Abstract
Background
The treatment options for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii (MDR Acb) complex are limited, and the optimal treatment has not been established.
Methods
To compare the efficacy of tigecycline-based with sulbactam (or ampicillin/sulbactam)-based therapy for pneumonia involving MDR Acb complex, we conducted a retrospective study comparing 84 tigecycline-treated adult patients during the period August 2007 to March 2010 with 84 sulbactam or ampicillin/sulbactam-treated adult patients during the period September 2004 to July 2007. Both groups had the matched Acute Physiology and Chronic Health Evaluation (APACHE) II score and received treatment for at least 7 days.
Results
The mean APACHE II score was 20.1 for both groups. More patients in sulbactam group had ventilator use (89.3 % versus 69.0 %), bilateral pneumonia (79.8 % versus 60.7 %) and combination therapy (84.5 % versus 53.6 %), particularly with carbapenems (71.4 % versus 6.0 %), while more patients in tigecycline group had delayed treatment (41.7 % versus 26.2 %) (P <0.05). At the end of treatment, more patients in sulbactam group had airway MDR Acb complex eradication (63.5 % versus 33.3 %, P <0.05). The clinical resolution rate was 66.7 % for both groups. The mortality rate during treatment was 17.9 % in sulbactam group, and 25.0 % in tigecycline group (P = 0.259). The multivariate analysis showed that bilateral pneumonia was the only independent predictor for mortality during treatment (adjusted odds ratio, 2.717; 95 % confidence interval, 1.015 to 7.272).
Conclusions
Patients treated with either tigecycline-based or sulbactam-based therapy had a similar clinical outcome, but tigecycline group had a lower microbiological eradiation rate.
Background
Pneumonia involving multidrug-resistant (MDR) Acinetobacter calcoaceticus-Acinetobacter baumannii (Acb) complex usually occurs in critically ill patients and is associated with unfavorable outcomes [1–3]. For MDR Acb complex resistant to most currently available antibiotics, including β-lactams, fluoroquinolones, and aminoglycosides, there are only a few treatment options, such as tigecycline, sulbactam, and colistin [4, 5].
Tigecycline is a glycylcycline with in vitro activity against MDR Acb complex [6]. The comparison analysis from the U.S. Food and Drug Administration showed that tigecycline treatment had a higher mortality rate than other antimicrobials in ventilator associated pneumonia (VAP) [7]. A recent study also reported a significantly lower cure rate in clinically evaluable patients with VAP treated with tigecycline when compared to imipenem (47.9 % versus 70.1 %) [8]. However, for pneumonia caused by MDR Acb complex resistant to carbapenems and other classes of antibiotics, off label use of tigecycline was common in clinical practice, and the clinical response rates ranged from 60 to 88 % in prior studies [9–11]. Sulbactam is a β-lactamase inhibitor with antimicrobial activity against Acinetobacter species [12]. It is available alone or in combination with ampicillin, and ampicillin doesn’t contribute activity or synergism against A. baumannii [12]. Sulbactam or ampicillin/sulbactam had clinical response rates ranging from 67 to 75 % for pneumonia involving MDR A. baumannii (MDRAB) or MDR Acb complex in prior studies [13–15].
In our hospital, tigecycline was not available until August 2007. Before that, sulbactam or ampicillin/sulbactam might be the only treatment option with in vitro activity against MDR Acb complex. Thus, we conducted a retrospective study to compare the efficacy of tigecycline-based with sulbactam (or ampicillin/sulbactam)-based treatment for pneumonia involving MDR Acb complex. With a match in the Acute Physiology and Chronic Health Evaluation (APACHE) II score for both groups, a comparison was made between tigecycline-treated adult patients during the period August 2007 to March 2010 and sulbactam (or ampicillin/sulbactam)-treated adult patients during the period September 2004 to July 2007. The clinical efficacy, outcomes and microbiological eradication were included for analyses.
Methods
Setting
Chang Gung Memorial Hospital (CGMH)-Linkou is a university-affiliated medical center providing both primary and tertiary health care in northern Taiwan. This retrospective study has been approved by institutional review boards of CGMH- Linkou (Number: 99-1478B and 100-0294B). The ethics committee granted a waiver for informed consent to be obtained.
Study design, patients and treatments
All hospitalized patients who were ≧ 18 years old and had pneumonia involving MDR Acb complex treated with tigecycline between August 2007 and March 2010, and sulbactam or ampicillin/sulbactam between September 2004 and July 2007, were reviewed. Each tigecycline-treated patient was matched to one sulbactam or ampicillin/sulbactam-treated patient based on identical values of APACHE II score and chart number sequence. Patients were excluded if they did not have a matched control or had a combination therapy with tigecycline and sulbactam (or ampicillin/sulbactam). Patients with initial bacteremia were also excluded since tigecycline treatment for bacteremia was controversial.
Pneumonia was diagnosed if the patient had a radiographic infiltrate that was new or progressive, along with at least two of the following clinical characteristics: new onset of fever (≧ 38 °C) or hypothermia (< 35.5 °C), leucocytosis (leucocyte count > 12000 cells/mm3) or leucopenia (leucocyte count < 4000 cells/mm3), decline in oxygenation (O2 saturation < 90 %), and increasing amount of purulent sputum [16]. Pneumonia involving MDR Acb complex was defined as clinical evidence of pneumonia with sputum or tracheal aspirate cultures positive for MDR Acb complex from 1 week before to 3 days after the first dose of tigecycline or sulbactam or ampicillin/sulbactam. Tracheal aspirate and sputum specimens were sent for bacterial culture only if their Gram’s stains showed at least 25 neutrophils and less than 10 epithelial cells per low-power field. Growth was assessed semi-quantitatively. The etiologic pathogen of pneumonia was determined if the tracheal aspirate or sputum culture had an at least moderate growth, i.e., the growth confined up to primary streaking line and > 5 colonies in secondary streaking zone [17]. Polymicrobial pneumonia was defined as one or more additional etiologic bacterial species concurrently isolated from the respiratory tract during treatment.
