Abstract
Objectives
To address the limited long-term outcome data for catheter ablation (CA) of persistent atrial fibrillation (PeAF), we analysed consecutive ablations performed at our centre from 1 January 2008 to 31 December 2010 and followed patients prospectively until January 2014.
Methods
Both arrhythmia recurrence and symptom relief were assessed. Follow-up data were collected from hospital records, supplemented by data from general practitioners and referring hospitals. At the end of the follow-up period, all patients were contacted by phone to determine their up-to-date clinical condition.
Results
188 consecutive patients with PeAF (157 male, mean age 57.3±9.7 years, 20% with long-standing PeAF) underwent a mean of 1.75 procedures (range 1–4). Telephone follow-up was achieved for 77% of surviving patients. Over a mean follow-up of 46±16 months (range 4–72), 139 (75%) patients experienced arrhythmia recurrence after a single procedure and 90 (48%) after their final procedure. Median time to first recurrence was 210 days (range 91–1850). 71% of recurrences were within the first year following ablation and 91% within 2 years. At final follow-up, 82% of patients reported symptomatic improvement. 7 (2.3%) major complications occurred, and there was no procedure-related death or stroke.
Conclusions
CA for PeAF is safe with a low rate of complications. Over a follow-up period of up to 6 years, a large majority of patients experience significant symptomatic improvement but recurrence after the initial procedure is the norm rather than the exception. 2 years' follow-up is sufficient to observe 90% of AF recurrences, but recurrence can occur even after 5 years' remission.
Keywords: Electrophysiology, Cardiac arrhythmias and resuscitation science
Key questions.
What is already known about this subject?
Freedom from recurrent atrial tachyarrhythmia is less common after catheter ablation of persistent, rather than paroxysmal, atrial fibrillation (AF). There is a belief that short-term success frequently is not maintained over time but there are few published studies of long-term results of persistent AF (PeAF) ablation, and the results in these studies vary greatly.
What does this study add?
This study provides data on outcomes after catheter ablation of PeAF over a 6-year period and is likely to more closely match real-life experience than the few previous studies which have originated from highly specialised and internationally renowned centres. In addition to arrhythmic outcomes, this is the first such study to look at the impact of ablation on patients' health status.
How might this impact on clinical practice?
The term curative AF ablation is often used but, from our results, would appear to be misleading for PeAF. Patients should be aware that complete freedom from recurrent arrhythmia is an exception, rather than the norm. That notwithstanding, the indication for catheter ablation is the relief of AF symptoms rather than freedom from recurrent AF and our results show that this is achievable for the majority of patients. Operators may consider using three-dimensional mapping techniques that combine integration of CT left atrial images, as our results suggest that this has a significant impact on long-term success.
Background
Catheter ablation (CA) for atrial fibrillation (AF) is now commonly used for a disease that has been described as an emerging medical epidemic.1 Between 2005 and 2009, the number of CAs performed in the UK increased by a factor of 10 (UK National Cardiac Audit data, personal communication). The current European Society of Cardiology guidelines give a class 1a recommendation for its use in drug refractory paroxysmal AF (PAF).2 The evidence base for PAF is well established with superior efficacy over antiarrhythmic drugs demonstrated in several clinical trials.3 For persistent AF (PeAF), the situation is less clear as the evidence base is weaker and a recent Cochrane review found insufficient evidence to support the use of CA for PeAF.4 However, more than 40% of procedures performed worldwide are reported to be for patients with PeAF.5
Although recent randomised controlled trials (RCTs) have shown superior efficacy of CA compared with medical therapy for patients with PeAF, the follow-up for these trials is short.6 7 A recent meta-analysis of randomised and non-RCTs of CA in PeAF found that the mean follow-up was 13.5±6 months.8 One issue for both forms of the disease is that early CA success does not always translate into long-term freedom from AF.9 We reviewed consecutive CA procedures performed for PeAF over a 3-year period at a single, high-volume centre in the UK and followed up patients for a further 3 years in order to provide up to 6 years of outcome data.
Methods
Patients
Consecutive patients who underwent a first-time CA for PeAF at Liverpool Heart and Chest Hospital (LHCH) during a 3-year period (01 January 2008 to 31 December 2010) were identified retrospectively and clinical notes reviewed for procedural data. Patients were classified as having PeAF if they had AF present continuously or had episodes of AF lasting for longer than 7 days at a time or requiring cardioversion, as specified by the published guidelines at the time the study was initiated.10 Comprehensive demographic, medical history and procedural details were collected from the hospital notes and electronic data storage systems. Data verification was performed for all outlying data points.
Follow-up
Clinical follow-up data were collected prospectively until January 2014. Two clinicians, both of whom were independent of the original procedure, reviewed the follow-up data. Missing follow-up data were obtained by contacting the patient's base hospital and/or general practitioner. Recurrence of AF was deemed to have occurred whenever AF or atrial tachycardia was documented on resting ECG or during a period of monitoring, or—in keeping with the real-world nature of this study—if the responsible clinician treated the patient for recurrence of AF without definitive proof, for example, by performing repeat CA or starting new antiarrhythmic medication because of the return of the patient's typical symptoms. Mortality data were obtained from the UK's central healthcare database (National Health Service (NHS) Spine). For any patient who died during follow-up, their general practitioner was contacted for cause of death and further details. At the end of the study, all patients were contacted by phone to invite them to provide final follow-up data. Telephone interviews were carried out using a standardised interview template. To avoid loss of follow-up due to working patterns or holidays, initial unsuccessful daytime contact was followed up by repeated attempts in the evening and at the weekend, spread out over a period of several weeks. The study was approved by both the hospital and regional research ethics committees.
