Summary
Incidental detection of pancreatic neuroendocrine tumors (PNETs) has substantially increased over the last decade due to widespread use of advanced imaging studies. Reliable initial imaging-based characterization is crucial for the differential diagnosis from other exocrine neoplasms and to determine the appropriate management plan. Measurements of chromogranin A, pancreatic polypeptide, and calcitonin are recommended for the biochemical evaluation. A thorough medical history needs to be performed to rule out multiple endocrine neoplasia (MEN) type 1. The European Neuroendocrine Tumor Society (ENETS)/Tumor Node Metastasis (TNM) staging system together with a grading based on the Ki-67 proliferation index and mitotic counts has proven to give more appropriate prognostic information than the World Health Organization (WHO)/American Joint Committee on Cancer (AJCC) TNM staging but may still fail to safely differentiate benign from malignant lesions. Poorly differentiated PNETs generally present with metastases and are rarely amenable for resection. Well- or intermediately differentiated tumors ≥2 cm with imaging evidence of malignancy or with a Ki-67 >2% should be resected. It has been suggested that non-MEN related, nonfunctioning, and asymptomatic PNETs <2 cm with a Ki-67 index ≤2% carry a low risk of metastasis and may be observed in the absence of clinical or radiologic criteria of malignancy or progression, especially in older patients. However, because metastases may occur with long delay with smaller PNETS, physicians should consider patient age, lesion location, and the risks of operation, and patients not undergoing surgery need to be closely followed closely.
Introduction
Knowledge on the biology, diagnosis, localization, and treatment of pancreatic neuroendocrine tumors (PNETs) has dramatically grown in recent years, and several papers have been published addressing different areas. In a recent publication in this journal, Vinik provided a comprehensive review and described advances in PNET diagnosis and treatment (1). In this review, we aim to focus on the current state of image characterization, biochemical evaluation, molecular categorization, and management of PNETs that present as pancreatic incidentalomas (PIs).
PIs were first described by Kostiuk et al as a pancreatic tumor discovered serendipitously when performing an imaging test for symptoms unrelated to the pancreatic mass (2). Most PIs are discovered by percutaneous ultrasound (US) or computed tomography (CT), while some tumors may be discovered by endoscopic US (EUS). Incidental detection of pancreatic lesions has substantially increased over the last decade due to widespread use of advanced imaging studies such as multidetector row CT (MDCT) and magnetic resonance imaging (MRI) for diagnosing various conditions in the abdomen. In a recent study of 15,185 patients, the incidence of nonfunctioning PNETS increased twofold in the last few years (3).
The overall PI incidence is between 0.01 and 0.6% (4); however, when analyzing the pancreatic resection series, between 6 and 23% are incidentally discovered pancreatic neoplasms (5). Approximately 48 to 60% of the lesions are solid, 24 to 50% are malignant, and 20 to 47% are considered premalignant or potentially malignant. Cystic neoplasms (including intraductal papillary mucinous neoplasms [IPMNs], mucinous cystic neoplasms [MCNs], and serous cystadenomas [SCAs]) or solid lesions such as PNETs and intrapancreatic accessory spleen are commonly encountered PIs. The true incidence of neuroendocrine PI is not known; however, based on the largest series of PI, we can estimate that PNETs account for 9 to 19% of cases. Table 1 shows the diagnostic distribution of PIs reported in different series (6-9).
Table 1. Final Pathologic Diagnosis in Recent Series of PIa.
