Abstract
The selective Aurora-A kinase inhibitor MLN8237 is in clinical trials for hematologic malignancies, ovarian cancer and other solid tumors. We previously showed that MLN8237 is potently antiproliferative toward standard monolayer cultured glioblastoma cells. We have now investigated the effect of MLN8237 with and without temozolomide or ionizing radiation on the proliferation of glioblastoma tumor stem-like cells (neurospheres) using soft agar colony formation assays and normal human astrocytes by MTT assay. Western blotting was utilized to compare MLN8237 IC50s to cellular Aurora-A and phospho-Thr288-Aurora-A levels. MLN8237 was more potently antiproliferative to neurosphere cells than to standard monolayer glioma cells, and was non-toxic to normal human astrocytes. Western blot analysis revealed that MLN8237 treatment inhibits phospho-Thr288-Aurora–A levels providing proof of drug target-hit in glioblastoma cells. Furthermore, phospho-Thr288-Aurora-A levels partially predicted the antiproliferative efficacy of MLN8237. We also found that Aurora-A inhibition by MLN8237 was synergistic with temozolomide and potentiated the effects of ionizing radiation on colony formation in neurosphere glioblastoma tumor stem-like cells. These results further support the potential of Aurora-A inhibitors as primary chemotherapy agents or biological response modifiers in glioblastoma patients.
Keywords: MLN8237, alisertib, glioblastoma, neurospheres, Aurora-A, temozolomide, radiation
Introduction
MLN8237 (alisertib) is a selective Aurora-A kinase inhibitor currently in clinical trials for hematopoietic neoplasms, neuroblastoma, ovarian cancer, melanoma and other solid tumors [1–5]. Aurora-A is a serine-threonine kinase important in centrosome maturation and mitotic entry and exit [6–8]. Its inhibition is antiproliferative and leads to mitotic arrest, often resulting in tetraploidy, cellular senescence and/or apoptosis [3, 9–10]. Aurora-A kinase activity also affects multiple signalling pathways involved in growth and differentiation [11–14]. Aurora-A inhibitors thus represent a unique class of anti-neoplastic agents that act as classical direct inhibitors of cellular proliferation machinery and disruptors of kinase signalling pathways.
Because new therapies for gliomas continue to be urgently needed, we have pursued preclinical studies of MLN8237 as a potential chemotherapy agent against glioblastoma. Aurora-A kinase is variably overexpressed in gliomas and MLN8237 inhibits colony formation in standard glioblastoma cell lines [14]. Here we show that MLN8237 also potently inhibits colony formation by neurosphere-forming glioblastoma tumor stem cells and is relatively nontoxic to normal human astrocytes. MLN8237 also inhibited Aurora-A autophosphorylation at Thr288 and its antiproliferative efficacy was partially predicted by phosho-Thr288-Aurora-A protein levels in vitro. Additionally, we demonstrate that MLN8237 potentiates the effects of standard glioma treatments, i.e., temozolomide (TMZ) and radiation in glioblastoma monolayer and neurosphere stem cells.
Methods
Neurosphere culture
Samples from resected brain tumors were collected at Henry Ford Hospital (Detroit, MI) with patient written consent in accordance with institutional guidelines, and histopathologically graded based on WHO criteria. Glioblastoma tumors were dissociated, as previously described (15]. Dissociated cells were plated in T-25 flasks at a density of 2 × 104 cells/ml in neurosphere medium (NM): DMEM/F12 medium containing 2 mM L-glutamine and supplemented with N-2 (Gibco Invitrogen Cell Culture, Grand Island, NY), 0.05% BSA, 25 µg/ml gentamicin, 50 units/ml penicillin G sodium, 50 µg/ml streptomycin sulfate, 20 ng/ml EGF and 20 ng/ml bFGF (PeproTech, Rocky Hills, NJ) and grown in standard conditions (5% C02/air at 37°C). After 1 to 3 weeks, multicellular floating neurospheres formed and were dissociated in Mg2+-Ca2+-free PBS and replated in NM for the formation of secondary spheroids. Glioblastoma neurospheres of less than 20 passages were used in experiments.
Neurosphere cell colony formation in soft agar
Neurosphere cells established from two separate glioblastoma patient tumors were dissociated into single cells. A total of 1x104 cells were suspended in 0.3% low melt agarose (Cambrex, Rockland, USA) with DMSO alone or the investigational Aurora-A inhibitor MLN8237 provided by Millennium Pharmaceuticals (Cambridge, MA) diluted in DMSO with and without TMZ. Cells were then seeded on 0.5% low melt agarose-precoated six-well plates (Corning, Corning, NY, USA). A total volume of 4 ml medium was added on top of the agarose layer containing the cells. Medium was changed every 3 days. For drug treatments, both agarose layers and top medium were mixed with MLN8237 and/or TMZ. After 14 days of culture, the plates were stained by 0.05% crystal violet for quantification of the colonies. Colonies with more than 20 cells were counted under an inverted light microscope. For radiation experiments, freshly plated cells with or without drugs were exposed to 1 Gy or 2 Gy of gamma radiation on the following day in a JL Shepherd & Associates Mark I-688 5000 Ci cesium (Cs-137) irradiator.
