Abstract
Objective
Previous studies suggested that the treatment response to Selective-Serotonin Reuptake Inhibitors (SSRIs) in Major Depressive Disorder (MDD) follows a flat response curve within the therapeutic dose range. Our study was designed to clarify the relationship between dosage and treatment response in MDD.
Methods
We searched PubMed for randomized placebo-controlled trials examining the efficacy of SSRIs for treating adults with MDD. Trials were also required to assess improvement in depression severity at multiple time points. Additional data was collected on treatment response and all cause and side effect-related discontinuation. All medication doses were transformed into imipramine equivalent doses. The longitudinal data was analyzed with a mixed regression model. Endpoint and tolerability analyses were analyzed using meta-regression and stratified subgroup analysis by predefined SSRI dose categories in order to assess the effect of SSRI dosing on the efficacy and tolerability of SSRIs for MDD.
Results
We included 40 studies involving 10,039 participants.. Longitudinal modeling [dose × time interaction=0.0007(95%CI:0.0001–0.0013;p=0.0196)] and endpoint analysis (meta-regression β=0.00053,95%CI:0.00018–0.00088,z=2.98,p=0.0029) demonstrated a small, but statistically significant positive association between SSRI dose and efficacy. Higher doses of SSRIs were associated with an increased likelihood of dropouts due to side-effects (meta-regression β=0.00207,95%CI:0.00071–0.00342,z=2.98,p=0.003) and decreased likelihood of all-cause dropout (meta-regression β=−0.00093,95% CI−0.00165–(−0.00021),z=−2.54,p=0.01).
Conclusions
Higher doses of SSRIs appear slightly more effective in MDD. This benefit appears to plateau around 250mg of imipramine equivalents (50mg of fluoxetine). The slightly increased benefits of SSRIs at higher doses are somewhat offset by decreased tolerability at high doses.
Keywords: Major Depressive Disorder, Meta-Analysis, Serotonin Reuptake Inhibitors, Tolerability
Introduction
While the efficacy of SSRI medications and their widespread use is generally accepted in Major Depressive Disorder (MDD) (especially more severe cases), there remains some uncertainty as to the optimal dose for SSRI pharmacotherapy of MDD (3, 4). Current APA Practice Guidelines state that “optimizing the medication dose is a reasonable first step if the side effect burden is tolerable and the upper limit of a medication dose has not been reached.” This recommendation is based on Level II evidence indicating that escalating antidepressant doses was “recommended based on moderate clinical confidence”(3).
Based on currently available evidence, the APA Practice Guidelines regarding antidepressant dosing appears reasonable. A previous meta-analysis examining dosing of antidepressant medications in MDD demonstrated a flat dose-response curve within the therapeutic range for antidepressant medications (≥100mg imipramine equivalents)(10). Furthermore, the meta-analysis demonstrated a greater side effect burden at higher doses as evidenced by an escalating adverse events rate with increasing dose of antidepressants(10). Although this meta-analysis employed quite advanced methodology for the time, the findings may be somewhat antiquated for use in clinical practice for several reasons: 1) the authors grouped other classes of antidepressants (MAOIs, TCAs, and atypical antidepressants) alongside SSRIs. Other antidepressants likely have a different dose-response relationship and tolerability profile with dose when compared to SSRIs. 2) The authors examined dose as a categorical rather than continuous outcome, which may reduce overall power to detect a dosing effect in the meta-analysis. In contrast, another meta-analysis, which was quite stringent in its inclusion criteria, examined the dose-response of SSRI medication in only 4 fixed-dose and 4 dose-escalation trials in MDD. This meta-analysis demonstrated a weak positive association between higher doses and treatment response(11). This meta-analysis examining the dose-response curve in SSRI suggests the possibility that SSRIs may behave differently than other antidepressants.
The goal of the current meta-analysis is to improve the existing evidence-base regarding the dose-response relationship of SSRIs in MDD. Specifically, our goal is to determine whether there exists any evidence in SSRI trials of MDD to suggest that higher doses are associated with improved outcome. We conducted a meta-analysis and used meta-regression to examine the relationship between target SSRI dose in trials and the measured efficacy (and tolerability) of SSRI treatment compared to placebo.
Methods
Search Strategy and Study Selection
A literature search was conducted on October 10, 2013 on PubMed and CENTRAL, The Cochrane Collaboration database of controlled trials (in the Cochrane Library). Published randomized controlled trials comparing all SSRIs vs placebo in short term treatment of unipolar depression were sought by two reviewers (ALV and MHB), using the search term: (“SSRI”[MESH] OR “fluoxetine”[MESH] OR “fluvoxamine”[MESH] OR “citalopram”[MESH] OR “escitalopram”[MESH] OR “sertraline”[MESH] OR “paroxetine”[MESH]) AND “placebo”[MESH] AND “depression”[MESH]. Trials were included if: 1) efficacy data were available for both SSRI and placebo-treated participants for at least one time point other than baseline and endpoints; 2) they utilized standardized, validated outcome measurements of depression; and excluded if: 1) age <19 years or >60; 2) a cross-over design; 3) studied psychiatric diagnosis other than MDD or a dual diagnosis; 4) did not study an SSRI; 5) were not randomized; 6) were not placebo-controlled; and 7) provided adjunctive psychotherapy to active or control group.
Data Extraction
Included trials provided depression ratings reported on the Hamilton Depression Rating Scale (HDRS) or the Montgomery-Asberg Depression Rating Scale (MADRS) at least 3 time points (baseline, endpoint, and at least one intermediate time point). If trials reported outcomes in a figure rather than in a table, a computer program (Dexter; German Astrophysical Virtual Observatory, University of Heidelberg, Germany) was used to extract weekly data points from figures (this software is available here: http://dc.zah.uni-heidelberg.de/sdexter). Additionally, the number of treatment responders (as defined by study criteria) and participants who discontinued during the course of the study was recorded (all-cause dropouts and dropouts due to side effects). Additional data was collected on type of SSRI, maximum dosage of medication, duration of the trial and year of the trial. All SSRI doses were transformed into imipramine equivalent doses using previously described methodology(10).