| Type of tumor | Winter et al (6) | Sachs et al (7) | Bruzoni et al (8) | Lahat et al (9) |
|---|---|---|---|---|
|
| ||||
| Adenocarcinoma | 19 (16) | 20 (18) | 17 (30) | 16 (25) |
|
| ||||
| PNETs | 11 (9) | 14 (13) | 11 (19) | 10 (16) |
| Benign | 7 | N/A | 7 | 7 |
| Malignant | 4 | N/A | 4 | 3 |
|
| ||||
| IPMN | 42 (36) | 22 (20) | 5 (9) | 15 (23) |
| Adenoma | 23 | 19 | 2 | 13 |
| Carcinoma | 19 | 3 | 3 | 2 |
|
| ||||
| Cystadenoma | 20 (17) | 20 (18) | 11 (19) | 10 (16) |
| Serous | N/A | 15 | 7 | -- |
| Mucinous | N/A | 5 | 4 | 10 |
|
| ||||
| Other | 26 (22) | 34 (31) | 13 (23) | 13 (20) |
|
| ||||
| Total | 118 | 110 | 57 | 64 |
Abbreviations: IPMN = intraductal papillary mucinous neoplasm; PI = pancreatic incidentaloma; PNET = pancreatic neuroendocrine tumor.
Data are shown as n or n (%)
PNETs are rare tumors that arise from the islet cells. They represent 2% of all pancreatic neoplasms, with a reported incidence of about 3 per million. PNETs ≤5 mm, called microadenomas, may occur in up to 10% in autopsy studies and account for 9 to 19% of PIs (10,11). PNETs may be functioning or nonfunctioning. Functioning tumors secrete a variety of hormones that cause specific syndromes. The clinical syndromes related to these tumors are specific enough to guide the biochemical work-up. Nonfunctioning tumors produce nonspecific peptides such as chromogranin A or may secrete low amounts of hormones, with pancreatic polypeptide and calcitonin as the most frequent. The lack of specific symptoms for nonfunctioning PNET may result in a late diagnosis with regional compressive or invasive symptoms (1,12).
Most PNETs are sporadic, but they may be associated to familial syndromes including multiple endocrine neoplasia (MEN) type 1, von Hippel-Lindau, type 1 neurofibromatosis (von Recklinghausen), and tuberous sclerosis complex. PNETs occurring as part of these syndromes are not considered PIs.
Imaging Characteristics
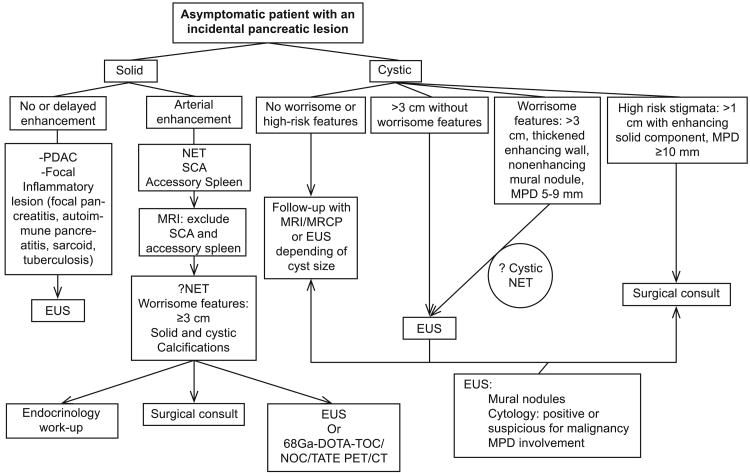
Reliable initial imaging-based characterization of PI is crucial to determine appropriate management (Fig. 1). Many nonfunctioning PNETs are detected on imaging studies and are now more common than functioning tumors. In a cohort of 148 patients with surgically verified PNET lesions, 60 (40%) were detected as PIs (13). In various clinical investigations assessing PNET diagnosis, MDCT and MRI have shown mean sensitivities of 73% (ranging from 63-82%) and 93% (ranging from 85-100%), respectively, and corresponding specificities of 96% (ranging from 83-100%) and 88% (ranging from 75-100%) (14).
Fig. 1.
Flow-chart for imaging work-up of incidental pancreatic lesions. CT = computed tomography; MPD = main pancreatic duct; MRI = magnetic resonance imaging; NET = neuroendocrine tumor; PDAC = pancreatic ductal adenocarcinoma; PET = positron emission tomography; SCA = serous cystadenoma.