Monolayer glioblastoma cell colony formation assays
Established glioma monolayer cell lines were cultured in DMEM with 10% fetal calf serum in 5% C02/air at 37°C using standard methods. For colony formation assays, 600 cells were seeded per 60 mm dish and treated the following day with various concentrations of MLN8237 with and without TMZ in triplicate dishes for 72 hr. Seven days later the culture was aspirated and the dishes were washed with DPBS, fixed with methanol, stained with Giemsa, rinsed in deionized water and air dried. Colonies containing 20 or more cells were counted using a dissecting microscope. Percent survival was calculated as the average number of colonies in 3 dishes for a given drug concentration time point divided by the average number of colonies in 3 untreated control dishes.
Cell proliferation assays
Normal human astrocytes (Invitrogen) were plated in 96 well plates at 3,000 cells per well, and cells were treated with the indicated concentration of MLN8237 or with DMSO vehicle in triplicate wells. After three days, 10 l of 5 mg/ml MTT Reagent (Roche) was added per well and plates were incubated four additional hours. One volume of cell solubilizing solution was added and the plate was incubated overnight to allow crystals to dissolve. Absorbance at was read 570 nm on a BMG LabTech Omega plate reader.
Western Blotting
Cell pellets were suspended in RIPA buffer (Thermo Scientific, Rockford, IL)] containing 1 µM dithiothreitol and protease inhibitors [16 µg/ml aprotinin, 1 µg/ml each of leupeptin A pepstatin and chymostatin (Sigma, St Louis, MO) and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (MP Biomedicals, Solon, OH)] and centrifuged at 16,100×g at 4°C for 20 min. Lysate total protein was determined using the Pierce BCA method (Thermo Scientific). Lysates (10 µg) were electrophoresed on 10% polyacrylamide gels and electrotransferred to Imobilon™ PVDF membranes (Millipore, Billerica, MA). Membranes were blocked with 4% dried milk in tris-buffered saline with 0.1% Tween-20 (TBST) and incubated with clone 35C1 anti-human Aurora-A antibody (Abcam ab13824, 1:500), rabbit anti-human phospho-Aurora-A (Thr288) (Abcam ab58494, 1:500), or mouse monoclonal anti-actin (Sigma A2228, 1:4000) for 90 min at room temperature. Secondary antibodies were goat anti-mouse or goat anti-rabbit IgG-HRP (Santa Cruz Biotechnologies, Santa Cruz, CA) incubated for 30 min. Blots were developed with Pierce ECL (Thermo Scientific) and exposed to X-ray film. Ratios of Aurora-A protein signal to -actin signal within samples were determined by densitometry using NIH Image J software.
RT-PCR
Real-time PCR for Aurora-A was performed using ABI TaqMan (Applied Biosystems) and a Roche LightCycler 480. The internal control gene was 2-microglobulin. Primers and reaction conditions were as previously described [14].
Statistics
Data are expressed as the mean ± the standard deviation. Colony count data were examined statistically using a Poisson regression model while Western blot and cell proliferation assays were examined using ANOVA models with the response transformed to the log scale. A probability value of p<0.05 was considered statistically significant between two groups. Residual plots from each analysis did not show violations of underlying assumptions.
Results
MLN8237 potently inhibits neurosphere colony formation of glioblastoma tumor stem cells
We previously showed that MLN8237 is cytotoxic for traditional monolayer glioma cell lines, with IC50s ranging from 60 to 225 nM for ten cell lines in clonogenic assays [14]. Glioma cells cultured as neurospheres in a defined medium have been shown to have stem cell-like properties, and to represent a better model of glioma than traditional serum-grown monolayer cells [15–18]. Therefore, we tested the effects of MLN8237 against colony formation by tumor stem cell neurosphere cultures from human patient tumors. Continuous MLN8237 exposure potently inhibited colony formation by HF2303SP and HF2587SP neurosphere tumor stem cells in soft agar with IC50’s of approximately 5.9 nM and 2.8 nM, respectively (Fig. 1).
Fig. 1. MLN8237 potently decreases colony formation of glioblastoma neurosphere cells.
