To the Editor
The clinical diagnosis of certain primary immunodeficiency diseases (PID) may be challenging due to the narrow phenotypes initially published. With the advent of whole exome sequencing (WES), accurate diagnosis of PID and extension of published phenotypes is more easily accomplished.
We describe a young woman initially diagnosed with common variable immunodeficiency disease (CVID), who developed monocytopenia and Mycobacterium avium complex infection (MonoMAC) syndrome. Prior to identification of her molecular diagnosis, she underwent a haploidentical hematopoietic stem cell transplant (HSCT) from her clinically healthy sister. Following HSCT, complete reversal of the hematological, immunological, and clinical manifestations of disease occurred. She was subsequently found to have mutations in CECR1 (encoding ADA2) by WES. (1–3) This case illustrates the phenotypic overlap among several PIDs, the role for WES in establishing the correct diagnosis, and the efficacy of HSCT in reversing the phenotype.
Case Description
A 20 year-old Hispanic female presented to the National Institutes of Health Clinical Center with an absolute neutropenia, anemia, and an invasive Fusarium proliferatum sinusitis. She had been diagnosed with CVID at age 7 with low IgG levels and frequent respiratory infections. She had frequent infections throughout childhood and received intravenous immune globulin (IVIG) on two occasions at age 15. At age 19 she was hospitalized for febrile neutropenia, right maxillary sinusitis secondary to Fusarium species, sepsis and respiratory failure due to an extended spectrum β-lactamase producer Escherichia coli. She required ICU care, mechanical ventilation, and treatment with broad-spectrum antibiotics, liposomal amphotericin B, posaconazole, granulocyte colony stimulating factor-1, and corticosteroids.
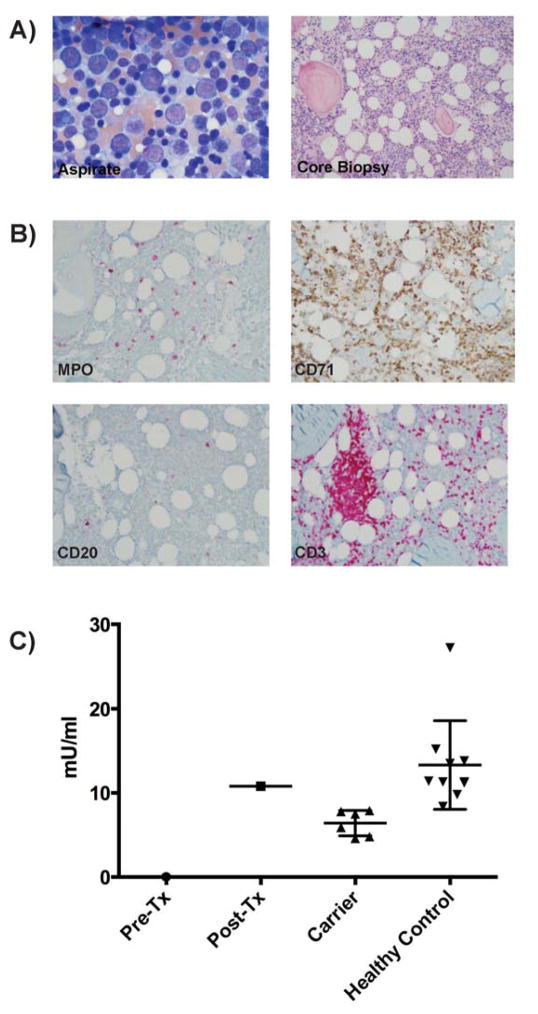
Upon admission to the NIH she had right maxillary Fusarium proliferatum sinusitis. Her absolute neutrophil count was 0 cells/μl, monocytes 1 cell/μl, NK cells 32 cells/μl, and CD20+ B-cells 0 cells/μl (Table 1). The bone marrow was mildly hypocellular for age with no neutrophils or neutrophil precursors (promyelocytes, myelocytes and/or metamyelocytes), erythroid predominance, myeloid hypoplasia, very low CD20+ B-cells, and increased T-cells with lymphohistiocytic aggregates (Fig. 1). There was no evidence of dysplasia. Consistent with GATA2 deficiency (4), flow cytometric analysis of the marrow aspirate showed 4% myeloblasts, absence of maturing neutrophil precursors, monocytopenia, severe B-lymphopenia, and increased T-cells with a CD4:CD8 ratio of 0.48. The patient’s clinical history, peripheral blood and bone marrow findings were compatible with GATA2 deficiency, although no mutation in GATA2 was identified. (1, 5) Bone marrow cytogenetic analysis was normal. Unlike GATA2 deficient marrow, both dendritic cells and B cell precursors were present.
