Summary
Objectives
While surgery with or without adjuvant radiation therapy (RT) is the standard of care for oral cavity cancer (OCC), a select group requires nonsurgical treatment. We provide a single-institution experience using definitive chemotherapy and RT for primary OCC.
Materials and methods
We examined 73 patients with previously untreated, non-metastatic primary OCC treated definitively from 1990 to 2011. There were 39 male and 34 female, with a median age of 63 years (range, 35–89). The disease distribution was Stage I and II (7% each), Stage III (14%), and Stage IV (73%). Oral tongue was the most common (48%), followed by floor of mouth (19%), retromolar trigone (13.7%), and others (8.2%). Median tumor dose was 70 Gy. Sixty-two percent of patients (n = 45) were treated with concurrent chemotherapy, predominantly platinum-based.
Results
Median follow-up among surviving patients was 73.1 months (interquartile range 14.2– 81.4 months). Actuarial 5-year overall survival was 15%. Incidences of locoregional and distant failures were 41.1% and 20.5%, respectively. Kaplan-Meier estimated 5-year rates of locoregional control and freedom from distant metastasis were 37% and 70%, respectively. Mucositis was the most common ≥ Grade 3 acute toxicity (49%). Incidences of Grade 3 late dysphagia and trismus were 15% and 13%, respectively.
Conclusion
This study demonstrates over 20 years of experience using definitive chemoradiation for OCC at our institution. Our results illustrate the challenges in treating patients with advanced disease who are not surgical candidates, and the need for adequate and early treatment to prevent distant disease and improve survival outcomes.
Keywords: Oral cavity cancer, Head and neck cancer, Definitive radiation
Introduction
Although the worldwide incidence of oral cavity cancer (OCC) has fallen considerably in developed countries in recent years, largely due to the decreased use of tobacco, it remains one of the more common cancers worldwide, with an incidence of 300,000 in 2012 [1–3]. Whereas oropharyngeal cancer (OPC) has been directly linked to the presence of human papillomavirus (HPV), and consequently, there has been a rise in HPV-associated malignancies, the link between OCC and HPV is less clear [1,4]. This is a notable distinction from the established connection to tobacco and alcohol use, which have both been found to be strong risk factors for OPC as well as OCC [4].
National guidelines recommend surgery, often with the addition of postoperative radiotherapy (RT) with or without chemotherapy if adverse pathologic features are present [5]. The 5-year overall survival rates for these tumors have not shown significant improvement with these regimens, remaining between 50% and 60% [5,6]. As continued advancements in reconstructive surgery have led to better cosmetic and functional results, surgical management remains the primary modality of treatment [7].
In patients who are not surgical candidates, either due to medical comorbidity, unresectable disease, or patient preference, definitive RT-based approaches are possible [7,8]. Although treatment employing concurrent chemotherapy and RT (CCRT) has been shown to be advantageous in terms of both local control and overall survival versus RT alone, clinical trials utilizing CCRT for advanced OCC patients are limited, largely due to perceptions of unacceptable toxicity and worse efficacy compared to surgery [7,9,10]. A recent single institution retrospective series that evaluated definitive CCRT for patients with advanced (stage III–IV) OCC reported an overall survival rate that exceed 65% with acceptable rates of toxicity [9]. Other studies that have examined primary CCRT also reported promising rates of organ preservation and overall survival, including those patients who presented with tumor invasion of the bone or cartilage [9,11,12].
In our institution, patients who are not candidates for surgery – either with unresectable tumors, locally advanced disease, or concerns about local morbidity – are treated with CCRT. Herein we reviewed our experience in treating locally advanced OCC with primary RT with or without concurrent chemotherapy.
Materials and methods
After obtaining approval from our Institutional Review Board, we retrospectively reviewed the charts of patients at our institution who were diagnosed with previously untreated non-metastatic primary OCC, and subsequently received definitive RT from 1990 to 2011. All oral cavity sites and all stages were included. Charts were reviewed via a computerized database, and data on patient demographics, tumor histology, stage, acute and late toxicity, and radiation and chemotherapy treatments were collected.