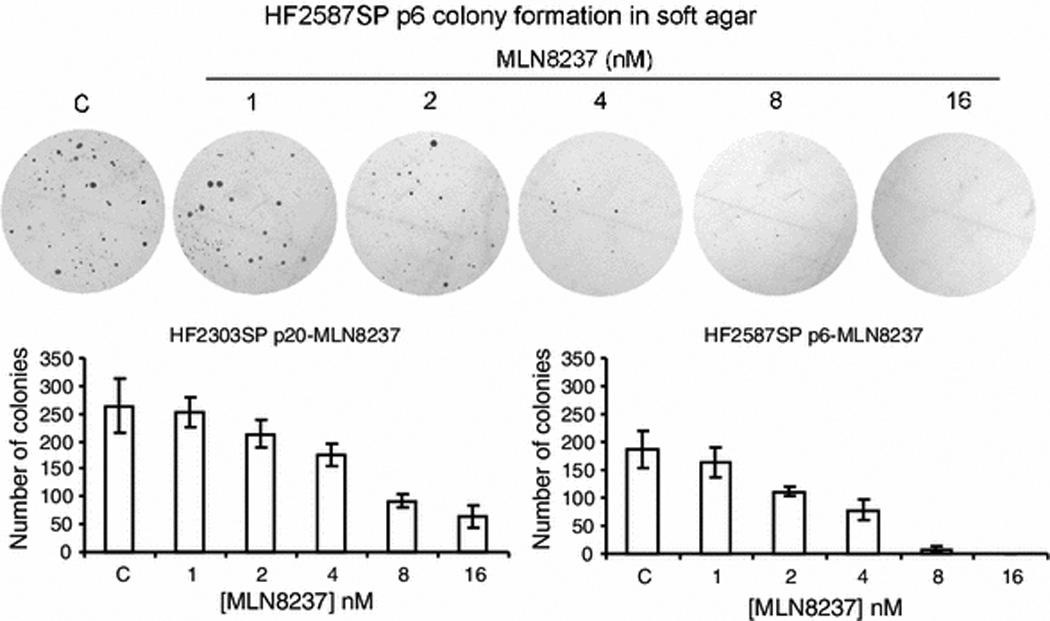
Colony formation of HF2303SP and HF2587SP cells treated with MLN8237. Dispersed glioblastoma neurospheres were seeded in soft agar and treated with the indicated concentration of MLN8237 for 14 days. Colony numbers were counted and used to calculate anchorage- independent growth of tumor cells under specified treatment conditions.
MLN8237 is not significantly toxic to normal human astrocytes
To examine whether MLN8237 has toxicity toward normal astrocytes, cultured human astrocytes were exposed to MLN8237 concentrations of up to 800 nM and metabolically active cells were quantitated by MTT assay. Concentrations of MLN8237 up to 200 nM caused no decrease in MTT metabolism compared to control untreated cells (Fig. 2). Even 800 nM resulted in little measured toxicity (10%).
Fig. 2. Effect of MLN8237 on human astrocytes.
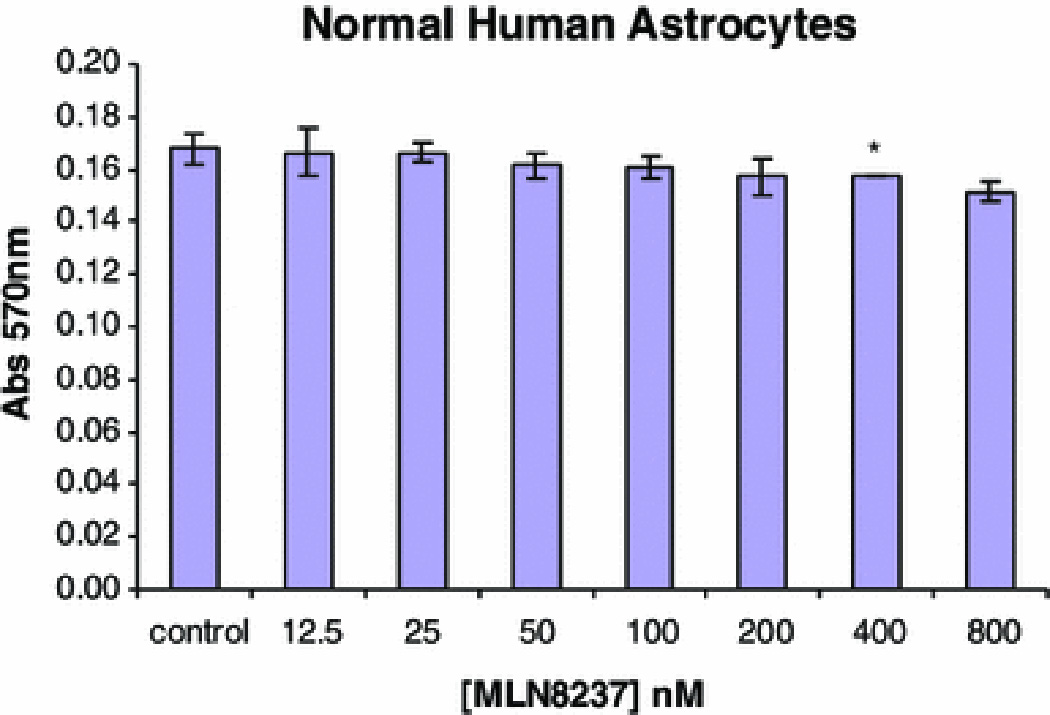
Normal human astrocytes were treated with the indicated concentrations of MLN8237 for three days and the number of metabolically active viable cells per well was measured by MTT assay. Data are means ± standard deviations of triplicate wells. Two independent experiments provided similar results (*, p<0.05, compared with DMSO alone).