Table I.
Clinical Characteristics of complete CECR1 Deficiency and Her Kindred
| patientc | fathera | motherb | sisterd | sistere | sisterf | sisterg | brotherh | reference range | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Clinical | |||||||||
|
| |||||||||
| Age (years) | 20 | 39 | 40 | 17 | 12 | 8 | 8 | 5 | |
| CECR1 status | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/+ |
|
| |||||||||
| infection | Fusarium proliferatum sinusitis | none | none | none | none | none | none | none | none |
|
| |||||||||
| vasculitis | no | no | no | no | no | no | no | no | no |
|
| |||||||||
| Lab | |||||||||
|
| |||||||||
| WBC | 0.7 ↓ | 3.6 ↓ | 6.8 | 4.2 | 6.8 | 5.1 | 4.8 | ND | 4.2–9.0 × 103 cells/μl |
|
| |||||||||
| ANC | 0 ↓ | 1.69 ↓ | 4 | 2.4 | 4.3 | 2.2 | 2.1 | ND | 1.7–5.3 × 103 cells/μl |
|
| |||||||||
| AMC | 0 ↓ | 0.22 ↓ | 0.43 | 0.24 ↓ | 0.41 | 0.26 ↓ | 0.12 ↓ | ND | 0.3–0.8 × 103 cells/μl |
|
| |||||||||
| Hb | 10.2 ↓ | 16.5 | 12.3 | 13.5 | 13.6 | 13.4 | 13.4 | ND | 11.2–15.7 g/dL |
|
| |||||||||
| platelets | 125 ↓ | 155 ↓ | 183 | 188 | 194 | 143 | 268 | ND | 173–369 × 103 cells/μl |
|
| |||||||||
| ABS CD19+ | 0 ↓ | 126 | 224 | 145 | 250 | 242 | 223 | ND | 61–321 cells/μl |
|
| |||||||||
| ABS NK(CD56+) | 32 ↓ | 140 | 213 | 175 | 426 | 416 | 488 | ND | 126–729 cells/μl |
|
| |||||||||
| ABS CD3+ | 645 ↓ | 1190 | 1860 | 939 | 1279 | 1887 | 1633 | ND | 714–2266 cells/μl |
FIG. 1.
Bone marrow findings and ADA2 enzyme activity. A, Left: Bone marrow aspirate. Right: Bone marrow core biopsy B, Immunohistochemistry: Myeloperoxidase (MPO); Erythroid (CD71); B cells (CD20), T cells (CD3); C, ADA2 plasma enzyme activity levels from the patient, pre and post transplant, her 1st degree carrier relatives, and 10 healthy, non-related controls.
Due to her recent intensive care hospitalization, invasive fusariosis, and 3-month history of refractory neutropenia, the patient underwent haploidentical HSCT from her 17 year-old sister, who had normal blood cell counts (Table 1), and no history suggestive of immunodeficiency. Her immediate post-transplant course was complicated by methicillin-resistant Staphylococcus aureus (MRSA) pneumonia and bacteremia on day +8 requiring intubation and mechanical ventilation. She had neutrophil engraftment on day +19 and was extubated on day +20. Her invasive fungal infection resolved. By day +30 her CD3+ and myeloid chimerisms were 100% donor and her monocyte count and NK cells returned to normal. CD19+ B-cells required one year to normalize. Immunosuppression was tapered after day +180. More than 2 years post-transplant she is off all immunosuppression with complete normalization of her hematologic and clinical abnormalities (see Methods in this article’s Online Repository).
WES was pursued due to the failure to detect a mutation in GATA2. After filtering out common variants, we identified a novel, homozygous change leading to a premature stop codon, c.794C>G, p.Gln265Stop, in CECR1 encoding adenosine deaminase 2 (ADA2). Sanger sequencing of parents and siblings demonstrated all to be heterozygous for CECR1 c.794C>G (Supplemental Figure 1). No consanguinity is reported by the family, however they are from a rural village and there is a homozygous region of 650kb surrounding CECR1 suggesting a common ancestral allele. No other family member had any clinical manifestations of disease. However, both the father and two twin sisters had slightly low neutrophil counts (Table 1). Plasma ADA2 levels were undetectable in this patient and intermediate in both her parents and siblings; the patient’s plasma ADA2 activity normalized post-transplant (Figure 1). ADA2 enzyme activity has been shown to be elevated in several primary immunodeficiencies.(6) The post-transplant ADA2 activity level could be the result of a single time point sample or due to underlying interactions with non-hematopoietic host cells, consistent with other immunodeficient patients.