Data Analysis
Data collection and preparation was conducted in Microsoft Excel 2007, and the effects of dose on time course of SSRI response was analyzed in SAS 9.3 (SAS Institute, Cary NC). We used generalized estimating equations to examine the effects of trial, treatment, modeling different forms of the treatment effect, accounting for different periods within trials as repeated measures, and defining a new covariance structure for each trial by defining these as random effects. For each trial and week, the standardized mean difference in outcome scores between SSRI and placebo groups was calculated and weighted by number of randomized patients in the trial. Previous research has demonstrated that a logarithmic model provided the best fit for the time course of SSRI response compared to placebo(13). The effects of SSRI were modeled using an autoregressive variance function and the model with the lowest values on the Akaike Information Criterion (AIC) was selected(14). Further details on this technique can be found elsewhere(13). We then examined the moderating effects of SSRI dosage using similar methodology. A mixed model was conducted that included the main effect of time and an interaction between SSRI dosage (in imipramine equivalents) and time. The main effect of SSRI dose was not included in the model since this effect should be trivial. There should be no differences in depression severity (compared to placebo) at baseline. Differences should only be seen later when different SSRI doses start taking effect. Dose of SSRI was converted into imipramine equivalents based on previously defined methodology based on each medications’ therapeutic dose range(10, 15). Imipramine dose equivalents were chosen as the standard for antidepressants since it was the first medication introduced in the class. For SSRI analysis: 100mg of imipramine = 120mg of sertraline = 100mg of fluvoxamine = 20mg of paroxetine or fluoxetine=33.3 mg of citalopram=16.7 mg of escitalopram. We additionally tested SSRI dose model with an additional term to account for a delayed effect of SSRI dosing. We examined models where the dosing effect of SSRI was only included after a lag of 2, 3 and 4 weeks and the initial model with no lag to see if a modified log model can fit the data better. For this we coded each week as a dummy variable and ran four models adding in a three-way-interaction between week, dose and time. The goal of analysis was to determine if the interaction between dose and time was significant, which would indicate that there exists a delayed effect of dose response relationship for SSRI in MDD.
As an alternative method of analysis, we also examined endpoint data from included trials. We examined (1) standardized mean difference between endpoint depression scores and (2) odds ratio (OR) of treatment response between SSRI and placebo using Comprehensive Meta Analysis Version 3. We conducted a meta-regression in CMA Version 3 using a fixed-effects model that plotted standardized mean difference (or OR) for each trial against SSRI-dose (in imipramine equivalents). A statistically significant meta-regression result would indicate an association between SSRI dose (in imipramine equivalents) and reported effect size of SSRI treatment compared to placebo. Additionally, in order to examine how our data replicated previous analysis in the area, we conducted an analysis examining previously utilized categories of SSRI dose. A stratified subgroup analysis was conducted using endpoint data with studies stratified by SSRI dosing (dose range categories (<100mg, 100–199mg, 200–250mg, and >250mg). This analysis examined the possibility that there might not be a linear association between SSRI dose and therapeutic response or that a linear relationship might exist but only up to or after a certain dose threshold. For clinician-friendly interpretation of the resultant data, we additionally converted all SMD outcomes to OR in CMA. We also calculated number needed to treat (NNT) or number needed to harm (NNH) for each outcome based on the OR and control event rate using the Center for Evidence-Based Medicine OR to NNT converter(16).
The analyses described so far used all available data; the following sensitivity analyses were added to examine time and dose effects specific to intent-to-treat (ITT) studies. The treatment effect was compared in ITT and completer studies both in the logarithmic model - by including an ITT status × time interaction - and in the endpoint data meta-analysis –via subgroup analysis. Further, in the logarithmic model, the robustness of effect of dose (time × dose interaction) was tested by controlling for the ITT/completer study × time interaction. Finally, endpoint data from only ITT studies was used to conduct a meta-regression testing the effect of dose, as well as to conduct a subgroup analysis comparing the above-mentioned dose ranges.
We additionally examined the relationship between tolerability and SSRI dose in MDD trials using fixed-effects meta-regression in CMA version 2.2. Specifically we examined the association between all-cause dropout (and dropouts due to side effects) as expressed in pooled odds ratio (OR) and SSRI dosage (in imipramine equivalents). A statistically significant meta-regression result would indicate an association between SSRI dose (in imipramine equivalents) and likelihood of participant dropout compared to placebo. Subgroup analysis was also performed between the 4-imipramine equivalent SSRI dose ranges.
For all analyses, we conducted an additional sensitivity analyses excluding trials involving fluvoxamine. We choose to include fluvoxamine in our primary analysis as fluvoxamine is an SSRI with an indication for MDD in many countries (e.g. United Kingdom, Australia and Russia). However, fluvoxamine does not possess an FDA indication for MDD and could have a different dose-response relationship compared to other SSRIs so we decided to present our findings without fluvoxamine trials as a sensitivity analysis (and in supplementary figures).
Results
Included Studies
A flowchart describing the selection of eligible trials is provided in Figure 1. Our search identified 1707 studies, and an additional 4 studies were identified in references of other included trials and meta-analyses in the area. Forty studies met our inclusion criteria (17–55). The included studies reported 49 active treatment arms involving 10,039 adult patients with MDD. The supplementary table depicts the characteristics of the included studies. Six different SSRIs were studied in placebo-controlled trials with major depressive disorder: fluoxetine (k=9, n= 2386) (17, 21, 29, 31, 46, 47, 49, 51, 56), fluvoxamine (k=8, n=910)(18, 24, 27, 38, 39, 42, 45, 54), paroxetine (k=16, n=3424)(19, 20, 22, 23, 25, 26, 28, 30, 33, 34, 36, 44, 48, 52, 55), sertraline (k=3, n=865)(43, 50, 57), citalopram (k=4, n=1349)(32, 37, 40, 50), and escitalopram (k=3, n=1105)(37, 41, 53).
Figure 1. Selection of Studies.
