children from the region, which has reported rates of severe dengue up to 18 times higher than in the Americas 20.
Also in the Americas, dengue transmission resurged in the late 1970s, and now many countries are hyperendemic, with epidemics occurring cyclically every 3-5 years, as in SEA; these epidemics are also increasing in frequency and size, particularly in Latin America 20. Similarly, since the early 1980s, dengue has spread throughout Brazil, occurring as annual recurrent seasonal epidemics mainly in the summer, with an increasing number of complicated cases and hospitalizations. All four serotypes of DENV circulate in Brazil, and there is a clear tendency towards an increase in the number of children and youth affected (see Figure 3), as previously observed in SEA. In 2013 and 2015 two of the largest epidemics of dengue ever recorded in a single country occurred in Brazil, with both involving more than 1,300,000 cases and with the second peaking at more than 1,600,000 cases notified to the Brazilian MoH 11.
This paper describes age and regional differences in the clinical presentation of dengue among more than 5.4 million cases reported to the SINAN in the period between 2000 and 2014. It also presents an analysis of the risk of hospitalization due to dengue. We found that the highest frequencies of CD, DHF and DSS were related to younger age classes, to DENV types 2 and 3, to the North and Northeast regions, to a delay to first attendance greater than 2 days and to less than 4 years of formal education. In addition, being younger than 10 years old nearly quintupled the risk of hospitalization. Infection by DENV 2 was associated with a risk of hospitalization six times larger than that for DENV 4, and DENV 3 was associated with a risk nearly twice as large as for DENV 4. Regarding the different Brazilian regions, the Northeast region was associated with a 38% increase in the risk of hospitalization relative to the South region. In contrast, both the Southeast and the Central-West regions had lower risks (88% and 41%, respectively) of hospitalization. Finally, delaying the first attendance by more than 5 days was associated with a nearly four times greater risk of hospitalization compared with a delay of less than 2 days.
Dengue usually presents as recurrent, seasonal, yearly epidemics, which may increase the awareness of the health sector, which may in turn promptly suspect and identify clinical cases 4. However, Brazil is a large country, with dengue occurring differently in different regions, states and municipalities 4-6. In certain areas, recurrent yearly dengue epidemics and outbreaks are common, occurring approximately during the same period year after year. In other regions, however, dengue presents as irregular large epidemics occurring sparsely over time and with a large number of cases occurring year round (but mainly throughout the summer and autumn), but without relation to recognizable outbreaks or epidemics. In these regions, the clinical awareness that prompts dengue suspicion and diagnosis may not be as present as needed. Whether dengue occurred during an outbreak/epidemic seems to be an important factor, with our study showing that the risk of hospitalization nearly doubles for dengue cases not related to recognizable outbreaks/epidemics. This finding indicates per se the need for adequate descriptions of age, gender and regional differences in the clinical presentation of dengue, as presented in this paper.
Timely and appropriate monitoring and clinical management of dengue cases depend on many parameters; among the most important are the socio-educational level of the population, the clinical awareness of physicians and other health personnel and the adequate infrastructure of the health system. Our results, sadly but not surprisingly, point to the Northeast region, which is the poorest and the second most populated in the country, as the region with the highest risk of hospitalization due to dengue. It is followed by the North region, which is nearly equally poor and destitute of basic conditions for adequate population care. Health conditions related to impoverishment and low socio-economic development, such as malnutrition and poor urban conditions and public services (including poor waste collection, water supply and sanitation), together with a poor basic public health structure constitute a nearly ideal amalgam for the breeding of Aedes spp. mosquitoes and the development of severe dengue cases as dengue outbreaks and epidemics subsequently occur.
Previous studies have described and critically commented on the clinical presentation of dengue in Brazil and elsewhere 12,14,18. Socio-demographic characteristics have also been previously studied in relation to the occurrence of dengue, but not in relation to its clinical presentation or the risk of hospitalization due to dengue 21-25. Therefore, to the best of our knowledge, this is the first study to systematically analyze and describe regional differences in dengue’s clinical presentation and associated risk of hospitalization and to relate these different presentations and outcomes to distinct socio-demographic variables.
Certain limitations of this study are related to its retrospective nature because information related to important data may be incomplete, which is a typical limitation of this type of study. In addition, several other important characteristics, such as nutritional status and individual socio-economic variables, that could be associated with the risk of hospitalization could not be assessed in the present analysis. Finally, dengue mortality and the risk factors related to the lethality of dengue were not analyzed here, as they will be specifically analyzed in a future publication.
AUTHOR CONTRIBUTIONS
M.N. Burattini planned the study, analysed data, discussed results and write the manuscript; L.F. Lopez, F.A.B. Coutinho and E. Massad analysed data, discussed results and write the manuscript; J.B. Siqueira-Jr formatted the data base and contributed to data analysis, discussion of the results and writing of the manuscript; S. Homsani and E. Sarti contributed in the discussion of results and writing of the manuscript.