Radiotherapy
Patients treated with 2D or 3DCRT were treated with opposed laterals (n = 50, 68.5%). Intensity-modulated RT (IMRT) began to be incorporated routinely for patients treated after 2004, and was utilized in all patients by 2006 (n = 23, 31.5%). There were eight patients who also received a brachytherapy boost to a median dose of 25 Gy, with a median total tumor dose of 74 Gy (range 70.8–81 Gy). All patients were simulated and treated with the use of an Aquaplast head/neck mask (Aquaplast, Wyckoff, NJ). When cervical lymph nodes (LN) were treated, the shoulders were included in the mask for immobilization. For patients receiving IMRT or 3DCRT, cross-axial images were used to individually outline 3-mm interval slices for delineating target volumes. As patients were not treated surgically, the gross visible tumor on clinical exam and imaging defined the gross tumor volume (GTV). The clinical target volume (CTV) represented areas at high-risk for sub-clinical disease, and was established by evaluation of the primary tumor size along with the extent of involvement of regional LN to establish a margin around the GTV. Typically, there was a 1.0–1.5 cm CTV60-66 margin outlined around primary tumor, and involved nodal regions were included as well. The CTV54 included LN areas that were uninvolved and at lower risk for microscopic spread. At the discretion of the treating radiation oncologist, LN level V was excluded for those with node-negative disease; levels Ib, II, III, IV were always included in the radiation portal.
Margins of 0.3 cm were added to define the planning target volume (PTV): the gross tumor constituted the PTV70, high-risk subclinical disease established the PTV60-66, while low-risk subclinical disease was included in the PTV54. For those who received 3DCRT or IMRT, normal structures were outlined, including the brainstem, spinal cord, optic nerves and chiasm, right and left cochlea, parotid glands, and mandible.
Chemotherapy
Patients treated since 2000 were given concurrent systemic chemotherapy. The majority of patients who were treated with chemotherapy received single-agent cisplatin (n = 23, 31.5%) during RT, with planned two to three cycles (100 mg/m2) on days 1, 22, and 43; an additional 4% of patients received cisplatin with a second agent. As an alternative, based on potential toxicities, preexisting medical conditions, and patient preference, carboplatin was given alone (70 mg/m2), or in combination with either 5-fluorouracil (600 mg/m2) or paclitaxel (50 mg/m2), to 19% of patients, for 4 days as a daily continuous infusion. Other patients were given single-agent cetuximab, or in combination with paclitaxel, with an initial loading dose (400 mg/m2), followed by seven weekly cycles (250 mg/m2).
Follow-up
Patients were evaluated on a weekly basis by the treating radiation oncologist while undergoing RT. Post-treatment, patients were evaluated every 2–3 months for 2 years, and every 4– 6 months thereafter in coordination with the radiation oncologist, medical oncologist, and surgical oncologist. Each follow-up visit consisted of a comprehensive head and neck examination and a flexible fiberoptic endoscopy when indicated. Toxicities at each visit were graded utilizing the Common Toxicity Criteria for Adverse Events (CTCAE) v4.0. Approximately 3 months after treatment, patients received cross-axial imaging, which typically consisted of a positron emission tomography/computed tomography scan or magnetic resonance imaging in the past decade.
A total of 55 (73%) of patients received a percutaneous endoscopic gastrostomy (PEG) tube: 41 (56%) prophylactically (reflecting the prior institutional practice of routine PEG placement prior to treatment), seven acutely during treatment (10%), and six post-treatment (8%). While PEG tubes are no longer placed prophylactically, they are still placed during treatment if a patient cannot tolerate sufficient oral intake. Otherwise, patients were encouraged to continue mouth exercise and swallowing, which can also potentially reduce stricture formation and other late complications.
Statistical analysis
Utilizing the first day of RT as a starting point, locoregional control (LRC), freedom from distant metastasis (FFDM), and overall survival (OS) were calculated using the Kaplan–Meier method. Univariate analyses using Cox proportional hazards model were performed on the following potential prognostic factors: age at diagnosis, T stage, N stage, and chemotherapy. All analyses were calculated using R statistical software version 3.1.2 (http://www.r-project.org/).
Results
Complete patient characteristics are summarized in Table 1. Out of our prospectively managed oral cavity database of 502 patients, we identified 73 who were treated definitively with RT and included in this analysis. There were 39 male (53.4%) and 34 female (46.5%) patients, with a median age of 63 years (range 35–89 years).
Table 1.
