Abstract
Background
Oncolytic viral efficacy may be limited by the penetration of the virus into tumors. This may be enhanced by intraoperative application of virus immediately after surgical resection.
Methods
Oncolytic vaccinia virus GLV-1h68 was delivered in silk-elastin-like protein polymer (SELP) in vitro and in vivo in anaplastic thyroid carcinoma cell line 8505c in nude mice.
Results
GLV-1h68 in SELP infected and lysed anaplastic thyroid cancer cells in vitro equally as effectively as in phosphate-buffered saline (PBS), and at 1 week retains a thousand fold greater infectious plaque-forming units. In surgical resection models of residual tumor, GLV-1h68 in SELP improves tumor control and shows increased viral β-galactosidase expression as compared to PBS.
Conclusion
The use of SELP matrix for intraoperative oncolytic viral delivery protects infectious viral particles from degradation, facilitates sustained viral delivery and transgene expression, and improves tumor control. Such optimization of methods of oncolytic viral delivery may enhance therapeutic outcomes.
Keywords: oncolytic virus, viral therapy, surgery, anaplastic thyroid carcinoma, vaccinia
Introduction
Oncolytic viral therapy has repeatedly demonstrated potent antitumoral effects against a variety of malignancies through novel mechanisms of activity mediated by viral replication and tumor cell lysis. Mutated herpes simplex virus, adenovirus, vaccinia virus, Newcastle disease virus, vesicular stomatitis virus, and other viruses have all demonstrated oncolytic therapeutic potential.1,2 However, the clinical application of oncolytic viral therapy has limited efficacy, even when multiple intratumoral injections are applied, because of difficulties of the virus to adequately distribute throughout the tumor mass.3 Intratumoral viral proliferation may be offset by immune clearance. In addition, tumor connective tissue was found in murine xenograft models to limit the ability of intratumorally injected virus to evenly distribute throughout the tumor, and lead to patchy areas of infection.4 Such challenges in viral distribution have prompted studies investigating the utility of applying matrix metalloproteinases, collagenase, and apoptosis-induction agents to facilitate viral penetration and efficacy after intratumoral injection.5–7
Oncolytic viral therapy is more effective when virus is delivered to low, rather than high, tumor volumes.8,9 The potential application of oncolytic viruses to a tumor bed immediately after surgical resection is an intuitively appealing strategy for scenarios in which a patient is considered at risk for harboring residual disease in the surgical field. In some clinical situations, surgical resection may remove the majority of disease, but residual disease may be left on critical anatomic structures that are considered unresectable. For these patients, the intraoperative, direct application of oncolytic viruses to a surface of low volume residual disease may optimize viral delivery and tumor penetration.
Silk-elastin-like protein polymers (SELPs) are genetically engineered copolymers consisting of peptide repeats of silklike (GAGAGS) and elastinlike (GVGVP) units.10 The silk units provide mechanical strength to the formed hydrogels, whereas the elastin units provide aqueous solubility, elasticity, and biodegradation. SELP-47K is a copolymer with 4 silk and 8 elastin blocks, including 1 lysine-substituted elastin, which undergoes irreversible transition from solution to hydrogel at body temperature.10,11 Recent studies have demonstrated that SELP allows for a controlled release of drugs, plasmid DNA, and adenovirus.12,13 SELP containing adenovirus has been shown to allow for release of virus over a 4-week period, while allowing for prolonged and localized adenoviral gene expression after intratumoral injection.14 SELP is also biodegradable without significant immunogenicity or toxicity. A recent study demonstrated that an injectable alginate gel allows for sustained delivery of an oncolytic adenovirus, prolonging adenoviral activity, and enhancing antitumoral effects.15
Our group recently demonstrated that a mutated, replication-competent, oncolytic vaccinia virus (GLV-1h68) is able to infect and impede the growth of human anaplastic thyroid cancer murine flank xenograft tumors grown in vivo.8,16 However, this is not a clinically accurate model. In patients, anaplastic thyroid cancer typically presents as a large tumor not amenable to adequate viral distribution with direct injection, and typically cannot be surgically resected with clear margins. To address this problem, we here explore the concept of partial surgical resection of an unresectable cancer, combined with the intraoperative application of GLV-1h68. We used an animal model mimicking incomplete surgical resection of anaplastic thyroid cancer, and studied the effects of intraoperative application of GLV-1h68 in either SELP or phosphate-buffered saline (PBS) to treat residual tumor.
Materials and Methods
Cell lines
The human anaplastic thyroid carcinoma cell line 8505c (Japanese Collection of Research Bioresources Cell Bank, Shinjuku, Japan) was maintained in minimal essential medium (MEM) with 10% fetal calf serum, penicillin, and streptomycin. CV-1 cells were maintained in Dulbecco's modified eagle medium. Cells were maintained at 37°C and 5% carbon dioxide.
Virus
GLV-1h68 is a recombinant, replication-competent vaccinia virus derived from the vaccinia virus LIVP strain (Lister strain from the Institute of Viral Preparations, Moscow, Russia).17 GLV-1h68 contains 4 inserted cassettes encoding Renilla luciferase-green fluorescent protein fusion (RUC-GFP cassette), a reverse-inserted human transferrin receptor (rTfr), β-galactosidase, and β-glucuronidase into the F14.5L, J2R (thymidine kinase), and A56R (hemagglutinin) loci of the viral genome, respectively.
Cytotoxicity assays
The 8505c cells were plated overnight at 2 × 104 cells per well in 12-well plates in 1 mL media. GLV-1h68 was added to each well at a multiplicity of infection (MOI) of 0, 0.01, 0.1, and 1. Cell viability was measured daily. Cells were washed with PBS and lysed with Triton X-100 (1.35%; Sigma, St. Louis, MO) to release intracellular lactate dehydrogenase (LDH), which was quantified using a Cytotox 96 Kit (Promega, Madison, WI) on a spectrophotometer (EL32le, BioTek Instruments) at 450 nm. Results were expressed as the percentage of surviving cells, determined by comparing the measured LDH of each infected sample relative to control cells. All samples were analyzed in triplicate.