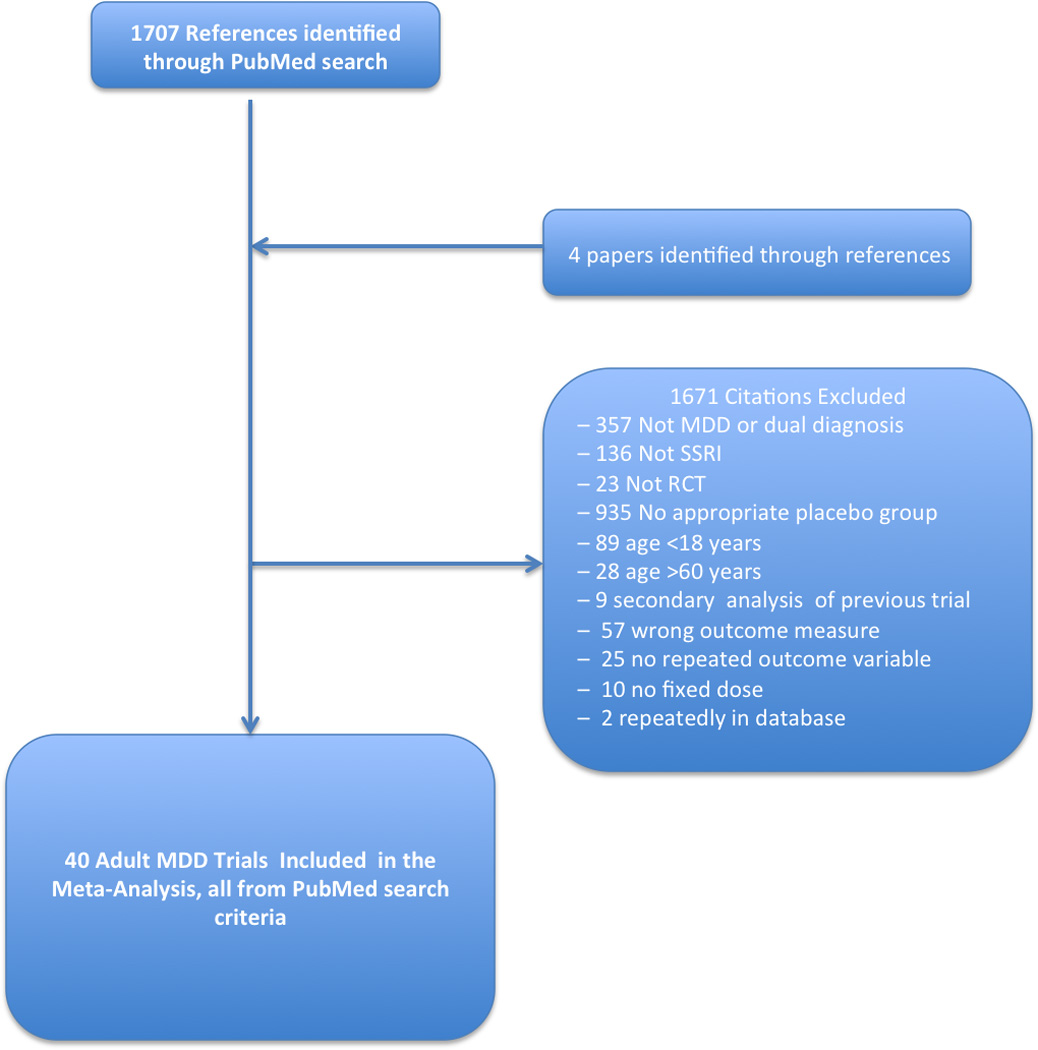
Figure 1 is a flowchart depicting the procedure for selection of eligible trials from identified references. Abbreviations: MDD=Major Depressive Disorder, SSRI=Selective-Serotonin Reuptake Inhibitor, RCT= Randomized Controlled Trial
SSRI Efficacy
Best-Fitting Model of SSRI Response
The natural logarithmic (loge) model of SSRI treatment response had the best model-fit. Based on Akaike information criterion, the logarithmic treatment model was significantly better than a model using the square root of week (χ2=4.9, p=0.03). The estimate of treatment effect by log (week+1) from the final model was 0.32 (95%CI: 0.27–0.37; p<0.001). A loge response curve indicates that the incremental SSRI benefit compared to placebo was greatest in the first week, and gradually declined in magnitude as time persisted in short-term treatment trials. Models that introduced delayed treatment effects all produced similar (but worse or equivalent) model fits to when the dosing effect was introduced at baseline (week 2: χ2=0, p=1; week 3: χ2=0.6, p=1; week 4: χ2=3.7 p=0.054).
Dose-Response Curve in Continuous Model of SSRI Response
Figure 2 depicts the logarithmic models at different imipramine equivalent dose isoquants. There was significant effect of time [log (week+1)=0.23 (95%CI:0.13–0.33; p<0.0001)], and a significant interaction between dose and time [interaction=0.0007 (95%CI: 0.0001–0.0013; p=0.0196)]. This result indicates that higher doses of SSRIs were associated with a greater therapeutic response. In sensitivity analysis, the dose by time interaction remained significant when use of non-ITT analysis was adjusted for in the model [interaction=0.0007, (95% CI 0.0000–0.0014, p=.00480)]. Similarly, when fluvoxamine trials were excluded from the analysis, there remained a significant dose by time interaction [interaction=0.0008 (95%CI: 0.0002–0.0014; p<0.001)] (Supplement Figure 2).
Figure 2. Effect of Dosage on Longitudinal Response Curve of SSRIs.
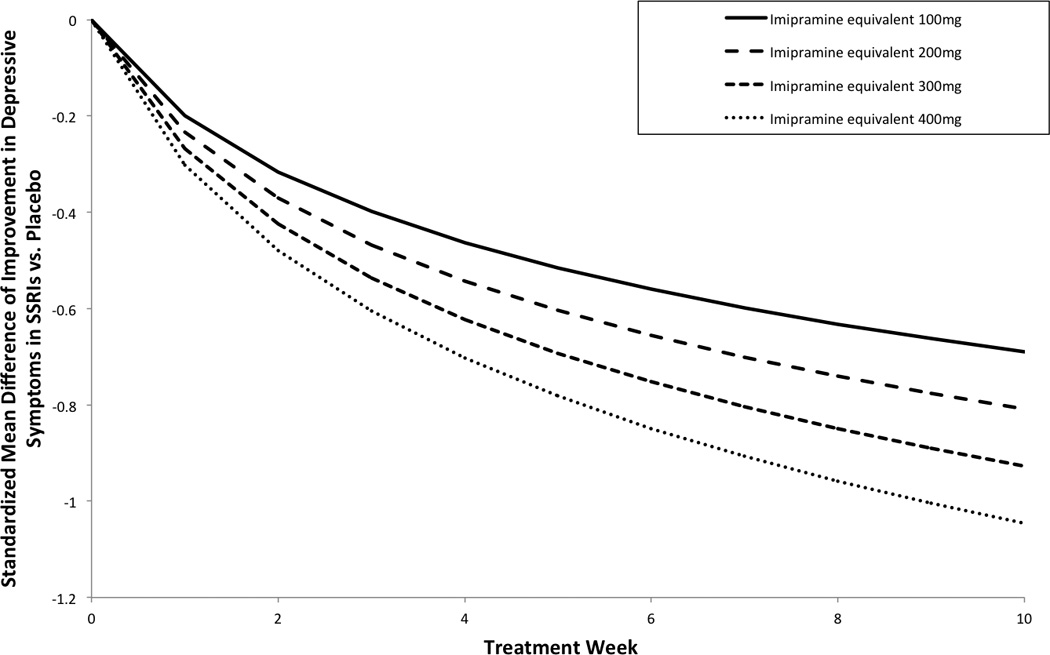
Figure 2 depicts the effects of dosage on the longitudinal response curve examining the efficacy of SSRIs compared to placebo over time. Each line represents the typical improvement in depressive symptoms experienced over time in SSRIs compared to placebo at a dosage isoquant. Dosages are expressed in imipramine equivalents. 100mg of imipramine = 120mg of sertraline = 100mg of fluvoxamine = 20mg of paroxetine or fluoxetine=33.3 mg of citalopram=16.7 mg of escitalopram. Abbreviations: SSRI= Selective-Serotonin Reuptake Inhibitor, SMD=Standardized Mean Difference between Active and Placebo
Traditional Meta-Analysis Examining Depression Severity
Meta-regression described a significant association between SSRI dose (in imipramine equivalents) and measured efficacy of SSRIs in reducing depression severity (β=0.00053, 95% CI 0.00018–0.00088, z = 2.98, p =0.0029). Figure 3A shows a scatterplot that depicts the relationship between imipramine equivalent dose of SSRIs and measured efficacy of SSRIs compared to placebo in terms of SMD. In sensitivity analysis, this result remained significant when restricted to trials using ITT analysis (β=0.00062, 95% CI 0.00025–0.00098, z = 3.32, p =0.00090) but not when trials involving fluvoxamine were excluded (β=0.00029, 95% CI −0.00010–0.00066, z = 1.44, p =0.15) (Supplement Figure 3A).