Patient and treatment characteristics.
| N | % | |
|---|---|---|
| Gender | ||
| Male | 39 | (53.4) |
| Female | 34 | (46.6) |
| Smoking | ||
| >10 pack years | 47 | (64.4) |
| <10 pack years | 19 | (26.0) |
| Unknown | 7 | (9.6) |
| Disease site | ||
| Oral tongue | 35 | (47.9) |
| Floor of mouth | 14 | (19.2) |
| Retromolar trigone | 10 | (13.7) |
| Buccal mucosa | 4 | (5.5) |
| Gingiva | 4 | (5.5) |
| Lip | 2 | (2.7) |
| Hard palate | 2 | (2.7) |
| Alveolar ridge | 2 | (2.7) |
| T stage | ||
| T1 | 5 | (6.8) |
| T2 | 10 | (13.7) |
| T3 | 11 | (15.1) |
| T4 | 47 | (64.4) |
| N stage | ||
| N0 | 24 | (32.9) |
| N1 | 12 | (16.4) |
| N2 | 31 | (42.5) |
| N3 | 6 | (8.2) |
| AJCC stage | ||
| I | 5 | (6.8) |
| II | 5 | (6.8) |
| III | 10 | (13.7) |
| IV | 53 | (72.6) |
| Histology | ||
| SCC | 73 | (100) |
| Chemotherapy | ||
| Concurrent | 45 | (61.6) |
| Cisplatin | 23 | (31.5) |
| Carboplatin/5-FU | 6 | (8.2) |
| Carboplatin/paclitaxel | 6 | (8.2) |
| Cetuximab | 4 | (5.5) |
| Other | 10 | (13.7) |
| None | 24 | (32.9) |
Abbreviations: SCC = squamous cell carcinoma; 5-FU = 5-fluorouracil.
Tumor characteristics
Most patients (72.6%, n = 53) were diagnosed with Stage IV disease, and a majority (64.4%, n = 47) had T4 tumors on presentation. All patients had SCC histology. While 42.5% (n = 31) of patients presented with N2 disease, there were nearly 33% who were N0 at presentation. Oral tongue cancer was most common subsite (47.9%; n = 35), followed by floor of mouth (19.1%, n = 14) and retromolar trigone (12.3%, n = 9, respectively). Full tumor details can be seen in Table 1.
Treatment
Forty-five (61.6%) patients received concurrent chemotherapy and four (5.5%) received induction therapy only, at the discretion of the medical oncologist. Patient refusal, inability to tolerate systemic therapy, advanced age, and comorbidities were the most common reasons for omission of chemotherapy. As chemotherapy was not given routinely with RT for locally advanced disease until after the year 2000, an additional 10 patients without the aforementioned reasons also did not receive systemic therapy.
RT doses ranged from 8.24 to 73.1 Gy, with a median dose of 70 Gy; one patient could not tolerate treatment as a result of tumor progression and therefore only received 8.24 Gy. Other reasons for subtherapeutic (<60 Gy) RT doses included: hospitalization and inability to complete RT (n = 4), noncompliance (n = 3), and death during RT (n = 1). Most (n = 49, 67.1%) patients received ≥66 Gy.
Locoregional control
There were 30 (41.1%) locoregional failures (LRF) in this cohort; 23 of which (31.5%) were local failures (LF) alone, four (5.5%) were regional failures (RF) alone, and three (4.1%) had both LF and RF. For those with local recurrences, the most common site was the oral tongue (n = 15), followed by the floor of mouth (n = 4). The vast majority (n = 20, 76.9%) of patients who failed locally presented with American Joint Committee on Cancer (AJCC) Stage IV disease (n = 44), while there were four LF that occurred in patients with early (Stage I–II) disease (n = 10).
In those with RF, the majority had an oral tongue primary tumor (n = 4). In total, there were nine patients (12.3%) who required a post-RT neck dissection, two of whom were subsequently found to have evidence of malignancy.
The 5-year Kaplan–Meier estimated locoregional control (LRC) was 37.4% (Fig. 1). Median time to LRF was 5.6 months (range 0.3–32.4 months). As an adverse prognostic factor for LRC, female gender trended toward statistical significance (p = 0.092). Other prognostic factors were not found to be significant, as shown in Table 2.
Fig. 1.
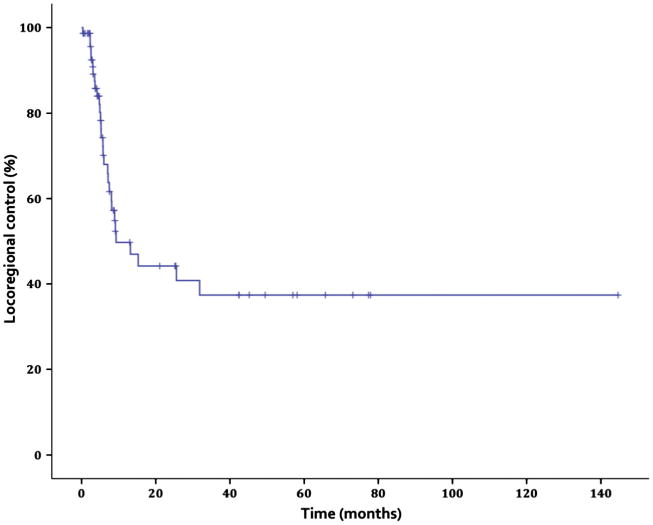
Locoregional control.