All patients in tigecycline group received tigecycline for at least 7 days, with a 100-mg loading dose followed by 50 mg administered intravenously every 12 h. All patients in sulbactam group received intravenous sulbactam 1 g or ampicillin/sulbactam 3 g (at a ratio 2:1) every 6 or 8 h for at least 7 days. Dose and dosing interval were adjusted according to serum creatinine levels. Combination therapy was defined as simultaneous use of another class of antibiotics for at least 3 days. These antibiotics included carbapenems (meropenem or imipenem), fluoroquinolones (ciprofloxacin or levofloxacin), amikacin, cephalosporins (ceftazidime or cefepime), piperacillin, piperacillin-tazobactam, colistimethate, and aztreonam. Delayed treatment was defined as more than 3 days between the detection of airway MDR Acb complex isolates and the first dose of tigecycline or sulbactam or ampicillin/sulbactam.
Microbiology
Identification of Acb complex depended upon Gram staining and conventional biochemical tests [18]. Briefly, the isolates were identified as species of the genus Acinetobacter based on the following properties: aerobic, Gram-negative, nonmotile coccobacillary rods with a nonfermentative, catalase-positive and oxidase-negative reaction. Acinetobacter species with glucose-oxidizing non-haemolytic characteristics were classified as Acb complex. Antimicrobial susceptibility was determined and interpreted according to the criteria of Clinical and Laboratory Standards Institute by disk diffusion method [19]. Susceptibility to tigecycline was determined using disk diffusion method with Mueller-Hinton agar (BD Microbiology Systems, Cockeysville, MD) with the resistant breakpoint at ≧ 16 mm and susceptible breakpoint at ≦ 12 mm [20]. An isolate with full or intermediate resistance to amikacin, gentamicin, cefepime, ceftazidime, aztreonam, piperacillin, piperacillin-tazobactam, ciprofloxacin, imipenem and meropenem was defined as MDR Acb complex [21].
Cultures were collected from 1 week before the first dose of tigecycline or sulbactam (or ampicillin/sulbactam) to the discharge of patients. Pathogens, sites of growth and susceptibility testing were recorded. Microbial eradication of MDR Acb complex was defined as no growth of Acb complex or susceptibility change from MDR strains to susceptible strains in Acb complex in follow-up respiratory tract cultures before and 7 days after treatment cessation. Relapse was defined as new isolation of MDR Acb complex from the respiratory tract cultures within 2 weeks after initial eradication. Initial bacteremia was defined as bacteremia at the beginning of treatment, which meant at least one positive blood culture 1 week before to 3 days after the first dose of tigecycline or sulbactam or ampicillin/sulbactam. Bacteremia during treatment was defined as at least one positive blood culture 3 days after to the end of treatment.
Demography and comorbidity
Data on age, sex, surgery, and co-morbid illness were gathered by reviewing in-patient medical records. Co-morbid illness included hepatic dysfunction of a serum total bilirubin level over 2.5 mg/dL or liver cirrhosis, renal insufficiency of a serum creatinine level above 2.0 mg/dL or requirement of dialysis, chronic pulmonary disease, heart disease, diabetes mellitus, immune compromise, and hematological or solid organ malignancy. Immune compromise was defined by at least one of the following: use of prednisone or equivalent over 20 mg per day for at least 2 weeks, organ transplant recipient, human immunodeficiency virus infection or acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count less than 500 cells/mm3), use of immunosuppressive agents, and concurrent hematological malignancy.
Clinical conditions and outcomes
Ventilator use, vital signs, and infections other than pneumonia during treatment were recorded. Defervescence was defined as normal body temperature for at least 3 days at the end of treatment. Severity of illness was assessed by a modified APACHE II score, which was recorded within 48 h before or after the first dose of tigecycline or sulbactam or ampicillin/sulbactam. The 30-day mortality was defined as death occurring within 30 days after treatment. The chest radiographs were evaluated by at least two investigators. A series of chest radiographs were evaluated during treatment. Clinical resolution of pneumonia at the end of treatment was defined as (1) decreased pulmonary infiltrate, and (2) survival with stationary findings on chest radiographs and defervescence. Thus, patients with persistent fever or death during treatment would be defined as clinical failure if infiltrates were stationary. Progressing infiltrates were defined as clinical failure.
Statistical methods
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (Version 15.0; SPSS Inc., Chicago, IL, USA). Categorical variables were compared using χ2 test or Fisher exact test, as appropriate. Continuous variables were tested for normality of distributions by Kolmogorov–Smirnov test, and then compared by Student’s t-test or the Mann-Whitney U test, as appropriate. Odds ratios (ORs) and 95 % confidence interval (CI) were calculated. The survival curve was plotted by means of the Kaplan-Meier method, and the log rank test was used to compare univariate survival distribution between tigecycline and sulbactam groups. Variables with a P value < 0.1 in univariate analysis and tigecycline use were included in a logistic regression model for multivariate analysis. All tests were two-tailed, and a P value of < 0.05 was considered significant.
Results
Patients, demography and concomitant diseases
One hundred and sixteen tigecycline-treated episodes of pneumonia involving MDR Acb complex were identified in 112 patients, while 177 sulbactam or ampicllin/sulbactam-treated episodes were identified in 173 patients. Finally, 84 tigecycline-treated patients were enrolled and matched to 84 patients treated with sulbactam (26 patients) or ampicillin/sulbactam (58 patients). The mean APACHE II score was 20.1 for both groups. In tigecycline group, 59 (70.2 %) and 25 (29.8 %) patients had positive MDR Acb complex cultures from tracheal aspirates and sputum, respectively. In sulbactam group, 73 (86.9 %) and 11 (13.1 %) patients had that from tracheal aspirates and sputum, respectively. There was no significant difference in age, gender, and concomitant diseases between the two groups (Table 1).
Table 1.
