Abstract
Lyme disease (LD) is a systemic disorder caused by Borrelia burgdorferi. Lyme spirochetes encode for a functional 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGR EC 1.1.1.88) serving as a rate limiting enzyme of the mevalonate pathway that contribute to components critical for cell wall biogenesis. Statins have been shown to inhibit B. burgdorferi in vitro. Using a mouse model of Lyme disease, we found that statins contribute to reducing bacterial burden and altering the murine immune response to favor clearance of spirochetes.
1. Introduction
Lyme disease is a multiphasic systemic disorder caused by the spirochetal pathogen Borrelia burgdorferi [1]. This bacterium is transmitted to mammalian hosts from arthropod vectors, specifically Ixodes spp. ticks [2]. Lyme disease is the most prevalent arthropod borne disease in the United States with over 25,000 cases confirmed by the Centers for Disease Control and Prevention (CDC) in 2014. The risk of infection is highest in areas where tick vectors are found in close association with infected reservoir hosts and humans [3, 4].
The genome of B. burgdorferi is very limited [5], and B. burgdorferi scavenges many required compounds from its arthropod and vertebrate host environments. Therefore, any intact metabolic pathway in B. burgdorferi serves as a potential target for inhibition of the bacterium. Sequence analysis of the borrelial genome indicates the presence of homologs (bb0683-bb0688) of the mevalonate pathway (MP) leading to the synthesis of isopentenyl-5-pyrophosphate (IPP) [5]. IPP is an essential component of several isoprenoids and a precursor for peptidoglycan synthesis contributing to the structural integrity of several organisms [6, 7]. Previous studies from our laboratory have shown that B. burgdorferi possesses a functional MP [8]. The rate-limiting step of the MP is the reduction of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) to mevalonate by HMG-CoA reductase (HMGR; EC 1.1.1.88) (8). We also determined that B. burgdorferi has a functional HMGR and that enzyme activity could be inhibited using two commercially available HMGR inhibitors (statins) [8]. Though the mevalonate pathway is found in many genera of bacteria known to cause human disease, including Staphylococcus, Streptococcus, Listeria, and Borrelia, the potential antimicrobial use of statins has not been fully explored [6, 5].
2. Materials and Methods
2.1. Bacterial strains and growth conditions
A clonal derivative of B. burgdorferi sensu stricto strain B31, MSK5 [9], which contains all plasmids was used for infectivity experiments. B. burgdorferi cultures were grown in 1% CO2 at 32°C in Barbour-Stoenner-Kelly II (BSK-II) liquid medium supplemented with 6% normal rabbit serum (Pel-Freez, Biologicals, Rogers, AR).
2.2. Statin inactivation
Statins were activated as previously described [8]. Briefly, 25mg of Lovastatin or Simvastatin (Sigma-Aldrich, St. Louis, MO) was dissolved in 500 μl of EtOH preheated to 55°C. 250 μl of 0.6 M NaOH and 5 ml of ddH2O were then added to the samples, which were then incubated at room temperature for 30 minutes. Following incubation, the pH was brought to 8.0 with 1 M HCl at which time ddH2O was added to the samples to bring them to a final concentration of 4 mg/ml. The statins were aliquoted and stored at −20°C for use.
2.3. Infectivity studies
All animal procedures were done in accordance with the approved animal use protocol from the Institutional Animal Care and Use Committee of The University of Texas at San Antonio. Groups (n=5) of mice were treated with activated simvastatin, lovastatin, or vehicle control at 5mg/kg every other day by oral gavage beginning at day 7 before infection. Groups (n=5) of 6-week-old female C3H/HeN mice (Charles River Laboratories, Wilmington, MA) were infected at a dose of 103 spirochetes per mouse intradermally. One group (n=5) was left uninfected. On day 14 postinfection, the spleen, left inguinal lymph node, heart, bladder, and a piece of abdominal skin were removed aseptically from infected mice and the tissues were processed to facilitate isolation of spirochetes in BSK-II growth medium [10]. All cultures were blind passed after 5 days into fresh BSK-II growth medium to minimize the toxicity associated with the degradation of host tissues and to facilitate growth of spirochetes. The cultures were scored for growth of B. burgdorferi after 2 to 3 weeks using dark-field microscopy [10].