Viral proliferation and titering
The 8505c cells were plated overnight at 2 × 104 cells per well in 12-well plates and infected with GLV-1h68 at MOI 0.1. Supernatants were collected daily and stored at − 80°C. After thawing and serial dilutions, standard plaque assays were performed on confluent CV-1 cells. All samples were measured in triplicate.
Silk-elastin-like protein polymer-47K hydrogel
SELP-47K was provided by Protein Polymer Technologies (San Diego, CA) as a 12% by weight solution, stored frozen at − 80°C. SELP was thawed at room temperature and diluted with PBS or MEM to a 4% concentration, and mixed with GLV-1h68 to the appropriate concentrations. Dilution was performed with culture media in cytotoxicity experiments, or PBS in other experiments. SELP-47K is a liquid at room temperature, but forms an irreversible gel at body temperature.
Green fluorescent protein expression
The 8505c cells were plated overnight at 2 × 104 cells per well in 24-well plates. The experimental groups were: MEM, 4% SELP diluted with MEM, GLV-1h68 (MOI 1) in MEM; GLV-1h68 (MOI 1) in 4% SELP; and GLV-1h68 (MOI 1) in 50 μl PBS under a layer of 4% SELP. In the last group, SELP was first layered over the cells, allowed to solidify for 10 minutes, and the virus was then injected by micropipette under the gel layer. At varying intervals, cells were examined with a fluorescence inverted microscope (Nikon Eclipse TS100, Nikon, Japan) for green fluorescent protein (GFP) expression.
Crystal violet staining
The 8505c cells were plated overnight at 2 × 104 cells per well in 24-well plates. The experimental groups were: MEM, 4% SELP diluted with MEM, GLV-1h68 (MOI 1) in MEM; GLV-1h68 (MOI 1) in 4% SELP; and GLV-1h68 (MOI 1) in 50 μL PBS under a layer of 4% SELP. Cells were fixed with 20% ethanol, stained with 0.1% crystal violet for 10 minutes, washed with H2O, and room air dried. Photographs were taken with an inverted microscope (Nikon Eclipse TS100). All samples were assessed in triplicate.
In vitro viral elution studies
Cylindrical gels of GLV-1h68 (1 × 106 pfu) in 50 μL of 4% SELP in PBS were formed using insulin syringes. A solution of GLV-1h68 (1 × 106 pfu) in 50 μL PBS was used as control. Samples were placed in 2 mL cryogenic tubes in 1.5 mL PBS, and incubated at 37°C in a shaking incubator to facilitate viral elution. Triplicate samples were removed at varying time points and stored at − 80°C. To disrupt the SELP gels for viral titer studies, SELP samples were shaken (Qiagen TissueLyser) at 30 Hz for 2 minutes to create tiny gel particles. GLV-1h68 titers were determined by plaque assay, as described above.
In vivo flank tumor silk-elastin-like protein polymer and GLV-1h68 injections
All animal procedures were approved by the Memorial Sloan–Kettering Institutional Animal Care and Use Committee. Orthotopic xenografts of human thyroid cancer in mice demonstrate aggressive and variable tumor growth that is difficult to surgically resect in a consistent fashion. Therefore, a flank tumor model was selected for these studies. Tumors were established by injecting 5 × 106 8505c cells into the subcutaneous flanks of 6-week-old female nude athymic mice (NCI, Bethesda, MD) under inhalational anesthesia with isoflurane (Baxter, Deerfield, IL). Tumor volumes were calculated as the shape of an ellipsoid: (4/3*π)*(a/2)*(b/2).2 At a mean tumor volume of 100 mm3, mice (n = 5 per group) underwent a single intratumoral injection with 50 μL volume of: (1) 1 × 107 pfu of GLV-1h68 in PBS, (2) 1 × 107 pfu of GLV-1h68 in 4% SELP, or (3) PBS. Tumor volumes and body weights were measured.
In vivo luciferase activity
At varying times, 5 μL coelenterazine (0.5 μg/μL; Biotium, Hayward, CA) in 95 μL PBS was injected via the retro-orbital sinus (n = 5 mice per group) of mice under inhalational anesthesia. Luciferase activity was detected with a cooled CCD camera (Xenogen IVIS; Xenogen Corp, Caliper Life Sciences, Hopkinton, MA). Emitted photons were measured for 60 seconds. Images were analyzed using Living Image software (Xenogen; Caliper Life Sciences).
In vivo green fluorescent protein expression
At varying times, mice (n = 3 mice per group) under inhalational anesthesia were imaged with a Maestro In Vivo Fluorescence Imaging System (Cambridge Research & Instrumentation, Woburn, MA). GFP signals were quantified by using Maestro 2.4 software.
In vivo post-resection model of high-volume residual tumor
To mimic an intraoperative scenario of an incomplete surgical resection with high-volume residual disease, animals with 8505c flank tumors underwent general anesthesia and surgical resection, leaving approximately 120 mm2 of the deep portion of the tumor as residual disease. Tumors (n = 7 mice per group) were then treated with an intraoperative direct application of 300 μL volume topically over the residual tumor of: (1) PBS, (2) 4% SELP, (3) 1 × 107 pfu of GLV-1h68 in PBS, or (4) 1 × 107 pfu of GLV-1h68 in 4% SELP. After application of the treatment to the surface of the residual tumor, the skin flap was closed with staples. Tumors were measured every other day, beginning 7 days after the surgery to allow for resolution of tissue edema and fluid/gel volume. Animals underwent in vivo luciferase imaging, as described above.
In vivo post-resection model of low-volume residual tumor
To mimic an intraoperative scenario of surgical resection with low-volume residual disease, animals with 8505c flank tumors underwent surgical resection of the superficial tumor, leaving approximately 50 mm3 of tumor remaining as a flat surface. Tumors were then treated with an intraoperative direct application of 50 μL volume topically over the residual tumor of: (1) PBS (n = 7), (2) 1 × 107 pfu of GLV-1h68 in PBS (n = 9), (3) 1 × 107 pfu of GLV-1h68 in 4% SELP (n = 9), or (4) 1 × 107 pfu of GLV-1h68 in tiny 4% SELP particles (n = 9). The 4% SELP particles were created by shaking the SELP gel at 30Hz (Qiagen TissueLyser) for 2 minutes to create tiny gel particles, as described in the viral elution studies above. After application to the surface of the residual tumor, the skin flap was closed with staples. Tumors were measured and animals underwent in vivo luciferase imaging, as described above.