The comparison analysis of demography, concomitant diseases, clinical features, and outcomes between tigecycline (TG) and sulbactam (SB) groups
| Variables | TG groupa | SB groupa | p | OR (95 % CI) |
|---|---|---|---|---|
| n = 84 | n = 84 | |||
| Demographic parameters | ||||
| Age, yr | 69.6 (15.9) | 70.6 (15.6) | 0.689 | |
| Male gender | 57 (67.9) | 58 (69.0) | 0.868 | 0.946 (0.494–1.814) |
| Concomitant diseases | ||||
| Hepatic dysfunction | 12 (14.3) | 6 (7.1) | 0.134 | 2.167 (0.773–6.075) |
| Renal insufficiency | 32 (38.1) | 32 (38.1) | 1.000 | 1.000 (0.536–1.864) |
| Chronic pulmonary disease | 22 (26.2) | 20 (23.8) | 0.722 | 1.135 (0.564–2.284) |
| Heart disease | 13 (15.5) | 7 (8.3) | 0.153 | 2.014 (0.761–5.333) |
| Diabetes mellitus | 26 (31.0) | 35 (41.7) | 0.149 | 0.628 (0.333–1.183) |
| Immune compromise | 13 (15.5) | 11 (13.1) | 0.659 | 1.215 (0.511–2.891) |
| Malignancy | 15 (17.9) | 20 (23.8) | 0.342 | 0.696 (0.328–1.474) |
| Surgery | 22 (26.2) | 15 (17.9) | 0.193 | 1.632 (0.778–3.423) |
| Clinical conditions | ||||
| APACHE II Score | 20.1 (6.1) | 20.1 (6.1) | 1.000 | |
| Ventilator use | 58 (69.0) | 75 (89.3) | 0.001 | 0.268 (0.117–0.615) |
| Pneumonia involving bilateral lung | 51 (60.7) | 67 (79.8) | 0.007 | 0.392 (0.197–0.781) |
| Polymicrobial pneumonia, overall | 66 (78.6) | 62 (73.8) | 0.469 | 1.301 (0.638–2.654) |
| Polymicrobial pneumonia, coinfection with | ||||
| MRSA | 26 (31.0) | 34 (40.5) | 0.198 | 0.659 (0.349–1.245) |
| Pseudomonas aeruginosa | 33 (39.3) | 23 (27.4) | 0.102 | 1.716 (0.896–3.285) |
| Klebsiella spp. b | 12 (14.3) | 5 (6.0) | 0.073 | 2.633 (0.884–7.840) |
| Escherichia coli | 2 (2.4) | 2 (2.4) | 1.000 | 1.000 (0.138–7.270) |
| Enterobacter spp. c | 2 (2.4) | 2 (2.4) | 1.000 | 1.000 (0.138–7.270) |
| Serratia marcescens | 10 (11.9) | 1 (1.2) | 0.005 | 11.216 (1.402–89.724) |
| Stenotrophomonas maltophilia | 6 (7.1) | 15 (17.9) | 0.036 | 0.354 (0.130–0.962) |
| Multisite infections, overall | 33 (39.3) | 35 (41.7) | 0.753 | 0.906 (0.489–1.678) |
| With urinary tract infection | 13 (15.5) | 19 (22.6) | 0.238 | 0.626 (0.287–1.369) |
| With catheter related infection | 2 (2.4) | 10 (11.9) | 0.017 | 0.180 (0.038–0.851) |
| With soft tissue and wound infection | 10 (11.9) | 5 (6.0) | 0.176 | 2.135 (0.697–6.540) |
| With intra-abdominal infection | 8 (9.5) | 4 (4.8) | 0.231 | 2.105 (0.609–7.279) |
| With invasive fungal infectiond | 12 (14.3) | 4 (4.8) | 0.035 | 3.333 (1.029–10.799) |
| Bacteremia during treatment | 4 (4.8) | 0 (0.0) | 0.121 | 9.447 (0.501–178.291) |
| With TG or SB-resistant MDR Acb complexe | 16 (19.0) | 43 (51.2) | < 0.0001 | 0.224 (0.112–0.448) |
| Treatment | ||||
| Duration, days | 14.6 (5.4) | 16.4 (7.6) | 0.150 | |
| Combination therapy, overall | 45 (53.6) | 71 (84.5) | < 0.0001 | 0.211 (0.102–0.439) |
| With cephalosporins | 20 (23.8) | 8 (9.5) | 0.013 | 2.969 (1.226–7.192) |
| With colistin | 12 (14.3) | 0 (0.0) | < 0.0001 | 29.138 (1.695–500.773) |
| With carbapenems | 5 (6.0) | 60 (71.4) | < 0.0001 | 0.025 (0.009–0.070) |
| With aminoglycosides | 7 (8.3) | 1 (1.2) | 0.064 | 7.545 (0.907–62.744) |
| With fluoroquinolones | 12 (14.3) | 4 (4.8) | 0.035 | 3.333 (1.029–10.799) |
| Delayed treatment | 35 (41.7) | 22 (26.2) | 0.034 | 2.013 (1.049–3.863) |
| Outcomes | ||||
| Airway eradication of MDR Acb complex without relapsef | 26 (33.3) | 47 (63.5) | < 0.0001 | 0.287 (0.147–0.560) |
| Defervescence | 54 (64.3) | 76 (90.5) | < 0.0001 | 0.189 (0.081–0.445) |
| Image study of lung | ||||
| Improvement | 37 (44.0) | 39 (46.4) | 0.757 | 0.908 (0.495–1.668) |
| Stationary | 32 (38.1) | 22 (26.2) | 0.099 | 1.734 (0.900–3.342) |
| Deterioration | 15 (17.9) | 23 (27.4) | 0.140 | 0.577 (0.276–1.204) |
| Clinical resolution of pneumonia | 56 (66.7) | 56 (66.7) | 1.000 | 1.000 (0.526–1.899) |
| Mortality during treatment | 21 (25.0) | 15 (17.9) | 0.259 | 1.533 (0.728–3.231) |
| 30-day mortality | 28 (33.3) | 25 (29.8) | 0.618 | 1.180 (0.615–2.264) |
Abbreviations: TG tigecycline, SB sulbactam, OR odd ratio, CI confidence interval, APACHE acute physiology and chronic health evaluation, MRSA methicillin resistant Staphylococcus aureus, MDR Acb multidrug resistant Acinetobacter calcoaceticus-Acinetobacter baumannii, ESBL extended-spectrum beta-lactamase
aCategorical data are no. (%) of subject, continuous data are expressed as mean (standard deviation)
b16 patients had coinfection with Klebsiella pneumoniae, including 10 with ESBL strains, and 1 had Klebsiella oxytoca-ESBL
c3 patients had coinfection with Enterobacter cloacae, and 1 had Enterobacter aerogenes
d15 patients had candidemia, and 1 had possible invasive aspergillosis diagnosed with positive serum galactomannan
eWith TG-resistant MDR Acb complex during TG treatment in TG group, or with SB-resistant MDR Acb commplex during SB treatment in SB group
f78 patients in TG group and 74 in SB group had available data for evaluation
Clinical conditions
Patients in sulbactam group had more ventilator use (89.3 % versus 69.0 %) and bilateral pneumonia (79.8 % versus 60.7 %) than those in tigecycline group. There were no significant differences between these two groups in the overall rates of polymicrobial pneumonia and multisite infections. Pseudomonas aeruginosa and Methicillin-resistant Staphylococcus aureus were the most common concurrent pathogens for pneumonia, and urinary tract infection was the most common concurrent infection. However, more patients had Serratia marcescnes coinfection and invasive fungal infection in tigecycline group, and more patients had Stenotrophomonas maltophilia coinfection and catheter related infection in sulbactam group. Among the 168 enrolled patients, bacteremia during treatment was observed in four patients, and all of them were from tigecycline group (P = 0.121). During treatment, tigecycline-resistant MDR Acb complex was isolated in 16 (19.0 %) tigecycline-treated patients, and sulbactam-resistant MDR Acb complex was isolated in 43 (51.2 %) sulbactam or ampicllin/sulbactam-treated patients (19.0 % versus 51.2 %, P < 0.0001) (Table 1). In tigecycline group, 71 patients (84.5 %) had airway MDR Acb complex isolates with full or intermediate resistance to sulbactam. Tigecycline susceptibility testing was not performed in sulbactam group.
