Abstract
Cognitive impairment and Alzheimer’s Disease are linked with intake of a Western Diet, characterized by high levels of saturated fats and simple carbohydrates. In rats, these dietary components have been shown to disrupt hippocampal-dependent learning and memory processes, particularly those involving spatial memory. Using a rat model, the present research assessed the degree to which consumption of a high-energy (HE) diet, similar to those found in modern Western cultures, produces a selective impairment in hippocampal function as opposed to a more global cognitive disruption. Learning and memory performance was examined following 90-days consumption of an HE-diet in three nonspatial discrimination learning problems that differed with respect to their dependence on the integrity of the hippocampus. The results showed that consumption of the HE-diet impaired performance in a hippocampal-dependent feature negative discrimination problem relative to chow-fed controls, whereas performance was spared on two discrimination problems that do not rely on the hippocampus. To explore the mechanism whereby consuming HE-diets impairs cognitive function, we investigated the effect of HE-diets on the integrity of the blood-brain barrier (BBB). We found that HE-diet consumption produced a decrease in mRNA expression of tight junction proteins, particularly Claudin-5 and -12, in the choroid plexus and the BBB. Consequently, an increased blood-to-brain permeability of sodium fluorescein was observed in the hippocampus, but not in the striatum and prefrontal cortex following HE-diet access. There results indicate that hippocampal function may be particularly vulnerable to disruption by HE-diets, and this disruption may be related to impaired BBB integrity.
Keywords: Hippocampus, blood-brain barrier, learning, occasion setting, high fat diet, choroid plexus, Western diet, dementia, beta-amyloid, Alzheimer’s Disease
INTRODUCTION
Saturated fats and refined carbohydrates are believed to be the principal components of a modern Western diet that contribute to excessive energy intake and body weight gain [1]. A growing body of evidence suggests that these dietary factors can also impede cognitive performance. In human populations, dietary saturated fat has been associated with increased incidence of Alzheimer’s Disease (AD) [2-4] and with milder forms of cognitive dysfunction [5, 6]. Consumption of simple carbohydrates (e.g., mono and disaccharides) has been linked with impaired cognitive performance relative to complex carbohydrates in both adults [7] and children [8]. Learning and memory impairments have also been demonstrated in laboratory rodents following consumption of diets high in saturated fat [9-11], high in simple carbohydrates (e.g., sucrose) [12, 13], or a combination of the two [14-16].
The hippocampus, a brain structure widely known to play a critical role in learning and memory function, is one of the most vulnerable brain regions in the early phases of neurodegenerative dementias, including AD [17, 18] and vascular dementia [19, 20]. Recent evidence suggests that the hippocampus may also be particularly susceptible to disruption by dietary factors. For instance, several studies using rodent models have demonstrated that diets similar to those found in modern Western cultures can impair learning and memory problems that rely on the integrity of the hippocampus, including those that involve the utilization of both spatial [15, 21, 22] and nonspatial [14, 23] information. However, the extent to which a Western-type diet produces a selective impairment in hippocampal function as opposed to a more global disruption in cognitive function is not well established.
There are also gaps in knowledge about the neurophysiological mechanisms underlying the effects of Western-type diets on cognitive function. . Some recent evidence suggests that this type of diet can influence cognitive function by interfering with the integrity of the blood-brain barrier (BBB) [24]. Two barrier systems prevent the blood from direct contact with brain parenchyma: the BBB separates the blood from brain extracellular fluid and the blood-cerebrospinal fluid (CSF) barrier located in the choroid plexus separates the blood from the CSF circulation [25]. These barrier systems shield the brain from toxic substances in the blood. BBB failure has been shown to precede the development of cognitive impairment in AD [26] and stroke [27]. Several recent findings have linked BBB disruption with metabolic and dietary factors. In humans, longitudinal studies have demonstrated that body mass index (BMI) and obesity in middle-aged subjects are strongly correlated with an increased risk of both BBB disruption [28] and dementia [29] later in life. In rabbits, a cholesterol-enriched diet produces increased BBB permeability in the hippocampus [30], as well as increased hippocampal accumulation of β-amyloid (Aβ), a major pathological hallmark of AD. Banks and colleagues have shown that triglycerides can alter BBB active transport mechanisms of feeding-related hormones, including the adipostat hormone leptin [31] and the gut-derived hormone ghrelin [32] in rodents . These findings link dietary and metabolic factors to BBB disruption; however, the effects of a Western-type diet on BBB permeability are not well established.
One objective of the present research was to assess the effects of maintenance on a high-energy (HE) diet, high in saturated fat and glucose, on learning and memory performance in nonspatial Pavlovian discrimination problems that differ in their sensitivity to hippocampal damage. Two of the discrimination problems involved a hierarchical Pavlovian learning process known as occasion setting, in which animals learn to depend on the presence of a stimulus for information about the relationship between other stimuli. For example, in a serial feature negative discrimination, a task that is frequently used to examine what is known as “negative occasion setting”, a discreet “target” stimulus (A) (e.g., brief tone) is followed by food reinforcement when presented alone (A+ trials); however, the target stimulus is not followed by food when preceded by the presentation of another, “feature” stimulus (X) (e.g., brief light). The feature stimulus “sets the occasion” for when the target stimulus will not be followed by reinforcement. The converse of this task is called a feature positive discrimination, in which a target stimulus is reinforced when preceded by a feature stimulus (X➔A+ trials), but not when presented alone (A− trials). Holland and colleagues [33] demonstrated that rats with selective lesions to the hippocampus were impaired in learning a feature negative discrimination, displaying increased appetitive responding on nonrewarded trials (X➔A− trials) compared to control rats. On the other hand, hippocampal lesions had much less of an impact on both a feature positive and a nonconditional discrimination task (B+, C−), where one stimulus is paired with food and another is not. Here we assess the effects of 90-days HE-diet maintenance on learning these discriminations.
