Abstract
Background
Bipolar disorder (BD) is a severe mental illness that can have high costs for youths (<18 years old) and adults. Relative to healthy controls (HC), individuals with BD often show impaired attention, working memory, executive function, and cognitive flexibility (the ability to adapt to changing reward/punishment contingencies). In our study of youths and young adults with BD, we investigated 1) how cognitive flexibility varies developmentally in BD, and 2) whether it is independent of other executive function deficits associated with BD.
Methods
We measured errors on a reversal-learning task, as well as spatial working memory and other executive function, among participants with BD (N=75) and HC (N=130), 7–27 years old. Regression analyses focused on the effects of diagnosis on reversal-learning errors, controlling for age, gender, IQ, spatial span, and executive function. Similar analyses examined nonreversal errors to rule out general task impairment.
Results
Participants with BD, regardless of age, gender, or cognitive ability, showed more errors than HC on the response reversal stages of the cognitive flexibility task. However, participants with BD did not show more errors on non-reversal stages, even when controlling for other variables.
Limitations
Study limitations include the cross-sectional, rather than longitudinal, design; inability to measure non-linear age effects; and inclusion of medicated participants and those with psychiatric comorbidity.
Conclusions
Individuals with BD show a specific impairment in reversing a previously rewarded response, which persists across the transition from childhood to young adulthood. Tailored interventions targeting this deficit may be effective throughout this developmentally turbulent time.
Keywords: bipolar disorder, cognitive flexibility, development, executive function, reversal learning
Introduction
Bipolar disorder (BD) is a highly impairing psychiatric illness, with health care costs estimated to be twice those of depression (Keck et al., 2008; Kleinman et al., 2003) and a prevalence of 1–4% in the general population (Merikangas et al., 2007; Merikangas et al., 2012). BD often leads to serious health and psychosocial problems, and tragically even suicide (Holma et al., 2014; Keck et al., 2008; Kleinman et al., 2003). While many assume BD solely affects adults, ample research especially during the past two decades demonstrates that BD may also affect children and adolescents (hereafter “youths” age <18 years old). As in adults, BD in youths can be a devastating illness associated with high health care costs, poor psychosocial outcomes, and suicide (Dusetzina et al., 2012; Hauser et al., 2013; Leverich et al., 2007; Romero et al., 2009). Increasing numbers of youths are being diagnosed with and treated for BD – a 40% increase in one study - as evidenced by both inpatient and outpatient clinical data from the US and abroad (Blader and Carlson, 2007; Holtmann et al., 2010; Moreno et al., 2007). Furthermore, while BD symptoms may start in childhood (Leboyer et al., 2005), many patients are not formally diagnosed with BD until adulthood, potentially creating substantial delays in receiving BD-specific treatments (Leverich et al., 2007). Altogether, a critical need exists for more studies to include both children and young adults with BD so as to examine how the phenomenology and pathophysiology of BD change across the lifespan.
In studies involving either youths (Dickstein et al., 2004; Joseph et al., 2008; Kyte et al., 2006; Pavuluri et al., 2006a; Pavuluri et al., 2006b) or adults with BD (Badcock et al., 2005; Green, 2006; Jabben et al., 2010; Roiser et al., 2009; Sweeney et al., 2000), but not both groups in the same study, BD is associated with cognitive deficits, including impaired attention, working memory, executive function, and response inhibition—all compared to healthy controls (HC) without psychopathology. Although several functional magnetic resonance imaging (fMRI) studies have compared youths and adults with BD on facial emotion recognition tasks (e.g., Brotman et al., 2013), fewer studies have directly compared cognitive deficits between youths and adults with BD (Wegbreit et al., 2014). Two such fMRI studies involving response inhibition tasks found that youths with BD showed more neural alterations than adults with BD in the inferior frontal gyrus and the anterior cingulate cortex, which are involved in cognitive control (Weathers et al., 2013; Weathers et al., 2012). Moreover, in an fMRI meta-analysis, youths with BD showed more consistently decreased anterior cingulate activation during cognitive tasks than adults with BD (Wegbreit et al., 2014). These cognitive problems are important to study because many are associated with reduced psychosocial functioning and do not remit during euthymia (Andreou and Bozikas, 2013; Buoli et al., 2014; Mora et al., 2013; Pavuluri et al., 2006a; Pavuluri et al., 2009; Peters et al., 2014). Better knowledge of their pathophysiology could provide a cost-effective way to improve the lives of individuals with BD by spurring the development of novel pharmacological agents (Miskowiak et al., 2014) and cognitive remediation treatments (Dickstein et al., 2015b).