Table 2.
Univariate analysis: treatment outcomes.
| Variable | OS | LRF | DM |
|---|---|---|---|
|
| |||
| p value | |||
| Age (>65 vs. <65) | 0.092 | 0.526 | 0.790 |
| Gender (female vs. male) | 0.083 | 0.092 | 0.363 |
| KPS (≥90 vs. <90) | 0.004 | 0.435 | 0.173 |
| T stage (T3–4 vs. T1–2) | 0.063 | 0.313 | 0.018 |
| Stage IV vs. Stage I-III | 0.032 | 0.107 | 0.007 |
| N stage (N2–3 vs. N0–1) | 0.911 | 0.842 | 0.057 |
| RT dose <60 Gy vs. ≥60 Gy | 0.015 | 0.927 | 0.444 |
Abbreviations: OS = overall survival; LRF = locoregional failure; DM = distant metastases; RT = radiotherapy.
Distant metastasis
There were 15 patients (20.5%) who developed distant metastases (DM), with most metastasizing to the lung (n = 10). The 5-year Kaplan–Meier estimated freedom from distant metastasis (FFDM) was 70.2%, with a median time to DM of 4 months (Fig. 2).
Fig. 2.
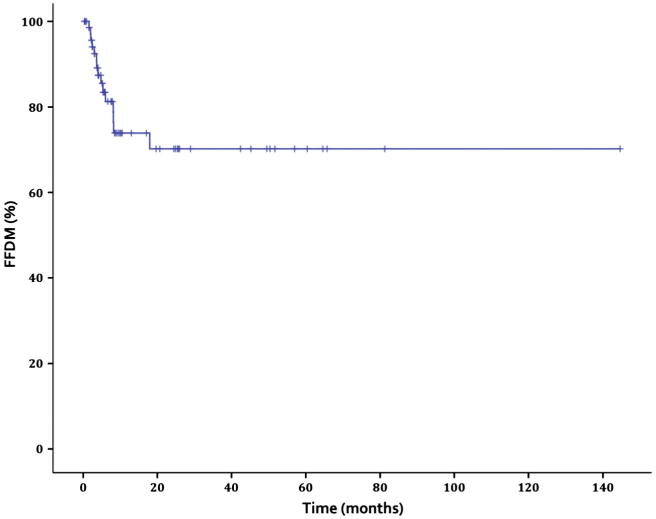
Freedom from distant metastasis.
Advanced T stage (T3–T4) and AJCC Stage IV disease were both found to be significant adverse prognostic factors for DM on univariate analysis (p = 0.018 and p = 0.007, respectively). The presence of N2–N3 disease as well trended toward statistical significance for development of DM (p = 0.057). Age greater than 65 was not found to be a significant prognostic factor (p = 0.790). Full DM prognostic data is shown in Table 3.
Table 3.
Acute toxicities.
| Total | Grade 0 | (%) | Grade 1 | (%) | Grade 2 | (%) | Grade 3 | (%) | Grade 4 | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dysphagia | 51 | 20 | (39) | 16 | (31) | 10 | (20) | 5 | (10) | 0 | (0) |
| Xerostomia | 53 | 15 | (28) | 21 | (40) | 15 | (28) | 2 | (4) | 0 | (0) |
| Voice changes | 44 | 32 | (73) | 9 | (20) | 3 | (7) | 0 | (0) | 0 | (0) |
| Nausea | 42 | 32 | (76) | 8 | (19) | 0 | (0) | 2 | (5) | 0 | (0) |
| Dermatitis | 55 | 10 | (18) | 19 | (35) | 21 | (38) | 5 | (9) | 0 | (0) |
| Mucositis | 62 | 6.0 | (10) | 10 | (16) | 16 | (26) | 27 | (44) | 3 | (5) |
Abbreviation: Total = number of patients with documentation of absence/presence of toxicity; analysis limited to these patients.