MLN8237 decreases Aurora-A phosphorylation and is partially predicted by phospho-Thr288-Aurora-A levels in glioblastoma cells in vitro
In order to begin to investigate the determinants of glioblastoma cell sensitivity to Aurora-A, we performed quantitative western blotting for total Aurora-A and phosphoThr288-Aurora-A in glioblastoma cell lines (Fig. 3). Treatment of U251 glioblastoma cells with MLN8237 decreased phospho-Aurora-A levels by approximately 4-fold (Fig. 3a). Total Aurora-A protein levels showed low correlation with MLN8237 IC50’s in glioblastoma cell lines r=0.55 (confidence intervals −0.12, 0.88) and was not significant (p=0.0975).
Fig. 3. MLN8237 inhibits Aurora-A phosphorylation and its antiproliferative efficacy is partially predicted by phospho-Thr288-Aurora-A levels in glioblastoma cells in vitro.
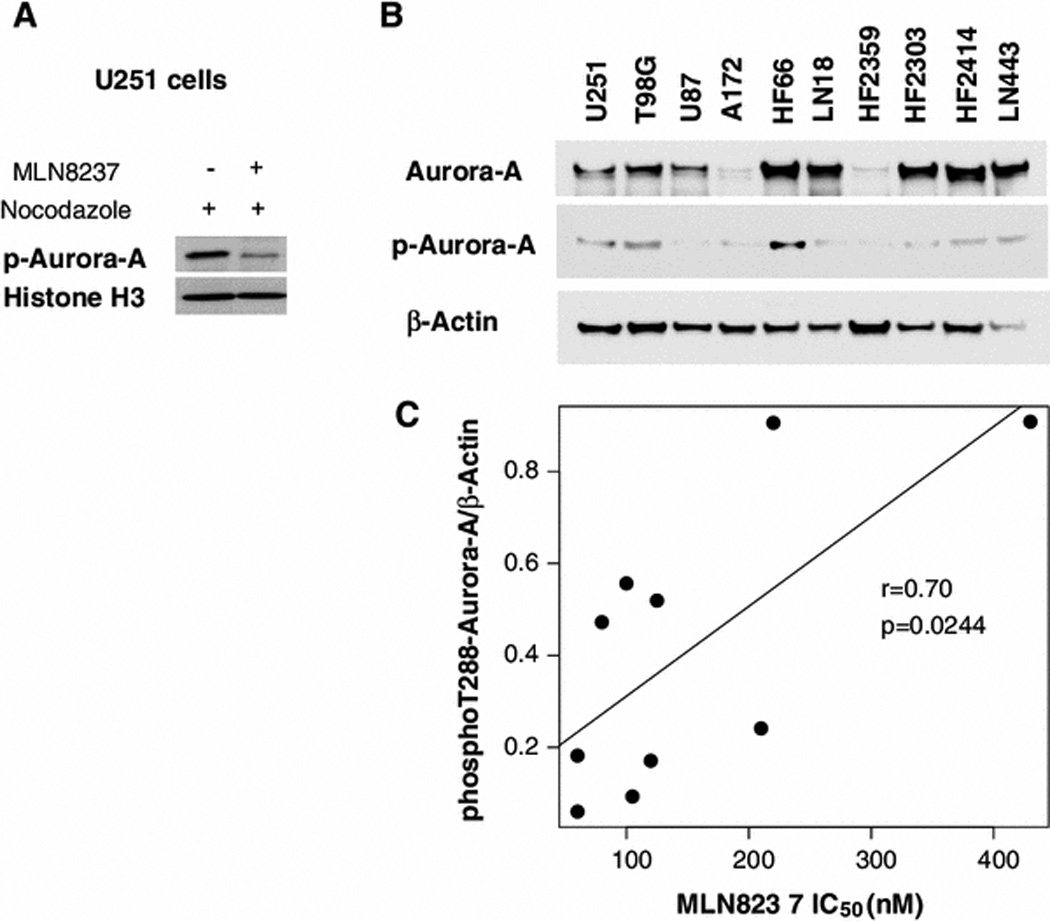
a. U251 cells were synchronized with 100 ng/ml nocodazole and then treated 18 hours with or without 5 M MLN8237 and phosphoT288-Aurora-A was examined by western analysis. b. Quantitative western blotting of glioblastoma cell lines for total Aurora-A and phosphoT288-Aurora-A. c. Linear regression analysis of phosphoT288-Aurora-A expression in glioblastoma cell lines compared to their respective 24hr MLN8237 IC50 values.