β-galactosidase histochemical staining
Mice (n = 2 mice per group) from the low volume postresection residual tumor model were sacrificed at varying time points after treatment. Residual tumors were excised, frozen in Tissue Tek (Sakura Finetek USA, Torrance, CA), and sectioned. Slides were fixed with 1% glutaraldehyde, stained with X-Gal (bromo-chloro-indolyl-galactopyranoside) at 1 mg/mL in 5 mM K4Fe(CN)6 and 2 mM MgCl2, and counterstained with nuclear fast red. Sections were digitally photographed using an inverted microscope (Nikon Eclipse TS100).
Results
GLV-1h68 infects, replicates within, and lyses 8505c anaplastic thyroid cancer cells
To assess the sensitivity of 8505c cells to GLV-1h68, virus at varying concentrations was added to cells in media and cell viability measured daily for 1 week by LDH assay. At an MOI 1, 0.1, and 0.01, there was just 1%, 5%, and 23% remaining cell viability, respectively, by day 7 (Figure 1A), demonstrating dose–response effects. Viral proliferation was assessed by measuring daily viral titers from the supernatants of the MOI 0.1 wells. Titers reveal logarithmic viral growth from days 1 through 6 (Figure 1B), demonstrating that 8505c cells support GLV-1h68 infection and effective viral proliferation.
Figure 1.
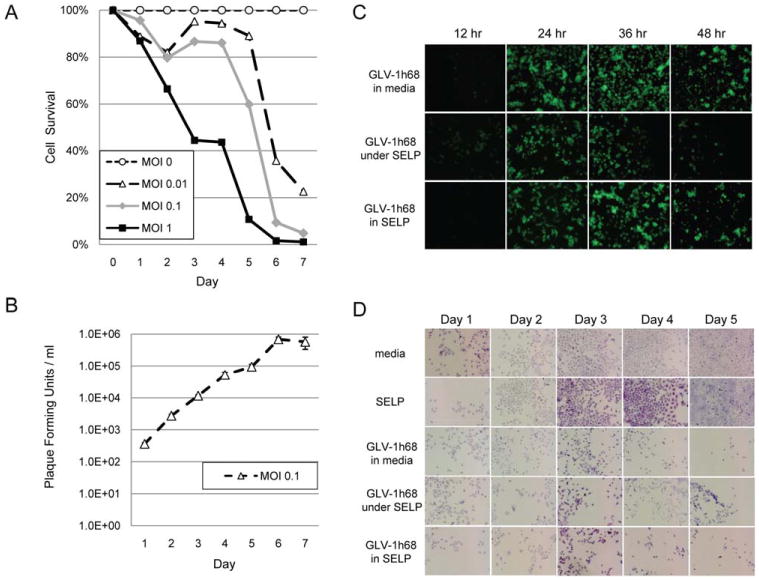
GLV-1h68 infects and lyses 8505c anaplastic thyroid cancer cells equally well in silk-elastin-like protein polymer (SELP) as in solution. (A) To assess the sensitivity of 8505c cells to GLV-1h68, virus at varying multiplicities of infection was added to cells in media and cell viability measured daily for 1 week by lactate dehydrogenase (LDH) assay. (B) Viral proliferation was assessed by measuring daily viral titers from the supernatants of the multiplicity of infection (MOI) 0.1 wells. (C) The ability of GLV-1h68 at MOI 1 to infect a monolayer of 8505c cells was assessed by green fluorescent protein (GFP) expression between (A) GLV-1h68 in media versus (B) GLV-1h68 in media under a solidified layer of SELP gel versus (C) GLV-1h68 in a matrix of SELP gel. (D) The same groups were used to assess cytotoxicity by assessing cell viability over 5 days using crystal violet staining. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
GLV-1h68 in silk-elastin-like protein polymer gel infects cells and expresses transgenes in vitro as effectively as in solution
We compared the ability of GLV-1h68 at MOI 1 in a SELP gel versus in media to infect a monolayer of 8505c cells in vitro. Cells infected by GLV-1h68 in either SELP or media exhibited identical intensity of GFP expression by microscopy at 12, 24, 36, and 48 hours (Figure 1C). Decreased GFP expression at 48 hours reflects expected decreased cell viability from viral cytotoxic effects. We also assessed the potential utility of applying SELP as a barrier to contain GLV-1h68 in media over the cell layer. However, an identical amount of GLV-1h68 in media injected by micropipette under a solidified layer of SELP resulted in diminished GFP expression at all time points as compared with the 2 other groups (Figure 1C), reflecting a decreased efficiency of infection with this approach.
GLV-1h68 in silk-elastin-like protein polymer lyses cells as effectively as GLV-1h68 in phosphate-buffered saline in vitro
We studied the ability of GLV-1h68 at MOI 1, in a SELP gel as compared to in media, to lyse a monolayer of 8505c cells in vitro. Cell viability was assessed visually with crystal violet staining. Untreated wells with either media or SELP gel exhibited progressive 8505c growth to confluence by day 5 (Figure 1D). GLV-1h68 in SELP versus in media both exhibited identical patterns of cell lysis by microscopy over a 5-day period. An identical dose of GLV-1h68 injected by micropipette under a solidified layer of SELP resulted in slightly diminished cell lysis as compared with the other 2 virally treated groups.