A second objective of the present research was to assess the impact of HE-diet consumption on BBB integrity. At the conclusion of the behavioral testing, BBB integrity was evaluated by measuring mRNA expression in the blood-brain and blood-cerebrospinal fluid (CSF) barriers of the structural proteins occludin, claudin 5, claudin 12, and the cytoplasmic zona occludin proteins, Zo-1 and Zo-2. These proteins are some of the primary molecular components that comprise the tight junctions of the blood-brain and blood-CSF barriers, and are considered to be critical in the maintenance of permeability restriction [34]. BBB integrity was further evaluated by measuring permeability to Sodium Fluorescein (NaFl), a tracer that is normally precluded across an intact BBB [35]. Leakage of NaFl from the vasculature to brain parenchyma was evaluated by semi-quantitiative fluorescence analysis. To further evaluate the hypothesis that the hippocampus is particularly vulnerable to disruption by an HE-diet, we examined BBB permeability to NaFl in three areas of the brain, including the hippocampus and the prefrontal cortex, two brain regions that are sensitive to diet-induced reductions in BDNF [14, 15], and the striatum, which has been shown to be susceptible to BBB disruption by pharmacological manipulations (e.g., [36, 37]).
MATERIALS AND METHODS
SUBJECTS
The subjects were 32 naϊve, male Sprague-Dawley albino rats (Harlan Inc., Indianapolis, IN), approximately 60 days of age upon arrival in the laboratory, and weighing between 275-300g. Subjects were housed individually in stainless steel cages and maintained on a 12- hr light/dark cycle with lights on at 0800 h. All procedures for the care and treatment of the rats during this experiment were reviewed and approved by the Purdue Animal Care and Use Committee.
DIETS
A diet high in saturated fat (lard-based) and glucose (Harlan Teklad, TD.04489) was used as the HE-diet. This diet was nutritionally adequate, with a caloric density of approximately 4.5 kcal/g (≈40% kcal from fat), and contained the following (g/kg): 270g casein, 220.5g glucose, 200g cornstarch, 50g cellulose, 170g lard, 15g safflower oil. A standard laboratory rodent chow diet (LabDiet, formula 5001) was used for the control diet. This control diet has a caloric density of approximatley 3.0 kcal/g (≈13% kcal from fat). Both diets were in powdered form, presented in glass jars fastened inside of the home cage of each rat. Water was available ad libitum throughout the entire study for all rats.
APPARATUS
The pretraining and training procedures were conducted in eight identical conditioning chambers, constructed of aluminum end walls and clear plexiglas side walls, measuring 21.6 × 21.6 × 27.9 cm. The floors of each conditioning chamber consisted of stainless steel bars spaced 1.9 cm apart, measuring 0.48 cm in diameter. A recessed food magazine was located in the center of one end wall of each chamber. The auditory stimuli (i.e., tones “T1”, “T2”, and white noise, “N”) were produced by auditory stimulus generators (Med Associates, ANL-926) located outside the conditioning chamber near the end wall opposite of the food magazine. A 6-W jeweled panel light 6 cm to the left of and above the food cup served as the light conditioned stimulus (stimulus “L”). A computer controlled infrared monitoring system with a photo transmitter and receptor in the food magazine was used to record food magazine entries. The conditioning chambers were controlled with computer software (MED PC IV, Med Associates). The food pellets that were used as unconditioned stimuli (USs) were 45mg sucrose pellets (Research Diets). The index of appetitive behavior was the percentage of 10ms periods that the photobeam inside the food magazine was interrupted during each 5-sec stimulus presentation period.
PROCEDURES
Dietary Treatment
Following a two-week acclimation period, the rats were divided into four groups, matched according to body weight: HE-FN, C-FN, HE-FP, and C-FP. The rats in groups HE-FN and HE-FP were fed the HE-diet (powdered) ad libitum for 90-days. The rats in groups C-FN and C-FP were switched from a pelleted to a powdered version of the 5001 (LabDiet) chow formula for the same 90-day ad libitum period. Body weights were recorded every 48-hrs during the 90-day period. At the end of the 90-day ad libitum period, the rats were given rationed food once daily in order to gradually reduce their body weight to 85% of their ad libitum body weights. The 85% of ad lib body weight criterion was achieved after 7-days of food rationing; The rats were maintained at this weight throughout the remainder of the experiment. During training, the rats received their designated food ration after the end of each conditioning session.
Magazine Training
The rats were assigned to squads and conditioning boxes for behavioral training according to the following criteria: 1) the first and third training squads contained rats that would receive FN training procedures (groups HE-FN and C-FN), while the second and fourth squads contained rats that would receive FP training procedures (groups HE-FP and C-FP), 2) assignment to conditioning boxes was counterbalanced with respect to the four experimental groups.
After 85% ad lib body weight criterion had been achieved, the rats received one 15-min magazine training session. During the 15-minute magazine training session, all rats received 10 presentations of two sucrose pellets on a variable-time 60-sec schedule (i.e., one presentation per min, on average). No conditioned stimuli (CSs) were presented during magazine training.
Discrimination Training
All animals received one training session per day, five-days per week. Both FN and FP training sessions contained four different trial types, two of which made up the conditional discrimination task (feature negative for FN sessions, feature positive for FP sessions), whereas the other two trial types made up the nonconditional discrimination, which was the same for both FN and FP sessions. Training continued for 8 four-session blocks of training.
The rats in groups HE-FN and C-FN received “FN” training sessions involving a feature negative discrimination and a nonconditional discrimination. In each FN training session the conditional discrimination (i.e., serial feature negative) contained one presentation of stimulus T1 (a 5-sec tone, 1500-Hz, 78-db) immediately followed by the presentation of 2 sucrose pellets (T1+ trial), and three nonreinforced presentations of the serial compound trials (L→T1− trials). Each L➔T1− trials consisted of, in order: a 5-sec panel light stimulus (stimulus L), a 5-sec empty interval, and a 5-sec tone stimulus (the same T1 stimulus used in T1+ trials). The nonconditional discrimination consisted of one T2+ trial presentation (a 5-sec 300-Hz tone, 78-db, followed by 2 sucrose pellets) and three presentations of N− trials (a nonreinforced 5-sec white noise, 78-db). Each session was 90 minutes with randomly selected trial orders and random intertrial intervals ranging between 300 and 900 seconds (average = 600 seconds).