2.4. Quantitative real-time PCR analysis
A portion of skin, spleen, right inguinal lymph node, and right tibiotarsal joint was collected aseptically, and total DNA was extracted using the High Pure PCR template preparation kit (Roche Applied Bioscience, Piscataway, NJ). The manufacturer's suggested protocol for extracting nucleic acids from the tail of the mouse was adapted to obtain total genomic DNA from different infected tissues. Briefly, the tissue samples were homogenized in 200 μl of lysis buffer containing proteinase K (final concentration, 2 mg/ml) and collagenase (final concentration, 1 mg/ml; Sigma Chemicals, St. Louis, MO). After incubation at 56°C overnight in a water bath, total genomic DNA was extracted according the manufacturer's suggested protocol. Known amounts of mouse or spirochete genomic DNA were used as standards to determine the total numbers of spirochetes in different mouse tissues. Total genomic DNA isolated from different infected mouse tissues was subjected to quantitative real-time PCR using SYBR green PCR master mix with a final concentration of 0.3 μM of oligonucleotides using the ABI Prism 7300 system (Applied Biosystems). Spirochetes were enumerated by real-time PCR analysis using primers specific to a borrelial gene, flaB (F-5’ TCTTTTCTCTGGTGAGGGAGCT; R-5’ TCCTTCCTGTTGAACACCCTCT), and normalized the total DNA extracted from different tissues to the number of copies of a mouse β-actin (F-5’ CAAGTCATCACTATTGGCAACGA; R-5’ CCAAGAAGGAAGGCTGGAAAA). The spirochete burden was expressed as the number of borrelial flaB copies per 106 mouse β-actin copies.
2.5. Enzyme-linked immunosorbent assays
A clonal isolate of B. burgdorferi strain B31, MSK5, was grown under conditions mimicking the fed-tick (pH 6.8/37°C) to a density of 1 × 108 spirochetes/ml. The cells were harvested by centrifugation and washed four times with in HBSS. The final pellet was disrupted by sonication and the cells were resuspended in ELISA coating buffer (50 mM sodium carbonate, pH 9.6) at a final concentration of 100 μg/ml following quantification using the BCA protein assay (Pierce, Thermo Fisher Scientific, Rockford, IL). 96-well MaxiSorp ELISA plates (Thermo Fisher Scientific, Rochester, NY) were coated with 100 μl of total sonicate in coating buffer and incubated overnight at 4°C. Following incubation, the coated plates were washed three times in ELISA wash buffer (0.80 mM Na2HPO4, 137 mM NaCl, 0.27 mM KCl, 0.15 mM KH2PO4 containing 0.5% Tween-20) and blocked for two hours at room temperature in ELISA wash buffer supplemented with 3% bovine serum albumin (BSA). After blocking, the wells were washed three times with ELISA wash buffer, then coated with serum derived from infected or control mice which was serially diluted in ELISA wash buffer supplemented with 1% BSA and the plates were incubated for 1 hour at room temperature. The plates were washed three times with ELISA wash buffer. The wells were coated with secondary antibody ([α–IgG and α–IgM) diluted in ELISA wash buffer supplemented with 1% BSA and incubated at room temperature for 1 hour. The plates were washed three times with ELISA wash buffer and the wells were coated with OPD buffer (50 mM Na2HPO4, 50 mM citric acid, pH 5.0, OPD tablets (Thermo Fisher Scientific), H2O2). The plates were then incubated in the dark for 15 minutes at room temperature. The absorbance was measured using a Synergy HT Plate Reader (BioTek, Winooski, VT) at 450nm. 50% binding titers were calculated using non-linear regression curve fit analysis with SlideWrite 7.0 software [11].