Figure 3. Effect of Dose on Measured Efficacy of SSRIs compared to Placebo at Trial Endpoint.
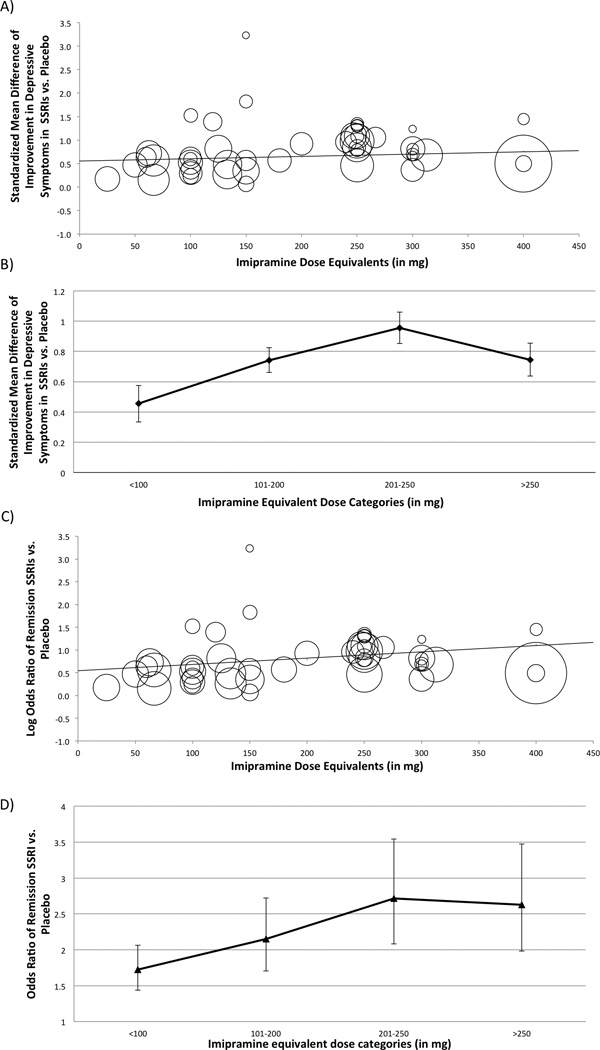
Figure 3A is a scatterplot depicting the association between SSRI dosage in imipramine equivalents and measured effect size of SSRIs compared to placebo (standardized mean difference). Within the scatterplot, circles represent individual studies with the size of the circle corresponding to its weight in the meta-analysis. The regression line reflects the significant positive relationship between SSRI dosage and measured efficacy compared to placebo (β=0.00053, 95% CI 0.00018–0.00088, z = 2.98, p =0.0029). Figure 3B depicts the association between SSRI dose and measured effect size in 4 dose categories of SSRIs. The 4 chosen dose categories of SSRIs: <100mg, 100–199mg, 200–250mg and >250mg were based on a meta-analysis that failed to demonstrate a dose-response relationship in antidepressant medications (not exclusively SSRIs). Dosages are expressed in imipramine equivalents. 100mg of imipramine = 120mg of sertraline = 100mg of fluvoxamine = 20mg of paroxetine or fluoxetine=33.3 mg of citalopram=16.7 mg of escitalopram. Abbreviations: SMD=Standardized Mean Difference. Figure 3C is a scatterplot depicting the association between SSRI dosage in imipramine equivalents and response in SSRIs compared to placebo (Odds Ratio). The regression line reflects the non-significant positive relationship between SSRI dosage and response compared to placebo (β=0.00029, 95% CI −0.00010–0.00066, z = 1.44, p =0.15). Figure 3D depicts the association between SSRI dose and response in 4 dose categories of SSRIs. Abbreviations: SMD=Standardized Mean Difference
When SSRI dose was examined as specific dosing categories rather than as a continuous variable, there remained a significant effect of dose (test for subgroup differences χ2=54.4, df=3, p<0.001). Figure 3B describes the estimated efficacy of each SSRI dose category compared to placebo. The greatest measured efficacy of SSRIs was observed in the dosing range of 200–250 imipramine equivalents. In sensitivity analysis, the differences between groups remained significant when restricted exclusively to trials employing ITT analysis (χ2=56.2, df=3, p<0.001) or when fluvoxamine trials were excluded (χ2=42.4, df=3, p<0.001) (Supplement Figure 3B).
Traditional Meta-Analysis Examining Treatment Response
Meta-regression demonstrated a significant association between SSRI dose (in imipramine equivalents) and measured efficacy of SSRIs with regards to OR of treatment response (β=0.0016, 95% CI 0.0005–0.0027, z = 2.86, p =0.004). Figure 3C shows a scatterplot that depicts the relationship between imipramine equivalent dose of SSRIs and measured efficacy of SSRIs compared to placebo in terms of OR of treatment response. In sensitivity analysis, this result remained significant when restricted to trials using ITT analysis (β=0.00062, 95% CI 0.00025–0.00098, z = 3.32, p =0.00090) and when trials involving fluvoxamine were excluded (β=0.0015, 95% CI 0.0003–0.0026, z = 2.47, p =0.013) (Supplement Figure 3C).
When SSRI dose was examined as specific dosing categories rather than as a continuous variable, there remained a significant effect of dose (test for subgroup differences χ2=14.5, df=3, p=0.002). Figure 3D depicts the odds ratio of each SSRI dose category compared to placebo. The greatest measured efficacy of SSRIs was again observed in the dosing range of 200–250 imipramine equivalents. In sensitivity analysis, the differences between groups remained significant when restricted exclusively to trials employing ITT analysis (χ2=56.2, df=3, p<0.001) or when fluvoxamine trials were excluded (χ2=11.4, df=3, p=0.01) (Supplement Figure 3D).
SSRI Tolerability
Higher SSRI dose was slightly, but significantly, associated with a lower likelihood of all cause dropout (β=−0.00093, 95% CI −0.00165– (−0.00021), z = −2.54, p =0.0110) in meta-regression analysis. Figure 4A depicts the association between SSRI dose (in imipramine equivalents) and likelihood of all-cause drop out compared to placebo. However, when SSRI dose was divided into previously defined categories, there was no significant association between SSRI dose and likelihood of all-cause dropout (test for subgroup differences χ2=4.8, df=3, p=0.19). The likelihood of all-cause dropout compared to placebo was highest in the 100–200 imipramine equivalent group and was slightly, but not significantly, lower if the dose was lowered or raised from this dose. Figure 4C depicts the association between SSRI dose and likelihood of all-cause dropout for each of the dosing categories. In the sensitivity analysis, excluding fluvoxamine trials, results remained similar for likelihood of all cause dropout in the meta-regression (β=−0.00092, 95% CI −0.00174– (−0.00010), z = −2.20, p =0.03), but became non-significant in the subgroup analysis (χ2=3.7, df=3, p=0.29) (Supplement Figure 4A and C).