The rats in groups HE-FP and C-FP received “FP” training sessions involving a feature positive discrimination and a nonconditional discrimination. In each FP training session, the conditional discrimination (i.e., serial feature positive) contained one presentation of the serial compound immediately followed by the presentation of two sucrose pellets (L➔T1+ trial), and three nonreinforced presentations of the T1 stimulus alone (T1− trials). The nonconditional discrimination problem in FP training sessions was the same as that used for FN training seessions. As with FN sessions, each FP session was 90 minutes with randomly selected trial orders and random intertrial intervals ranging between 300 and 900 seconds (average = 600 seconds).
Blood-Brain Barrier Integrity
At the conclusion of FN and FP training, rats were chosen for either tight junction protein mRNA analysis (n=15), or for NaFl permeability analysis (n=17) according to the following criterion: 1) at least seven rats were chosen from each dietary treatment (HE, C) for each analysis, 2) at least three rats were chosen from each of the four behavioral treatment conditions (HE-FN, C-FN, HE-FP, C-FP) for each analysis, and 3) the rats were divided for subsequent BBB analysis matched for body weight and training performance.
Tight Junction Protein mRNA Analysis: Capillary Separation
The capillary separation for analysis of mRNA encoding tight junction proteins was carried out as previously described [38, 39]. Briefly, at the conclusion of behavioral procedures, the rats were sacrificed by CO2 decapitation, the brains were removed rapidly, and the thalamus and midbrain were dissected and kept at 4°C. The brain tissues were homogenized in 3 ml volume of a cold buffer solution. The buffer solution contains (mmol/L): HEPES, 10; NaCl, 141; KCl, 4; MgSO4, 1; NaH2PO4, 1; CaCl2, 2.5; and glucose, 10 (pH= 7.4). Dextran 70 (15%) was then added and the solution was homogenized. The homogenate solution was then spun at 5,400×g for 15 min at 4 °C. The pellet (capillary-enriched fraction) was separated carefully from the supernatant and washed two times with a mixture of homogenized buffer and Dextran 70 (3:4). Pellets were then spun again and stored at −80°C for RNA extraction. For tight junction protein mRNA analysis of the blood-csf barrier, the choroid plexus was extracted from the lateral ventricle following CO2 decapitation.
Tight Junction Protein mRNA Analysis: Quantitative Real-Time RT-PCR
Total RNAs were isolated from brain capillaries and choroid plexuses using acid guanidium-phenol-chloroform method with TRIzol® Reagent (invitrogen , Carlsbad CA). Then mRNA was reverse transcribed following standard procedure. The reaction volume of 100 μL contained 1 μg of total RNA, 2.5 μM of oligo dT primer, 2 mM dNTP mixtures, 0.4 U of RNase inhibitor, 1.25 U of MuLV reverse transcriptase, 5 mM MgCl2 and 1× PCR buffer according to the manufacturer’s instruction (Applied Biosystems, Foster City, CA). Real-time RT-PCR using the Mx3000p (Stratagene, Cedar Creek, TX) was used to quantify mRNA levels. The primers for checked genes were designed using Primer 3 software. Each PCR reaction contained 3 μL of cDNA, 7.5 μL of 2×FastStart Universal SYBR Green Master (Rox) (Roche Dignostics Indianapolis IN), and 400 nM of the forward and reverse primers. After an initial denaturation at 95 °C for 15 min, the amplification program consisted of 40 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 1 min, and extension at 72 °C for 30 s. The relative differences in mRNA expression among brain capillaries and choroid plexus were expressed using cycle time (Ct) values. The relative differences between control and treatment group were calculated and expressed as relative increase by setting the control as 100% [40] [40].
Sodium Fluorescein Permeability Analysis
The rats were anesthetized with intraperitoneal injections of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Under full anesthesia, the right femoral artery was exposed from linear incision and a polyethylene catheter fixed by ligation was introduced into the artery. Sodium fluorescein (10% NaFl, 0.6 ml/kg in saline) was applied from one syringe into the catheter at a rate of approximately .2 ml/min. After the NaFl had been applied, the artery was tied with a suture to prevent backflow and the dye was allowed to circulate for 45 min. The rats were then perfused intracardially with 150 ml of sterile saline at a rate of 10 ml/min. Following perfusion the rats were decapitated and the brains were quickly removed. After removing the choroid plexus and capillaries by hand, the hippocampus, striatum, and prefrontal cortex were bilaterally extracted over ice and stored at −80°C.
For NaFl fluorescence analysis, the tissue samples were weighed, homogenized in 200 μl phosphate buffered saline, and centrifuged for 10 min at 5000 rpm. Supernatants were diluted with 400 μl trichloracetic acid (20%) and then centrifuged for 15 min at 13,600 rpm. Supernatants were then neutralized with sodium hydroxide (NaOH) and fluorescence was measured in duplicates with ELISA scan at 480 excitation wavelength and 525 emission wavelength. Tissue samples were processed under minimal lighting conditions. Because the total number of samples with duplicates exceeded the number of wells in one 96-well ELISA plate, each of the three brain areas were processed and analyzed separately. This analysis allowed comparison of the two dietary groups within each brain area, but did not allow cross-brain area analyses. A negative control sample lacking brain tissue was used with each brain area scan; the fluorescence value of the negative control was subtracted from the fluorescence intensity value for each sample. To calculate dependent variables for NaFl analysis, absolute intensity values were divided by the tissue weight for each separate sample. The relative differences between control and treatment group were calculated and expressed as a relative increase by setting the control as 100% (Arbitrary Units, % of controls).
STASTICAL ANALYSIS
Overall ANOVAs were performed for each discrimination problem across the entire training phase using Diet (HE, C) as a between-subjects factor, Trial Type (+, −) as a within-subjects factor, with repeated measures for the variable Training Block (1-8) and Training Session (1-4). Discrimination learning was defined as a significant main effect of Trial Type across a two-block period of training. To determine when in training significant discrimination learning was achieved, additional analyses were performed across each two-block period of training for each discrimination problem, using Diet as a between-subjects variable, Trial Type as a within-subjects variable, and Training Block and Training Session as repeated measures variables. To determine which type of trial (i.e., rewarded or nonrewarded) any potential group differences in discrimination learning were based on, ANOVAs were performed separately for each Trial Type across the entire training session with Diet as a between-subjects factor and Training Block as a repeated measures factor. For body weight analyses, Diet (HE, C) and Training Group (FN, FP) were used as between-subjects variables, with Week (1-13) as a repeated measures variable. Separate ANOVAs with Diet and Training Group as between-subjects variables were used to analyze body weights at the onset of powdered feeding and at the end of the 90-day ad libitum period. Dietary differences in NaFl fluorescence and tight junction protein expression were evaluated using Student’s t-tests. Alpha level for significance was set at 0.05 for all analyses.