The majority of PNETs are discovered in the body or tail and often display arterial enhancement. Approximately 17% have calcifications, and are generally solid. Cystic tumors are relatively rare, comprising 6.9% in a recent large series of PNETs (15). Larger lesions and PNETs in patients with familial syndromes more often show cystic changes (13). The latter can masquerade as other cystic pancreatic lesions. Unlike other pancreatic cysts, cystic PNETs present a uniform thick or irregular enhancing wall. Rarely (3%), these may present as thin-walled unilocular cysts that morphologically overlap with other cystic lesions such as pseudocysts, IPMNs, MCNs, or oligocystic SCAs. In these cases, further work-up with EUS and fluid analysis might be needed to reach a diagnosis (16). At presentation, 50 to 90% of nonfunctioning PNETs are malignant, but unlike ductal adenocarcinomas, they have a more indolent behavior and better prognosis. (17) In a solid tumor, size >3 cm, duct dilatation, vascular invasion, enlargement of peripancreatic lymph nodes, and calcification are suggestive of malignancy. A cutoff size of 2 cm has a positive predictive value (PPV) for malignancy of 44%, whereas 3 cm increases the PPV to 61% (13).
Other incidental pancreatic lesions that should be considered in the differential diagnoses of solid PNETs are solid SCAs and intrapancreatic accessory spleen. On MRI, intrapancreatic accessory spleen can be diagnosed reliably because its signal and enhancement patterns follow that of the spleen (18). SCAs are benign cystic lesions with variable features such as polycystic, honeycomb, or oligocystic morphology. SCA with unusually small microcysts and hypervascular cyst linings can appear as hypervascular solid lesions on contrast-enhanced MDCT. On MRI, hyperintensity on T2-weighted images and subtle microcystic patterns on delayed contrast-enhanced images can help distinguish SCA from PNETs and intrapancreatic accessory spleen; nevertheless, it can be difficult to differentiate them in preoperative images (19).
Because 50 to 90% of pancreatic PNETs express somatostatin receptors, functional imaging such as octreotide scintigraphy or single-photon emission computed tomography (SPECT) or positron emission tomography (PET)/CT with radioactively labeled somatostatin analogs such as 68Ga-DOTA-TOC/TATE/NOC can be helpful in diagnosing PNETs or their metastases when CT or MRI findings are equivocal; their sensitivities for PNET detection range from 75 to 100% (20-21).
Cystic lesions constitute approximately 52% of incidental pancreatic lesions, with a prevalence of 19.9% on MRI and 1.2 to 2.6% on MDCT (22-25). Though the majority of incidental pancreatic cysts are benign, premalignant and frankly malignant lesions do occur. Therefore, characterization of the cystic lesion is equally essential to appropriately stratify patients with a higher risk of malignancy (26). Pancreatic cysts are broadly categorized into mucinous and nonmucinous cysts, with the common mucinous lesions having lower potential for aggressive behavior. Infrequent nonmucinous cysts including cystic PNETs and solid pseudopapillary epithelial neoplasms (SPENs) also present malignant potential. Detailed morphologic assessment of each cystic lesion is beyond the scope of this review; however, overall morphologic features on imaging that may apply to most lesions suggestive of aggressive behavior are summarized in Figure 1.
Most cystic lesions are benign or low grade unless any worrisome or high-risk features are noted. On the other hand, solid lesions need to be pursued for PNETs and may require functional imaging, clinical assessment, or invasive procedures.
Biochemical Diagnosis of PNETS
Once the presence of a PNET has been suggested by imaging studies as a pancreatic incidentaloma, 2 main questions arise: (1) Is it functioning? and (2) Is it benign or malignant? Most patients with incidental PNETs are asymptomatic; however, it is difficult to define whether the tumors are truly nonfunctional or are being detected at an earlier stage in which hormonal secretion is still insufficient to result in an overt clinical syndrome. A recent study from a single institution revealed that biochemical evaluation that included fasting serum levels of chromogranin A, pancreatic polypeptide, glucose, insulin, gastrin, and glucagon could detect functional PNETS in asymptomatic patients. Among 119 patients with PNETs, 53 (44.5%) were found to have functional tumors by endocrine evaluation, and only 28 (24%) presented with symptoms of hormonal hypersecretion. In the 53 asymptomatic patients with biochemical and immunohistochemical analysis results consistent with functioning tumors, 31 (58%) had insulin-producing tumors (27).