In 2014 mutations in CECR1/ADA2 were shown to underlie a complex autoinflammatory syndrome of intermittent fevers, early onset lacunar strokes and other neurovascular manifestations including livedo rash, hepatosplenomegaly, and systemic vasculopathy.(2, 3) In the first study, 6 patients were compound heterozygous for 8 CECR1 mutations, while 3 patients with polyarteritis nodosum (PAN) or small vessel vasculitis were homozygous for a p.Gly47Arg mutation. (3) All 9 patients had low ADA2 enzyme activity in the blood. Skin, liver, and brain biopsies revealed vasculopathic changes. These patients had modest decreases in B-lymphocytes with 2 patients treated with IVIG. Knockdown of the zebrafish ADA2 homologue caused intracranial hemorrhage and neutropenia. The second study described 6 families with inherited PAN with homozygous or compound heterozygous missense mutations in CECR1. (2)A subsequent report emphasized B-cell deficiency and hypogammaglobulinemia in two siblings with compound heterozygous, missense CECR1 mutations. (7)
The initial report of ADA2 deficiency noted that systemic immunosuppressive treatment did not control the inflammatory state.(3) However, they suggested that HSCT might reverse the phenotype since monocytes and macrophages are the two main cell types that secrete ADA2. Subsequently, Van Eyck, et. al (8) reported the successful use of HLA matched sibling HSCT in a 3 year-old boy with homozygous ADA2 p.Arg169Gln mutation who had lymphadenopathy, splenomegaly and autoimmunity. Five years post-transplant the patient has had complete reversal of the ADA2 immunologic phenotype and is off all medications. A 4 year-old patient with ADA2 was also successfully transplanted with an unrelated donor after myeloablative conditioning. (9)
As more ADA2 deficient patients are identified, the phenotype will continue to expand. ADA2 deficiency should be considered in the setting of immunodeficiency, even without vasculopathy. Interestingly, it is possible that whereas aberrant ADA2 proteins (due to missense mutations) may cause additional complications, the complete absence of ADA2, as in this case, may lead to profound cytopenias rather than the vascular complications seen with missense mutations. This case highlights the need for WES analysis in patients with a phenotype suspicious for a known genetic disorder yet no mutation identified in the candidate gene.
Supplementary Material
Acknowledgments
Funding sources
Funded by the NCI Contract No. HHSN261200800001E.
This research was supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research
This research was supported, in part, by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
We thank the patient and her family for participating in this research study.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations used
- CECR1
cat eye syndrome chromosome region, candidate 1)
- ADA2
adenosine deaminase type 2
- MonoMAC
monocytopenia with atypical mycobacterial infection
- GVHD
graft versus host disease
- HSCT
hematopoietic stem cell transplantation
- ND
not done
- NIH
National Institutes of Health
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–5. doi: 10.1182/blood-2011-05-356352. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–31. doi: 10.1056/NEJMoa1307362. Epub 2014/02/21. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–20. doi: 10.1056/NEJMoa1307361. Epub 2014/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganapathi KA, Townsley DM, Hsu AP, Arthur DC, Zerbe CS, Cuellar-Rodriguez J, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125(1):56–70. doi: 10.1182/blood-2014-06-580340. Epub 2014 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquet M, Bellanne-Chantelot C, Tavitian S, Prade N, Beaupain B, Larochelle O, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822–9. doi: 10.1182/blood-2012-08-447367. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poursharifi P, Saghiri R, Ebrahimi-Rad M, Nazem H, Pourpak Z, Moin M, et al. Adenosine deaminase in patients with primary immunodeficiency syndromes: the analysis of serum ADA1 and ADA2 activities. Clin Biochem. 2009 Sep;42(13–14):1438–43. doi: 10.1016/j.clinbiochem.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Santiago TM, Zavialov A, Saarela J, Seppanen M, Reed AM, Abraham RS, et al. Dermatologic Features of ADA2 Deficiency in Cutaneous Polyarteritis Nodosa. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2015.1635. Epub 2015/07/02. [DOI] [PubMed] [Google Scholar]
- 8.Van Eyck L, Jr, Hershfield MS, Pombal D, Kelly SJ, Ganson NJ, Moens L, et al. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135(1):283–7. e5. doi: 10.1016/j.jaci.2014.10.010. Epub 2014/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Montfrans J, Zavialov A, Zhou Q. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):478. doi: 10.1056/NEJMc1405506. Epub 2014/07/31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.