Aurora-A mRNA levels as measured by RT-PCR showed virtually no correlation to MLN8237 potency. In contrast, phosphoThr288-Aurora-A showed moderate correlation with MLN8237 IC50’s, r=0.70 (0.12, 0.92) and was statistically significant (p=0.0244) (Fig. 3c). Although these results provide evidence that phosphoT288-Aurora-A levels are relevant to MLN8237 sensitivity, the only moderate correlation (r=0.70) suggests that other factors may be important in determining cell susceptibility to MLN8237.
MLN8237 antiproliferative activity is synergistic with temozolomide in glioblastoma cells
We first investigated this question using standard monolayer glioblastoma tumor cell lines by colony formation assays. We previously showed that the IC50 for inhibition of U87 glioblastoma cell colony formation by MLN8237 is approximately 100 nM [14]. Concentrations at and above the IC50 (100 nM to 200 nM) substantially potentiated inhibition of U87 colony formation by TMZ (Fig. 4). In addition, concentrations of MLN8237 as low as 12.5 nM, which did not significantly affect colony formation by U87 alone, significantly enhanced inhibition of colony formation by TMZ, suggesting a synergistic effect of low concentrations of MLN8237 with TMZ on these cells.
Fig. 4. Effect of MLN8237 and TMZ on proliferation of monolayer glioma cells.
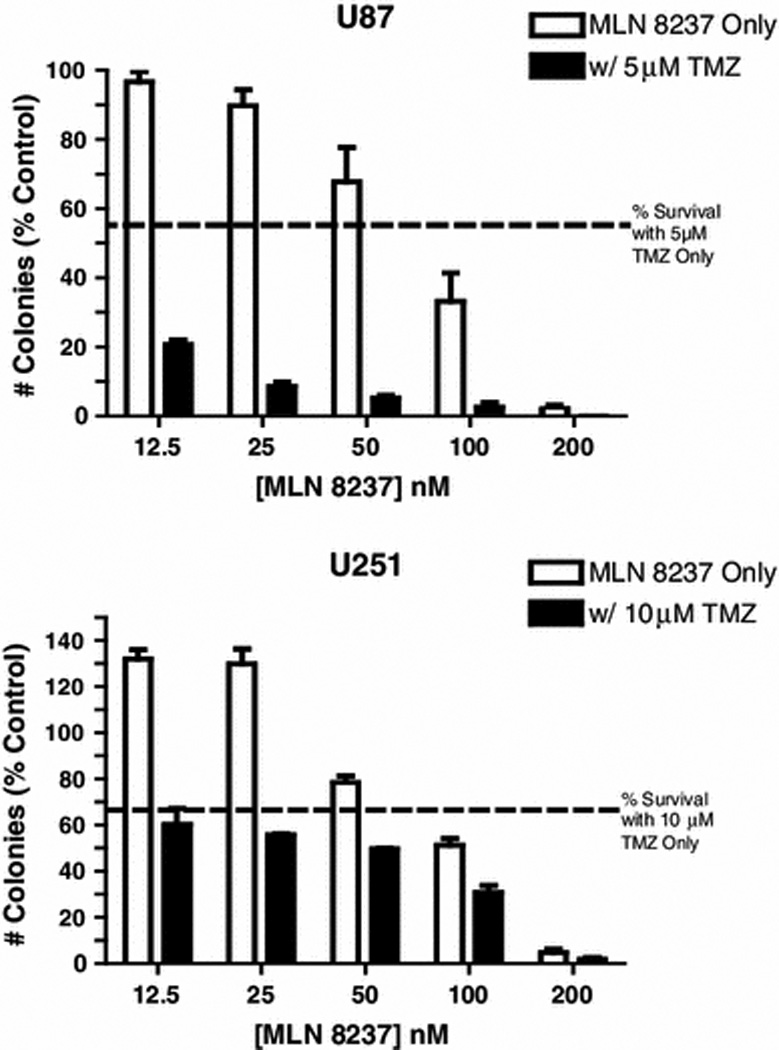
U87 and U251 cells were seeded in 60mm dishes containing DMEM and treated with MLN8237 or TMZ for 14 days. Colony numbers were counted and used to calculate anchorage- independent growth of tumor cells under different treatment conditions.
In U251 cells 100 nM or higher MLN8237 potentiated the effect of TMZ on colony formation in an additive fashion (Fig. 4). Similar effects were seen on cell proliferation as measured by MTT assay (Fig. 4c). Both MLN (ANOVA p < 0.001) and TMZ (p <0.001) were associated with statistically significant reductions in cell proliferation by the MTT assay. The effects of MLN8237 and TMZ are essentially additive with TMZ reducing the measured values by about 46% at each MLN8237 dose level except at the highest dose where there may not have been enough to measure accurately (41% dose 0; 42% dose 0.01; 52% dose 0.05; 49% dose 0.1; and 7% at dose 1).