Another cognitive construct that has been investigated in separate studies of BD adults or BD youths is cognitive flexibility, defined as adapting to changes in rewards and punishments (Cools et al., 2002; Cools et al., 2004). Reversal-learning tasks are one laboratory measure of cognitive flexibility, whereby participants use trial-and-error learning to determine which of two objects is rewarded versus punished. Then, without warning, the stimulus/reward association reverses, so that the previously rewarded stimulus is now punished, and vice versa. During reversal learning, youths with BD make more errors than HC youths (Dickstein et al., 2010a; Dickstein et al., 2007; Dickstein et al., 2004; Gorrindo et al., 2005) and show specific alterations in regions involved in cognitive control, including ventral prefrontal cortex (vPFC) and ventral striatum (VS) (Adleman et al., 2011; Dickstein et al., 2010b). Adults with BD are less consistent, as some studies revealed behavioral deficits in reversal learning versus adult HCs and associated vPFC and VS alterations (Clark et al., 2001, 2002; Kozicky et al., 2013; Linke et al., 2013; Linke et al., 2012; McKirdy et al., 2009), but others have not (Roiser et al., 2009; Rubinsztein et al., 2000; Sweeney et al., 2000). To the best of our knowledge, no study has examined altered response reversal in BD using a developmental framework including both youths and adults with BD.
Consequently, we examine response reversal in participants with childhood-onset BD, including both young adults (those ≥18) and youths (those <18). Specifically, we enrolled adults who had been followed for BD since childhood by the Brown University site of the Course and Outcome of Bipolar Youth (COBY) study to ensure that retrospective recall bias did not affect these participants’ BD diagnosis (Birmaher et al., 2009; Leboyer et al., 2005). This strategy also eliminates another potential confound because all participants had early-onset BD, rather than comparing youths with childhood-onset BD to adults with adult-onset BD. We employed age as a continuous variable to search for specific diagnosis-by-age interactions, as our prior work suggests that younger people with BD show delayed development in their facial emotion recognition ability (Wegbreit et al., 2015). Thus, we hypothesized that younger participants with BD would also show worse reversal-learning performance than expected for their age relative to older participants with BD (Jarcho et al., 2012; Wegbreit et al., 2015). Moreover, we conducted additional analyses to determine how cognitive flexibility deficits relate to broader deficits in executive function, given that spatial span predicts planning ability in participants with BD (Badcock et al., 2005). These extended analyses investigated whether cognitive flexibility deficits in BD are independent of other executive functioning deficits, including mental storage capacity (spatial span) and planning ability (tested by the Stockings of Cambridge task).
Methods
Participants
All participants were enrolled in Institutional Review Board-approved research studies conducted at Bradley Hospital and Brown University. After written informed consent and assent were obtained, participants’ psychiatric symptoms and history were assessed using the Child Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997) administered to participants under 18 years old and their parents separately, or the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002) for participants 18 years and older. All interviews were conducted by either a board-certified child/adolescent psychiatrist or a licensed clinical psychologist with established inter-rater reliability (DPD, KLK; κ ≥0.85).
Inclusion criteria for all BD participants were: (1) age between 7–30 years, (2) English fluency, and (3) meeting DSM-IV-TR criteria for BD type-I. More specifically, participants needed to have at least one manic episode (≥7 days) with abnormally elevated/expansive and/or irritable mood and ≥3 DSM-IV criterion “B” mania symptoms (≥4 if predominantly irritable mood). All adults with BD were originally enrolled as youths in the Brown University site of the aforementioned COBY study (Birmaher et al., 2009). Therefore, all participants were diagnosed with child onset type-I BD. No participants were biologically related.
Inclusion criteria for all HC were: (1) age between 7–30 years, (2) no current or lifetime psychiatric illness or substance abuse/dependence in themselves or any first-degree relatives, and (3) English fluency.
Exclusion criteria for participants with BD were: (1) Autism spectrum disorder or primary psychosis, (2) Full Scale IQ (FSIQ)<80 on the Wechsler Abbreviated Scale of Intelligence (WASI)(Wechsler, 2005), (3) medical/neurological conditions potentially mimicking BD.
Exclusion criteria for HC were: (1) WASI FSIQ <80, (2) serious non-psychiatric medical disorders (e.g., epilepsy), and (3) learning disorders or pervasive developmental disorders.