Overall survival
Median follow-up time among surviving patients was 73.1 months (interquartile range 14.2–81.4 months). The actuarial 5-year OS was 15% (Fig. 3). Disease-specific survival was 52%, 43.8%, and 38.1% at 1, 2, and 3 years, respectively (Fig. 4). There were a total of 62 deaths, 32 (51.6%) of which were secondary to either the primary tumor or to distant metastases. Eight deaths occurred during or within three months of treatment, and were attributed to disease progression (n = 3), respiratory failure unrelated to RT (n = 3), or failure to thrive (n = 2).
Fig. 3.
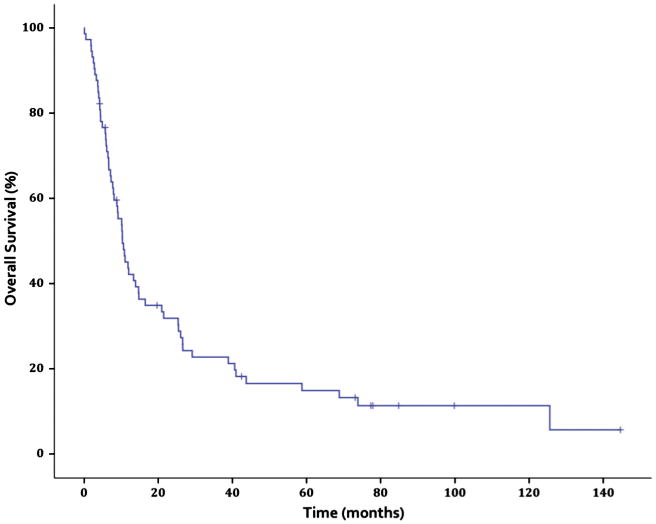
Overall survival.
Fig. 4.
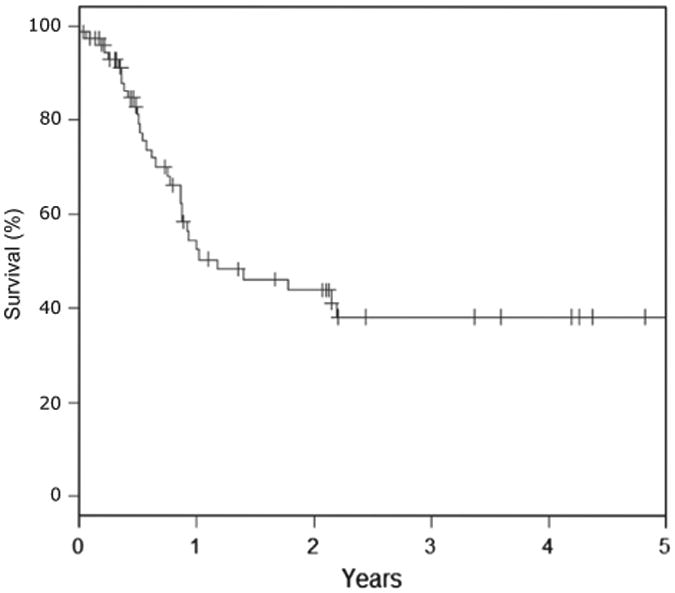
Disease-specific survival.
On univariate analysis, treatment with <60 Gy was associated with a greater risk of death compared to treatment with ≥60 Gy (p = 0.015). A Karnofsky performance status (KPS) of <90 was found to be associated with a greater risk of death as well (p = 0.004). As adverse prognostic factors, advanced T stage, age, and female gender trended toward statistical significance (p = 0.063, 0.092, and 0.083, respectively). Nodal status was not associated with poorer OS (p = 0.911). See Table 2 for full details.
Acute and late toxicity
Full acute toxicity details can be seen in Table 3. Mucositis was the most common Grade ≥3 acute toxicity present in 49% of patients (n = 23 for conventional RT; n = 7 for IMRT). Three patients experienced Grade 4 mucositis during treatment requiring hospitalization. Other significant Grade 2 acute toxicities included dermatitis (38%), xerostomia (28%), mucositis (26%), and dysphagia (20%). While undergoing treatment, two patients experienced respiratory distress that was unrelated to RT, and deceased prior to treatment completion.
There were 28 patients with data available for late toxicity evaluation (Table 4). There were no late grade 4 toxicities. Eight patients experienced significant Grade 3 late toxicity: dysphagia (n = 4), trismus (n = 2), xerostomia (n = 1), and mucositis (n = 1). Post-RT PEG placement was required for six patients: two were due to severe dysphagia, a third developed severe oral pain secondary to ulcerative tongue lesions, and one was a result of a bulky tumor recurrence; there were two additional patients PEG placement after treatment for unknown reasons. Osteoradionecrosis was reported in a total of 5 patients (6.8%).