2.6. Cytokine analysis
On day 14 postinfection, serum was collected from all mice. Cytokine levels in the serum of individual mice were analyzed using the Bio-Plex Protein Array System (Luminex-based technology) (Bio-Rad Laboratories, Hercules, CA) as described previously [12] for the presence of interferon (IFN)- γ, interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-17, tumor necrosis factor (TNF)-α, and granulocyte-colony stimulating factor (G-CSF) expression as well as chemokines (macrophage inflammatory protein [MIP]-1α (CCL3), MIP-1β (CCL4), macrophage chemoattractant protein [MCP]-1 (CCL2), KC (CXCL1), and regulated upon activation, normal T cell expressed and secreted [RANTES] (CCL5)). The data reported are the averages of all mice per group for all cytokines that were significantly differentially expressed.
2.7. Statistical analysis
The quantitative real-time PCR were analyzed using a one-way analysis-of-variance test with Bonferroni's multiple-comparison test. The unpaired Student's t test (two-tailed) was used to analyze cytokine/chemokine and ELISA data. All statistical data were calculated using GraphPad Prism version 4.0 for Macintosh (GraphPad Prism Software, San Diego, CA). Statistical significance was accepted when P values were less than 0.05 [10].
3. Results
3.1. Infectivity analysis of statin treated C3H/HeN mice
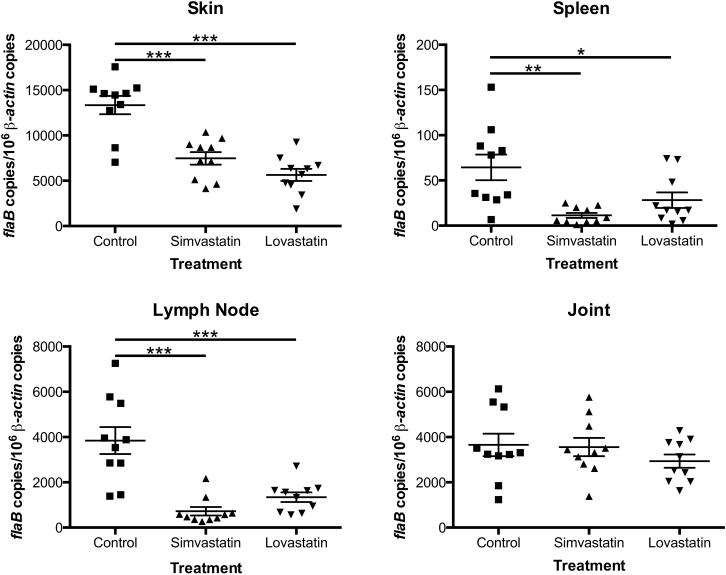
C3H/HeN mice, with or without statin treatment, infected with 103 spirochetes per mouse, exhibited dissemination of spirochetes to all tissues with a few exceptions (Table 1). Though there was no significant difference in bacterial dissemination to distal tissues between statin-treated and untreated mice, we wanted to determine whether there were differences in the numbers of bacteria migrating to specific tissues. To that end, total genomic DNA was extracted from a portion of skin, spleen, right inguinal lymph node, and right tibiotarsal joint and subjected to quantitative real-time PCR analysis using primers specific for a B. burgdorferi gene (flaB) and a mouse gene (β-actin). As shown in Figure 1, there was a significant decrease in the numbers of bacteria in each of the tissues tested with the exception of the joints. There were higher levels of reduction seen in the lymph nodes and spleens of mice treated with simvastatin when compared to the same tissues from mice treated with lovastatin, while there was a higher level of reduction in the skin of lovastatin-treated mice, though these data were not significant.
Table 1.