Silk-elastin-like protein polymer preserves GLV-1h68 viral integrity at body temperature
Virus extrinsic to a host cell degrades at body temperature. We studied GLV-1h68 viral integrity, in SELP as compared to in PBS, when maintained in an incubator at 37°C over a 4-week period in the absence of any cells. Viral integrity was measured by measuring infectious viral titers. Viral elution from the SELP gel for plaque assays was facilitated by the use of a shaking incubator and by gel disruption by shaking at 30 Hz for 2 minutes to create microscopic gel particles. The shaking gel disruption enhanced viral recovery from SELP gel, as compared with other techniques examined including vortexing, or shaking with homogenizer beads (Figure 2A).
Figure 2.
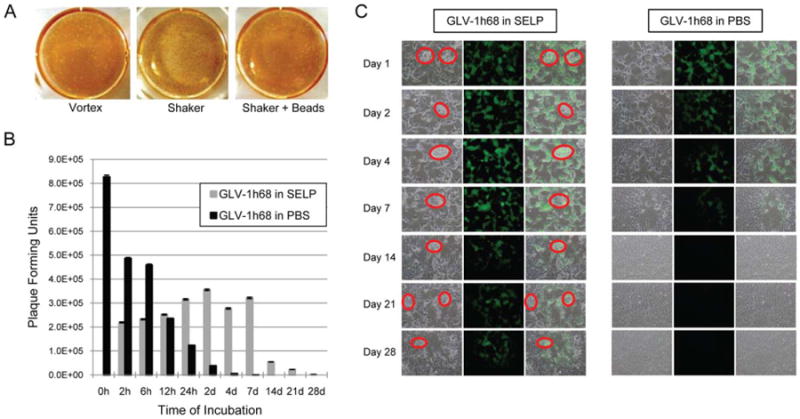
Silk-elastin-like protein polymer (SELP) preserves GLV-1h68 viral integrity at body temperature. (A) Viral elution from the SELP gel for plaque assays was optimized by the use of a shaking incubator to disrupt the gel. Shaking enhanced viral recovery from SELP gel, as compared with vortexing, or shaking with homogenizer beads. (B) GLV-1h68 viral integrity was measured in SELP versus phosphate-buffered saline (PBS), when maintained in an incubator at 37°C over a 4-week period in the absence of any cells. Viral integrity was measured by measuring infectious viral titers. (C) Green fluorescent protein (GFP) expression by plaque assay samples demonstrates enhanced late viral integrity and transgene expression by the GLV-1h68 in SELP as compared with PBS. The red circles in the SELP group denote tiny gel particles created by the shaking incubator. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
GLV-1h68 in PBS exhibits a rapid decline of infectious viral plaque-forming units over time, dropping from over 8 × 105 pfu to below 2.5 × 105 pfu by 12 hours. In sharp contrast, GLV-1h68 in SELP permitted relatively stable infectious particle retention from 2 hours to 7 days, retaining over the 3.2 × 105 pfu at 7 days, in contrast to just 2.9 × 102 pfu for GLV-1h68 in PBS at 7 days (Figure 2B and 2C). GLV-1h68 in PBS could not be detected past 1 week, whereas GLV-1h68 in SELP remained detectable at 4 weeks. Note that the actual number of infectious viral particles within the SELP gel specimens is very likely higher than the quantities that we could detect, because of our limited ability to elute all viral particles from the solid gel matrix. Therefore, these results probably underrepresent this protective effect. These studies demonstrate that SELP gel confers a protective effect against normal viral degradation at body temperature.
Intratumoral injection of GLV-1h68 in silk-elastin-like protein polymer into established flank tumors results in enhanced late transgene expression and equivalent tumor volume regression as compared to GLV-1h68 in phosphate-buffered saline
We performed a single intratumoral injection of GLV-1h68 in either SELP or PBS, into established 8505c murine flank tumors. At day 10 and later, viral transgene expression was found to be enhanced by SELP, as measured by GFP (n = 3 mice per group; Figure 3A and 3B) and luciferase (n = 5 mice per group; Figure 3C and 3D) quantification. Such enhanced late transgene expression likely reflects a continuous slow viral release from the SELP matrix and subsequent cancer cell infection. In contrast, most of the virus in PBS has already degraded by later time points (see Figure 2). A single intratumoral injection of GLV-1h68, in either SELP or PBS, into established flank tumors induced equivalent, near-complete, tumor regression (n = 5 mice per group; Figure 3E). There was no advantage of SELP over PBS observed in this model. The high sensitivity of these tumors to GLV-1h68 may not have permitted a differentiation of therapeutic effect at these conditions.
Figure 3.
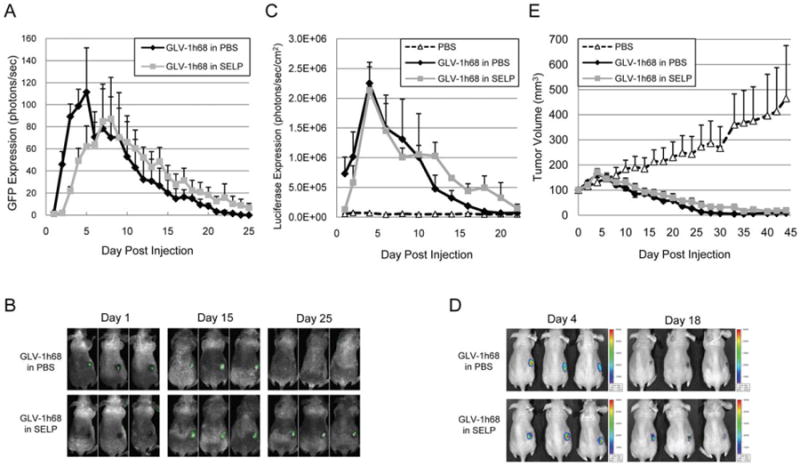
Intratumoral injection of GLV-1h68 in silk-elastin-like protein polymer (SELP) into flank tumors results in equivalent tumor volume regression as compared to GLV-1h68 in phosphate-buffered saline (PBS). A single intratumoral injection of GLV-1h68, in either SELP or PBS, as delivered into 8505c murine flank tumors. At day 10 and later, viral transgene expression was enhanced by SELP over PBS, as measured by green fluorescent protein (GFP; n = 3 per group) (A) and luciferase (n = 5 per group) (C) quantification. Representative animals were imaged for GFP (B) or luciferase (D). Tumor volumes were measured and demonstrate equivalent, near-complete, tumor regression for GLV-1h68 in both PBS and SELP (n = 8 per group) (E). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Topical application of GLV-1h68 in silk-elastin-like protein polymer to high-volume residual disease after surgical resection enhances tumor control as compared with GLV-1h68 in phosphate-buffered saline
We hypothesized that the greatest of utility of SELP matrix might be in a setting of topical application, where a sustained contact of virus in SELP to a flat tumor surface, combined with a slow continuous release of virus, may optimize therapeutic efficacy. To mimic a clinical scenario of intraoperative application of virus after incomplete surgical resection, we applied GLV-1h68, in either SELP or PBS (n = 7 mice per group), topically to a partially resected tumor surface in the flanks of mice (Figure 4A). In this model of high volume residual disease, approximately 120 mm of tumor was the starting volume. In vivo luciferase imaging at day 12 demonstrated more than double peak expression levels for GLV-1h68 in SELP as compared to GLV-168 in PBS, demonstrating a higher infection efficacy (Figure 4B). A time course of tumor response showed significantly smaller tumor volumes after day 12 for GLV-1h68 in SELP as compared to GLV-1h68 in PBS. At day 14, the mean volume of control tumors treated with topical PBS alone was 398 ± 142 mm, and SELP alone was 424 ± 86 mm, whereas the mean volume of tumors treated with topical virus was 104 ± 5 mm for GLV-1h68 in PBS, and 30 ± 14 mm for GLV-1h68 in SELP (Figure 4D; p < .05; t test; 2-tailed).