RESULTS
DISCRIMINATION TRAINING
Feature Negative Discrimination
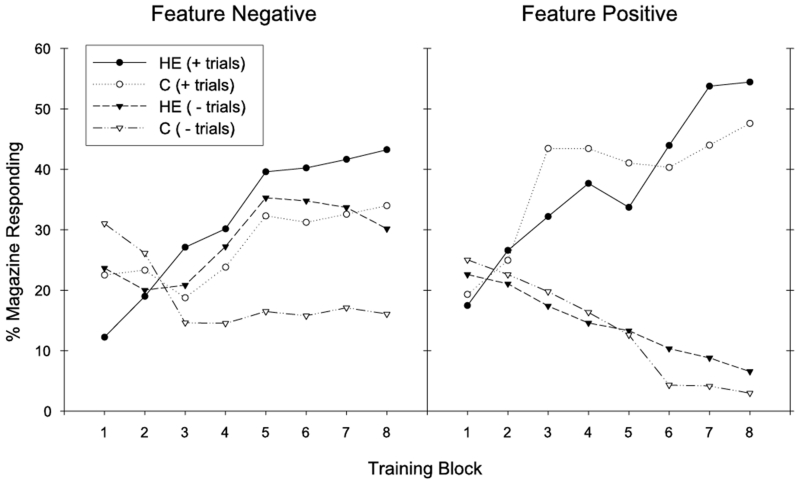
Rats maintained on the HE diet exhibited impaired serial feature negative discrimination performance compared to rats maintained on chow. Discrimination performance across training for the feature negative problem is presented in Figure 1 (left) for groups HE-FN and C-FN. Whereas both groups learned to increase appetitive responding on rewarded T1+ trials across training, Group C-FN showed a greater reduction in appetitive responding on nonrewarded L➔T1− trials across training compared to Group HE-FN. Furthermore, differences in responding on rewarded (T+) and nonrewarded (L➔T−) were slower to emerge across training for Group HE-FN compared to Group C-FN.
Figure 1.
Performance during training is shown for the feature negative (left) and feature positive (right) discrimination problems. Discrimination performance was impaired for group HE-FN in the feature negative discrimination, whereas the HE diet did not impair feature positive discrimination for group HE-FP.
An overall ANOVA for the feature negative discrimination demonstrated a significant main effect of Trial Type (F(1,14) = 13.35, p < .01) and a significant Trial Type × Training Block interaction (F(7,98) = 8.16, p < .01). The main effect of Diet was not significant (F(1,14) = 1.05), nor were any significant interactions obtained with the variable Diet (largest F for Diet × Trial Type interaction, F(1,14) = 2.32). Additional analyses were performed across each two-block period of training to determine when discrimination learning emerged. These analyses showed that significant discrimination emerged over the period containing the 5th and 6th blocks of training, which was confirmed by a significant main effect of Trial Type (F(1,14) = 17.16, p < .01). However, discrimination performance across this period differed by Diet, which was confirmed by a significant Diet × Trial Type interaction (F(1,14) = 4.71, p < .05). When this interaction was broken down, a significant main effect of Trial Type was obtained for Group C-FN (F(1,7) = 26.23, p < .01), but not for Group HE-FN (F(1,7) = 1.57). For the period containing the 7th and 8th training blocks, the main effect of Trial Type was significant (F(1,14) = 34.54, p < .01); however, in this case, the Diet × Trial Type interaction was not significant (F(1,14) = 1.77). This analysis confirmed that discrimination occurred earlier in training for Group C-FN (5th and 6th blocks) than for Group HE-FN (7th and 8th blocks).
Separate analyses by Trial Type demonstrated that the groups differed in appetitive responding on nonrewarded L➔T1− trials across the training phase. For this trial type, a significant Training Block × Diet interaction was obtained (F(7,98) = 3.95, p < .01). When this interaction was broken down, a significant main effect of Training Block was observed for group C-FN (F(7,49) = 4.73, p < .01), but not for group HE-FN (F(7,49) = 1.85). On the other hand, the two groups did not differ in terms of responding on rewarded T1+ trials. For this trial type, both groups demonstrated an increase in appetitive responding on T1+ trials across the training phase, which was confirmed by a significant main effect of Training Block for T1+ trials (F(7,98) = 5.68, p < .01). Both the Training Block × Diet interaction (F(7,98) < 1.2) and the main effect of Diet (F1,14) < 0.4) were not significant for T1+ trials. These results show that the HE-FN rats were impaired relative to the C-FN rats in learning to reduce responding to the T1 stimulus on nonrewarded L➔T1− trials.
Feature Positive Discrimination
Rats receiving the HE-diet (group HE-FP) were not impaired in solving the feature positive discrimination relative to the chow-fed rats (group C-FP). As seen in Figure 1 (right), both dietary groups learned to respond more on rewarded L➔T1+ trials and less on T1− trials across training. These results were confirmed by ANOVA. The main effect of Trial Type (F(1,14) = 65.75, p < .01) and the Trial Type × Training Block interaction (F(7,98) = 26.39, p < .01) were significant. The overall main effect of Diet was not significant (F(1,14) = .04), nor were any interactions with the variable diet (largest F for the Diet × Training Block interaction, F(7,98) = 1.31). Significant discrimination between rewarded and nonrewarded trials was first present across the 3rd and 4th blocks of training, demonstrated by a significant main effect of Trial Type across this period (F(1,14) = 72.97, p < .01). Discrimination performance did not differ according to diet group across this period (Diet × Trial Type interaction, F(1,14) = 1.54; Diet main effect, F(1,14) = 0.55).