Table 4.
Late toxicities.
| Total | Grade 0 | (%) | Grade 1 | (%) | Grade 2 | (%) | Grade 3 | (%) | Grade 4 | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dysphagia | 27 | 11 | (41) | 6 | (22) | 6 | (22) | 4 | (15) | 0 | (0) |
| Xerostomia | 28 | 3 | (11) | 18 | (64) | 6 | (21) | 1 | (4) | 0 | (0) |
| Mucositis | 13 | 7 | (54) | 3 | (23) | 2 | (15) | 1 | (8) | 0 | (0) |
| Trismus | 16 | 3 | (19) | 6 | (38) | 5 | (31) | 2 | (13) | 0 | (0) |
Abbreviation: Total = number of patients with documentation of absence/presence of toxicity; analysis limited to these patients.
Discussion
This series evaluated the outcomes and prognostic factors for patients with OCC who were not surgical candidates, with a significant majority of whom had advanced-stage disease. The 5-year OS was 15%, which was lower than other recent studies examining similarly large proportions of advanced-stage cancer, with OS rates between 37% and 76% (Table 5) [9,14,15]. Importantly, our series contained a significant proportion of patients with medically unresectable disease or who were medically unfit for surgery. Of the 35 patients in our cohort with oral tongue cancer, the majority, 30, had advanced disease not amenable to brachytherapy; the remaining five patients received brachytherapy. Additionally, due to either comorbidities or the initiation of treatment before the routine use of chemotherapy along with RT for locally advanced disease in the year 2000, only 62% of patients were treated with CCRT, which also likely resulted in inferior outcomes. Accordingly, in other definitive series in which OS was greater (between 67% and 76% at 5 years), all patients had received CCRT, which has been shown to result in significantly better rates of OS and LRC [9,14,16]. Our 5-year rates of LRC and FFDM were 37% and 70%, respectively, which were still comparable to similar studies of definitive treatment for OCC (Table 5).
Table 5.
Definitive OCC published studies.
| Author | Year | Number primary RT | Number primary CCRT | Median F/U (mo) | T3-T4 (%) | N0-N1 (%) | N2-N3 (%) | Chemo (%) | LC% (y) | LRC% (y) | OS% (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fazekas | 1980 | 73 | 73 | n/a | 96 | N0: 29 | N1-N3: 70 | 50 | n/a | n/a | RT:11 (5) CCRT: 12 (5) |
| Turner | 1996 | 274 | 59 | n/a | 47 | 94 | 6 | 18 | 61 (5) | n/a | 55 (5) |
| Murthy | 2010 | 159 | 64 | 24 | 52 | 78 | 19 | 29 | 34 (3) | 31 (3) | DFS: 30 (3) |
| Stenson | 2010 | 0 | 111 | 39 | 78 | n/a | n/a | 100 | n/a | n/a | 67 (5) |
| Pederson | 2011 | 21 | 21 | 53 | 76 | 48 | 52 | 100 | n/a | 90 (5) | 76 (5) |
| Studer | 2012 | 44 | 30 | 12 | 62 | N0: 27 | N1a-N2c: 64 | 79 | n/a | 37 (4) | 37 (4) |
| Present study | 2015 | 28 | 45 | 73 | 80 | 49 | 51 | 62 | n/a | 37 (5) | 15 (5) |
Abbreviations: RT = radiation therapy; OCC = oral cavity cancer; CCRT = concurrent chemoradiation; F/U = follow-up; mo = months; y = years; LC = local control; LRC = locoregional control; OS = overall survival; DFS = disease free survival.
While others have found T stage, age, and nodal status to be prognostic factors for LRC, the present study did not find these to have a significant effect. [17]. However, our cohort had over 75% of patients with T3–T4 disease on presentation, and over 85% with AJCC Stage III or greater, which may have masked the influence of most prognostic factors. Advanced T stage (T3–T4) at initial diagnosis, however, was found to be significant risk factor for DM in our series (p = 0.018). Studies have shown that advanced pathologic or clinical T stage alone can increase the likelihood of DM; even in patients who have locally controlled disease, the development of subsequent DM can subsequently lead to a reduced rate of survival [18–20]. Alternatively, some have suggested that the overall AJCC tumor stage – factoring in nodal status – plays a more significant role in the increasing the likelihood of DM [21,22]. Accordingly, in our series, all 15 cases of DM occurred exclusively in patients with AJCC Stage IV disease, a result that demonstrated statistical significance when compared to those with Stage I–III disease (p = 0.007).