Figure 4. Relationship between SSRI Dosage and Likelihood of Dropout.
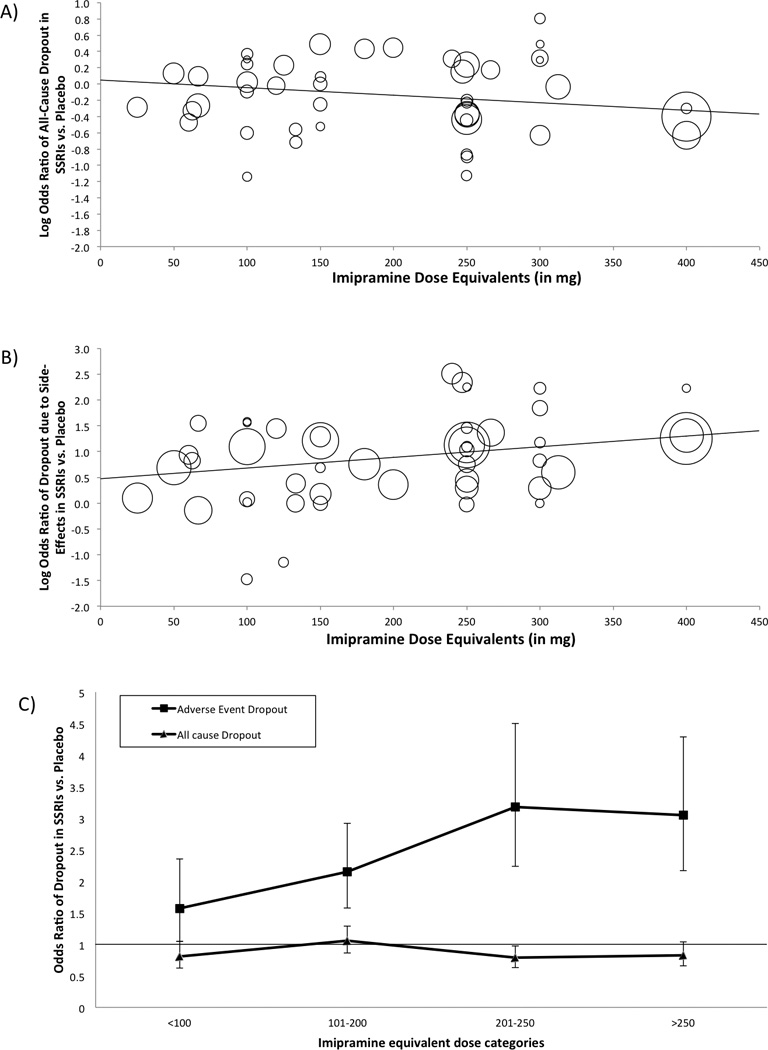
Figure 4A is a scatterplot of a meta-regression analysis that examines the association between SSRI dose and likelihood of all-cause dropout. Higher SSRI dose were associated with a lower rate of all-cause dropouts (β=−0.00093, 95% CI −0.00165– (−0.00021), z = −2.54, p =0.0110). Figure 4B is a scatterplot of a meta-regression analysis that examines the association between SSRI dose and likelihood of dropout due to side-effects. Higher doses of SSRIs were associated with a higher rate of dropouts due to side-effects (β=0.00207 95% CI 0.00071– 0.00342, z = 2.98, p =0.0028). Within the scatterplot, circles represent individual studies with the size of the circle corresponding to its weight in the meta-analysis. Lines represent the results of meta-regression analysis. Figure 4C depicts the association between SSRI dose and all-cause dropouts and dropouts due to side-effects in 4 dose categories. The 4 chosen dose categories of SSRIs: <100mg, 100–199mg, 200–250mg and >250mg were based on a meta-analysis that failed to demonstrate a dose-response relationship in antidepressant medications (not exclusively SSRIs). Dosages are expressed in imipramine equivalents. 100mg of imipramine = 120mg of sertraline = 100mg of fluvoxamine = 20mg of paroxetine or fluoxetine=33.3 mg of citalopram=16.7 mg of escitalopram. Abbreviations: SMD=Standardized Mean Difference, SSRI=Selective-Serotonin Reuptake Inhibitor, LogOR= Logarithm of odds ratio
Meta-regression described a significant association between higher SSRI dose and increased likelihood of dropout due to side effects (β=0.00207 95% CI 0.00071– 0.00342, z = 2.98, p =0.0028). Figure 4B depicts a scatterplot demonstrating the relationship between SSRI dose and the likelihood of dropout due to side effects. Stratified subgroup analysis by SSRI dose category also demonstrated a significant association between SSRI dose and likelihood of dropout due to side effects. All dosing categories of SSRIs were associated with a greater likelihood of dropout due to side effects compared to placebo. Higher dosing categories of SSRIs were associated with a greater likelihood of dropout due to side effects. Figure 4B depicts the association between SSRI dose and likelihood of dropout due to side effects for each of the dosing categories. In the sensitivity analysis, excluding fluvoxamine trials, results remained mostly unchanged for the likelihood of dropout due to side effects in the meta-regression (β=0.00249 95% CI 0.00073– 0.00425, z = 2.77, p =0.006), however became non-significant in the subgroup analysis (χ2=7.7, df=3, p=0.052) (Supplement Figure 4B).
Discussion
Meta-analysis demonstrated a significant association between higher SSRI doses and greater measured efficacy of SSRIs in placebo controlled trials. This significant association between SSRI dose and measured efficacy was demonstrated in 1) longitudinal mixed model meta-analysis; 2) endpoint meta-regression and 3) stratified subgroup analysis by dose. These findings remained significant if the analysis was restricted to only data from studies employing ITT analysis and when fluvoxamine trials were excluded. Meta-analysis suggests that there may also be a consequence associated with escalating the dose of SSRI associated with reduced tolerability, as evidenced by a greater likelihood of dropout due to side effects with higher SSRI dose.
The results of this meta-analysis both extend upon and contradict previous meta-analysis in this area (10). We replicated previous evidence suggesting a reduced tolerability of SSRIs at higher doses as evidenced by a higher likelihood of dropouts due to side effects. However, we demonstrated a significant positive association between SSRI dose and measured efficacy that flattened out only at the higher end of the recommended dosing range (greater than 250mg imipramine equivalents). Specifically, meta-analysis demonstrates that using a higher dose of SSRI for MDD is associated with increased likelihood of response. Table 1 depicts OR and NNT comparisons for different initial dosing strategies of SSRIs. Our results suggest a modest improvement in efficacy of high ((200–250 or >250mg imipramine equivalents) as compared to low-dose (100–200 imipramine equivalents) with ORs of approximately 1.3 and NNTs in the 14–16 range.
Table 1.