Treatment
The mean treatment duration was 14.6 and 16.4 days for tigecycline and sulbactam group, respectively. Compared to the tigecycline group, more patients in sulbactam group had combination therapy (84.5 % versus 53.6 %), particularly with carbapenems (71.4 % versus 6.0 %). In sulbactam group, the most common co-administered agent were carbapenems (60/71, 84.5 %), followed by cephalosporins (8/71, 11.3 %), and 32 patients (38.1 %) had glycopeptides use. In tigecycline group, the most common co-administered agent was cephalosporins (20/45, 44.4 %), followed by colistin (12/45, 26.7 %) and fluoroquinolones (12/45, 26.7 %). Colistin was not available until May 2007 in our hospital, and co-use of colistin was only noted in tigecycline group. More patients in tigecycline group had delayed treatment (41.7 % versus 26.2 %) (Table 1).
Outcomes
Sulbactam group had a higher rate of airway MDR Acb complex eradication (63.5 % versus 33.3 %) and defervescence (90.5 % versus 64.3 %) than tigecycline group at the end of treatment. There was no significant difference between these two groups in the rates of clinical resolution, 30-day mortality and mortality during treatment (66.7 % versus 66.7 %; 33.3 % versus 29.8 %; 25.0 % versus 17.9 %, respectively) (Table 1). The cumulative survival rate at 30 days was similar between the two groups by Kaplan-Meier method (Fig. 1).
Fig. 1.
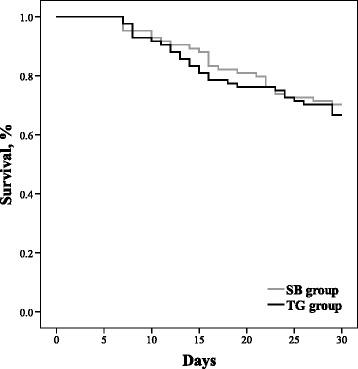
Comparative survival curves for tigecycline (black line) and sulbactam (gray line) groups; Log-rank test: p = 0.605. Abbreviations: SB sulbactam, TG tigecycline
The predictor for mortality during treatment
In the univariate analysis, the survivors were more likely to have tigecycline or sulbactam-resistant MDR Acb complex than the deceased (39.4 % versus 19.4 %) (Table 2). And most of the resistant isolates in survivors were from sulbactam group (39/52, 75 %). In the multivariate analysis including tigecycline-based treatment and variables with a P value < 0.1 in the univariate analysis, bilateral pneumonia was the only independent predictor for mortality during treatment (adjusted OR, 2.717; 95 % CI, 1.015 to 7.272) (Table 2). Other models of multivariate analysis including polymicrobial pneumonia, combination therapy, and combination with carbapenem or colistin also showed that bilateral pneumonia was the only independent predictor (Table 3).
Table 2.
Univariate and multivariate analyses of the predictors for mortality during treatment of tigecycline or sulbactam or ampicillin/sulbactam for pneumonia involving multidrug resistant Acinetobacter calcoaceticus-Acinetobacter baumannii (MDR Acb) complex
| Variables | Deceaseda | Survivorsa | Univariate | Multivariateb | |
|---|---|---|---|---|---|
| n = 36 | n = 132 | p | p | Adjusted OR (95 % CI) | |
| Demographic parameters | |||||
| Age, yr | 69.8 (14.7) | 70.2 (16.0) | 0.658 | ||
| Male gender | 26 (72.2) | 89 (67.4) | 0.583 | ||
| Concomitant diseases | |||||
| Hepatic dysfunction | 6 (16.7) | 12 (9.1) | 0.224 | ||
| Renal insufficiency | 15 (41.7) | 49 (37.1) | 0.619 | ||
| Chronic pulmonary disease | 6 (16.7) | 36 (27.3) | 0.193 | ||
| Heart disease | 6 (16.7) | 14 (10.6) | 0.383 | ||
| Diabetes mellitus | 14 (38.9) | 47 (35.6) | 0.717 | ||
| Immune compromise | 5 (13.9) | 19 (14.4) | 0.939 | ||
| Malignancy | 11 (30.6) | 24 (18.2) | 0.105 | ||
| Surgery | 11 (30.6) | 26 (19.7) | 0.163 | ||
| Clinical conditions | |||||
| APACHE II Score | 20.3 (7.3) | 20.1 (5.8) | 0.858 | ||
| Ventilator use | 26 (72.2) | 107 (81.1) | 0.247 | ||
| Bilateral pneumonia | 30 (83.3) | 88 (66.7) | 0.053 | 0.047 | 2.717 (1.015–7.272) |
| Polymicrobial pneumonia | 29 (80.6) | 99 (75.0) | 0.488 | ||
| With MRSA | 10 (27.8) | 50 (37.9) | 0.262 | ||
| With Pseudomonas aeruginosa | 14 (38.9) | 42 (31.8) | 0.425 | ||
| With Klebsiella spp. | 6 (16.7) | 11 (8.3) | 0.207 | ||
| With Serratia marcescens | 3 (8.3) | 8 (6.1) | 0.704 | ||
| With Stenotrophomanas maltophilia | 6 (16.7) | 15 (11.4) | 0.400 | ||
| Multisite infections | 18 (50.0) | 50 (37.9) | 0.189 | ||
| With urinary tract infection | 8 (22.2) | 24 (18.2) | 0.584 | ||
| With catheter related infection | 2 (5.6) | 10 (7.6) | 1.000 | ||
| With skin and soft tissue infection | 6 (16.7) | 9 (6.8) | 0.094 | 0.214 | 2.070 (0.657–6.521) |
| With intra-abdominal infection | 3 (8.3) | 9 (6.8) | 0.721 | ||
| With invasive fungal infection | 3 (8.3) | 13 (9.8) | 1.000 | ||
| Microbiology | |||||
| MDR Acb complex with TG or SB resistancec | 7 (19.4) | 52 (39.4) | 0.026 | 0.079 | 0.426 (0.164–1.103) |
| Airway eradication of MDR Acb complexd | 11 (42.3) | 62 (49.2) | 0.521 | ||