Patients who received RT doses of 60 Gy or greater were found to have significantly better OS than those who received less than 60 Gy (p = 0.015). As doses of 60 Gy or less are more typical of postoperative patients, it follows that those receiving this dose as a definitive treatment performed more poorly; even in postoperative patients, doses less than 60 Gy demonstrated inferior efficacy [23,24]. While this may be a reflection of greater efficacy with doses higher than 60 Gy, it may also be confounded by the fact that in this cohort, those who received lower, subtherapeutic doses had clinically worse or progressive disease that sometimes necessitated shortened treatment courses.
Limiting factors in this study included the disproportionately high amount of advanced-stage disease, which likely mitigated the effect of multiple prognostic factors. Additionally, further prospective trials need to establish the most effective treatment regimen for delivery of definitive CCRT for OCC patients who are not surgical candidates. Although outcomes in this series were relatively poor, the vast majority of patients had advanced disease, and outcomes were likely influenced by the proportion of patients who did not receive radiosensitizing chemotherapy as a result of the year in which they were treated. The addition of chemotherapy to a definitive RT regimen offers improved recurrence-free survival, and patients can also often be spared significant functional impairments (such as a total glossectomy for T4 oral tongue cancer) [9].
The use of IMRT has shown promise in improving patient outcomes; rates of LRC have even exceeded 90% in smaller studies utilizing CCRT with IMRT rather than 3DCRT [14]. The rate of osteoradionecrosis in the present study was 6.8%, which falls in line with other studies – ranging from 5.9% to 18.4% – in which both conventional and IMRT were utilized [9,25]. However, as IMRT has been shown to afford lower rates osteoradionecrosis, rates in modern IMRT series have dropped to between 1% and 14% [14,15,26,27]. In patients who are surgical candidates, however, post-operative IMRT rather than IMRT alone may provide the greatest benefit [15]. For reasons not clearly defined, however, the rates of LRC for OCC treated with either definitive or post-operative IMRT are generally poorer than those of OPC [15,28].
In determining a more effective and comprehensive treatment plan to improve outcomes in those with OCC, an approach that hinges on the genetic pillars of these tumors may lead to greater efficacy. The presence of HPV DNA or p16 positivity in those with OPC plays a significant beneficial role in treatment outcomes, although the role of HPV positivity in OCC remains less clearly defined [29–31]. A recent analysis did demonstrate that those with p16-positive tumors of the oral cavity, hypopharynx, and larynx had significantly better overall and progression-free survival than those with p16-negative disease; the implications of these results must be taken in context, however, as the significance of p16 decoupled from HPV is not fully established [32]. The direction of genetic sequencing has uncovered many of the mutations unique to OCC – but it has also illustrated their complex genetic heterogeneity [29]. While this may complicate the horizon of future therapeutic avenues to an extent, these alterations can also lead to the development of targeted therapies to improve treatment for a disease that has not yet shown significant response to the current standards of care [29,33].
Conclusion
Although definitive CCRT is a viable option for those with either unresectable disease, or who are not surgical candidates, steps need to be taken in order to help improve survival and disease control in this cohort. Earlier detection, increasing the use of multi-modality therapy and targeted radiation, as well as gaining a greater understanding of the clinical utility of the biology behind these tumors should be pursued, in order to continue to improve future patient outcomes.
Acknowledgments
Funding: This report is not supported by specific funding; there are no financial disclosures or conflicts of interest from any authors.