Electrophysiological study
CA was performed under conscious sedation or general anaesthesia. Antiarrhythmic drugs were stopped at least five half-lives prior to the procedure, except for amiodarone which was generally continued. Postprocedural anticoagulation was with warfarin with bridging low molecular weight heparin (LMWH) used until the patient's international normalised ratio was ≥2. Preprocedural transoesophageal echocardiography was carried out for patients with AF without at least four preceding weeks of therapeutic anticoagulation. In general, vascular access was exclusively via the right femoral vein, with other routes only used as required. A deflectable decapolar catheter was positioned within the coronary sinus. Trans-septal access was gained using a Brockenbrough or Endrys needle under fluoroscopic guidance with either a single or double puncture. A variety of long sheaths were used. Unfractionated heparin was used to maintain an activated clotting time above 250–300 s. In all cases, pulmonary vein isolation (PVI) was first performed, predominantly using a 3.5–4 mm irrigated tip radiofrequency catheter with flow rates between 12 and 30 mL/min, using a segmental ostial or wide area circumferential ablation (WACA) pattern. A 20-pole circular mapping catheter was used to measure electrical activity within the pulmonary veins. The addition of linear lesions and complex fractionated atrial electrogram (CFAE) ablation was according to operator preference. Temperature limits were set to 50°C and ablation power was limited to 25–30 W on the posterior left atrial (LA) wall, 30–35 W on the anterior wall, roof and the intervenous carina, 25 W in the coronary sinus and 50 W for cavotricuspid isthmus ablation. Ablation was carried out either using a continuous dragging technique or individual point-by-point lesions of 20–40 s duration to achieve >75% attenuation of the local electrogram. In three cases, PVI was performed using the HD Mesh Ablator (C.R. Bard, Murray Hill, New Jersey, USA) and in one case with the Arctic Front cryoablation system (Medtronic, Minneapolis, Minnesota, USA). In keeping with published guidance, PVI was defined as proven entrance block. For linear lesions, excluding non-anchored lesions, the desired end point was bidirectional conduction block, as verified with appropriate pacing manoeuvres.11 The overall procedural end point was completion of the attempted lesion sets rather than termination of AF. Patients were monitored overnight and routinely discharged the following day.
Statistical analysis
Discrete variables, including change in health status which was treated as a dichotomous variable, are described in terms of the frequency and proportion and compared using the χ2 or Fisher's exact test. Continuous variables are described as mean±SD, and compared using unpaired t-tests for normally distributed data, or median (IQR) and compared using Mann-Whitney U test. Analyses were performed using IBM SPSS Statistics software V.21. Survival data were plotted using the Kaplan-Meier estimator. The log-rank test was used to compare survival between groups. Univariable and multivariable predictors of AF recurrence were examined using logistic regression using forward conditional modelling. We prespecified that variables with a p value ≤0.1 would be included in the multivariable model and, if necessary to maintain a minimum of 10 events per variables, selected on a hierarchical basis. 12 Where applicable, two-tailed tests were used in all analyses. A p value ≤0.05 was considered significant for all tests.
Results
Patients and baseline characteristics
We identified 189 patients in whom first-time percutaneous CA for PeAF was attempted during the study period. Baseline characteristics are provided in table 1. The mean duration of the current episode at the time of CA was 7±14 months. Thirty-seven (20%) patients had long-standing PeAF, defined as 12 months' continuous AF with no period of sinus rhythm (SR) lasting >24 hours. One hundred and forty-three (76%) patients had at least one attempt at electrical cardioversion prior to their CA, although only 70 (46%) maintained SR for >1 month. The mean CHA2DS2-VASc score was 1.45±1.46 (median=1). One patient did not undergo ablation as LA access was not possible, leaving 188 patients who were eligible for follow-up.
Table 1.
Baseline characteristics
| Characteristic | |
|---|---|
| Male gender, n (%) | 157 (83%) |
| Age (years) | 57.3±9.7 |
| Time since first AF diagnosis (months) | 48±52 |
| Long-standing PeAF, n (%) | 37 (20%) |
| Left atrial anteroposterior diameter (mm) | 44±6 |
| Left ventricular systolic function, n (%) | |
| Good (EF >50%) | 156 (83%) |
| Mildly impaired (EF 40–49%) | 12 (6%) |
| Moderately impaired (EF 30–39%) | 9 (5%) |
| Severely impaired (EF <30%) | 4 (2%) |
| Hypertension, n (%) | 85 (45%) |
| Diabetes, n (%) | 12 (6%) |
| Obstructive sleep apnoea, n (%) | 11 (6%) |
| Previous stroke/transient ischaemic attack, n (%) | 8 (4%) |
| Ischaemic heart disease, n (%) | 15 (8%) |
AF, atrial fibrillation; EF, ejection fraction; PeAF, persistent AF.