Statins do not affect bacterial dissemination.
| Treatment and Dose of MSK5 | No. of cultures positive/No. tested | No. of mice infected/No. tested | |||||
|---|---|---|---|---|---|---|---|
| Skin | Spleen | Lymph Node | Heart | Bladder | All Sites | ||
| Control | |||||||
| 0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/50 | 0/10 |
| 103 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 50/50 | 10/10 |
| Simvastatin | |||||||
| 0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/50 | 0/10 |
| 103 | 10/10 | 10/10 | 10/10 | 10/10 | 9/10 | 49/50 | 10/10 |
| Lovastatin | |||||||
| 0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/50 | 0/10 |
| 103 | 10/10 | 9/10 | 10/10 | 10/10 | 10/10 | 49/50 | 10/10 |
FIGURE 1. Statins lower bacterial burden in infected C3H/HeN mice.
Quantitative real-time PCR analysis of the spirochetal burden in mice infected with B. burgdorferi and treated with statins. Groups (n = 5) of 6-week-old C3H/HeN female mice were infected intradermally with B. burgdorferi strain B31 isolate MSK5 at 103 spirochetes per mouse. Each group was treated with 5 mg/kg of lovastatin, simvastatin, or vehicle control, every other day by oral gavage. Total genomic DNA was isolated from tissues (skin, spleen, lymph node, and joint) using the High Pure PCR template preparation kit, and quantitative real-time PCR was performed. Numbers of borrelial flaB copies were normalized against total mouse β-actin copies. Data shown represent 2 independent experiments. All tissues showed a significant decrease in burden with the exception of the joints. The asterisks indicate levels of significance as follows: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
3.2. Statins affect antibody titers in infected C3H/HeN mice
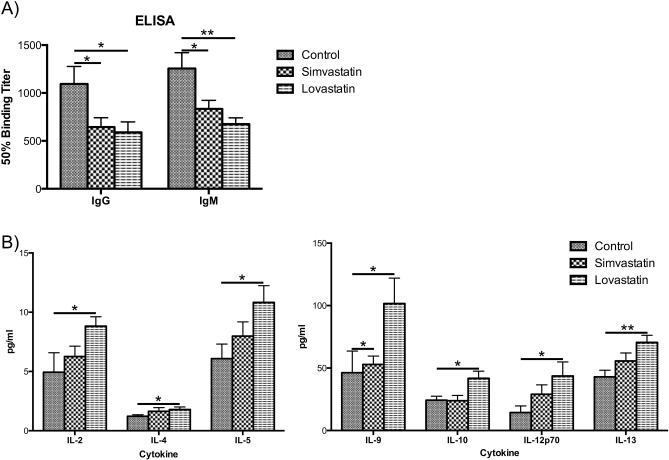
Enzyme-linked immunosorbent assays against B. burgdorferi whole cell sonicates were performed to determine the antibody profile present in the mice after 14 days of infection with B. burgdorferi and treatment with simvastatin or lovastatin. As shown in Figure 2A, there was a significant reduction in total IgG antibodies following treatment with simvastatin and lovastatin. IgM titers were also significantly reduced in statin-treated mice (Fig. 2A).
FIGURE 2. Statins affect antibody titers in treated mice and lovastatin elicits a TH2 type response in mice infected with B. burgdorferi.
A) Enzyme-linked immunosorbent assays using individual sera from C3H/HeN mice against whole cell B. burgdorferi strain B31 clonal isolate MSK5 with a-IgG, a-IgG2a, a-IgG2b, and a-IgM secondary antibodies. Levels of IgG and IgM were significantly lower in statin-treated mice when compared to levels in untreated mice. There were no significant differences seen in titers of IgG2a and IgG2b (data not shown). Asterisks indicated antibody titers significantly different between statin-treated and untreated mice. B) Bio-Plex cytokine/chemokine analysis of individual sera from C3H/HeN mice treated with statins. Only cytokines and chemokines showing significant differences are shown. The y-axis gives cytokine and chemokine levels in pg/ml. IL-4, IL-5, IL-9, IL-10, and IL-13 are TH2 cytokines whose levels are significantly higher in lovastatin-treated mice. IL-2 and IL-12p70, important for clearance of B. burgdorferi, are also upregulated in lovastatin-treated mice. All data shown are average of 2 independent experiments. The asterisks indicate levels of significance as follows: **, P < 0.01; *, P < 0.05.