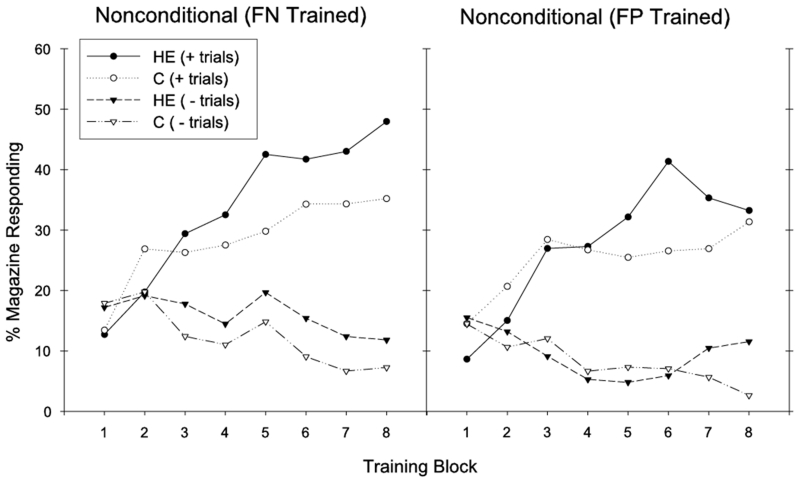
Nonconditional Discrimination
Like the feature positive discrimination, the HE-diet was not associated with impaired nonconditional discrimination learning, regardless of whether it was embedded in sessions containing a feature negative (FN-trained rats) or a feature positive (FP-trained rats) discrimination. As can be seen in Figure 2, all treatment groups learned to increase appetitive responding on rewarded T2+ trials, and to decrease responding on nonrewarded N− trials across training. For FN and FP trained rats (groups HE-FN and C-FN, Figure 2 left), overall ANOVAs demonstrated significant main effects of Trial Type (Fs(1,14) > 23, ps < .01) and significant Trial Type × Training Block interactions (Fs(7,98) > 12, ps < .01). Significant discrimination emerged across the 3rd and 4th blocks of training for both FN- and FP-trained rats (significant main effects of Trial Type, Fs(1,14) > 15, ps < .01). However, the main effects of Diet (Fs(1,14) < 1), and all interactions containing the variable Diet (Fs(7,98) < 1.2) were not significant for this and all other 2-block periods of training. Thus, we found no evidence that maintenance on the HE diet was accompanied by impaired performance on the simple nonconditional discrimination problem.
Figure 2.
Performance during training is shown for the nonconditional discrimination problem for FN (left) and FP (right)-trained rats. The HE diet did not influence nonconditional discrimination performance.
BLOOD-BRAIN BARRIER INTEGRITY
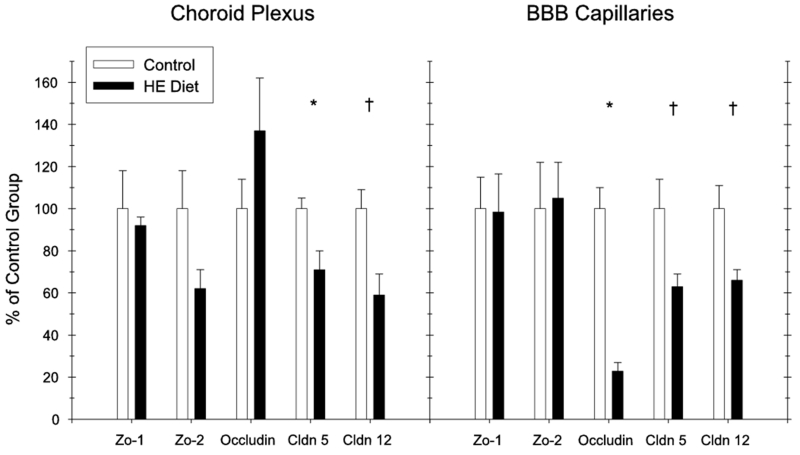
Tight Junction Protein mRNA: Quantitative real-time RT-PCR
The data for the tight junction protein analyses are presented in Figure 3 (choroid plexus, left; BBB capillaries, right) for the submembranous proteins Zo-1 and Z0-2, and the transmembrane proteins occludin, claudin-5, and claudin-12. In the choroid plexus, a significant HE-diet induced reduction in mRNA expression was observed for claudin-5 (t(13) = 3.22, p < .05). Marginally significant reductions were also observed for both Zo-2 (t(13) = 2.61, p = .07) and claudin-12 (t(13) = 2.3, p = .08). No differences were observed for Zo-1 and occludin (ts < 1). In the BBB capillaries, a significant HE-diet induced mRNA expression reduction was observed for occludin (t(13) = 2.44, p < .05), with nearly significant differences based on diet obtained for both Claudin-5 (t(13) = 2.39, p = .053) and Claudin-12 (t(13) = 2.54, p = .051). No differences based on diet were obtained for Zo-1 and Zo-2 (ts < 1).
Figure 3.
Tight junction protein mRNA expression (Quantitative RT-PCR) results for the blood-csf (left) and the blood-brain barrier (right) for HE diet and standard chow-fed rats (Experiment 2) (* = p<.05, † = p<.071) (error bars depict S.E.M.).