Patient journey
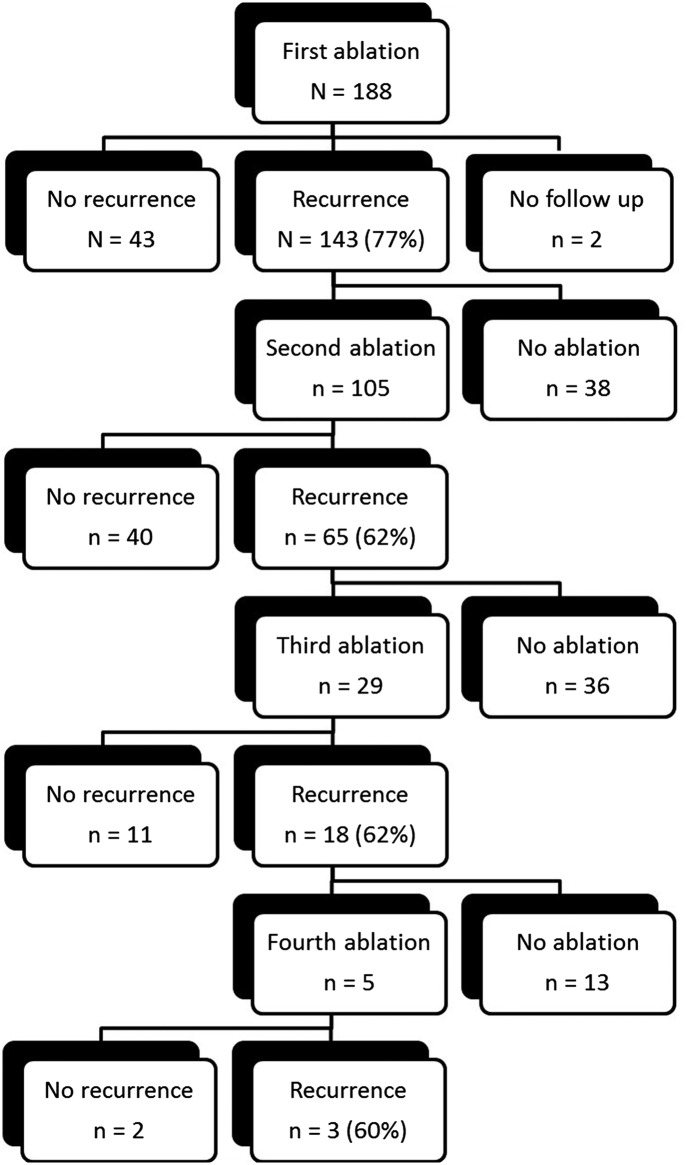
In total, 332 CA procedures were performed or attempted. Four procedures were abandoned before any ablation was performed due to complications of trans-septal puncture. Of these, three had no sequelae and one resulted in a small pericardial effusion that was managed conservatively. These procedures were included in complication data but excluded when calculating arrhythmic outcome data. Excluding abandoned procedures, 105 (56%) patients underwent more than one CA for AF. The ‘patient journey’ experienced in our patient cohort is illustrated in figure 3. Eight patients (4%) were eventually treated by implantation of a permanent pacemaker and atrioventricular node ablation.
Figure 3.
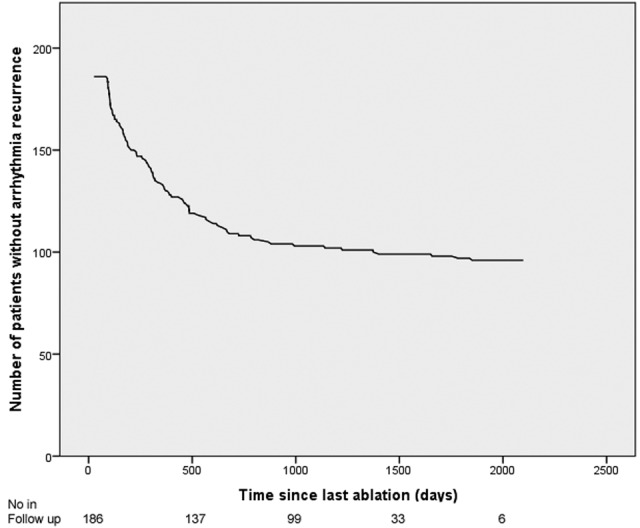
Recurrence curve showing number of patients remaining free from documented recurrence over time after their last procedure.