Evidence-Based Medicine Estimates for Risks and Benefits of SSRI Doing Strategies for Major Depression
| Dose Category | Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | |
| Subtherapeutic | <100mg | 1.72 (1.44–2.07) | 2.23 (1.91–2.62) | ||||
| Low-Dose | 100–199mg | 2.02 (1.69–2.41) | 1.17 (0.98–1.40) | 3.16 (2.76–3.62) | 1.42 (1.24–1.62) | ||
| Medium Dose | 200–249mg | 2.72 (2.08–3.54) | 1.58 (1.17–2.06) | 1.35 (1.00–1.70) | 5.07 (4.34–5.91) | 2.27 (1.95–2.65) | 1.60 (1.37–1.87) |
| High Dose | >250mg | 2.65 (2.22–3.17) | 1.54 (1.29–1.84) | 1.31 (1.07–1.52) | 3.06 (2.65–3.52) | 1.37 (1.18–1.58) | 0.97 (0.84–1.11) |
| Dose Category | Actual NNT of Treatment Response* | Estimated NNT based on Effect Size* | |||||
| Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | ||
| Subtherapeutic | <100mg | 8 (6–11) | 5 (4–6) | ||||
| Low-Dose | 100–199mg | 6 (5–8) | 27 (12–∞) | 4 (3–4) | 12 (9–20) | ||
| Medium Dose | 200–249mg | 4 (3–6) | 9 (6–27) | 14 (8–∞) | 3 (2–3) | 5 (4–6) | 9 (7–13) |
| High Dose | >250mg | 4 (4–5) | 10 (7–17) | 16 (10–64) | 4 (3–4) | 13 (9–26) | No Benefit* |
| Dose Category | Actual OR of Dropout Due to Side-Effects | Actual OR of All Cause Dropout | |||||
| Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | ||
| Subtherapeutic | <100mg | 1.56 (1.08–2.25) | 0.82 (0.67–1.01) | ||||
| Low-Dose | 100–199mg | 2.22 (1.68–2.94) | 1.42 (1.08–1.88) | 1.09 (0.92–1.29) | 1.33 (1.12–1.57) | ||
| Medium Dose | 200–249mg | 3.16 (2.32–4.32) | 2.02 (1.49–2.77) | 1.42 (1.04–1.95) | 0.80 (0.68–0.95) | 0.98 (0.83–1.16) | 0.73 (0.62–0.87) |
| High Dose | >250mg | 3.08 (2.29–4.14) | 1.97 (1.47–2.65) | 1.39 (1.03–1.86) | 0.75 (0.64–0.87) | 0.91 (0.78–1.07) | 0.69 (0.59–0.80) |
| Dose Category | Actual NNH of Dropout Due to Side-Effects | ||||||
| Placebo |
Subtherapeutic SSRI |
Low-dose SSRI | |||||
| Subtherapeutic | <100mg | 34 (16–230) | |||||
| Low-Dose | 100–199mg | 16 (10–28) | 45 (22–230) | ||||
| Medium Dose | 200–249mg | 10 (7–15) | 19 (11–38) | 45 (20–459) | |||
| High Dose | >250mg | 10 (7–15) | 20 (12–40) | 48 (22–611) | |||
Table 1 describes the Odds Ratios (OR) and Number Needed to Treat/Harm (NNT/NNH) for SSRI dosing strategies compared to each other and placebo.
Previous meta-analysis and fixed-dose trials in this area have provided no evidence for escalating dose beyond the minimum recommended therapeutic dose(10, 57). Our meta-analysis differed in methodology in several important ways from this previous meta-analysis that likely explain the difference in results. 1) We restricted our analysis to SSRI trials and did not include other antidepressants which likely have different dose-response and dose-tolerability curves. 2) We examined symptom improvement as a continuous measure rather than examining clinical improvement (yes/no) as the primary outcome of the meta-analysis. This decision likely increased power of the meta-analysis by increasing sensitivity of the primary outcome measure and reducing heterogeneity by eliminating differences in definition of therapeutic response. 3) We additionally examined the dosing effects of SSRIs not only with treatment response as a dichotomous outcome but also as a continuous measure. Meta-regression with a continuous measure is more sensitive to a change in SSRI benefit with dose. 4) We also included several trials published after the first meta-analysis. The additional trials provided more power to conduct this analysis.
Our meta-analysis demonstrates that there is substantial evidence for a modest increase in efficacy when higher doses of SSRIs starting from the point of initial titration. We also demonstrate that this benefit is at the cost of reduced tolerability. Given this tradeoff between the risks and benefits, another potential prudent clinical strategy is to raise SSRI doses in non-responders to low-dose treatment. Other systematic reviews, have previously examined whether dose-escalation strategies are effective in non-responders to low-dose antidepressant treatment. A systematic review that examined dose-escalation studies in low-dose SSRI non-responders suggested that SSRIs have a flat dose-response relationship within the therapeutic range and that higher SSRI doses were only associated with a greater side-effect burden (58). By contrast, a later systematic review that examined the efficacy of dose escalation strategies in SSRI non-responders suggested a modest benefit (NNT range: 12–82 in trials) of increasing to a higher dose SSRI if subjects had received previous low-dose SSRI treatment for at least 4 weeks (59). By contrast, this systematic review suggested that when the dose-escalation strategy was initiated before 4 weeks of SSRI treatment, there was no evidence of benefit to raising SSRI dose on likelihood of treatment response (59). Our results extend upon these previous dose-escalation studies and systematic reviews by demonstrating that the dose-response relationship of SSRI is mildly positive and not flat within the SSRI therapeutic range even when started from the initial point of treatment. Further research is needed to extend upon our results in order to (1) better gauge the risk/benefit of SSRI dose-escalation in low-dose SSRI non-responders and (2) determine the ideal time-point for starting SSRI dose escalation.
Given the potential clinical implications of the results of our meta-analysis, it is important to be clear in its limitations. Publication bias is a well-identified problem in trials involving antidepressant agents (6). We employed a comprehensive search strategy to try to identify all available published and unpublished trials of SSRIs. Given that our meta-analysis examined the difference in efficacy of different doses of SSRIs rather than the overall efficacy of the underlying therapeutic class, it is not clear how publication bias could have influenced the relationship between SSRI dosing and measured efficacy. Assuming that the positive association between SSRI dose and measured efficacy is true, then publication bias, if present, would likely have dampened our measured association. Publication bias would have likely caused the suppression of negative trials, which based on the findings of this meta-analysis would be more likely to occur at lower SSRI doses, and potentially lead to a reduced measured association between dose and efficacy in the meta-analysis. Other limitations were present in our meta-analysis examining tolerability of SSRI agents at different doses. We would have liked to have analyzed the frequency of different side effects (e.g. sexual dysfunction, nausea, sedation etc.) at different SSRI doses. However, measurement and reporting of side effects have changed dramatically over the 3 decades during which these trials were published. Selective reporting of side effects in earlier manuscripts and changes in how side effects are screened for over time made this analysis not feasible. We would have also liked to examine how timing of dose titration affected likelihood of subject dropout in the trials employing higher SSRI doses but titration schedules are also variably reported in trials. Another general limitation is the generalizability to the community population. Most SSRI trials included in this meta-analysis have strict inclusion criteria. Therefore, many patients seen in typical clinical practice with depression such as those with significant comorbid medical or psychiatric conditions or taking adjunctive medications would be specifically excluded from these trials. Clinical patients with additional comorbid illness or concomitant medication use may respond differently to SSRI dose escalation both in terms of efficacy and side-effects as compared to clinical trials samples (11, 58, 59).