Footnotes
Conflict of interest statement: None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 2011;9 doi: 10.1002/14651858.CD006205.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer [n/a–n/a] 2014 doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 2.2013. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 6.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–51. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brizel DM, Esclamado R. Concurrent chemoradiotherapy for locally advanced, nonmetastatic, squamous carcinoma of the head and neck: consensus, controversy, and conundrum. J Clin Oncol. 2006;24:2612–7. doi: 10.1200/JCO.2005.05.2829. [DOI] [PubMed] [Google Scholar]
- 8.Yao M, Smith RB, Graham MM, Hoffman HT, Tan H, Funk GF, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biology Phys. 2005;63:991–9. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 9.Stenson KM, Kunnavakkam R, Cohen EEW, Portugal LD, Blair E, Haraf DJ, et al. Chemoradiation for patients with advanced oral cavity cancer. Laryngoscope. 2010;120:93–9. doi: 10.1002/lary.20716. [DOI] [PubMed] [Google Scholar]
- 10.Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J Clin Oncol. 2005;23:1125–35. doi: 10.1200/JCO.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Giralt JL, Gonzalez J, del Campo JM, Maldonado J, Sanz X, Pamias J, et al. Preoperative induction chemotherapy followed by concurrent chemoradiotherapy in advanced carcinoma of the oral cavity and oropharynx. Cancer. 2000;89:939–45. doi: 10.1002/1097-0142(20000901)89:5<939::aid-cncr1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Samant S, Robbins KT, Kumar P, Ma JZ, Vieira F, Hanchett C. Bone or cartilage invasion by advanced head and neck cancer: intra-arterial supradose cisplatin chemotherapy and concomitant radiotherapy for organ preservation. Arch Otolaryng Head Neck Surg. 2001;127:1451–6. doi: 10.1001/archotol.127.12.1451. [DOI] [PubMed] [Google Scholar]
- 14.Pederson AW, Salama JK, Witt ME, Stenson KM, Blair EA, Vokes EE, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for organ preservation of locoregionally advanced oral cavity cancer. Am J Clin Oncol. 2011;34:356–61. doi: 10.1097/COC.0b013e3181e8420b. [DOI] [PubMed] [Google Scholar]
- 15.Studer G, Brown M, Bredell M, Graetz KW, Huber G, Linsenmeier C, et al. Follow up after IMRT in oral cavity cancer: update. Radiat Oncol. 2012;7:84. doi: 10.1186/1748-717X-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama JK, Seiwert TY, Vokes EE. Chemoradiotherapy for locally advanced head and neck cancer. J Clin Oncol. 2007;25:4118–26. doi: 10.1200/JCO.2007.12.2697. [DOI] [PubMed] [Google Scholar]
- 17.Murthy V, Agarwal J, Laskar SG, Gupta T, Budrukkar A, Pai P, et al. Analysis of prognostic factors in 1180 patients with oral cavity primary cancer treated with definitive or adjuvant radiotherapy. J Cancer Res Ther. 2010;6:282. doi: 10.4103/0973-1482.73360. [DOI] [PubMed] [Google Scholar]
- 18.León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–6. doi: 10.1002/1097-0347(200010)22:7<680::aid-hed7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Leemans CR, Tiwari R, Nauta J, Van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–6. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Liao CT, Wang HM, Chang JTC, Ng SH, Hsueh C, Lee LY, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer. 2007;110:1501–8. doi: 10.1002/cncr.22959. [DOI] [PubMed] [Google Scholar]
- 21.Troell RJ, Terris DJ. Detection of metastases from head and neck cancers. Laryngoscope. 1995;105:247–50. doi: 10.1288/00005537-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck. 1997;19:500–5. doi: 10.1002/(sici)1097-0347(199709)19:6<500::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Soo KC, Tan EH, Wee J, Lim D, Tai BC, Khoo ML, et al. Surgery and adjuvant radiotherapy vs concurrent chemoradiotherapy in stage III/IV nonmetastatic squamous cell head and neck cancer: a randomised comparison. Br J Cancer. 2005;93:279–86. doi: 10.1038/sj.bjc.6602696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Harrison LB, Fass DE, Armstrong JG, Shah JP, Strong EW. Postoperative radiation therapy for squamous cell carcinomas of the oral cavity and oropharynx: impact of therapy on patients with positive surgical margins. Int J Radiat Oncol Biol Phys. 1993;25:17–21. doi: 10.1016/0360-3016(93)90139-m. [DOI] [PubMed] [Google Scholar]
- 25.Turner SL, Slevin NJ, Gupta NK, Swindell R. Radical external beam radiotherapy for 333 squamous carcinomas of the oral cavity—evaluation of late morbidity and a watch policy for the clinically negative neck. Radiother Oncol. 1996;41:21–9. doi: 10.1016/s0167-8140(96)91785-5. [DOI] [PubMed] [Google Scholar]
- 26.Ben-David MA, Diamante M, Radawski JD, Vineberg KA, Stroup C, Murdoch-Kinch CA, et al. Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys. 2007;68:396–402. doi: 10.1016/j.ijrobp.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez DR, Estilo CL, Wolden SL, Zelefsky MJ, Kraus DH, Wong RJ, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e207–13. doi: 10.1016/j.ijrobp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 29.Riaz N, Morris LG, Lee W, Chan TA. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes Dis. 2014;1(1):75–86. doi: 10.1016/j.gendis.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.54.5228. JCO. 2013.54. 5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Disc. 2013;3:770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]