| Bacteremia during treatment | 2 (5.6) | 2 (1.5) | 0.201 | ||
| Treatment | |||||
| Tigecycline-based treatment | 21 (58.3) | 63 (47.7) | 0.259 | 0.451 | 1.371 (0.604–3.116) |
| Duration, days | 15.6 (7.9) | 15.5 (6.2) | 0.519 | ||
| Combination therapy | 25 (69.4) | 91 (68.9) | 0.954 | ||
| With cephalosporins | 6 (16.7) | 22 (16.7) | 1.000 | ||
| With carbapenems | 12 (33.3) | 53 (40.2) | 0.457 | ||
| With fluoroquinolones | 2 (5.6) | 14 (10.6) | 0.527 | ||
| With colistin | 3 (8.3) | 9 (6.8) | 0.721 | ||
| Delayed treatment | 9 (25.0) | 48 (36.4) | 0.202 | ||
Abbreviations: MDR Acb multidrug resistant Acinetobacter calcoaceticus-Acinetobacter baumannii, OR odd ratio, CI confidence interval, APACHE acute physiology and chronic health evaluation, MRSA methicillin resistant Staphylococcus aureus, TG tigecycline, SB sulbactam
aCategorical data are no. (%) of subject, continuous data are expressed as mean (standard deviation)
bAll variables included in the final multivariable model are shown
c The initial airway MDR Acb complex isolates with resistance to TG in TG group, or with resistance to SB in SB group
d26 patients in the deceased group and 126 in the survivors group had available data for evaluation
Table 3.
Multivariate analyses of the predictors for mortality during treatment including combination therapy, carbapenems or colistin use, and polymicrobial pneumonia
| Variables | Odds ratio | 95 % confidence interval | p |
|---|---|---|---|
| Model Aa | |||
| With skin and soft tissue infection | 2.041 | 0.644–6.466 | 0.225 |
| MDR Acb complex with TG or SB resistance | 0.418 | 0.160–1.092 | 0.075 |
| Bilateral pneumonia | 2.663 | 0.987–7.186 | 0.053 |
| Tigecycline-based treatment | 1.405 | 0.608–3.245 | 0.426 |
| Combination therapy | 1.133 | 0.472–2.720 | 0.779 |
| Model Bb | |||
| With skin and soft tissue infection | 2.071 | 0.657–6.523 | 0.214 |
| MDR Acb complex with TG or SB resistance | 0.426 | 0.163–1.114 | 0.082 |
| Bilateral pneumonia | 2.717 | 1.007–7.329 | 0.048 |
| Tigecycline-based treatment | 1.373 | 0.497–3.795 | 0.541 |
| Combination with carbapenem | 1.002 | 0.346–2.905 | 0.997 |
| Model Cc | |||
| With skin and soft tissue infection | 2.002 | 0.624–6.425 | 0.243 |
| MDR Acb complex with TG or SB resistance | 0.420 | 0.161–1.090 | 0.075 |
| Bilateral pneumonia | 2.795 | 1.028–7.600 | 0.044 |
| Tigecycline-based treatment | 1.430 | 0.608–3.363 | 0.413 |
| Combination with colistin | 0.783 | 0.178–3.444 | 0.746 |
| Model D | |||
| With skin and soft tissue infection | 2.035 | 0.639–6.485 | 0.230 |
| MDR Acb complex with TG or SB resistance | 0.428 | 0.165–1.111 | 0.081 |
| Bilateral pneumonia | 2.711 | 1.013–7.254 | 0.047 |
| Tigecycline-based treatment | 1.362 | 0.598–3.102 | 0.462 |
| Polymicrobial pneumonia | 1.109 | 0.426–2.884 | 0.833 |
| Model E | |||
| With skin and soft tissue infection | 1.979 | 0.612–6.405 | 0.254 |
| MDR Acb complex with TG or SB resistance | 0.422 | 0.160–1.110 | 0.080 |
| Bilateral pneumonia | 2.781 | 1.014–7.624 | 0.047 |
| Tigecycline-based treatment | 1.422 | 0.498–4.056 | 0.510 |
| Polymicrobial pneumonia | 1.086 | 0.413–2.853 | 0.868 |
| Combination with colistin | 0.797 | 0.179–3.557 | 0.767 |
| Combination with carbapenem | 1.006 | 0.346–2.921 | 0.991 |
Abbreviations: MDR Acb multidrug resistant Acinetobacter calcoaceticus-Acinetobacter baumannii, TG tigecycline, SB sulbactam
aNo significant predictor was revealed when model A included polymicrobial pneumonia
bBilateral pneumonia was the only significant predictor when model B included polymicrobial pneumonia (p = 0.049, adjusted odds ratio, 2.709; 95 % confidential interval, 1.004–7.305)
cBilateral pneumonia was the only significant predictor when model C included polymicrobial pneumonia (p = 0.045, adjusted odds ratio, 2.783; 95 % confidential interval, 1.023–7.569)
Monotherapy of tigecycline and sulbactam
Thirty-nine (46.4 %) patients had tigecycline monotherapy and 13 (15.5 %) had sulbactam or ampicillin/sulbactam monotherapy. Tigecycline group had significant lower rates of ventilator use, bilateral pneumonia, and airway eradication of MDR Acb complex. Both groups had similar clinical resolution rates. However, tigecycline group had lower rates of 30-day mortality and mortality during treatment (25.6 % versus 53.8 %, 17.9 % versus 30.8 %, P >0.05). In the univariate analysis for the patients with monotherapy, both the survivors and the deceased during treatment had no significant difference in demography, concomitant diseases, clinical conditions, and treatment (Table 4).