3.3. Statins elicit a TH2 type response in treated mice
The typical cytokine profile of C3H/HeN mice infected with B. burgdorferi is a pro-inflammatory, TH1/TH17 response, with a downregulation in the TH2 response [13-16]. Cytokine analysis of all serum samples was performed to determine if there was a differential cytokine profile in response to statin therapy. Statin therapy alone had little to no effect on the levels of various cytokines, however in infected mice, lovastatin treatment significantly upregulated IL-4, IL-5, IL-9, IL-10, and IL-13, cytokines involved in a TH2 immune response (Fig. 2B). Lovastatin-treated mice also showed an upregulation in TH1 cytokines IL-2 and IL-12p70. Simvastatin had no significant effect on cytokine levels, with the exception of IL-9.
4. Discussion
The inhibitory property of stains has several implications for interactions of B. burgdorferi with its hosts. As our in vitro data showed that statins were able to exert an inhibitory effect on B. burgdorferi, we wanted to test the effects of statins on a susceptible C3H/HeN model of Lyme disease. Though there was no defect in dissemination, we did see a significant decrease in bacterial burden in all tissues tested, with the exception of the joint (Fig. 1) in statin-treated mice.
Though we have previously found that statins are able to inhibit survival of B. burgdorferi in vitro [8], the levels of statins in the blood of treated animals do not reach the concentrations necessary for complete bactericidal effects in vitro [17]. However, the numbers of bacteria reaching distal tissues are significantly lower in statin-treated mice, providing evidence that even some bactericidal activity can have an impact on the course of a borrelial infection.
Statins have also been previously shown to negatively affect bacterial growth and survival, even in bacteria that do not encode an hmgr homolog due to their cholesterol lowering properties [18]. Bacteria which require host cholesterol have defects in growth when cholesterol levels are lowered. As B. burgdorferi absolutely require cholesterol for their outer membranes [19], it is likely that the lowered levels of cholesterol would provide less cholesterol for B. burgdorferi to sequester, thus preventing B. burgdorferi from properly forming its membrane.
In the vertebrate host, there is a robust antibody response to B. burgdorferi infection that plays a role in bacterial clearance. When infected mice were treated with statins, we saw a significant decrease in the titers of whole IgG and IgM, two classes of Abs known to be elevated during the response against B. burgdorferi [20, 21]. We saw no difference in Ab titers in statin-treated uninfected mice (data not shown).
The typical cytokine profile of C3H/HeN mice infected with B. burgdorferi is a pro-inflammatory, TH1/TH17 response, with a downregulation in the TH2 response. This has been shown to contribute to increased disease severity as measured by increased arthritis [13]. In infected mice treated with lovastatin, there was significant upregulation of TH2 cytokines, some of which have been shown to be upregulated during convalescence in humans and have roles in controlling inflammation [22]. Previously, it has been demonstrated that statins can drive a TH2 response in other disease models [23, 24]. Lovastatin-treated mice also showed an upregulation in TH1 cytokines IL-2 and IL-12p70, implicated in clearance of B. burgdorferi, and whose loss is shown to lead to increased Lyme arthritis [25].
We have previously shown that simavastatin and lovastatain inhibit borrelial growth under in vitro growth conditions [8]. It appears that statin treatment contributes to decreased bacterial burden in infected mice, which could be due to either direct interference with spirochetal growth or by limiting available cholesterol to the bacteria [19]. Moreover, it is also possible that alterations in the immune response to the spirochetes could lead to increased bacterial clearance. It is interesting to speculate that statins could potentially have a number of as yet uncharacterized primary or secondary anti-borrelial effects. These pharmacological properties can be further exploited to enhance bacterial clearance and/or reduce persistence in reservoir hosts to subsequently reduce the incidence of Lyme disease.