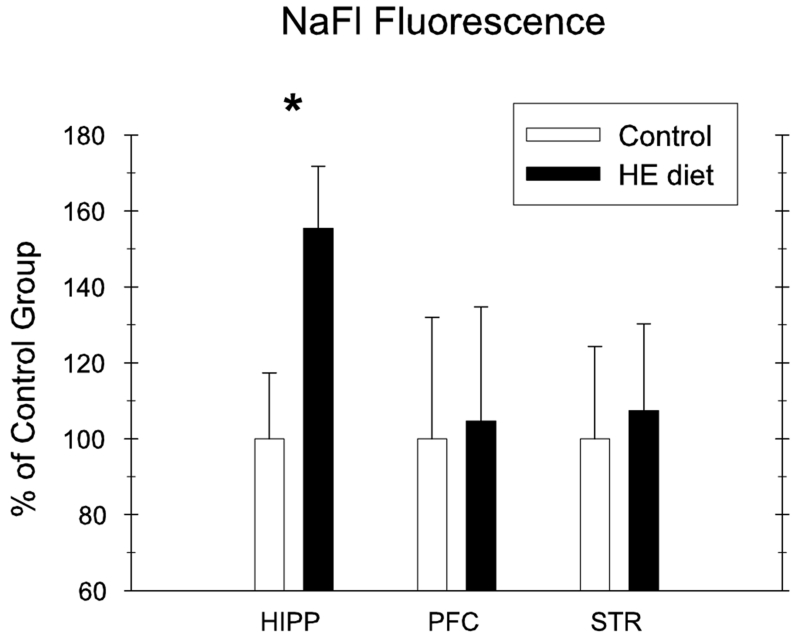
BBB Permeability to Sodium Fluorescein
Exposure to the HE-diet for 90-days prior to discrimination training was accompanied by an increase in permeability of NaFl into brain parenchyma in the hippocampus; however, no difference was observed in both striatum and prefrontal cortex. Figure 4 demonstrates NaFl fluorescence intensity in the three different brain areas, expressed as arbitrary units (% of control group for each brain area separately). Differences based on diet were supported statistically for the hippocampus (t(15) = 2.32, p < .05), but not for the striatum and the prefrontal cortex (t-scores <1, df=15).
Figure 4.
Permeability to Sodium Fluorescein (NaFl) in the hippocampus, prefrontal cortex, and striatum for HE-diet and chow-fed rats. Permeability was significantly increased in the hippocampus for HE-diet relative to chow-fed controls (* = p<.05) (error bars depict S.E.M.).
BODY WEIGHTS
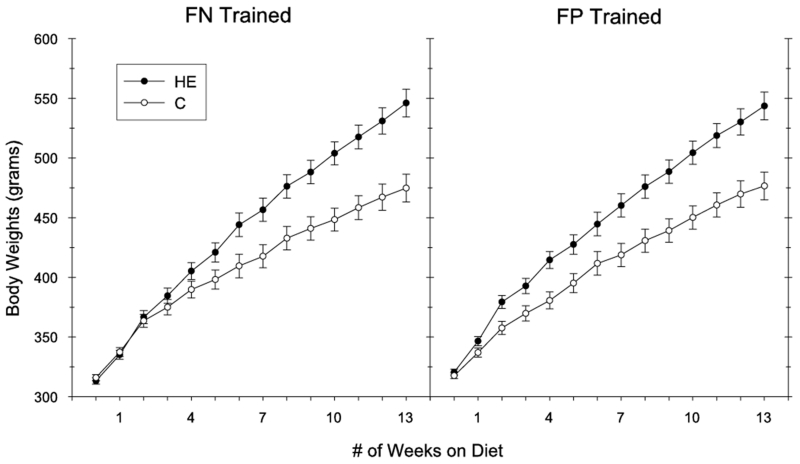
Intake of the HE-diet produced significant increases in body weight during the 90-day ad libitum period relative to intake of the C diet (see Figure 5). This was confirmed by a significant main effect of Diet (F(1,28) = 20.37, p < .01) and a significant Diet × Week interaction (F(13,364) = 35.67, p < .01). Body weights did not differ according to training, which was confirmed by a nonsignificant main effect of Training Group (F(1,28) = 0.1), as well as nonsignificant Training Group × Week (F(13,364) = 0.3), Training Group × Diet (F(1,28) = 0.2), and Training Group × Week × Diet (F(13,364) = 1.2) interactions. The body weights of the rats did not differ between the four treatment groups at the onset of powdered HE and chow feeding, which was confirmed by nonsignificant main effects of Diet (F1,28) = 0.01) and Training Group (F(1,28) = 3.0), and a nonsignificant Training × Diet interaction (F(1,28) = 1.4). At the end of the 90-day ad libitum feeding period, the rats fed the HE diet weighed significantly more than the chow-fed rats (significant main effect of Diet, F(1,28) = 35.57, p < .01). Body weights at the end of the 90-day ad libitum period did not differ, however, according to training (nonsignificant main effect of Training Group, F(1,28) = .01; nonsignificant Training Group × Diet interaction (F(1,28) = .04)).
Figure 5.
Average body weights for rats receiving the HE diet (HE-FN and HE-FP) or the standard chow diet (C-FN and C-FP) during the 90-day pre-training period. Significant body weight differences were obtained based on diet, but not based on assigned training groups (error bars depict S.E.M.).
DISCUSSION
Previous research demonstrated that diets high in saturated fat and simple carbohydrates can impair learning and memory function, particularly in tasks that rely on the utilization of spatial cues. One of the goals of the present research was to advance what is known about the detrimental effects of this type of diet on cognitive function by comparing learning and memory performance in rats on nonspatial discrimination learning tasks that vary in terms of their dependence on the integrity of the hippocampus. Impairments were observed following 90-days of maintenance on an HE-diet in a hippocampal-dependent feature negative discrimination problem, whereas performance was not affected by diet in two different discrimination tasks that do not rely on the hippocampus. Furthermore, the nature of the HE-diet induced impairment in the feature negative discrimination problem was similar to what has previously been observed in rats with selective hippocampal lesions [33]; both long-term HE-diet maintenance and hippocampal lesions are associated with increased appetitive responding on nonrewarded trials (e.g., L➔T−) relative to standard chow fed, and nonlesioned controls, respectively. These results support the hypothesis that some learning and memory processes, particularly those that rely on the integrity of the hippocampus, are more susceptible than others to disruption by diets containing saturated fat and refined carbohydrates.
Our results also demonstrated that HE-diet maintenance compromised blood-brain barrier integrity relative to a standard chow diet. Rats fed the HE-diet showed reduced mRNA expression of the tight junction proteins Claudin-5, and Claudin-12 in the choroid plexus, as well reduced expression of occludin, Claudin-5, and Claudin-12 in the BBB capillary system. Of the tight junction proteins, the claudins are believed to be the principle proteins that establish the tight junction properties of the endothelial cells, and are considered to be important in permeability restriction [34]. Consistent with reduced expression of these proteins, the HE-diet was also associated with an increase in permeability of sodium fluorescein (NaFl) from the vasculature to the hippocampal parenchyma, whereas no group differences were observed in the prefrontal cortex and the striatum. Overall, these results support the hypothesis that this type of diet can impact the regulation of the brain’s internal environment by impairing the blood-CSF and blood-brain barriers. Furthermore, the sensitivity of the hippocampus to increased NaFl permeability taken together with our behavioral results suggest that the hippocampus may be a particularly vulnerable brain structure to the detrimental effects of saturated fats and refined carbohydrates on both BBB permeability and cognitive function.