Figure 4.
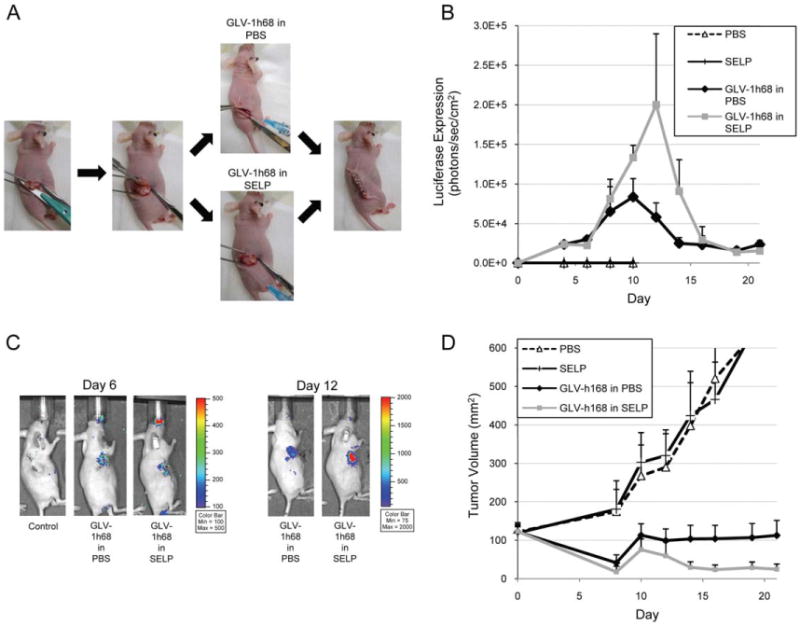
Topical application of GLV-1h68 in silk-elastin-like protein polymer (SELP) to high-volume residual disease after surgical resection enhances tumor control as compared with GLV-1h68 in phosphate-buffered saline (PBS). (A) To mimic a clinical scenario of intraoperative application of virus after incomplete surgical resection, GLV-1h68 was applied, in SELP or PBS, topically to a partially resected tumor surface in murine flanks (n = 7 per group). Approximately 120 mm of tumor was the starting volume. (B and C) In vivo luciferase expression was quantified, with representative images shown. (D) Tumor volumes demonstrate significantly smaller volumes for GLV-1h68 in SELP as compared with GLV-1h68 in PBS (p < .05; t test; 2-tailed; day 14). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Topical application of GLV-1h68 in silk-elastin-like protein polymer to low-volume residual disease after surgical resection enhances tumor control as compared with GLV-1h68 in phosphate-buffered saline
To test the ability of topical GLV-1h68 to treat low volume residual disease, we modified our flank model to leave a very thin layer of residual tumor after surgical resection. In this model, approximately 50 mm of residual tumor is the starting volume. Furthermore, we tested the efficacy of applying GLV-1h68 in microscopic gel particles, as generated in the in vitro viral elution studies described above. We reasoned that the small diameter gel particles might enhance viral release from the gel and increase the potential areas of contact with tumor. In vivo imaging demonstrated more than a 2.5-fold increase in peak luciferase expression for GLV-1h68 in SELP as compared with GLV-168 in PBS at day 10 (Figure 5A and 5B). In addition, GLV-1h68 in the SELP particles resulted in 50% higher luciferase expression at day 12 as compared to GLV-1h68 in SELP gel, showing that the gel particles may enhance viral infection as compared to a solid gel surface. A time course of tumor volumes demonstrated greater tumor regression for GLV-1h68 in SELP particles (n = 9 in each group), followed by GLV-1h68 in SELP gel, and GLV-1h68 in PBS (Figure 5C). At day 13, there was a trend toward lower tumor volumes for GLV-1h68 in SELP particles (8.0 ± 3.0 mm) as compared with GLV-1h68 in PBS (31.5 ± 10.8 mm; p = .05; t test; 2-tailed). By day 19, there was slight tumor regrowth in several of the virally treated groups. The GLV-1h68 in the SELP particles or gel also led to a higher number of animals being rendered free of disease, as compared with GLV-1h68 in PBS (Figure 5D). Although differences between these groups were not statistically significant using 2-tailed Fisher's exact tests, these pilot animal studies were not powered for significance for such clinical endpoints.
Figure 5.