Table 4.
The comparison and outcome analyses of the patients with monotherapy of tigecycline or sulbactam
| Variables | TG groupa | SB groupa | p | Deceasedab | Survivorsa | p |
|---|---|---|---|---|---|---|
| n = 39 | n = 13 | n = 11 | n = 41 | |||
| Demographic parameters | ||||||
| Age, yr | 71.4 (15.0) | 68.7 (19.9) | 0.899 | 75.3 (12.0) | 69.5 (17.1) | 0.439 |
| Male gender | 25 (64.1) | 10 (76.9) | 0.506 | 8 (72.7) | 27 (65.9) | 1.000 |
| Concomitant diseases | ||||||
| Hepatic dysfunction | 3 (7.7) | 0 (0.0) | 0.564 | 1 (9.1) | 2 (4.9) | 0.518 |
| Renal insufficiency | 10 (25.6) | 4 (30.8) | 0.729 | 1 (9.1) | 13 (31.7) | 0.251 |
| Chronic pulmonary disease | 11 (28.2) | 4 (30.8) | 1.000 | 4 (36.4) | 11 (26.8) | 0.709 |
| Heart disease | 6 (15.4) | 1 (7.7) | 0.664 | 2 (18.2) | 5 (12.2) | 0.630 |
| Diabetes mellitus | 9 (23.1) | 4 (30.8) | 0.714 | 3 (27.3) | 10 (24.4) | 1.000 |
| Immune compromise | 8 (20.5) | 1 (7.7) | 0.420 | 2 (18.2) | 7 (17.1) | 1.000 |
| Malignancy | 8 (20.5) | 3 (23.1) | 1.000 | 4 (36.4) | 7 (17.1) | 0.216 |
| Surgery | 9 (23.1) | 1 (7.7) | 0.419 | 2 (18.2) | 8 (19.5) | 1.000 |
| Clinical conditions | ||||||
| APACHE II Score | 17.0 (6.1) | 18.2 (6.0) | 0.557 | 17.1 (6.8) | 17.3 (5.9) | 0.904 |
| Ventilator use | 21 (53.8) | 11 (84.6) | 0.048 | 6 (54.5) | 26 (63.4) | 0.730 |
| Bilateral pneumonia | 18 (46.2) | 12 (92.3) | 0.004 | 8 (72.7) | 22 (53.7) | 0.319 |
| Polymicrobial pneumonia | 31 (79.5) | 9 (69.2) | 0.466 | 9 (81.8) | 31 (75.6) | 1.000 |
| With MRSA | 20 (51.3) | 6 (46.2) | 0.749 | 5 (45.5) | 21 (51.2) | 0.734 |
| With Pseudomonas aeruginosa | 14 (35.9) | 3 (23.1) | 0.506 | 5 (45.5) | 12 (29.3) | 0.470 |
| With Klebsiella spp. | 7 (17.9) | 1 (7.7) | 0.662 | 1 (9.1) | 7 (17.1) | 1.000 |
| With Serratia marcescens | 5 (12.8) | 1 (7.7) | 1.000 | 1 (9.1) | 5 (12.2) | 1.000 |
| With Stenotrophomanas maltophilia | 3 (7.7) | 3 (23.1) | 0.157 | 1 (9.1) | 5 (12.2) | 1.000 |
| Multisite infections | 14 (35.9) | 5 (38.5) | 1.000 | 4 (36.4) | 15 (36.6) | 1.000 |
| With urinary tract infection | 7 (17.9) | 3 (23.1) | 0.697 | 2 (18.2) | 8 (19.5) | 1.000 |
| With catheter related infection | 0 (0.0) | 2 (15.4) | 0.059 | 1 (9.1) | 1 (2.4) | 0.382 |
| With skin and soft tissue infection | 3 (7.7) | 1 (7.7) | 1.000 | 1 (9.1) | 3 (7.3) | 1.000 |
| With intra-abdominal infection | 4 (10.3) | 0 (0.0) | 0.561 | 0 (0.0) | 4 (9.8) | 0.567 |
| With invasive fungal infection | 6 (15.4) | 0 (0.0) | 0.317 | 1 (9.1) | 5 (12.2) | 1.000 |
| Microbiology | ||||||
| MDR Acb complex with TG or SB resistancec | 6 (15.4) | 4 (30.8) | 0.244 | 1 (9.1) | 9 (22.0) | 0.668 |
| Airway eradication of MDR Acb complexd | 12 (34.3) | 8 (88.9) | 0.006 | 4 (57.1) | 16 (43.2) | 0.684 |
| Bacteremia during treatment | 2 (5.1) | 0 (0.0) | 1.000 | 1 (9.1) | 1 (2.4) | 0.382 |
| Treatment | ||||||
| Tigecycline-based treatment | 7 (63.6) | 32 (78.0) | 0.435 | |||
| Duration, days | 13.8 (5.1) | 12.7 (5.6) | 0.293 | 11.9 (3.0) | 14.0 (5.6) | 0.398 |
| Delayed treatment | 20 (51.3) | 5 (38.5) | 0.423 | 5 (45.5) | 20 (48.8) | 0.845 |
| Outcomes | ||||||
| Clinical resolution of pneumonia | 26 (66.7) | 8 (61.5) | 0.747 | |||
| Mortality during treatment | 7 (17.9) | 4 (30.8) | 0.435 | |||
| 30-day mortality | 10 (25.6) | 7 (53.8) | 0.089 | |||
Abbreviations: TG tigecycline, SB sulbactam, APACHE acute physiology and chronic health evaluation, MRSA methicillin resistant Staphylococcus aureus, MDR Acb multidrug resistant Acinetobacter calcoaceticus-Acinetobacter baumannii
aCategorical data are no.(%) of subject, continuous data are expressed as mean (standard deviation)
bMortality during treatment
cThe initial airway MDR Acb complex isolates with resistance to TG in TG group, or with resistance to SB in SB group
d35 patients in TG group and 9 in SB group; 7 patients in the deceased group and 37 in the survivors group had available data for evaluation
Discussion
Prior case series studies reported clinical response rates ranging from 60 to 88 % in tigecycline treatment [9–11], and 67 to 75 % in sulbactam or ampicillin/sulbactam treatment for pneumonia involving MDRAB or MDR Acb complex [13–15]. There were only a few comparative studies investigating the efficacy of tigecycline or sulbactam, and usually they were compared with colistin or polymyxin, the other major treatment option for MDR Acb complex. Betrosian AP et al. reported that high-dose ampicillin/sulbactam monotherapy and colistin were comparably safe and effective treatment for critically ill patients with MDRAB VAP. The clinical success and improvement rate was 76.9 % for ampcillin/sulbactam group and 73.3 % for colistin group [22]. Oliveira MS et al. reported another study comparing ampicillin/sulbactam with polymyxins in treating infections caused by carbapenem-resistant Acinetobacter spp. [23]. In the study, about half of the enrolled patients had Acinetobacter bacteremia, and quarter of them had pneumonia. The mortality rate during treatment was 33 % in ampicillin/sulbactam group and 50 % in polymyxin group, and polymyxin use was an independent factor associated with mortality during treatment [23]. Chuang YC et al. reported a study comparing tigecycline-based to colistin-based therapy for MDRAB pneumonia in intensive care units. The tigecycline group has an excess mortality of 16.7 % (60.7 % versus 44 %, P = 0.04). The excess mortality of tigecycline is significant only among those with minimal inhibitory concentration (MIC) > 2 μg/mL, but not for those with MIC ≦ 2 μg/mL [24].