Follow-up
Clinical follow-up data were available for 186 of 188 eligible patients (98.5%). Mean follow-up time was of 46±16 months and ranged between 4 and 72 months. Follow-up of >1 year was available for >95% of patients. Eight patients died during follow-up. All deaths were remote from CA, with the earliest occurring after 121 days. Five deaths were non-cardiovascular (two from malignancy, one each from pulmonary fibrosis, pneumonia and renal failure) and three were cardiovascular in aetiology (one each of aortic dissection, heart failure and myocardial infarction). Three patients suffered a stroke or transient ischaemic attack (TIA) during follow-up, of whom two had complete neurological recovery. All three had had warfarin stopped prior to their event, in one because of intolerable side effects, one because of low-risk profile (CHA2DS2-VASc=0) and one by the original referring physician despite a previous history of TIA. Data on antithrombotic medication were available for 139 patients (75%), of whom 75 (54%) were taking oral anticoagulation (warfarin in 69, direct thrombin/factor Xa inhibitor in 6), 27 (19%) an antiplatelet agent and 37 (27%) no antithrombotic therapy. Mean CHA2DS2-VASc was significantly lower for those on no therapy or an antiplatelet agent (no therapy 0.65±0.75, antiplatelet 1.07±1.00, combined 0.83±0.88) compared with those on an oral anticoagulant (1.52±1.12, p<0.001).
Procedural complications
Seven patients experienced major complications. There were three pericardial effusions requiring percutaneous (n=2) or surgical (n=1) drainage, two inadvertent aortic punctures, one phrenic nerve paralysis and one femoral arterial pseudoaneurysm. Overall, this represents an incidence of major complications of 2.1% per procedure and 3.7% per patient. There were 18 minor complications of which 11 related to vascular access and 4 were pericardial effusions managed conservatively. One patient developed constrictive pericarditis 4 years after his CA, which had been felt at the time to be uncomplicated. He was found to have a calcified pericardial haematoma on pericardiectomy that may have been an unrecognised consequence of his CA procedure.
Ablation procedures
Of 188 index procedures, 91% were performed under conscious sedation and 9% under general anaesthesia. Conventional radiofrequency energy was used in 98% of procedures. Ninety per cent of cases used three-dimensional (3D) mapping systems: CARTO (Biosense Webster, Diamond Bar, California, USA) in 60% and Ensite NavX (St Jude Medical, St Paul, Minnesota, USA) in 30%. In 96 (51%) cases, the 3D map of the LA was integrated with a preoperative CT scan. Mean procedure duration was 200±41 min, and mean ablation and fluoroscopy times were 57±22 and 40+31 min, respectively. Average radiation dose, in terms of dose-area-product, was 5796±7634 mGy cm2.
PVI was performed in all cases, with additional linear lesions deployed in 146 (78%) cases and CFAE ablation in 62 (33%). PVI was by means of WACA in 109 cases, segmental isolation in 75 and a mixed approach in three. The most common LA linear lesion was a roof line (117, 62%) followed by a floor line (72, 38%). A mitral isthmus line was created in 37 (20%) cases. A right atrial flutter line was performed in 97 (52%) patients. CFAE ablation was performed in the LA in 56 (30%) cases and in the right atrium in 9 (5%).
At the start of the procedure, 112 (60%) patients were with AF or flutter and a further 23 patients developed sustained AF intraoperatively. Of these 135 patients, 28 (21%) were ablated to SR but the majority (104, 77%) were cardioverted either electrically or pharmacologically. Three patients remained in AF.
Outcomes after a single procedure
Antiarrhythmic medication was continued until the first follow-up appointment (median 3 (IQR 2–3) months after CA (IQR 2–3)) for 75% of patients (47% amiodarone, 17% flecainide, 8% sotalol and 3% others). Allowing for a 3-month blanking period, 139 (75%) patients experienced recurrence of AF after a single procedure during extended follow-up. The initial recurrence mechanism was paroxysmal in 55 (39%) patients, persistent in 81 (58%) and unclear in 4. AF was the recurrence arrhythmia in 104 (74%) patients with atrial tachycardia or flutter seen in 29 (21%). The recurrence arrhythmia was unknown for seven patients. The median time to first recurrence was 210 days (range 91–1850). A graphical representation of recurrence over time is shown in figures 1 and 2. Although first recurrence was seen to occur as late as 5 years after a hitherto successful procedure, 71% of AF recurrences occurred within the first year following CA and 91% within 2 years.
Figure 1.
The procedural journey for our cohort of patients with persistent atrial fibrillation. Patients underwent a maximum of four ablations and these are detailed in the centre of the diagram as well as the number with postprocedural recurrence. To the left are the patients without recurrence and to the right are those with recurrence who did not have further ablation.
Figure 2.
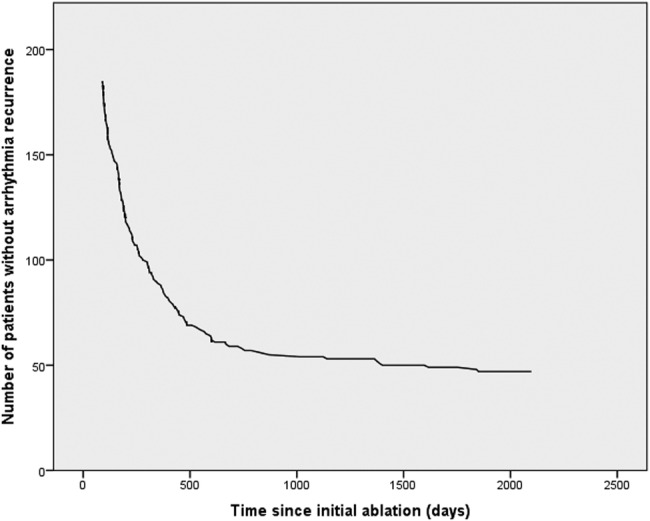
Recurrence curve showing number of patients remaining free from documented recurrence over time after a single procedure.