ACKNOWLEGMENTS
This work was partially supported by Fundo Nacional de Saúde of the Brazilian Ministry of Health (FNS, Grant#777588/2012); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP): MNB, LFL, EM; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): MNB, LFL, EM; LIM 01-HCFMUSP: MNB, LFL, EM; Hospital São Paulo (HSP) and EPM-UNIFESP: MNB; Sanofi Pasteur: SH & ES; and Dengue TOOLS (under the health theme of the Seven Framework Program of the European Community, Grant #282589): EM. The sponsors did not participate in the data collection, data analysis, or data interpretation or in the writing of the manuscript.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.World Health Organization. Dengue and severe dengue. Fact sheet N°117; Updated March 2014. Available from. http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Cattand P, Desjeux P, Guzmán MG, Jannin J, Kroeger A, Medici A, et al. Tropical Diseases Lacking Adequate Control Measures: Dengue, Leishmaniasis, and African Trypanosomiasis. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries. 2nd edition. 2006. Chapter 23. [PubMed] [Google Scholar]
- 3.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, Tan LH, et al. Cost of Dengue Cases in Eight Countries in the Americas and Asia: A Prospective Study. Am J Trop Med Hyg. 2009;80((5)):846–55. [PubMed] [Google Scholar]
- 4.Teixeira MG, Siqueira JB, Jr, Ferreira GLC, Bricks L, Joint G. Epidemiological Trends of Dengue Disease in Brazil (2000–2010): A Systematic Literature Search and Analysis. PLoS Negl Trop Dis. 2013;19;7((12)):e2520. doi: 10.1371/journal.pntd.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viana DV, Ignotti E. The occurrence of dengue and weather changes in Brazil: a systematic review. Rev Bras Epidemiol. 2013;16((2)):240–56. doi: 10.1590/S1415-790X2013000200002. [DOI] [PubMed] [Google Scholar]
- 6.Coelho GE, Burattini MN, Teixeira MG, Coutinho FAB, Massad E. Dynamics of the 2006/2007 dengue outbreak in Brazil. Mem Inst Oswaldo Cruz. 2008;103((6)):535–9. doi: 10.1590/S0074-02762008000600004. [DOI] [PubMed] [Google Scholar]
- 7.Massad E, Wilder-Smith A, Ximenes R, Amaku M, Lopez LF, Coutinho FA, et al. Risk of symptomatic dengue for foreign visitors to the 2014 FIFA World Cup in Brazil. Mem Inst Oswaldo Cruz. 2014;109((3)):394–7. doi: 10.1590/0074-0276140133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Dengue. Guidelines for Diagnosis, Treatment, Prevention and Control [Internet] Geneva: 2009. Available from: http://www.who.int/tdr/publications/training-guideline-publications/dengue-diagnosis-treatment/en/ [PubMed] [Google Scholar]
- 9.Tomashek KM, Margolis HS. Dengue. IN: Yellow Book | Travelers' Health | CDC; 2014. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/dengue. [Google Scholar]
- 10.Ministério da Saúde. Secretaria de Vigilância em Saúde. Diretoria Técnica de Gestão-Programa Nacional de Controle da Dengue. Dengue Diagnóstico e Manejo Clínico: adulto e criança. 4a ed. 2013. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/dengue_manejo_adulto_crianca__4ed_2011.pdf.
- 11.Health Surveillance Secretary (SVS) of the Brazilian Ministry of Health. Sistema de Informação de Agravos de Notificação (SINAN) Available from: http://dtr2004.saude.gov.br/sinanweb/ (acessed in December, 17, 2014).
- 12.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2((1)):1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deen J, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368((9530)):170–3. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 14.Rigau-Perez J. Severe dengue: the need for new case definitions. Lancet Infect Dis. 2006;6((5)):297–302. doi: 10.1016/S1473-3099(06)70465-0. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue hemorrhagic fever. Trop Med Intern Health. 2006;11((8)):1238–55. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 16.Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, et al. Multicentre prospective study on dengue classification in four South-east Asian and three Latin American countries. Trop Med Intern Health. 2011;16((8)):936–48. doi: 10.1111/j.1365-3156.2011.02793.x. [DOI] [PubMed] [Google Scholar]
- 17.Lima FR, Croda MG, Muniz DA, Gomes IT, Soares KR, Cardoso MR, et al. Evaluation of the traditional and revised world health organization classifications of dengue cases in Brazil. Clinics. 2013;68((10)):1299–304. doi: 10.6061/clinics/2013(10)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siqueira JB, Martelli CMT, Coelho GE, Simplicio AC da R, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerg Infect Dis. 2005;11((1)):48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Instituto Brasileiro de Geografia e Estatística (IBGE) Available from. ftp://ftp.ibge.gov.br/Indicadores_Sociais/Sintese_de_Indicadores_Sociais_2014/pdf/asp_demograficos.pdf. (February, 09, 2015).
- 20.Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira TRA, Medronho RA. Indicadores sócio-demográficos e a epidemia de dengue em 2002 no Estado do Rio de Janeiro, Brasil. Cad Saude Publica. 2008;24((9)):2160–70. doi: 10.1590/S0102-311X2008000900022. [DOI] [PubMed] [Google Scholar]
- 22.Castillo VMS. Epidemiological study in the Entre Rios Health Area. Rev Clin Med Fam. 2011;4((1)):25–31. [Google Scholar]
- 23.Cordeiro R, Donalisio MR, Andrade VR, Mafra AC, Nucci LB, Brown JC, et al. Spatial distribution of the risk of dengue fever in southeast Brazil, 2006-2007. BMC Public Health. 2011;11:355. doi: 10.1186/1471-2458-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dom NC, Ahmad AH, Ishak AR, Ismail R. Assessing the Risk of Dengue Fever Based On the Epidemiological, Environmental and Entomological Variables. Procedia-Social and Behavioral Sciences. 2013;105((3)):183–94. doi: 10.1016/j.sbspro.2013.11.019. [DOI] [Google Scholar]
- 25.Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014;129:1–14. doi: 10.1016/j.actatropica.2013.08.008. [DOI] [PubMed] [Google Scholar]