To our knowledge, the study was the first comparative study of tigecycline-based versus sulbactam or ampicillin/sulbactam-based treatment for pneumonia involving MDR Acb complex. Our two patient groups were from different but successive time periods, and the major treatment for MDR Acb complex was different in each time period in our hospital. Before August 2007, sulbactam or ampicillin/sulbactam was the only option probably with in vitro activity against MDR Acb complex in our hospital. After that, tigecycline became the major treatment option because of its high susceptibility rate to MDR Acb complex. However, the clinical and microbiological diagnostic criteria and definition, and standards of care and infection control were similar in both time periods. Covariate adjustment with multivariate analyses and matching with disease severity were performed to reduce bias of the historically controlled comparison.
The patients from both groups were aged with complicated underlying diseases and high disease severity. Higher rates of ventilator use and bilateral pneumonia reflected that sulbactam group might have a higher severity of pneumonia than tigecycline group. A higher rate of delay use in tigecycline group might reflect the early policy of tigecycline use in our hospital: usually tigecycline was not used as empiric or first-line regimen for nosocomial infection. Both groups had similar clinical outcomes. Bilateral pneumonia was the only independent predictor for mortality during treatment in different models of multivariate analysis. Combination therapy did not stand out as an independent predictor, which might be due to difference of combination strategies and regimens between the two patient groups. Most patients in sulbactam group had combination with carbapenem for synergistic effect against MDR Acb complex; however, tigecycline group mainly had anti-pseudomonal cephalosporins and fluoquinolones to cover Pseudomonas aeruginosa.
Because most patients in sulbactam group had concurrent carbapenem use, the study results in them might mainly reflect the efficacy of combination of sulbactam and carbapenem. In the comparative analyses of monotherapy, the patients with sulbactam or ampicillin/sulbactam monotherapy had relatively higher mortality rates than the patients with tigecycline monotherapy or the overall sulbactam group. The results implied that combination with carbapenem might improve clinical outcomes of sulbactam-based treatment. Besides, more than half of the sulbactam group had sulbactam-resistant MDR Acb complex isolates during treatment. Combination with carbapenem might play a role giving a high airway eradication rate. The patients with sulbactam monotherapy also had a high airway eradication rate, but most of them did not have sulbactam-resistant MDR Acb complex.
Synergistic effect against MDRAB with the combination of sulbactam and carbapenem had been reported [4, 25]. However, the synergistic effect was associated with the MICs of carbapenem and sulbactam. If the MICs exceeded achievable serum levels, the potential of sulbactam/carbapenem combination as treatment regimen for MDRAB infections might be limited [4]. In our study, full or intermediate sulbactam resistance was detected in 84.5 % of tigecycline-treated patients, therefore, physicians tended to use tigecycline for these patients with sulbactam-resistant MDB Acb complex. For these cases, the clinical outcomes of sulbactam group might not be achieved if they received sulbactam/carbapenem combination therapy.
There are some other limitations in this study. First, our respiratory specimens were clinical specimens from clinical practice, and they might not be obtained from deep sites in the lungs. Growths of etiologic pathogens were assessed semi-quantitatively if the specimens were qualified for culture. We cannot absolutely distinguish airway MDR Acb complex infections from colonization. However, our definition for pneumonia was practical, and our conclusion based on clinically relevant data and management could provide important information for clinical practice. Second, polymicrobial pneumonia and concomitant infections were common, and the clinical impact of other etiologic pathogens or extrapulmonary infections was not evaluated comprehensively. Third, we studied MDR Acb complex rather than MDRAB. Although prior studies reported that about 90 % of Acb complex with multidrug or carbapenem resistance was the genomic specie of A.baumannii, comparison with studies on A. baumannii isolates are not straightforward [26].
Conclusions
Tigecycline-based treatment had a similar clinical outcome to sulbactam or ampicillin/sulbactam-based treatment for pneumonia involving MDR Acb commplex, but tigecycline group had a lower microbiological eradiation rate. More comparison studies are essential to establish the optimal regimens for pneumonia involving MDR Acb complex.