Regression analysis identified AF as initial rhythm at the time of CA (OR 2.05, 95% CI 1.05 to 4.03, p=0.037) as the only univariable predictor of AF recurrence after a single procedure, while integration of CT imaging into 3D mapping reduced the risk of recurrence (OR 0.39, 95% CI 0.19 to 0.78, p=0.008). Female sex (p=0.092) met the prespecified criteria for inclusion in multivariable modelling, but was not conventionally significant. After multivariable analysis, only AF as initial in-laboratory rhythm (OR 2.59, 95% CI 1.27 to 5.31, p=0.009) remained a statistically significant predictor of recurrence, and CT integration (OR 0.33, 95% CI 0.16 to 0.69, p=0.003) remained an independent predictor of success. Details of single procedure univariable and multivariable analyses are given in table 1.
Outcomes after multiple procedures
In our cohort, patients underwent a mean of 1.75±0.79 procedures (range 1–4). Median follow-up time after patients' last CA was 35 months (IQR 15–45). Ninety (48%) had a further recurrence of AF following their final procedure. Median time to recurrence after the last procedure was 301 days (range 91–1850). A graphical representation of recurrence over time is shown in figure 3. Of those who remained free of recurrence, 31 (32%) remained on class I or III antiarrhythmic drugs.
The only univariable predictor of AF recurrence after the final procedure was age (OR 1.05, 95% CI 1.01 to 1.08, p=0.006). Both female sex (p=0.08) and time (in months) since first diagnosis of AF (p=0.07) also met the criteria for inclusion in multivariable modelling. CT integration (OR 0.33, 95% CI 0.18 to 0.61, p<0.001) and isolation of the pulmonary veins using a WACA technique (OR 0.49, 95% CI 0.27 to 0.88, p=0.018) were associated with a lower risk of recurrence. After controlling for confounding with multivariable modelling, only age (OR 1.05, 95% CI 1.01 to 1.09, p=0.018) and lack of CT integration (OR 0.30, 95% CI 0.15 to 0.60, p=0.001) remained statistically significant in terms of predicting recurrence.
Long-standing PeAF
The presence of long-standing (>1 year) PeAF has traditionally been associated with poorer outcomes after CA but was not a predictor of recurrence after single or multiple procedures in our cohort. To investigate this further, we performed a subgroup analysis and found that AF recurrence was no more likely for patients with long-standing PeAF than for those with shorter duration PeAF after either a single (73% vs 74%, p=0.9) or final (41% vs 48%, p=0.4) procedure.
Effect of CT integration on freedom from recurrent AF
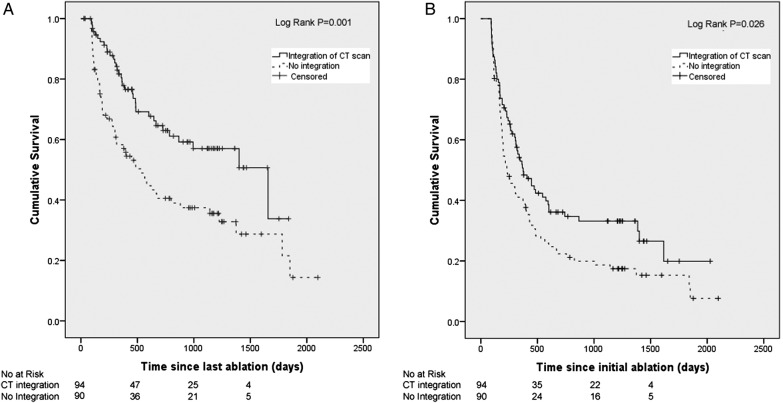
As shown in tables 1 and 2, integration of CT imaging into 3D mapping was the only significant predictor of outcome after multivariate modelling after both single and multiple procedure(s). We therefore undertook subgroup survival analysis grouping patients according to use of CT integration during their initial procedure. AF-free survival was significantly increased with CT integration after both initial (p=0.026) and final procedure (p=0.001) compared with patients whose ablation was performed without image integration, as shown in figure 4 (table 3).
Table 2.
Logistic regression analysis for recurrence of AF after a single procedure
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Variable | p Value | OR (95% CI) | p Value | OR (95% CI) |
| Age | 0.374 | 1.02 (0.98 to 1.05) | ||
| Female sex | 0.092 | 2.59 (0.86 to 7.84) | 0.063 | |
| Hypertension | 0.559 | 1.22 (0.62 to 2.39) | ||
| Body mass index | 0.671 | 0.98 (0.91 to 1.06) | ||
| Diabetes | 0.388 | 0.57 (0.16 to 2.04) | ||
| LA diameter (mm) | 0.564 | 1.02 (0.95 to 1.09) | ||
| Time since diagnosis* | 0.985 | 1.00 (0.99 to 1.01) | ||
| Current episode length* | 0.406 | 1.02 (0.98 to 1.06) | ||
| Long-standing PeAF | 0.776 | 0.88 (0.37 to 2.10) | ||
| General anaesthesia | 0.195 | 2.72 (0.60 to 12.37) | ||
| 3D mapping | 0.989 | 0.99 (0.30 to 3.24) | ||
| CT integration | 0.008 | 0.39 (0.19 to 0.78) | 0.003 | 0.33 (0.16 to 0.69) |
| WACA | 0.349 | 0.72 (0.36 to 1.44) | ||
| Linear ablation | 0.775 | 1.10 (0.56 to 2.19) | ||
| CFAE ablation | 0.192 | 1.64 (0.78 to 3.44) | ||
| AF as initial rhythm | 0.037 | 2.05 (1.05 to 4.03) | 0.009 | 2.59 (1.27 to 5.31) |
| Ablate to SR† | 0.163 | 0.52 (0.20 to 1.31) | ||
*Time in months.