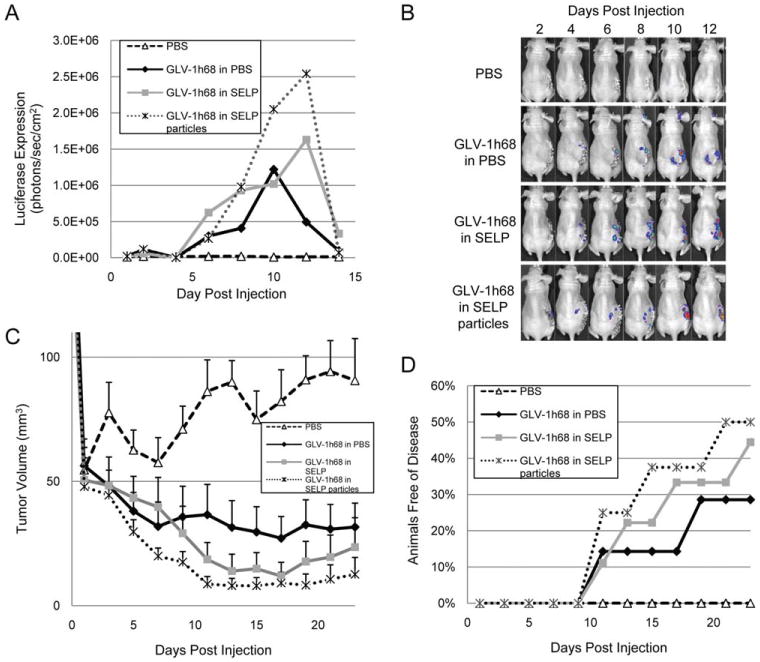
Topical application of GLV-1h68 in silk-elastin-like protein polymer (SELP) to low volume residual disease after surgical resection enhances tumor control as compared with GLV-1h68 in phosphate-buffered saline (PBS). In this postresection model, approximately 50 mm of residual tumor is the starting volume. The efficacy of applying GLV-1h68 in microscopic gel particles is also assessed. (A and B) In vivo luciferase expression was quantified, with representative images shown. (C) Tumor volumes trended lower for GLV-1h68 in SELP particles as compared with GLV-1h68 in PBS, although the difference was not statistically significant (p = .05; t test; 2-tailed; day 13). (D) GLV-1h68 in SELP particles or gel led to a higher number of animals being rendered free of disease, as compared with GLV-1h68 in PBS. There were no significant differences between the viral groups using 2-tailed Fisher's exact tests (n = 9 per group, n = 7 in control group). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Topical GLV-1h68 in silk-elastin-like protein polymer induces prolonged and enhanced β-galactosidase expression
A time course of β-galactosidase histochemical staining was performed on excised, low volume, postresection tumor specimens treated with topical GLV-1h68 in PBS, SELP, or SELP particles (n = 2 in each group) to assess lacZ expression as a measure of viral infection. GLV-1h68 in PBS resulted in maximal lacZ expression by day 8, with subsequent gradual loss of expression to nearly complete loss of expression by day 30. GLV-1h68 in SELP or SELP particles resulted in similar early expression of lacZ expression with maximal expression by day 8. However, in contrast to using virus with PBS, using SELP or SELP particles with virus led to wider and more intense areas of lacZ expression over the next 22 days. No appreciable difference in β-galactosidase expression was noticed between virus with SELP and virus with SELP particles. LacZ expression was dramatically stronger and more durable for SELP or SELP particles, with intense expression even at day 30. These findings demonstrate more potent and sustained viral infection in cancer cells treated with GLV-1h68 in SELP or SELP particles as compared with PBS.
Discussion
Genetically engineered oncolytic viruses represent a type of biologic therapy that harnesses a virus's lytic life cycle for therapeutic effects targeted against malignant tumors.1,2 A variety of different viral types have demonstrated significant therapeutic efficacy in experimental models of solid tumor malignancies. However, oncolytic viral efficacy may be limited by an inability of the virus to effectively penetrate into a solid tumor, evenly distribute within that tumor, and adequately infect cancer cells. Intratumoral connective tissue may limit viral penetration, leading to a patchy distribution of infection, and the immune system facilitates viral clearance.4 Recent studies have emphasized the value of exploiting strategies to facilitate oncolytic viral distribution throughout a tumor to enhance therapeutic effects. For example, the expression of matrix metalloproteinases by tumor cells (MMP1 and 8)5 or the exogenous delivery of collagenase6 may degrade tumor matrix and potentiate viral distribution, penetration, and oncolytic efficacy in a 3D solid tumor. Similarly, the initial administration of apoptosis-inducing chemotherapy agents may induce cancer cell death that may enhance subsequent delivery and penetration of intratumoral oncolytic virus.7 These studies collectively demonstrate the importance of optimizing adequate oncolytic viral delivery to a 3D tumor target for maximal therapeutic efficacy.
The surgical resection of a cancer is perhaps the most direct strategy to immediately reduce tumor volume. However, with larger tumors, or tumors adjacent to critical anatomic structures, microscopic or gross residual disease may be left behind despite a surgeon's best efforts to technically achieve complete surgical resection. In this setting, the application of an oncolytic virus to the postsurgical resection bed is an intuitively attractive strategy. The surgical resection would remove the large bulk of the tumor and leave behind presumably only low volume or microscopic disease, likely shaped as a sheet or a thin layer. This seems to be the exact setting in which locally delivered oncolytic viral therapy may have its greatest chance at maximal efficacy, with problems pertaining to viral distribution after injection into a spherical tumor avoided. The topical application of an oncolytic virus intraoperatively would facilitate immediate and direct contact of high titer oncolytic virus with low volume residual cancer cells.