Abbreviations
AB, Acinetobacter baumannii; Acb, Acinetobacter calcoaceticus-Acinetobacter baumannii; APACHE, acute physiology and chronic health evaluation; CGMH, Chang Gung Memorial Hospital; CI, confidence interval; MDR, multidrug-resistant; MIC, minimal inhibitory concentration; OR, odds ratio; VAP, ventilator associated pneumonia
Acknowledgments
This retrospective study has been approved by institutional review boards of CGMH- Linkou (Number: 99-1478B and 100-0294B). The ethics committee granted a waiver for informed consent to be obtained. No funding sources had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Authors’ contributions
JJY, and HSL reviewed the medical records and drafted the manuscript; CFY, YMW, PYH, and CCY analyzed and interpreted the data. JJY, MHL, and CTH designed and oversaw the study, analyzed and interpreted the data, and revised the manuscript. All authors have read and approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Jung-Jr Ye, Email: loyalwise@gmail.com.
Huang-Shen Lin, Email: linhuangshen@gmail.com.
Chun-Fu Yeh, Email: MR7828@cloud.cgmh.org.tw.
Yen-Mu Wu, Email: b001089106@tmu.edu.tw.
Po-Yen Huang, Email: pyhuang@gmail.com.
Chien-Chang Yang, Email: bears112@gmail.com.
Ching-Tai Huang, Email: chingtaihuang@gmail.com.
Ming-Hsun Lee, Phone: 886-3-3281200, Email: drharrylee@gmail.com.
References
- 1.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65(3):204–11. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, Srinivasan A. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13(1):97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye JJ, Huang CT, Shie SS, Huang PY, Su LH, Chiu CH, Leu HS, Chiang PC. Multidrug resistant Acinetobacter baumannii: risk factors for appearance of imipenem resistant strains on patients formerly with susceptible strains. PLoS One. 2010;5(4):e9947. doi: 10.1371/journal.pone.0009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh PR, Teng LJ, Chen CY, Chen WH, Yu CJ, Ho SW, Luh KT. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg Infect Dis. 2002;8(8):827–32. doi: 10.3201/eid0805.020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs. 2009;69(14):1879–901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Hoban DJ, Bouchillon SK, Johnson BM, Johnson JL, Dowzicky MJ, Tigecycline Evaluation and Surveillance Trial (TEST Program) Group In vitro activity of tigecycline against 6,792 Gram-negative and Gram-positive clinical isolates from the global Tigecycline Evaluation and Surveillance Trial (TEST Program; 2004) Diagn Microbiol Infect Dis. 2005;52(3):215–27. doi: 10.1016/j.diagmicrobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.FDA Drug Safety Communication. Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed Sept 2010.
- 8.Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H, 311 Study Group Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68(2):140–51. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Curcio D, Fernández F, Vergara J, Vazquez W, Luna CM. Late onset ventilator-associated pneumonia due to multidrug-resistant Acinetobacter spp.: experience with tigecycline. J Chemother. 2009;21(1):58–62. doi: 10.1179/joc.2009.21.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, Nicolaou C, Yphantis D, Antoniadou A, Trikka-Graphakos E, Roussou Z, Clouva P, Maguina N, Kanellakopoulou K, Armaganidis A, Giamarellou H. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58(4):273–84. doi: 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Ye JJ, Lin HS, Kuo AJ, Leu HS, Chiang PC, Huang CT, Lee MH. The clinical implication and prognostic predictors of tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii. J Infect. 2011;63(5):351–61. doi: 10.1016/j.jinf.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Corbella X, Ariza J, Ardanuy C, Vuelta M, Tubau F, Sora M, Pujol M, Gudiol F. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1998;42(6):793–802. doi: 10.1093/jac/42.6.793. [DOI] [PubMed] [Google Scholar]
- 13.Betrosian AP, Frantzeskaki F, Xanthaki A, Georgiadis G. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand J Infect Dis. 2007;39(1):38–43. doi: 10.1080/00365540600951184. [DOI] [PubMed] [Google Scholar]
- 14.Levin AS, Levy CE, Manrique AE, Medeiros EA, Costa SF. Severe nosocomial infections with imipenem-resistant Acinetobacter baumannii treated with ampicillin/sulbactam. Int J Antimicrob Agents. 2003;21(1):58–62. doi: 10.1016/S0924-8579(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin HS, Lee MH, Cheng CW, Hsu PC, Leu HS, Huang CT, Ye JJ. Sulbactam treatment for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Infect Dis. 2015;47(6):370–8. doi: 10.3109/00365548.2014.995129. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani S, Cohen-Melamed MH, Tuttle RP, Delgado E, Taira Y, Darby JM. Comparison of semi-quantitative endotracheal aspirates to quantitative non-bronchoscopic bronchoalveolar lavage in diagnosing ventilator-associated pneumonia. Respir Care. 2009;54(11):1446–8. [PubMed] [Google Scholar]
- 18.Schreckenberger PC, Daneshvar MI, Weyant RS, Hollis DG. Acinetobacter, Achromobacter, Chryseobacerium, Moraxella and other nonformentative gram-negative rods. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Louise-Landry M, Warnock DW, editors. Manual of clinical microbiology. 10. Washington: American Society of Microbiology; 2011. pp. 714–38. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: twenty-first informational supplement M100-S21. Wayne: CLSI; 2011. [Google Scholar]
- 20.Jones RN, Ferraro MJ, Reller LB, Schreckenberger PC, Swenson JM, Sader HS. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol. 2007;45(1):227–30. doi: 10.1128/JCM.01588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterson DL. The Epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(S2):S43–48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 22.Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56(6):432–6. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61(6):1369–75. doi: 10.1093/jac/dkn128. [DOI] [PubMed] [Google Scholar]
- 24.Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, Chang SC. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14:102. doi: 10.1186/1471-2334-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee NY, Wang CL, Chuang YC, Yu WL, Lee HC, Chang CM, Wang LR, Ko WC. Combination carbapenem-sulbactam therapy for critically ill patients with multidrug-resistant Acinetobacter baumannii bacteremia: four case reports and an in vitro combination synergy study. Pharmacotherapy. 2007;27(11):1506–11. doi: 10.1592/phco.27.11.1506. [DOI] [PubMed] [Google Scholar]
- 26.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, Hsueh PR. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother. 2011;66(8):1839–46. doi: 10.1093/jac/dkr200. [DOI] [PubMed] [Google Scholar]