Recommendations on how to evaluate nonfunctioning and functioning PNETs are well established (1). Both the North American (NANETS) and European (ENETS) Neuroendocrine Tumor Societies recommend analysis of chromogranin A in both nonfunctioning and functioning tumors, as well as measurement of specific hormones such as insulin, C-peptide, pro-insulin, gastrin, glucagon, vasoactive intestinal peptide, parathyroid hormone-related protein, adrenocorticotropic hormone, and somatostatin based on clinical manifestations (28,29). However, recommendations on how to evaluate small, asymptomatic, incidental PNETs are not as clear. Until more experience is gained on how these small tumors present and a consensus is reached, measurement of chromogranin A is recommended in all patients, as well as perhaps pancreatic polypeptide and calcitonin. It is important to take a thorough medical history to rule out MEN type 1. Less clear is the measurement of specific hormones. While these measurements are currently recommended only upon clinical suspicion, physicians should be aware of a potential subclinical syndrome and take a detailed clinical history noting any incipient symptoms of hormonal hypersecretion. The high prevalence of asymptomatic insulin hypersecretion in a Canadian study (27), other reports of asymptomatic insulinoma (30), and the nonspecific characteristics of hypoglycemic symptoms raise the question whether measurement of fasting glucose, insulin, and pro-insulin should be done in all patients in addition to chromogranin A testing. Measurements of pancreastatin and neurokinin A have also been suggested to assess prognosis (1,31). Prospective studies are needed to determine the role of these tests in patients with PIs.
Staging and Grading
The disease spectrum of PNETs extends from poorly differentiated carcinomas, which are rapidly progressive and seldom resectable, to small, apparently innocent nodules that could remain unchanged for years (1). In 2006, the ENETS provided a TNM staging system for classification foregut NETs (stomach, duodenum, and pancreas), together with a grading based on the Ki-67 proliferation index and mitotic count (MC), which was followed by similar classifications for other NETs (32,33). Grade 1 (G1) depicted tumors with Ki-67 index ≤2% or MC <2/10 high-power fields (HPF); grade 2 (G2) tumors with Ki-67 index of 3 to 20%, or 2 to 20 MC/10 HPF; and grade 3 (G3) tumors with Ki-67 index >20% or >20 MC/10 HPF. The WHO 2010 (UICC/AJCC) classification used the ENETS Ki-67 and MC-based grading to distinguish low grade 1 (G1) and intermediate grade 2 (G2) well-differentiated NETs (WDNETs) from poorly differentiated grade 3 (G3) neuroendocrine carcinoma (PDNEC). The original Ki-67 cutoff index was changed to <3% for G1, and 3 to 20% for G2 (33,35). Modification of the ENETS T stage was done to facilitate inclusion of nonpathology criteria and permit accurate clinical staging (36). The WHO/AJCC TNM system adopted a different staging developed for pancreatic exocrine tumors, using peripancreatic extension as criteria for T3 tumors, whereas ENET staging relied on the more reproducible tumor size (37). When evaluated in a large international cohort of PNETS the ENETS TNM staging proved to be superior to the WHO/AJCC system in accurately predicting survival, and grading was the second most effective independent predictor of survival in the absence of staging information (38). Further revision of the Ki-67 cutoff values to 5% has been suggested to better separate G1 and G2 tumors and to correlate more accurately with prognosis for PNETs (and other GI-NETS) (36,39). The index to separate G2 and G3 has been suggested to change to 15% because there is a group of WDNETs with a Ki-67 index >15% that may benefit from platinum-based chemotherapy (37,40).
Fine Needle Aspiration Biopsy (FNAB)
FNAB has been used in the cytologic diagnosis of primary and metastatic PNETs for many years. It is typically guided by transcutaneous or increasingly also by EUS (EUS-FNAB), which is successful at identifying up to 90% of PNETs. The diagnosis of PNETs has been improved by a US-guided transcutaneous semifine needle cutting biopsy technique that allows immunostaining of tissue biopsies with Ki-67/MIB antibodies and other markers. An automatic tissue biopsy machine with a 1.2-mm needle (achieving a tissue block of 2,000 cells) has been routinely used for Ki-67 grading, often from metastases (41).