Traditionally, viral preparations in solution have been directly injected into 3D tumors as one method of local delivery. We sought to assess the utility of combining surgical resection followed by topical viral application to residual cancer cells. However, methods of applying virus to flat surfaces of residual disease after surgical resection have not been well investigated. The topical application of a viral solution intraoperatively might theoretically be hindered by: inadequate or only brief contact with the tumor surface, runoff of the viral solution away from the tumor surface to the surgical cavity, rapid viral degradation at body temperature, and immunologic clearance of virus that fails to infect cancer cells. We therefore hypothesized that intraoperative oncolytic viral delivery may be enhanced in a gel matrix that would meet several criteria. The matrix should ideally be safe and biodegradable, adhere to a 3D tumor surface for prolonged viral-tumor contact, protect viral integrity within the gel at body temperature, allow for sustained viral release in vivo, and facilitate effective viral infection of adjacent tumor cells.
SELPs are genetically engineered block copolymers consisting of peptide repeats of silklike (GAGAGS) and elastinlike (GVGVP) units.10 The silk units allow formation of hydrogen bonds to confer strength to the hydrogels, while the elastin units confer aqueous solubility and elasticity and biodegradation. SELP-47K is one such copolymer with 4 silk and 8 elastin blocks that undergoes irreversible solution to gel transition at body temperature.11 The formed SELP-47K hydrogels are able to allow for a localized, controlled release of plasmid DNA and viruses in vivo.12–14 Such a sustained release of adenovirus from SELP gels facilitates more effective viral gene expression in tumors.14 In addition, an oncolytic adenovirus recently was demonstrated to have enhanced antitumoral effects when delivered in an alginate gel by intratumoral injection.15 We reasoned that an oncolytic virus within SELP-47K might be applied intraoperatively as a liquid to a flat surface of residual disease within a surgical cavity, and then rapidly solidify to a gel that would then remain in continuous contact with that surface and permit controlled viral release over time. To study this concept, we selected the highly aggressive and often fatal anaplastic thyroid cancer, in which complete surgical resection is rarely achievable. We sought to determine the utility of SELP-47K as a matrix for the intraoperative delivery of an oncolytic vaccinia virus after incomplete anaplastic thyroid cancer resection.
GLV-1h68 is an attenuated, replication-competent, oncolytic vaccinia virus.17 GLV-1h68 has a mutation in its thymidine kinase that makes viral replication dependent on host cell thymidine kinase, increasing its selectivity for malignant tumors. GLV-1h68 also carries an insertional mutation in the hemagglutinin gene that reduces its virulence and carries the luciferase and GFP reporter genes. GLV-1h68 injections into established anaplastic thyroid tumors in murine flanks result in specific and prolonged luciferase expression within tumor tissues, but not in normal tissue sites.16 GLV-1h68 has also been shown to have therapeutic effects on both breast and head and neck squamous cell carcinoma.17,18
We demonstrate that GLV-1h68 in SELP gel is able to efficiently infect and lyse a monolayer of cancer cells in vitro as effectively as GLV-1h68 in PBS. These findings show that virus embedded in SELP gel retains its infectious ability, and virus in SELP is able to effectively infect cells in direct contact with this solid gel, rather than requiring a solution for viral-cell interactions to occur. Perhaps more importantly, the SELP gel clearly conferred dramatic protective effects against normal vaccinia viral degradation at body temperature. When incubated in vitro at 37°C, GLV-1h68 in SELP at 1 week retained over 1000-fold greater infectious plaque-forming units as compared with GLV-1h68 in PBS. GLV-1h68 in PBS could not be detected past 1 week, whereas GLV- 1h68 in SELP remained detectable at 4 weeks. These in vitro studies showed that SELP both protects GLV-1h68 integrity and permits effective viral infection of cancer cells in direct contact with the SELP gel.
Direct intratumoral injection of GLV-1h68 in SELP resulted in similar tumor volume regression as GLV-1h68 in PBS. The SELP does not seem to hold a significant advantage over PBS as a viral medium when applied as an intratumoral injection into the center of a 3D tumor sphere, in which early viral diffusion and tumor penetration is likely similar between SELP liquid (before its transition to a solid gel) and aqueous PBS (Figure 3C). The structure of an intact tumor likely facilitated containment of the injected virus, permitting equal infection efficiency by the virus in either a SELP gel or aqueous matrix.
In contrast, SELP clearly held an advantage over PBS when applied topically intraoperatively against a flat surface of disease. To maintain clinical relevance, we studied two different models of intraoperative residual disease. The first was a high volume model of residual disease, to reflect a surgical scenario of incomplete resection with gross residual disease left behind. The second was a low volume model of residual disease, to reflect a surgical scenario of near-complete resection with microscopic residual disease. Both models demonstrated increased peak transgene expression (Figures 4B, 4C, 5A, and 5B) and improved tumor volume reduction (Figures 4D and 5C) for GLV-1h68 in SELP as compared with GLV-1h68 in PBS. In this postsurgical resection setting, the SELP likely allows the virus to maintain prolonged contact with a residual layer of tumor, and also facilitates continuous release and infection. In contrast, virus in aqueous solution may have only transient contact with the tumor tissue, with excess virus escaping into the surgical cavity and then being cleared by the host immune system.
During our in vitro gel elution studies, we found that assessing viral titers inside SELP gel was made possible by the disruption of solid SELP to tiny SELP particles through mechanical shaking. We reasoned that these SELP particles may enhance viral infection by increasing gel surface area, viral contact with tumor tissue, and viral elution from the gel. Therefore, we assessed the ability of using these SELP gel particles, as compared to solid SELP gel and PBS, as a matrix for GLV-1h68 in treating low volume residual disease (see Figure 5). We noted that SELP particles supported the highest levels of luciferase expression and the greatest tumor volume responses in this model, with a slight but statistically insignificant advantage over solid SELP gel.
Tumor sections were stained for β-galactosidase expression by GLV-1h68 for histologic examination of the tumor surface adjacent to the viral matrix (see Figure 6). Both SELP solid gel and SELP gel particles facilitated more intense, widespread, and durable viral gene expression as compared to PBS as a viral medium. Tissue expression of viral β-galactosidase expression was similar between virus in SELP and virus in SELP particles, suggesting that the potential advantages of gel fragmentation into particles are minor. More importantly, these results suggest that the SELP allows for continued viral release, resulting in prolonged tumor infection. The sustained release of viral particles from the gradually biodegrading SELP, which also is able to preserve its infectious ability, results in an improved therapeutic effect.