†Compared with those cardioverted to SR.
3D, three-dimensional; AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; LA, left atrial; PeAF, persistent AF; SR, sinus rhythm; WACA, wide area circumferential ablation.
Figure 4.
(A) Kaplan-Meier curve showing AF-free survival after a single procedure for patients grouped according to use of CT integration. (B) Kaplan-Meier curve showing AF-free survival after the final procedure for patients grouped according to use of CT integration. AF, atrial fibrillation.
Table 3.
Logistic regression analysis for recurrence of AF after multiple (mean 1.75) procedures
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| p Value | OR (95% CI) | p Value | OR (95% CI) |
|||
| Age | 0.006 | 1.05 (1.01 to 1.08) | 0.018 | 1.05 (1.01 to 1.09) | ||
| Female sex | 0.080 | 2.03 (0.92 to 4.46) | 0.424 | |||
| Hypertension | 0.434 | 1.26 (0.71 to 2.25) | ||||
| BMI | 0.645 | 0.98 (0.92 to 1.05) | ||||
| Diabetes | 0.479 | 0.63 (0.18 to 2.24) | ||||
| LA diameter (mm) | 0.800 | 1.01 (0.95 to 1.06) | ||||
| Time since diagnosis* | 0.071 | 1.01 (1.00 to 1.01) | 0.211 | |||
| Current episode length* | 0.900 | 1.00 (0.98 to 1.02) | ||||
| Long-standing PeAF | 0.353 | 0.68 (0.30 to 1.53) | ||||
| General anaesthesia | 0.594 | 1.31 (0.48 to 3.56) | ||||
| 3D mapping | 0.801 | 0.88 (0.31 to 2.44) | ||||
| CT integration | <0.001 | 0.33 (0.18 to 0.61) | 0.001 | 0.30 (0.15 to 0.60) | ||
| WACA | 0.018 | 0.48 (0.27 to 0.88) | 0.370 | |||
| Linear ablation | 0.202 | 0.68 (0.37 to 1.23) | ||||
| CFAE ablation | 0.755 | 1.10 (0.60 to 2.03) | ||||
| AF as initial rhythm | 0.938 | 0.98 (0.54 to 1.76) | ||||
| Ablate to SR† | 0.138 | 0.51 (0.21 to 1.24) | ||||
| Time to first recurrence‡ | 0.767 | 1.00 (1.00 to 1.00) | ||||
*Time in months.
†Compared with those cardioverted to sinus.
‡For those patients who had a recurrence of AF after their first procedure.
3D, three-dimensional; AF, atrial fibrillation; BMI, body mass index; CFAE, complex fractionated atrial electrogram; LA, left atrial; PeAF, persistent AF; SR, sinus rhythm; WACA, wide area circumferential ablation.
Quality of life after CA of PeAF
As a surrogate for formal quality of life (QoL) measurement, patients' health status was assessed by the independent investigators for each follow-up clinic visit. We also asked patients to rate their own health state at the final telephone follow-up. Overall, 82% of patients felt better in terms of their arrhythmia with 62% of patients having considerable clinical improvement or arrhythmia cure and a further 20% gaining at least some improvement. These proportions did not differ between those cases adjudicated by the investigators and those reported directly by the patient. Although three-quarters of patients with ongoing episodes of AF still gained a benefit in terms of their health status, patients who remained free from recurrent AF were significantly more likely to gain symptomatic improvement (75% vs 93%, p<0.001).
Discussion
There are a number of conclusions to be drawn from the data presented. First, CA of PeAF is safe with a low rate of complications. Our 2.1% incidence of major complications, with no thromboembolic events, compares favourably with that reported in worldwide registries and other studies.5 13–16 Second, recurrence after the initial CA is the norm rather than the exception. Although 2 years' follow-up is sufficient to observe ∼90% of AF recurrence, recurrences can occur even after 5 years of remission. After multiple procedures (in our cohort, the mean number was 1.75, which is lower than that reported in many other series),16 over half of the patients can be rendered free of AF. Importantly, although many patients continue to experience episodes of AF, the vast majority gain clear symptomatic benefit, especially if persisting SR is achieved. Finally, there are few predictors of successful outcome. In our series, although being in AF was associated with a higher recurrence risk after the initial procedure and increased age predicted poorer long-term success, only integration of CT imaging into 3D mapping predicted both single and multiple procedure success.