A new technique for obtaining EUS-guided tissue samples has been developed, referred to as EUS-guided fine-needle tissue acquisition (EUS-FNTA) to distinguish it from EUS-FNAB, is also increasingly used. EUS-FNTA with a19-gauge needle has been shown to be safe, feasible, and highly accurate for both diagnosis and Ki-67 determination (41). The EUS-FNTA technique has shown diagnostic accuracy of 93.3% and capability of measuring Ki-67 expression in 86.6 to 92.9% of cases. It has been suggested to be especially valuable in evaluating nonfunctioning PNETs (42). Importantly, no complications have been reported with these needle biopsies, but tissue sampling from lesions in the head of the pancreas and uncinate process may require a transduodenal approach. Preoperative determination of the Ki-67 proliferation index may prove crucial to management decisions in many cases, and the technique should be considered for obtaining tissue core samples in patients with suspected nonfunctioning PNETs based on imaging studies. The same technique may also be useful for obtaining samples for molecular characterization.
Molecular Biology
Approximately 45% of sporadic PNETs show loss of protein expression of DAXX and ATRX, which corresponds to mutations in the DAXX and ATRX genes that are crucial for telomere maintenance (37,43-45). The nuclear transcription factor islet-1 (isl1) and pancreatic duodenal homeobox 1 (PDX-1) are additional markers (36). Cytokeratin 19 (CK19), regarded as a marker of ductal lineage in the pancreas, is sometimes expressed by PNETs and has been suggested as marker of more aggressive behavior (37).
Expression of p53 is positive in both small-cell (100%) and large-cell poorly differentiated neuroendocrine carcinoma (90%), but it is negative in well-differentiated PNETs (37,45). Rb protein expression is lost in 60 to 90% of PDNECs. In contrast, Bcl2 protein is overexpressed in PDNEC but negative in G1 tumors and variably expressed in G2 PNETS (37,45). The mammalian target of rapamycin (mTOR) pathway integrates signals from growth factor tyrosine kinases receptors for epidermal growth factor, insulin growth factor, vascular endothelial growth factor, and cytokines acting through the phosphoinositide 3-kinase (PI3K)/Akt system, in regulating cell growth, metabolism, cell division, and angiogenesis (37,45). Approximately 15% of PNETs have genetic mutations affecting mTOR cell signaling pathway proteins (37,43). PNETs have also high vascularity and overexpress VEGF and its receptors (37). Recently, elucidating PNET molecular biology has garnered considerable interest for the possibility of new treatment options and individualized treatment of unresectable tumors (37,43,46).
Surgical Management
Functional PNETs should be resected. If a solid PNET is not functional, the optimal treatment depends on its size. Although there is no clear size criterion to warrant surgery, considering their potential for malignancy, lesions ≥2 cm are most commonly recommended for resection (47).
The goal in any pancreatic resection is to effectively eradicate the tumor while preserving as much pancreatic parenchyma as possible. For small lesions not located near the pancreatic duct, enucleation may be effective and low risk. Larger lesions in the head of the pancreas or close to the pancreatic duct are best treated with a Whipple procedure (pancreaticoduodenectomy). Similarly, larger lesions or those near the pancreatic duct that are in the pancreas body and tail are best treated by distal pancreatectomy. For larger lesions in the body of the pancreas, a central pancreatectomy can be considered, but the risks of complications are higher than for a Whipple or distal pancreatectomy (48). It is generally accepted that resection of the primary tumor improves survival, even in the face of metastatic disease (1).