Against HF2303SP glioblastoma neurosphere stem cells, 10 nM MLN8237 potentiated the effects of 100 to 200 nM TMZ (Fig. 5). In HF2587SP neurosphere cells MLN8237 was synergistic with TMZ (p ≈ 0.006). These effects were seen at much lower concentrations of MLN8237 (1 – 10nM) with glioblastoma neurosphere cells, in comparison to monolayer glioma cells, in agreement with the greater sensitivity of neurosphere cells to MLN8237 seen above.
Fig. 5. Colony formation of glioblastoma neurosphere cells treated with MLN8237 and temozolomide (TMZ).
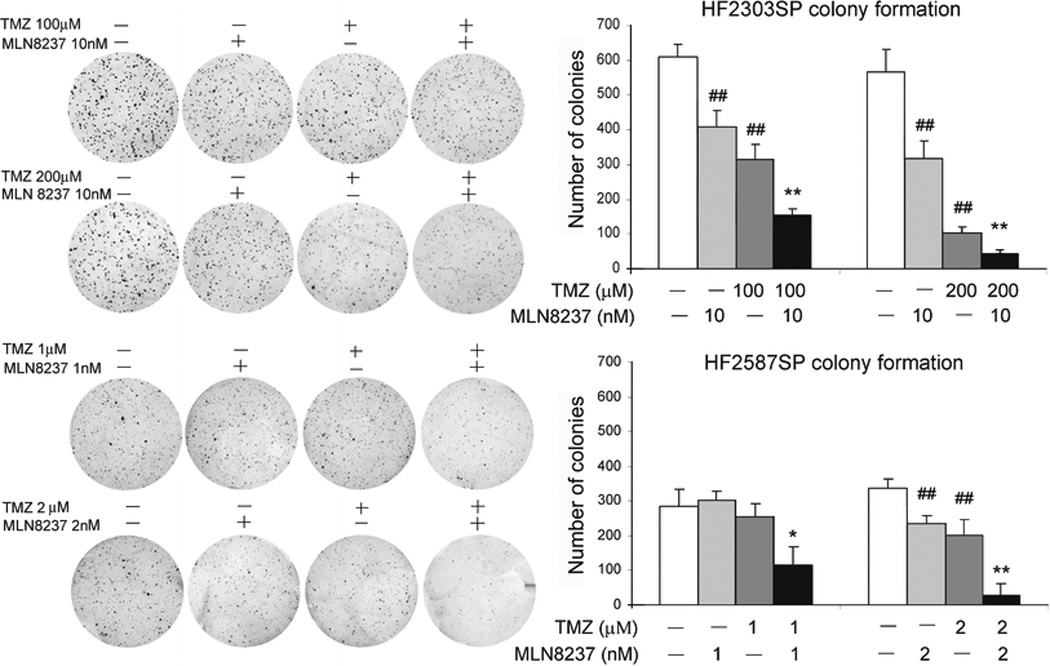
Dispersed HF2303SP and HF2587SP cells were seeded in soft agar and treated with MLN8237 or TMZ for 14 days. Colony numbers were counted and used to calculate anchorage- independent growth of tumor cells under different treatment conditions (##, P<0.01, compared control without drug *, p<0.05; **, p<0.01, compared with MLN8237 or TMZ treatment alone).
MLN8237 potentiates ionizing radiation in neurosphere tumor stem cells
We previously demonstrated that MLN8237 potentiated the effects of ionizing radiation in glioblastoma monolayer cell lines. For HF2303SP cells low dose MLN8237 (10 nM) potentiated the effect of radiation on glioblastoma neurosphere cell colony formation in the range of exposure tested (2 to 6 Gy). There was a statistically significant effect of both MLN8237 (p≈0.0003) and radiation (p<0.0001) with MLN8237 reducing colony counts by about 55% and radiation reducing the number of colonies by about 75 per Gy over this range. For HF2587SP cells a similar low toxicity MLN8237 concentration of 2 nM was synergistic with radiation at measurable doses (1 to 2 Gy) (Fig. 6). There was a statistically significant effect of both MLN8237 (p≈0.001) and radiation (p<0.0001) with MLN8237 reducing colony counts by about 40% and radiation having an approximate linear effect in the square root of the dose.
Fig. 6. Colony formation of glioblastoma neurosphere cells treated with MLN8237 and ionizing radiation.