Figure 6.
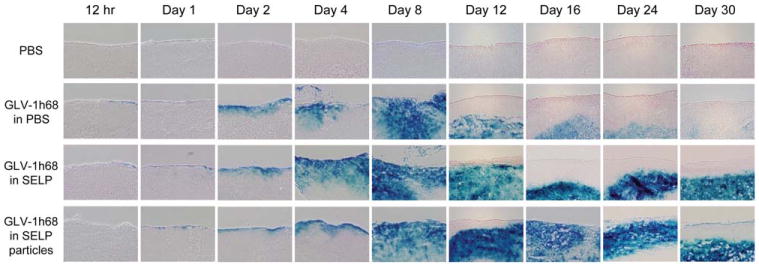
Topical GLV-1h68 in silk-elastin-like protein polymer (SELP) induces prolonged and enhanced β-galactosidase expression. A time course of β-galactosidase histochemical staining was performed on excised, low-volume residual disease, postresection tumor specimens treated with topical GLV-1h68 in phosphate-buffered saline (PBS), SELP, or SELP particles to assess lacZ expression as a measure of viral infection (n = 2 per group). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
GLV-1h68 has an ability to selectively infect malignant tumors, making it an attractive candidate as a therapeutic vector.8,17–19 Most oncolytic viruses are natural pathogens, and even with viral attenuation strategies their safety for clinical use remains a serious concern. In contrast, vaccinia virus has a unique history in its widespread application as a smallpox vaccine that has resulted in an established track record of safety in humans. Toxicities related to vaccinia administration occur in <0.1% of cases, and may be effectively addressed with immunoglobulin administration.20 The routine administration of vaccinia in the United States ended in 1972, and the historical application of vaccinia in humans provides strong evidence of its safety as a therapeutic agent. We previously injected GLV-1h68 directly into normal murine muscle, liver, thyroid, and testes. Virus was found to be rapidly cleared in these tissues, with no evidence of tissue toxicity on histologic examination after 10 days.8 Additional studies have shown a lack of histologic hepatic, brain, or lung toxicity after intravenous administration of oncolytic vaccinia in mice bearing widespread breast cancer metastases.21
In conclusion, we demonstrate that a SELP gel matrix significantly potentiates the efficacy of intraoperative oncolytic vaccinia therapy after incomplete tumor resection. The use of oncolytic viral therapy after surgical resection is intuitively attractive by permitting direct viral application to a layer of low volume residual disease. In this scenario, a cold SELP solution applied to the residual disease rapidly solidifies to a solid biodegradable gel at body temperature. The SELP gel matrix presents many advantages including: (1) prolonged physical contact of virus against the tumor surface, (2) effective viral infection of the tumor surface, (3) protection of viral integrity within the SELP gel, and (4) slow release of virus as the SELP gradually biodegrades over time. These promising preclinical findings support the design of future clinical trials aimed at optimizing methods of intraoperative oncolytic viral therapy.
Acknowledgments
Contract grant sponsor: This study was supported by grants T32CA009685 from the National Cancer Institute (Daniel Price) and R21DE019015 from the National Institute for Dental and Craniofacial Research and a Clinical Innovator Award from the Flight Attendant Medical Research Institute (Richard J. Wong).
Footnotes
Conflict of interest statement: Nanhai Chen is an employee, stockholder, and inventor of a relevant patent at Genelux Corporation; Aladar Szalay is an employee, stockholder, and inventor of a relevant patent at Genelux Corporation; Yong Yu is an employee and inventor of a relevant patent at Genelux Corporation; Joseph Cappello was an employee at Protein Polymer Technologies, and is currently an employee at Genelux Corporation.
References
- 1.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005;11:180–195. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 4.Sauthoff H, Hu J, Maca C, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systematically at late time points. Hum Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 5.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67:10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 6.McKee TD, Grandi P, Mok W, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 7.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–3802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SF, Price DL, Chen CH, et al. Oncolytic vaccinia virotherapy of ana-plastic thyroid cancer in vivo. J Clin Endocrinol Metab. 2008;93:4403–4407. doi: 10.1210/jc.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong RJ, Chan MK, Yu Z, et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic virus expressing interleukin 12. Clin Cancer Res. 2004;10(1 Pt 1):251–259. doi: 10.1158/1078-0432.CCR-0197-3. [DOI] [PubMed] [Google Scholar]
- 10.Cappello J, Crissman J, Dorman M, et al. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 11.Cappello J, Crissman JW, Crissman M, et al. In-situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. J Control Release. 1998;53:105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Adv Drug Deliv Rev. 2010;62:1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Hwang D, Moolchandani V, Dandu R, Haider M, Cappello J, Ghandehari H. Influence of polymer structure and biodegradation on DNA release from silk-elastinlike protein polymer hydrogels. Int J Pharm. 2009;368:215–219. doi: 10.1016/j.ijpharm.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24:773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 15.Choi JW, Kang E, Kwon OJ, et al. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther. 2013;20:880–892. doi: 10.1038/gt.2013.10. [DOI] [PubMed] [Google Scholar]
- 16.Lin SF, Yu Z, Riedl C, et al. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery. 2007;142:976–983. doi: 10.1016/j.surg.2007.09.017. discussion 984. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Yu YA, Wang E, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, Li S, Brader P, et al. Oncolytic vaccinia therapy of squamous cell carcinoma. Mol Cancer. 2009;8:45. doi: 10.1186/1476-4598-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 20.Sharp JC, Fletcher WB. Experience of anti-vaccinia immunoglobulin in the United Kingdom. Lancet. 1973;1:656–659. doi: 10.1016/s0140-6736(73)92215-0. [DOI] [PubMed] [Google Scholar]
- 21.Gholami S, Chen CH, Lou E, et al. Vaccinia virus GLV-1h153 is effective in treating and preventing metastatic triple-negative breast cancer. Ann Surg. 2012;256:437–445. doi: 10.1097/SLA.0b013e3182654572. [DOI] [PubMed] [Google Scholar]