Nonoperative Treatment
Dating back to 2006, Falconi et al (49) stated “no data exist with respect to a positive effect of surgery on overall survival in small (<2 cm), possibly benign or intermediate-risk pancreatic endocrine tumors,” a view supported in a 2012 review article by Minter and Simeone (50). A series of powerful publications from Italy has sequentially and logically laid a solid foundation for conservative management of these small, nonfunctioning, incidentally discovered PNETs. Starting in 2009, based on careful histologic and clinicopathologic criteria of 155 tumors, none of the proposed 49 stage 1 tumors developed malignancy during follow-up. The authors commented that “…small, well-demarcated tumors detected with modern imaging techniques raises the issue of whether an invasive therapeutic approach such as pancreatic surgery is indeed needed.” Using the improved TNM staging and a histopathologic grading system (ENETS-TNM stage with Ki-67 grading), Scarpa and colleagues reported 5-year survivals for stages 1 to 4 of 100, 93, 65, and 35% respectively in 274 operated patients. Falconi and colleagues (51) published results of pancreatic parenchyma-preserving resections including enucleations and middle pancreatic resections. Whereas the incidence of exocrine/endocrine insufficiency was limited to 8%, recurrence was also 8%, morbidity remained excessive at 58%, and 70% of the middle pancreatectomy patients suffered intra-abdominal complications. Bettini (52) reported on 90 patients with resected tumors ≤2 cm of whom 51 were incidentally discovered. Only 6% were demonstrated to be malignant, and 100% of the patients survived. They proposed “a nonoperative management could be advocated for tumors ≤2 cm when discovered incidentally.” Corroborating the Italian experience was a recent publication by Crippa and colleagues (53) that included 12 patients who were managed nonoperatively with a 36-month follow-up and no disease progression. A sobering note, however, was that 30% of incidentally discovered nonfunctioning PNETs present with stage 3 or 4 disease. Birnbaum et al (54) reported a 92% disease-free survival for asymptomatic, nonfunctioning PNETs <2 cm in size, although 9% had positive lymph nodes. They concluded that their experience could support a selective nonoperative approach.
Lee et al (55) reported on 77 PNET patients, followed nonoperatively, with a mean tumor size of 1.0 cm and a mean of 45 months of follow-up. Median tumor size did not change throughout the follow-up period, and there was no disease progression or disease specific mortality. However, the prevailing surgical opinion is probably best expressed by Haynes and colleagues (56). After reviewing the experience at the Massachusetts General Hospital, they wrote “Patients with incidentally discovered, nonfunctioning PETs should undergo neoplasm resection and careful postoperative surveillance, even if surgical pathologic findings suggest benign disease. No size cutoff exists beyond which malignancy can be safely excluded.” The data supporting a conservative, even nonoperative management recommendation is undeniable, but thoughtful criteria should be developed to guide clinical practice, optimally through a clinical trial. Patient age is an important consideration. The suggested criteria for conservative or nonoperative management are shown in Table 2.
Table 2. Suggested Criteria for Nonoperative Management of PNETs.
| • T ≤2 cm |
| • Ki-67 ≤2% (or equivalent by mitotic count) |
| • Asymptomatic |
| • No clinical or radiologic criteria of local invasion, LN, or distant metastasis (ENETS TNM stage 1) |
Abbreviations: ENETS = European Neuroendocrine Tumor Society; LN = lymph node; PNET = pancreatic neuroendocrine tumor; TNM = tumor node metastasis.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CT
computed tomography
- ENETS
European Neuroendocrine Tumor Society
- EUS
endoscopic ultrasound
- EUS-FNTA
EUS-guided fine-needle tissue acquisition
- FNAB
fine needle aspiration biopsy
- HPF
high-power feld
- MC
mitotic count
- MDCT
multidetector row CT
- MEN
multiple endocrine neoplasia
- MRI
magnetic resonance imaging
- PDNEC
poorly differentiated neuroendocrine carcinoma
- PI
pancreatic incidentaloma
- PNET
pancreatic neuroendocrine tumor
- SCA
serous cystadenoma
- TNM
tumor node metastasis
- US
ultrasound
- WHO
World Health Organization
Footnotes
The opinions represented in the AACE/ACE Disease State Clinical Review: Pancreatic Neuroendocrine Incidentalomas are the expressed opinions of the Neuroendocrine and Pituitary Scientific Committee of the American Association of Clinical Endocrinologists. AACE/ACE Disease State Clinical Reviews are systematically developed documents written to assist health care professionals in medical decision making for specific clinical conditions, but are in no way a substitute for a medical professional's independent judgment and should not be considered medical advice. Most of the content herein is based on literature reviews. In areas of uncertainty, professional judgment of the authors was applied.