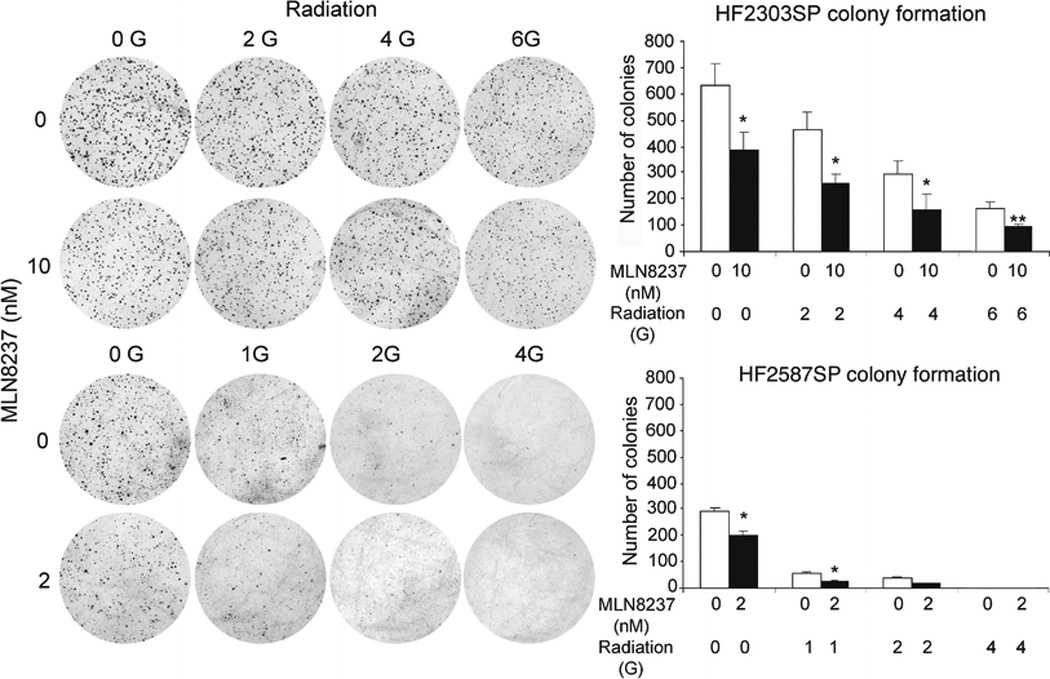
Dispersed HF2303SP and HF2587SP cells were seeded in soft agar and irradiated. MLN8237 was then added to cell culture medium and the culture continued for 14 days (*, p<0.05; **, p<0.01, compared with no MLN8237 treatment).
Discussion
Glioblastoma is an aggressive tumor with a poor prognosis for which novel approaches to clinical treatment are critically needed. Tumor stem cells represent the self renewing component of tumors and are thus an important target of therapy. Yet such tumor stem cells are also more resistant to chemotherapy and radiation compared to other tumor cells. Thus, the potential usefulness of an anti-cancer strategy hinges on its efficacy against tumor stem cells. We have previously demonstrated that the selective Aurora-A inhibitor MLN8237 potently inhibits colony formation of established and low-passage primary monolayer glioblastoma cell lines, and also potentiated the effects of ionizing radiation in these lines [14].
Monolayer tumor cell lines represent a mixed culture of tumor cells. In monolayer glioblastoma cultures tumor stem cells quickly revert to a more differentiated, non-stem-like state under standard adherent cell culture conditions including fetal calf serum [15–18]. Culture of glioblastoma tumor cells freshly isolated from patient surgical samples in serum-free media selects for tumor stem cells which grow in spherical conglomerations called neurospheres, similar to normal brain stem cells. Here we demonstrate that MLN8237 is also antiproliferative and potently inhibits colony formation of glioblastoma neurosphere stem cells, but showed no toxicity toward normal human astrocytes. Surprisingly, MLN8237 antiproliferative activity was even more potent in the glioblastoma tumor stem cells compared to monolayer cells, and is achieved at MLN8237 concentrations selective for Aurora-A kinase and below those in which inhibition of Aurora-B kinase was reported to occur, approximately 1500 nM in HeLa cells [4].
Preclinical studies of MLN8237’s efficacy in pediatric tumor xenograft models found correlation of drug efficacy with lower Aurora-A gene copy numbers. We found some correlation of MLN8237 potency with glioblastoma cell Aurora-A levels and better, yet still limited correlation with phospho-aurora-A levels. These findings suggest that other factors such as Aurora-A interacting proteins [19–22] or downstream targets of Aurora-A kinase signalling [11–14] are important in drug response.
MLN8237 antiproliferative activity also potentiated the activity of the standard clinical anti-glioma agent TMZ in either an additive or synergistic fashion in monolayer and neurosphere tumor stem cells. When we similarly looked at MLN8237’s ability to potentiate ionizing radiation in neurosphere tumor stem cells we found that MLN8237 was synergistic with ionizing radiation at low dosages. Similar additive potentiation or synergy with TMZ or radiation has been found with the selective (Aurora-A and Aurora-B) Aurora-B inhibitor ZM447439 against primary and standard glioblastoma monolayer cell lines [23]. MLN8237 has also been found to sensitize atypical teratoid rhabdoid tumor cells to radiation [24].
These in vitro studies support that MLN8237 is a potentially effective adjunctive therapy against glioblastoma alone or in combination with other agents, such as TMZ, and/or may be useful as a biochemical sensitizer to radiation therapy. We are currently testing these possibilities in a mouse orthotopic glioblastoma xenograft model to further evaluate this promising chemotherapy agent as a potential new therapy for glioblastoma.