Few other groups have reported long-term CA outcomes for large series of patients undergoing CA for PeAF. The largest reported study contained 676 patients with non-PAF.17 Success rates of 67% after a single procedure and 84% after multiple procedures were reported. However, in contrast to our study, they appear to have found very few recurrences occurring between 24 and 84 months after either single or multiple procedures, and none later than 32 months. Intensity of follow-up after the initial 12 months was unclear. Another leading European centre reported slightly lower single (20%) and multiple procedure (45%) success rates compared with our study, but that was in a population with long-standing PeAF over a slightly longer follow-up period (56 months).18 They found that only total AF duration, which was significant on univariate but not multivariate analysis in our study, predicted freedom from AF.
Other studies have generally been of short duration and/or contained small numbers of patients. In the largest previously published study from the UK, Hunter and colleagues reported on 125 patients with PeAF as part of a larger mixed cohort. Single and multiprocedure success rates for PeAF off antiarrhythmic drugs were 20% and 60%, respectively, over a follow-up period of 2.7 years.16 An important study by Bertaglia and colleagues highlighted the importance of long-term follow-up in this group of patients. In patients who had already remained in SR for 12 months after their initial CA, they found an actuarial recurrence rate of 55% at 6 years.9 A recent meta-analysis of long-term CA outcomes for PeAF has reported 42% (95% CI, 25% to 61%) success after a single procedure and 78% (95% CI 69% to 85%) after multiple procedures but with substantial heterogeneity between studies.19
One of the most striking findings from our study is the value of CT integration into a 3D mapping system in predicting procedural success. Since its introduction, there has been much interest in image integration due to the potential benefits of accurate anatomical visualisation, particularly of anomalous PV arrangements, leading to improved ablation delivery.20 A few small RCTs and observational studies yielded mixed results.21–26 A detailed review of these studies highlights several important limitations. In two of the RCTs in which no difference in outcome was found, CT imaging was performed in all patients and was available for review by operators, thereby negating part of the benefit of CT integration in recognising variant anatomy.22 24 A meta-analysis showed a non-significant reduction in risk of AF recurrence with image integration (RR=0.76, 95% CI 0.55 to 1.04, p=0.09).27 Crucially, the five studies included in the meta-analysis had follow-up durations of only 6–12 months. The only previous study with longer follow-up (283 patients with a median 37 months follow-up), which was not included in the meta-analysis, showed a significant improvement in single procedure success with CT integration compared with 3D mapping alone (p=0.018), in keeping with our findings.26
The use of implantable loop recorders to monitor for AF recurrence appears an attractive option, particularly to help guide decisions about long-term anticoagulation, etc. However, their use is not without cost and limitations. This study shows that AF can still recur up to 5 years after a successful ablation, a time period that exceeds the battery life of currently available monitors. In addition, they cannot protect against asymptomatic AF that may recur between device follow-up appointments. While their routine use cannot currently be justified by available evidence, use in selected patients may be of benefit.
Implications for clinical practice
The term curative AF ablation is often used but, from our results, would appear to be misleading for PeAF. Patients must be aware that recurrence is the norm rather than the exception. Operators may consider using 3D mapping techniques that combine integration of CT images, as our results suggest that this has a significant impact on long-term success. That notwithstanding, the indication for CA is the relief of AF symptoms rather than freedom from recurrent AF and our results show that this is achievable for the majority of patients. Although previously discouraged as a primary end point for clinical trials,28 formal assessment of QoL will help us to better inform patients and make more meaningful cost-effectiveness analyses than those based purely on arrhythmic outcome.
Limitations
Since the study was observational, follow-up and management were decided on clinical grounds by the responsible physician. As a result, there was some inevitable variation in the intensity of follow-up and monitoring and treatment strategies employed. ECGs were performed for all patients attending follow-up clinics, but more intense monitoring tended to be dependent on symptoms, and therefore it is possible that some patients with asymptomatic paroxysms of AF were missed. However, the long duration of follow-up and broad definition of recurrence go some way towards mitigating this risk. Our final follow-up was by telephone, which may have reduced the accuracy of reports of recurrence. However, by asking if patients had had an episode of AF confirmed by a doctor, we attempted to achieve a similar degree of diagnostic certainty as required at other follow-up points. We based our QoL assessment on patients' reported clinical state rather than a formal questionnaire that would have provided more objective information. It cannot be assumed that the results would have been the same with an anonymised interview/questionnaire. While other long-term studies have employed similar techniques, it is clearly suboptimal.16 We have since changed our practice to collect validated generic and AF-specific QoL data from all patients at each clinic visit. Finally, the CA procedures in our study predated the advent of contact force sensing technology that may result in more durable lesions and thus higher success rates for AF ablation than those found in our study. However, this is an inevitable limitation while reporting long-term results of a rapidly evolving procedure such as CA for AF.
Conclusion
CA of PeAF is safe, with a low incidence of major complications. During long-term follow-up, recurrence of AF is common, particularly after a single procedure, but considerable improvements in patient well-being can be achieved, especially in those who remain free of recurrent AF. Use of 3D mapping with CT image integration is associated with improved procedural success rates.
Footnotes
Competing interests: None declared.