This review article is a working document that reflects the state of the field at the time of publication. Because rapid changes in this area are expected, periodic revisions are inevitable. We encourage medical professionals to use this information in conjunction with, and not a replacement for, their best clinical judgment. The presented recommendations may not be appropriate in all situations. Any decision by practitioners to apply these guidelines must be made in light of local resources and individual patient circumstances.
Disclosure: The authors have no multiplicity of interest to disclose.
References
- 1.Vinik AI. Advances in diagnosis and treatment of pancreatic neuroendocrine tumors (PNETS) Endocr Pract. 2014;20:1222–1230. doi: 10.4158/EP14373.RA. [DOI] [PubMed] [Google Scholar]
- 2.Kostiuk TS. Observation of pancreatic incidentaloma [in Russian] Klin Khir. 2001;9:62–63. [PubMed] [Google Scholar]
- 3.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms, a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 4.Herrera MF, Pantoja JP, Sierra M, Velázquez-Fernandez D. Pancreatic incidentaloma (Chapter 41) In: Hubbard JGH, Inabnet WB, Lo CY, editors. Endocrine Surgery: Principles and Practice. New York City, NY: Springer; 2009. pp. 541–552. [Google Scholar]
- 5.Karatzas T, Dimitroulis D, Charalampoudis P, Misiakos EP, Vasileiadis I, Kouraklis G. Management of cystic and solid pancreatic incidentalomas: a review analysis. J BUON. 2013;18:17–24. [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Lillemoe KD, et al. Periampullary and pancreatic incidentaloma a single institution's experience with and increasingly common diagnosis. Ann Surg. 2006;243:673–683. doi: 10.1097/01.sla.0000216763.27673.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs T, Pratt WB, Callery MP, Vollmer CM., Jr The incidental asymptomatic pancreatic lesion: Nuisance or threat? J Gastrointest Surg. 2009;13:405–415. doi: 10.1007/s11605-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 8.Bruzoni M, Johnston E, Sasson A. Pancreatic incidentalomas: clinical and pathological spectrum. Am J Surg. 2008;195:329–332. doi: 10.1016/j.amjsurg.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Lahat G, Ben Haim M, Nachmany I, et al. Pancreatic incidentalomas: High rate of potentially malignant tumors. J Am Coll Surg. 2009;209:313–319. doi: 10.1016/j.jamcollsurg.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RB, Lattin GE, Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010;30:1445–1464. doi: 10.1148/rg.306105523. [DOI] [PubMed] [Google Scholar]
- 12.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallotti A, Johnston RP, Bonaffini PA, et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am J Roentgenol. 2013;200:355–362. doi: 10.2214/AJR.11.8037. [DOI] [PubMed] [Google Scholar]
- 14.Sundin A, Vullierme MP, Kaltsas G, Plöckinger U Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90:167–183. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 15.Baker MS, Knuth JL, DeWitt J, et al. Pancreatic cystic neuroendocrine tumors: preoperative diagnosis with endoscopic ultrasound and fine-needle immunocytology. J Gastrointest Surg. 2008;12:450–456. doi: 10.1007/s11605-007-0219-7. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto A, Johnston PT, Shi C, et al. Pancreatic neuroendocrine tumor with cystlike changes: Evaluation with MDCT. AJR Am J Roentgenol. 2013;200:W283–W290. doi: 10.2214/AJR.12.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockall AG, Reznek RH. Imaging of neuroendocrine tumors (CT/MR/US) Best Pract Res Clin Endocrinol Metab. 2007;21:43–68. doi: 10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sahani DV, Bonaffni PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013;266:38–61. doi: 10.1148/radiol.12112512. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Kim MJ, Lee JY, et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136–142. doi: 10.2214/AJR.08.1309. [DOI] [PubMed] [Google Scholar]
- 20.Haug AR, Cindea-Drimus R, Auernhammer CJ, et al. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. J Nucl Med. 2012;53:1686–1692. doi: 10.2967/jnumed.111.101675. [DOI] [PubMed] [Google Scholar]