Our meta-analysis provides evidence to support clinical guidelines that recommend raising SSRI dose in adults with MDD who fail to respond to SSRI medications at or below the lower-end of the therapeutic dose range. Higher doses of SSRIs are associated with increased efficacy (NNT for treatment response ≈14–16) but also reduced tolerability as evidenced by a higher likelihood of drop-outs due to side effects in trials (NNH≈22–24). However, overall dropout rates were reduced at higher doses of SSRIs, which is likely attributable to their greater efficacy. Further, research needs to be performed to examine the ideal timing of dose-escalation of SSRIs in MDD in order to maximize benefit while reducing unnecessary additional side-effects caused by higher dose SSRI treatment.
Supplementary Material
Acknowledgments
Dr. Taylor reports a family member is an employee of GlaxoSmithKline.
Michael Bloch gratefully acknowledges support from the National Institute of Mental Health (K23MH091240), the AACAP/ Eli Lilly Junior Investigator Award, NARSAD, the Rembrandt Foundation, the Tourette Syndrome Association and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research. Dr. Taylor is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The authors thank Jilian Mulqueen, BA and Catherine Coughlin, BS for their help with proofreading and formatting.
Footnotes
Disclosures: All other authors have no conflicts of interest to disclose.
Contributor Information
Ewgeni Jakubovski, Associated with the Yale Child Study Center.
Anjali L. Varigonda, University of Vermont College Of Medicine.
Nicholas Freemantle, Associated with the Department of Primary Care and Population Health at the UCL Medical School in London.
Matthew J. Taylor, Associated with the Department of Psychosis Studies of King’s College London.
Michael H. Bloch, Child Study Center and Department of Psychiatry of Yale University.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Alan J, Gelenberg MD, Chair, Marlene P, Freeman MD, John C, Markowitz MD, Jerrold F, Rosenbaum MD, Michael E, Thase MD, Madhukar H, Trivedi MD, Richard S, Van Rhoads MD., Consultant Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 2010 [Google Scholar]
- 4.NICE. London: National Institute for Health and Care Excellence; 2009. Depression - The treatment and management of depression in adults. [PubMed] [Google Scholar]
- 5.Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Archives of general psychiatry. 2010;67:1265–1273. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- 6.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. The New England journal of medicine. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 7.Machado M, Iskedjian M, Ruiz I, Einarson TR. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Current medical research and opinion. 2006;22:1825–1837. doi: 10.1185/030079906X132415. [DOI] [PubMed] [Google Scholar]
- 8.Papakostas GI, Nelson JC, Kasper S, Moller HJ. A meta-analysis of clinical trials comparing reboxetine, a norepinephrine reuptake inhibitor, with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18:122–127. doi: 10.1016/j.euroneuro.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. The Journal of clinical psychiatry. 2001;62:869–877. doi: 10.4088/jcp.v62n1106. [DOI] [PubMed] [Google Scholar]
- 10.Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. The British journal of psychiatry : the journal of mental science. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- 11.Baker CB, Tweedie R, Duval S, Woods SW. Evidence that the SSRI dose response in treating major depression should be reassessed: a meta-analysis. Depression and anxiety. 2003;17:1–9. doi: 10.1002/da.10079. [DOI] [PubMed] [Google Scholar]
- 12.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Molecular psychiatry. 2010;15:850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Archives of general psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akaike H. A New Look at the Statistical Model Identification. IEEE Transactions on Automatic Control. 1974;AC-19:716–723. [Google Scholar]
- 15.Benkert O, Szegedi A, Wetzel H. Minimum effective dose for antidepressants--an obligatory requirement for antidepressant drug evaluation? International clinical psychopharmacology. 1996;11:177–185. doi: 10.1097/00004850-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Center for Evidence-Based Medicine Toronto. OR to NNT converter. KT Clearinghouse [Google Scholar]
- 17.Byerley WF, Reimherr FW, Wood DR, Grosser BI. Fluoxetine, a selective serotonin uptake inhibitor, for the treatment of outpatients with major depression. Journal of clinical psychopharmacology. 1988;8:112–115. [PubMed] [Google Scholar]
- 18.Claghorn JL, Earl CQ, Walczak DD, Stoner KA, Wong LF, Kanter D, Houser VP. Fluvoxamine maleate in the treatment of depression: a single-center, double-blind, placebo-controlled comparison with imipramine in outpatients. Journal of clinical psychopharmacology. 1996;16:113–120. doi: 10.1097/00004714-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Claghorn JL, Kiev A, Rickels K, Smith WT, Dunbar GC. Paroxetine versus placebo: a double-blind comparison in depressed patients. The Journal of clinical psychiatry. 1992;53:434–438. [PubMed] [Google Scholar]
- 20.Cohn JB, Wilcox CS. Paroxetine in major depression: a double-blind trial with imipramine and placebo. The Journal of clinical psychiatry. 1992;53(Suppl):52–56. [PubMed] [Google Scholar]
- 21.Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depression and anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of general psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 23.Detke MJ, Wiltse CG, Mallinckrodt CH, McNamara RK, Demitrack MA, Bitter I. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2004;14:457–470. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez RA, Goldstein BJ, Jacobson AF, Steinbook RM. A double-blind placebo-controlled study of fluvoxamine and imipramine in depression. The Journal of clinical psychiatry. 1985;46:84–87. [PubMed] [Google Scholar]
- 25.Dunbar GC, Claghorn JL, Kiev A, Rickels K, Smith WT. A comparison of paroxetine and placebo in depressed outpatients. Acta psychiatrica Scandinavica. 1993;87:302–305. doi: 10.1111/j.1600-0447.1993.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JG, Goldie A. Placebo-Controlled trial of paroxetine in depressive illness. Human Psychopharmacology: Clinical and Experimental. 1993;8:203–209. [Google Scholar]
- 27.Fabre L, Birkhimer LJ, Zaborny BA, Wong LF, Kapik BM. Fluvoxamine versus imipramine and placebo: a double-blind comparison in depressed patients. International clinical psychopharmacology. 1996;11:119–127. [PubMed] [Google Scholar]
- 28.Fabre LF. A 6-week, double-blind trial of paroxetine, imipramine, and placebo in depressed outpatients. The Journal of clinical psychiatry. 1992;53(Suppl):40–43. [PubMed] [Google Scholar]
- 29.Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, Zajecka J, Murck H, Rosenbaum JF. A Double-blind, randomized trial of St John's wort, fluoxetine, and placebo in major depressive disorder. Journal of clinical psychopharmacology. 2005;25:441–447. doi: 10.1097/01.jcp.0000178416.60426.29. [DOI] [PubMed] [Google Scholar]