The mechanisms underlying the relationship between diet-induced BBB disruption and hippocampal dysfunction remain to be elucidated. One possible explanation is that dietary saturated fat is related to increases in circulating levels of Aβ, which can lead to compromised BBB integrity and exaggerated blood to brain delivery of peripherally-derived Aβ peptides. For example, a recent study demonstrated that mice maintained on a diet high in saturated fat had increased stimulation Aβ from the absorptive epithelial cells of the small intestines relative to control mice receiving a low fat diet [41, 42]. Elevations in peripheral levels of circulating Aβ has been shown to cause BBB disruption in rats [43]. Furthermore, BBB breakdown in a transgenic mouse model of AD can be reversed by Aβ immunization [44]. Collectively, these studies suggest that dietary saturated fat can stimulate intestinal production of Aβ, and that increases in circulating Aβ are related to BBB impairment. Our observation of increased BBB leakage of NaFl into the hippocampus of rats maintained on a diet high in saturated fat is consistent with these findings. Potentially, elevations in peripheral circulating Aβ peptides contribute towards BBB impairment, which can eventually lead to exaggerated blood to brain delivery of Aβ. Consistent with this possibility, it has recently been shown that Aβ peptides can enter the brain of mice from the periphery when the BBB was made defective by pharamacological manipulation, whereas Aβ peptides did not enter the brain of control mice with an intact BBB [45]. Future research is needed to determine if HE-diet induced BBB disruption can lead to elevations in Aβ levels in the hippocampus.
It seems unlikely that the observed diet-induced impairments in the feature negative problem are based on motivational mechanisms, such as altered taste, reward, etc, as opposed to disrupted learning processes. For example, while appetitive responding on nonrewarded trials in the feature negative problem was elevated for HE diet-fed relative to chow-fed control rats, this outcome was not obtained in the feature positive and nonconditional discrimination problems. This selective change in performance in the feature negative problem is inconsistent with the operation of a nonspecific motivational process as such a process would be expected to influence responding in each type of discrimination problem
Both dietary factors [2] and metabolic syndrome [46, 47] are linked with the development of cognitive impairment and AD onset. In the present study, several months of maintenance on the HE diet produced significantly greater body weight gain relative to the standard chow diet. This brings to question the possibility that the observed learning impairment is not based on the specific effects of the HE-diet, per se, but rather is a result of metabolic factors associated with diet-induced obesity (e.g., peripheral insulin resistance, dyslipidemia). While this account cannot be ruled out in the present study, there is evidence that this type of dietary manipulation can influence hippocampal function independent of body weight gain and obesity. For example, recent reports [48], including those from our laboratory [49], have shown that very brief consumption of a Western-type diet can impair hippocampal-dependent spatial memory . We previously reported that as little as 3-days consumption of the same HE-diet used in the present study impaired spatial memory in a radial arm maze paradigm in rats that did not differ in body weight from chow-fed controls. While these data suggest that diet-induced learning impairments can arise independent of factors associated with metabolic syndrome, the neurophysiological mechanisms underlying impairment following short- and long-term Western diet maintenance are likely different. Further research is needed to examine the respective contributions of dietary vs. metabolic factors on the development of cognitive impairment and AD, and to assess the extent to which cognitive disruption following short- and longer-term consumption of this type of diet involves similar or difference mechanisms.
The hippocampus is vulnerable to insult by neurodegenerative dementias [17-20], ishchemia [50, 51], and oxidative stress [52-54]. The present results in conjunction with our previous findings show that the hippocampus is also particularly vulnerable to diet-induced disruption following both short- and longer-term HE-diet consumption. Other findings are consistent with the possibility that this type of diet can also disrupt the function of the prefrontal cortex as well. For example Greenwood and Winocur (e.g., [10, 11]) have shown that 90-days maintenance on a diet high in saturated fat produced a pattern of impairments in an operant rule-learning task that was consistent with impaired function of both the hippocampus and the prefrontal cortex. Although we did not observe increased BBB permeability in the PFC in the present research, we have previously reported deficits in prefrontal cortical function following longer- (e.g., > 2 months), but not short-term HE diet consumption [14, 49]. These findings are interesting with respect to the temporal pattern of neurodegeneration observed in AD. For example, while hippocampus and adjacent tempero-cortical structures are vulnerable to degeneration in preclinical and early AD [18], the prefrontal and other neocortical regions are prone to atrophy during the later stages of AD [55]. Thus, the pattern of functional impairment observed in rodents with increasing exposure to an HE diet is consistent with the temporal pattern of neurodegeneration observed in AD.
The present results may also have implications for understanding the regulation of food intake and body weight. Recent research (e.g., [56]) shows that rats with selective hippocampal lesions show elevated food intake and body weight gain compared to sham- and non-lesioned controls. Based on the present findings, it may be that one mechanism though which intake of saturated fats and simple carbohydrates contribute to excess energy intake and body weight gain is by interfering hippocampal-dependent processes (see [57, 58]).
Obesity and AD are two of the most serious health challenges facing Western cultures. At present, there are no effective therapies for either disease. Although most clinicians and scientists view these disorders as having distinct etiologies and underlying pathologies, the present findings suggest that both may have common dietary origins and brain substrates. Our results join with other recent findings (see [46, 59, 60] for review) in pointing to the need for a more integrative research approach that could help describe shared neuronal and behavioral mechanisms that may underlie obesity and AD, and (b) help identify effective new treatments to prevent or ameliorate both of these serious threats to well-being.
ACKNOWLEDGMENTS
The authors would like to thank Mamta Behl, Andrea Tracy, Peter Urcuioli, and Kim Kinzig for their contributions. This work was done in partial fulfillment of the requirements for completion of a Doctor of Philosophy degree by Scott E. Kanoski at Purdue University, West Lafayette, IN, USA. Funding was supported by the National Institutes of Health, NICHDR01: grant numbers HD44179, R01 HD29792, P01 HD052112 to TLD, and NIEHS: grant numbers ES008146 and ES017055 to WZ.