- 21.Miederer M, Weber MM, Fottner C. Molecular imaging of gastroenteropancreatic neuroendocrine tumors. Gastroenterol Clin North Am. 2010;39:923–935. doi: 10.1016/j.gtc.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Sachs T, Pratt WB, Callery MP, Vollmer CM., Jr The incidental asymptomatic pancreatic lesion: nuisance or threat? J Gastrointest Surg. 2009;13:405–415. doi: 10.1007/s11605-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 24.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–659. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Morin E, Cheng S, Mete O, et al. Hormone profiling, WHO 2010 grading, and AJCC/UICC staging in pancreatic neuroendocrine tumor behavior. Cancer Med. 2013;2:701–711. doi: 10.1002/cam4.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 29.Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 30.Kishi S, Sakamoto K, Mori M, Isogawa A, Shiba T. Asymptomatic insulinoma: A case report and autopsy series. Diabetes Res Clin Pract. 2012;98:445–451. doi: 10.1016/j.diabres.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Sherman SK, Maxwell JE, O'Dorisio MS, O'Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21:2971–2980. doi: 10.1245/s10434-014-3728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rindi G, Klöppel G, Ahlman H, et al. TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchofs Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchofs Arch. 2007;451:757–762. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 34.Bosman FT World Health Organization, International Agency for Research on Cancer. 2010 WHO classification of tumours of the digestive system. 4th. Lyon, France: International Agency for Research on Cancer; 2010. p. 417. [Google Scholar]
- 35.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors. a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 36.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 37.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014;25:65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 38.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 39.Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer. 2013;108:1838–1845. doi: 10.1038/bjc.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hentic O, Couvelard A, Rebours V, et al. Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factors in patients with liver metastases of digestive endocrine carcinoma. Endocr Relat Cancer. 2011;18:51–59. doi: 10.1677/ERC-09-0319. [DOI] [PubMed] [Google Scholar]
- 41.Elvin A. Therapy evaluation and diagnostic accuracy in neuroendocrine tumours: assessment of radiological methods. Acta Radiol Suppl. 1993;390:1–25. [PubMed] [Google Scholar]
- 42.Larghi A, Capurso G, Carnuccio A, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc. 2012;76:570–577. doi: 10.1016/j.gie.2012.04.477. [DOI] [PubMed] [Google Scholar]
- 43.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karatzas T, Dimitroulis D, Charalampoudis P, et al. Management of cystic and solid pancreatic incidentalomas: a review analysis. J BUON. 2013;18:17–24. [PubMed] [Google Scholar]
- 48.Goudard Y, Gaujoux S, Dokmak S, et al. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg. 2014;149:356–363. doi: 10.1001/jamasurg.2013.4146. [DOI] [PubMed] [Google Scholar]
- 49.Falconi M, Plockinger U, Kwekkeboom D, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196–211. doi: 10.1159/000098012. [DOI] [PubMed] [Google Scholar]
- 50.Minter R, Simeone D. Contemporary management of nonfunctioning pancreatic neuroendocrine tumors. J Gastrointest Surg. 2012;16:435–446. doi: 10.1007/s11605-011-1693-5. [DOI] [PubMed] [Google Scholar]
- 51.Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 52.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Crippa S, Partelli S, Zamboni G, et al. Incidental diagnosis as prognostic factor in different tumor stages of nonfunctioning pancreatic endocrine tumors. Surgery. 2014;155:145–153. doi: 10.1016/j.surg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Birnbaum D, Gaujoux S, Cherif R, et al. Sporadic nonfunctioning pancreatic neuroendocrine tumors: prognostic significance of incidental diagnosis. Surgery. 2014;155:13–21. doi: 10.1016/j.surg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Lee LC, Grant CS, Salamao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965–974. doi: 10.1016/j.surg.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 56.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534–538. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]