- 30.Feighner JP, Cohn JB, Fabre LF, Jr, Fieve RR, Mendels J, Shrivastava RK, Dunbar GC. A study comparing paroxetine placebo and imipramine in depressed patients. Journal of affective disorders. 1993;28:71–79. doi: 10.1016/0165-0327(93)90035-i. [DOI] [PubMed] [Google Scholar]
- 31.Feighner JP, Boyer WF, Merideth CH, Hendrickson GG. A double-blind comparison of fluoxetine, imipramine and placebo in outpatients with major depression. International clinical psychopharmacology. 1989;4:127–134. doi: 10.1097/00004850-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Feighner JP, Overo K. Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. The Journal of clinical psychiatry. 1999;60:824–830. doi: 10.4088/jcp.v60n1204. [DOI] [PubMed] [Google Scholar]
- 33.Feighner JP, Boyer WF. Paroxetine in the treatment of depression: a comparison with imipramine and placebo. The Journal of clinical psychiatry. 1992;53(Suppl):44–47. [PubMed] [Google Scholar]
- 34.Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM. Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. The Journal of clinical psychiatry. 2002;63:577–584. doi: 10.4088/jcp.v63n0707. [DOI] [PubMed] [Google Scholar]
- 35.Heiligenstein JH, Tollefson GD, Faries DE. A double-blind trial of fluoxetine, 20 mg, and placebo in out-patients with DSM-III-R major depression and melancholia. International clinical psychopharmacology. 1993;8:247–251. doi: 10.1097/00004850-199300840-00007. [DOI] [PubMed] [Google Scholar]
- 36.Kiev A. A double-blind, placebo-controlled study of paroxetine in depressed outpatients. The Journal of clinical psychiatry. 1992;53(Suppl):27–29. [PubMed] [Google Scholar]
- 37.Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. International clinical psychopharmacology. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lydiard RB, Laird LK, Morton WA, Jr, Steele TE, Kellner C, Laraia MT, Ballenger JC. Fluvoxamine, imipramine, and placebo in the treatment of depressed outpatients: effects on depression. Psychopharmacology bulletin. 1989;25:68–70. [PubMed] [Google Scholar]
- 39.March JS, Kobak KA, Jefferson JW, Mazza J, Greist JH. A double-blind, placebo-controlled trial of fluvoxamine versus imipramine in outpatients with major depression. The Journal of clinical psychiatry. 1990;51:200–202. [PubMed] [Google Scholar]
- 40.Mendels J, Kiev A, Fabre LF. Double-blind comparison of citalopram and placebo in depressed outpatients with melancholia. Depression and anxiety. 1999;9:54–60. [PubMed] [Google Scholar]
- 41.Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson GD, Wohlreich MM. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Current medical research and opinion. 2007;23:401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- 42.Porro V, Fiorenzoni S, Menga C, De Cristofaro A. Single-blind comparison of the efficacy of fluvoxamine versus placebo in patients with depressive syndrome. Current therapeutic research. 1988 [Google Scholar]
- 43.Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, Masco HL, Mendels J. Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. The Journal of clinical psychiatry. 1990;51(Suppl B):18–27. [PubMed] [Google Scholar]
- 44.Rickels K, Amsterdam J, Clary C, Fox I, Schweizer E, Weise C. A placebo-controlled, double-blind, clinical trial of paroxetine in depressed outpatients. Acta psychiatrica Scandinavica Supplementum. 1989;350:117–123. doi: 10.1111/j.1600-0447.1989.tb07188.x. [DOI] [PubMed] [Google Scholar]
- 45.Roth D, Mattes J, Sheehan KH, Sheehan DV. A double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Progress in neuro-psychopharmacology & biological psychiatry. 1990;14:929–939. doi: 10.1016/0278-5846(90)90078-u. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. Journal of affective disorders. 1999;56:171–181. doi: 10.1016/s0165-0327(99)00067-1. [DOI] [PubMed] [Google Scholar]
- 47.Silverstone PH, Ravindran A. Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. Venlafaxine XR 360 Study Group. The Journal of clinical psychiatry. 1999;60:22–28. doi: 10.4088/jcp.v60n0105. [DOI] [PubMed] [Google Scholar]
- 48.Smith WT, Glaudin V. A placebo-controlled trial of paroxetine in the treatment of major depression. The Journal of clinical psychiatry. 1992;53(Suppl):36–39. [PubMed] [Google Scholar]
- 49.Sramek JJ, Kashkin K, Jasinsky O, Kardatzke D, Kennedy S, Cutler NR. Placebo-controlled study of ABT-200 versus fluoxetine in the treatment of major depressive disorder. Depression. 1995;3:199–203. [Google Scholar]
- 50.Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biological psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- 51.Tollefson GD, Holman SL. How long to onset of antidepressant action: a meta-analysis of patients treated with fluoxetine or placebo. International clinical psychopharmacology. 1994;9:245–250. doi: 10.1097/00004850-199400940-00003. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi MH, Pigotti TA, Perera P, Dillingham KE, Carfagno ML, Pitts CD. Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. The Journal of clinical psychiatry. 2004;65:1356–1364. doi: 10.4088/jcp.v65n1010. [DOI] [PubMed] [Google Scholar]
- 53.Wade A, Michael Lemming O, Bang Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. International clinical psychopharmacology. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Walczak DD, Apter JT, Halikas JA, Borison RL, Carman JS, Post GL, Patrick R, Cohn JB, Cunningham LA, Rittberg B, Preskorn SH, Kang JS, Wilcox CS. The oral dose-effect relationship for fluvoxamine: a fixed-dose comparison against placebo in depressed outpatients. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 1996;8:139–151. doi: 10.3109/10401239609147751. [DOI] [PubMed] [Google Scholar]
- 55.Perahia DG, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ. Duloxetine in the treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. European psychiatry : the journal of the Association of European Psychiatrists. 2006;21:367–378. doi: 10.1016/j.eurpsy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Heiligenstein JH, Tollefson GD, Faries DE. Response patterns of depressed outpatients with and without melancholia: a double-blind, placebo-controlled trial of fluoxetine versus placebo. Journal of affective disorders. 1994;30:163–173. doi: 10.1016/0165-0327(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 57.Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, Dube S, Small JG. Sertraline safety and efficacy in major depression: a double-blind fixed-dose comparison with placebo. Biological psychiatry. 1995;38:592–602. doi: 10.1016/0006-3223(95)00178-8. [DOI] [PubMed] [Google Scholar]
- 58.Adli M, Baethge C, Heinz A, Langlitz N, Bauer M. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. European archives of psychiatry and clinical neuroscience. 2005;255:387–400. doi: 10.1007/s00406-005-0579-5. [DOI] [PubMed] [Google Scholar]
- 59.Ruhe HG, Huyser J, Swinkels JA, Schene AH. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder: systematic review. The British journal of psychiatry : the journal of mental science. 2006;189:309–316. doi: 10.1192/bjp.bp.105.018325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