Ethics approval: NRES Committee North West–Greater Manchester West.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Any requests for data sharing should be made to the corresponding author.
References
- 1.Stewart S, Hart CL, Hole DJ et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–21. 10.1136/heart.86.5.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De CR et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 3.Parkash R, Tang AS, Sapp JL et al. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J Cardiovasc Electrophysiol 2011;22:729–38. 10.1111/j.1540-8167.2011.02010.x [DOI] [PubMed] [Google Scholar]
- 4.Chen HS, Wen JM, Wu SN et al. Catheter ablation for paroxysmal and persistent atrial fibrillation. Cochrane Database Syst Rev 2012;(4):CD007101 10.1002/14651858.CD007101.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappato R, Calkins H, Chen SA et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. 10.1161/CIRCEP.109.859116 [DOI] [PubMed] [Google Scholar]
- 6.Mont L, Bisbal F, Hernandez-Madrid A et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter RJ, Berriman TJ, Diab I et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–8. 10.1161/CIRCEP.113.000806 [DOI] [PubMed] [Google Scholar]
- 8.Wynn GJ, Das M, Bonnett LJ et al. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomised and non-randomised controlled trials. Circ Arrhythm Electrophysiol 2014;7:841–52. 10.1161/CIRCEP.114.001759 [DOI] [PubMed] [Google Scholar]
- 9.Bertaglia E, Tondo C, De SA et al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace 2010;12:181–7. 10.1093/europace/eup349 [DOI] [PubMed] [Google Scholar]
- 10.Fuster V, Rydén LE, Cannom DS et al. , American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006;8:651–745. 10.1093/europace/eul097 [DOI] [PubMed] [Google Scholar]
- 11.Calkins H, Kuck KH, Cappato R et al. , Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632–96. 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 12.Peduzzi P, Concato J, Kemper E et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 13.Cappato R, Calkins H, Chen SA et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100–5. 10.1161/01.CIR.0000157153.30978.67 [DOI] [PubMed] [Google Scholar]
- 14.Gunawardena R, Furniss SF, Shepherd E et al. Outcomes following catheter ablation of atrial fibrillation in the UK—a single-centre cohort analysis. Br J Cardiol 2010;17:271–6. [Google Scholar]
- 15.Hunter RJ, Schilling RJ. Long-term outcome after catheter ablation for atrial fibrillation: safety, efficacy and impact on prognosis. Heart 2010;96:1259–63. 10.1136/hrt.2010.194613 [DOI] [PubMed] [Google Scholar]
- 16.Hunter RJ, Berriman TJ, Diab I et al. Long-term efficacy of catheter ablation for atrial fibrillation: impact of additional targeting of fractionated electrograms. Heart 2010;96:1372–8. 10.1136/hrt.2009.188128 [DOI] [PubMed] [Google Scholar]
- 17.Bhargava M, Di Biase L, Mohanty P et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009;6:1403–12. 10.1016/j.hrthm.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 18.Tilz RR, Rillig A, Thum AM et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 2012;60: 1921–9. 10.1016/j.jacc.2012.04.060 [DOI] [PubMed] [Google Scholar]
- 19.Ganesan AN, Shipp NJ, Brooks AG et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549 10.1161/JAHA.112.004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLellan AJ, Ling LH, Ruggiero D et al. Pulmonary vein isolation: the impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm 2014;11:549–56. 10.1016/j.hrthm.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 21.Martinek M, Nesser HJ, Aichinger J et al. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol 2007;30:1215–23. 10.1111/j.1540-8159.2007.00843.x [DOI] [PubMed] [Google Scholar]
- 22.Tang K, Ma J, Zhang S et al. A randomized prospective comparison of CartoMerge and CartoXP to guide circumferential pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Chin Med J 2008;121:508–12. [PubMed] [Google Scholar]
- 23.Bertaglia E, Bella PD, Tondo C et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 2009;11:1004–10. 10.1093/europace/eup152 [DOI] [PubMed] [Google Scholar]
- 24.Kistler PM, Rajappan K, Jahngir M et al. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006;17:1093–101. 10.1111/j.1540-8167.2006.00594.x [DOI] [PubMed] [Google Scholar]
- 25.Caponi D, Corleto A, Scaglione M et al. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome? A randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 2010;12:1098–104. 10.1093/europace/euq107 [DOI] [PubMed] [Google Scholar]
- 26.Hunter RJ, Ginks M, Ang R et al. Impact of variant pulmonary vein anatomy and image integration on long-term outcome after catheter ablation for atrial fibrillation. Europace 2010;12:1691–7. 10.1093/europace/euq322 [DOI] [PubMed] [Google Scholar]
- 27.Liu SX, Zhang Y, Zhang XW. Impact of image integration on catheter ablation for atrial fibrillation using three-dimensional electroanatomic mapping: a meta-analysis. Pacing Clin Electrophysiol 2012;35:1242–7. 10.1111/j.1540-8159.2012.03492.x [DOI] [PubMed] [Google Scholar]
- 28.Kirchhof P, Auricchio A, Bax J et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J 2007;28:2803–17. 10.1093/eurheartj/ehm358 [DOI] [PubMed] [Google Scholar]