REFERENCES
- [1].Hu F, van Dam R, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44:805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- [2].Berrino F. Western diet and Alzheimer’s disease. Epidemiol Prev. 2002;26:107–115. [PubMed] [Google Scholar]
- [3].Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer’s disease. J Alzheimers Dis. 2002;4:179–189. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- [4].Pasinetti G, Eberstein J. Metabolic syndrome and the role of dietary lifestyles in Alzheimer’s disease. J Neurochem. 2008;106:1503–1514. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eskelinen M, Ngandu T, Helkala E, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- [6].Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- [7].Papanikolaou Y, Palmer H, Binns MA, Jenkins DJ, Greenwood CE. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49:855–862. doi: 10.1007/s00125-006-0183-x. [DOI] [PubMed] [Google Scholar]
- [8].Benton D, Maconie A, Williams C. The influence of the glycaemic load of breakfast on the behaviour of children in school. Physiol Behav. 2007;92:717–724. doi: 10.1016/j.physbeh.2007.05.065. [DOI] [PubMed] [Google Scholar]
- [9].Farr S, Yamada K, Butterfield D, Abdul H, Xu L, Miller N, Banks W, Morley J. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- [11].Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behavioral Neuroscience. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- [12].Jurdak N, Kanarek R. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav. 2009;96:1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [13].Jurdak N, Lichtenstein A, Kanarek R. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- [14].Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- [16].Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [17].Gomez-Isla T, Price JL, McKeel DW, Jr., Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- [19].Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Granholm A, Bimonte-Nelson H, Moore A, Nelson M, Freeman L, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stranahan A, Norman E, Lee K, Cutler R, Telljohann R, Egan J, Mattson M. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiology of Learning & Memory. 2001;75:179–189. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- [24].Persidsky Y, Ramirez S, Haorah J, Kanmogne G. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- [25].Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ujiie M, Dickstein D, Carlow D, Jefferies W. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- [27].Latour L, Kang D, Ezzeddine M, Chalela J, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- [28].Gustafson D, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–650. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- [29].Whitmer R, Gustafson D, Barrett-Connor E, Haan M, Gunderson E, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- [30].Chen X, Gawryluk J, Wagener J, Ghribi O, Geiger J. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Banks W, Coon A, Robinson S, Moinuddin A, Shultz J, Nakaoke R, Morley J. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- [32].Banks W, Burney B, Robinson S. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29:2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holland PC, Lamoureux JA, Han J-S, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [34].Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- [35].Kozler P, Pokorny J. Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52:607–614. [PubMed] [Google Scholar]
- [36].Chen X, Lan X, Roche I, Liu R, Geiger J. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem. 2008;107:1147–1157. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duran-Vilaregut J, Del Valle J, Camins A, Pallas M, Pelegri C, Vilaplana J. Blood-brain barrier disruption in the striatum of rats treated with 3-nitropropionic acid. Neurotoxicology. 2009;30:136–143. doi: 10.1016/j.neuro.2008.10.007. [DOI] [PubMed] [Google Scholar]
- [38].Choi B, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Deane R, Zheng W, Zlokovic B. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem. 2004;88:813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [41].Galloway S, Jian L, Johnsen R, Chew S, Mamo J. beta-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. J Nutr Biochem. 2007;18:279–284. doi: 10.1016/j.jnutbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [42].Galloway S, Pallebage-Gamarallage M, Takechi R, Jian L, Johnsen R, Dhaliwal S, Mamo J. Synergistic effects of high fat feeding and apolipoprotein E deletion on enterocytic amyloid-beta abundance. Lipids Health Dis. 2008;7:15. doi: 10.1186/1476-511X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Su G, Arendash G, Kalaria R, Bjugstad K, Mullan M. Intravascular infusions of soluble beta-amyloid compromise the blood-brain barrier, activate CNS glial cells and induce peripheral hemorrhage. Brain Res. 1999;818:105–117. doi: 10.1016/s0006-8993(98)01143-3. [DOI] [PubMed] [Google Scholar]
- [44].Dickstein D, Biron K, Ujiie M, Pfeifer C, Jeffries A, Jefferies W. Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer disease. FASEB J. 2006;20:426–433. doi: 10.1096/fj.05-3956com. [DOI] [PubMed] [Google Scholar]
- [45].Clifford P, Zarrabi S, Siu G, Kinsler K, Kosciuk M, Venkataraman V, D’Andrea M, Dinsmore S, Nagele R. Abeta peptides can enter the brain through a defective blood-brain barrier and bind selectively to neurons. Brain Res. 2007;1142:223–236. doi: 10.1016/j.brainres.2007.01.070. [DOI] [PubMed] [Google Scholar]
- [46].Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, Clarke K. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009 doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- [49].Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology: Animal Behavior Processes. 2009 doi: 10.1037/a0017228. in press. [DOI] [PubMed] [Google Scholar]
- [50].Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anat Rec (Hoboken) 2009;292:1914–1921. doi: 10.1002/ar.20969. [DOI] [PubMed] [Google Scholar]
- [51].Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- [52].Fitsanakis VA, Thompson KN, Deery SE, Milatovic D, Shihabi ZK, Erikson KM, Brown RW, Aschner M. A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the morris water maze. Neurotox Res. 2009;15:167–178. doi: 10.1007/s12640-009-9017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Haces ML, Montiel T, Massieu L. Selective vulnerability of brain regions to oxidative stress in a non-coma model of insulin-induced hypoglycemia. Neuroscience. 165:28–38. doi: 10.1016/j.neuroscience.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [54].Hota SK, Barhwal K, Singh SB, Ilavazhagan G. Differential temporal response of hippocampus, cortex and cerebellum to hypobaric hypoxia: a biochemical approach. Neurochem Int. 2007;51:384–390. doi: 10.1016/j.neuint.2007.04.003. [DOI] [PubMed] [Google Scholar]
- [55].McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr., Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [59].Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer’s Disease: A Link Between Body Weight and Cognitive Function in Old Age. Am J Alzheimers Dis Other Demen. 2009 doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]