Acknowledgements
This study was partly supported by Public Health Service grant SC1-AI-078559 from the National Institute of Allergy and Infectious Diseases, the Army Research Office of the Department of Defense under Contract No. W911NF-11-1-0136, predoctoral fellowships from the South Texas Center for Emerging Infectious Diseases (to TAV and SLRK) and predoctoral fellowships from the Center For Excellence in Infection Genomics (to TAV and CLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare they have no competing interests.
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–9. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, et al. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest. 2000;106:561–9. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–6. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 4.Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease--United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 5.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–6. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 6.Bochar DA, Stauffacher CV, Rodwell VW. Sequence comparisons reveal two classes of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol Genet Metab. 1999;66:122–7. doi: 10.1006/mgme.1998.2786. [DOI] [PubMed] [Google Scholar]
- 7.Wilding EI, Kim DY, Bryant AP, Gwynn MN, Lunsford RD, McDevitt D, et al. Essentiality, expression, and characterization of the class II 3-hydroxy-3-methylglutaryl coenzyme A reductase of Staphylococcus aureus. J Bacteriol. 2000;182:5147–52. doi: 10.1128/jb.182.18.5147-5152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Laar TA, Lin YH, Miller CL, Karna SL, Chambers JP, Seshu J. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PLoS One. 2012;7:e38171. doi: 10.1371/journal.pone.0038171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun. 2001;69:446–55. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruskova M, Esteve-Gassent MD, Sexton VL, Seshu J. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect Immun. 2008;76:391–402. doi: 10.1128/IAI.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine. 2011;29:2519–22. doi: 10.1016/j.vaccine.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anguita J, Rincon M, Samanta S, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased lyme arthritis. J Infect Dis. 1998;178:1512–5. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 14.Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–7. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straubinger RK, Straubinger AF, Summers BA, Erb HN, Harter L, Appel MJ. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect Immun. 1998;66:247–58. doi: 10.1128/iai.66.1.247-258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widhe M, Skogman BH, Jarefors S, Eknefelt M, Enestrom G, Nordwall M, et al. Up-regulation of Borrelia-specific IL-4- and IFN-gamma-secreting cells in cerebrospinal fluid from children with Lyme neuroborreliosis. Int Immunol. 2005;17:1283–91. doi: 10.1093/intimm/dxh304. [DOI] [PubMed] [Google Scholar]
- 17.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19:26–37. doi: 10.1016/s0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 18.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–4. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 19.Stubs G, Fingerle V, Wilske B, Gobel UB, Zahringer U, Schumann RR, et al. Acylated cholesteryl galactosides are specific antigens of borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J Biol Chem. 2009;284:13326–34. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdash N, Fernandes J. Lyme borreliosis: detecting the great imitator. J Am Osteopath Assoc. 1991;91:573–4, 7-8. [PubMed] [Google Scholar]
- 21.Lazarus JJ, McCarter AL, Neifer-Sadhwani K, Wooten RM. ELISA-based measurement of antibody responses and PCR-based detection profiles can distinguish between active infection and early clearance of Borrelia burgdorferi. Clin Dev Immunol. 2012;2012:138069. doi: 10.1155/2012/138069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glickstein L, Moore B, Bledsoe T, Damle N, Sikand V, Steere AC. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun. 2003;71:6051–3. doi: 10.1128/IAI.71.10.6051-6053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–35. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 25.Sjowall J, Carlsson A, Vaarala O, Bergstrom S, Ernerudh J, Forsberg P, et al. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-alpha and interleukin-12 in asymptomatic individuals in response to live spirochetes. Clin Exp Immunol. 2005;141:89–98. doi: 10.1111/j.1365-2249.2005.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]