Acknowledgments
The authors thank Lisa J. Whitely for performing RT-PCR. This work was supported in part by Takeda Pharmaceuticals International Co.
This work was supported in part by Takeda Pharmaceuticals International Co., which also supplied MLN8237. Coauthor Dr. Jeffrey A. Ecsedy is employed by Takeda.
Footnotes
Conflict of Interest
No other coauthors have a financial relationship with the company.
References
- 1. [accessed October 12, 2013]; ClinicalTrials.gov, a service of the U.S. National Institutes of Health. www.clinicaltrials.gov.
- 2.Kelly KR, Ecsedy J, Medina E, Mahalingam D, Padmanabhan S, Nawrocki ST, Giles FJ, Carew JS. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukemia and significantly increases the efficacy of nilotinib. J Cell Mol Med. 2011;15:2057–2070. doi: 10.1111/j.1582-4934.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Görgün G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, Nahar S, Zheng M, Bandi M, Carrasco RD, Raje N, Munshi N, Richardson P, Anderson KC. A novel aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell cycle arrest in multiple myeloma. Blood. 2010;115:5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, Stroud SG, Chen W, Shinde V, Huck JJ, Wysong DR, Janowick DA, Hyer ML, Leroy PJ, Gershman RE, Silva MD, Germanos MS, Bolen JB, Claiborne CF, Sells TB. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 5.Dees EC, Cohen RB, von Mehren M, Stinchcombe TE, Liu H, Venkatakrishnan K, Manfredi M, Fingert H, Burris HA, 3rd, Infante JR. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 7.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 8.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the Kinase Aurora A Cooperatively Activate the Kinase Plk1 and Control Mitotic Entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carol H, Boehm I, Reynolds CP, Kang MH, Maris JM, Morton CL, Gorlick R, Kolb EA, Keir ST, Wu J, Wozniak AE, Yang Y, Manfredi M, Ecsedy J, Wang J, Neale G, Houghton PJ, Smith MA, Lock RB. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huck JJ, Zhang M, McDonald A, Bowman D, Hoar KM, Stringer B, Ecsedy J, Manfredi MG, Hyer ML. MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8:373–384. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 11.Otto T, Horn S, Brockmann M, Eilers U, Schüttrumpf L, Popov N, Kenney AM, Schulte JH, Beijersbergen R, Christiansen H, Berwanger B, Eilers M. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, He S, Zhou X, Liu M, Zhu H, Wang Y, Zhang W, Yan S, Quan L, Bai J, Xu N. Suppression of Aurora-A oncogenic potential by c-Myc downregulation. Exp Mol Med. 2010;42:759–767. doi: 10.3858/emm.2010.42.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasayama T, Marumoto T, Kunitoku N, Zhang D, Tamaki N, Kohmura E, Saya H, Hirota T. Over-expression of Aurora-A targets cytoplasmic polyadenylation element binding protein and promotes mRNA polyadenylation of Cdk1 and Cyclin B1. Genes Cells. 2005;10:627–638. doi: 10.1111/j.1365-2443.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- 14.Lehman NL, O'Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2010;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, Mikkelsen T. Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells. 2010;28:181–190. doi: 10.1002/stem.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 17.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Lim SK, Gopalan G. Aurora-A kinase interacting protein 1 (AURKAIP1) promotes Aurora-A degradation through an alternative ubiquitin-independent pathway. Biochem J. 2007;403:119–127. doi: 10.1042/BJ20061272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fumoto K, Lee PC, Saya H, Kikuchi A. AIP regulates stability of Aurora-A at early mitotic phase coordinately with GSK-3beta. Oncogene. 2008;27:4478–4487. doi: 10.1038/onc.2008.92. [DOI] [PubMed] [Google Scholar]
- 21.Qin L, Tong T, Song Y, Xue L, Fan F, Zhang G. Aurora-A interacts with Cyclin B1 and enhances its stability. Cancer Lett. 2009;275:77–85. doi: 10.1016/j.canlet.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Asteriti IA, Rensen WM, Lindon C, Lavia P, Guarguaglini G. The Aurora-A/TPX2 complex: a novel oncogenic holoenzyme? Biochim Biophys Acta. 2010;1806:230–239. doi: 10.1016/j.bbcan.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Borges KS, Castro-Gamero AM, Moreno DA, da Silva Silveira V, Brassesco MS, de Paula Queiroz RG, de Oliveira HF, Carlotti CG, Jr, Scrideli CA, Tone LG. Inhibition of Aurora kinases enhances chemosensitivity to temozolomide and causes radiosensitization in glioblastoma cells. J Cancer Res Clin Oncol. 2012;138:405–414. doi: 10.1007/s00432-011-1111-0. [DOI] [PubMed] [Google Scholar]
- 24.Venkataraman S, Alimova I, Tello T, Harris PS, Knipstein JA, Donson AM, Foreman NK, Liu AK, Vibhakar R. Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012;107:517–526. doi: 10.1007/s11060-011-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