CANTAB NEUROPSYCHOLOGICAL PERFORMANCE TASKS
Intra-Dimensional/Extra-Dimensional Shift (ID/ED)
Reversal learning was assessed using the ID/ED task of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) (Cambridge Cognition Limited, 2012). The task has nine stages— including four reversal stages (simple, compound, intradimensional, and extra-dimensional). Depending on the stage, each trial displays two simple shapes (lone, color-filled shapes) or two compound shapes (white lines overlying color-filled shapes), and participants must identify the “correct” stimulus. On-screen feedback establishes an underlying rule, and participants move onto the next stage after completing six consecutive correct trials. The previously correct stimulus then becomes incorrect, and participants must reverse their responses. The first seven stages are intradimensional (ID) stages only involving the colored shapes, with other dimension (white lines) being entirely irrelevant. At the extradimensional (ED) shift stage, this previously irrelevant dimension becomes relevant, and participants must shift their attention from the previously relevant dimension. On any stage, if participants cannot meet the criterion of six consecutive correct responses after 50 trials, the whole task terminates.
Stockings of Cambridge (SOC)
Participants’ executive functioning and planning abilities were assessed with the SOC. The task displays two arrangements of three colored balls, one at the top of the screen and one at the bottom, and participants must move the balls in the bottom set to match the top set. Each trial requires two to five moves for correct completion. If participants make double the necessary moves, the trial terminates. The test ends after three terminations in a row, but all of our participants completed the SOC.
SOC performance is assessed by evaluating (1) total moves, (2) the number of problems solved in minimum moves, (3) thinking time before starting each problem, and (4) thinking time spent after starting. We created a single score for each variable by summing across all problems (weighting by the number of each type).
Spatial Span (SSP)
Participants’ spatial working memory capacity was assessed with the SSP. Nine squares are displayed on the screen, and change color one by one. Participants are then asked to touch the boxes in the order in which they changed color. The task starts with two boxes changing colors and increases by one box after each successful trial until all nine boxes are changing colors. If participants are not successful on their first try on a level, they receive two more attempts. After three failed attempts on a level, the test terminates. Outcome measures include (1) span length, (2) total errors, and (3) usage errors.
Mood/Functional Ratings
To characterize the BD sample, we evaluated depressive symptoms, manic symptoms, and overall functional impairment. Specifically, the Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski et al., 1985) and the Hamilton Depression Scale (HAM-D) (Hedlund and Vieweg, 1979) were administered to participants under 18 years old and over 18 years old, respectively. The CDRS-R and HAM-Dhave different scales, so for correlations between depression and task performance, sample-specific Depression Z-scores were created (Wegbreit et al., 2015). Manic symptoms were assessed using the Young Mania Rating Scale (YMRS) (Kaufman et al., 1997). Overall functioning was evaluated using the Children’s Global Assessment Scale (CGAS) (Shaffer et al., 1983) for <18 year olds, or the Global Assessment of Functioning (GAF) (Hall, 1995) for those >18. Both the CGAS and the GAF utilize a 1–100 scale with similar, but developmentally appropriate, cut-offs every 10 points (Schorre and Vandvik, 2004).
Analytic strategy
First, we investigated the simple relationship between diagnosis and ID/ED task performance using independent-samples t-tests. Next, we sought to identify which other variables might be of interest when evaluating the relationship between diagnosis and ID/ED task performance, focusing primarily on variables that differed between diagnostic groups. Finally, we ran regression models with diagnosis included along with other variables that could moderate the relationship between diagnosis and ID/ED performance. All statistical tests were conducted in SPSS 22 (IBM Corp., Armonk, NY) and were two-tailed with a 0.05 criterion for statistical significance.
For the ID/ED task, we focused on the intra-dimensional (ID) stages, which measure reversal learning, rather than the extra-dimensional (ED) stages, which measure attention shifting. In prior studies, researchers have summarized participants’ ID/ED performance in several ways, including the (1) number of stages participants completed (Sweeney et al., 2000), (2) number of participants who complete the ED shift stages (Roiser et al., 2009), (3) total errors before the ED shift or overall on the task (Dickstein et al., 2004), and/or (4) total reversal-stage errors (McKirdy et al., 2009). We were primarily interested in increasing task sensitivity versus distinguishing the effects on different types of response reversals, so we took the latter approach and summed errors across pre-ID reversal stages to create a Reversal-Stage Errors score. We were also interested in identifying deficits in general ID/ED performance, so we also summed errors on non-reversal pre-ID stages to create a Total Non-Reversal-Stage Errors score. If participants with BD are generally impaired on the task then they should show more errors on this measure relative to HC in addition to showing more errors on the reversal stages. However, if the impairment is specific to reversal learning then participants with BD should only show an increase in reversal-stage errors relative to HC. In follow up analyses, we also examined errors on each reversal stage (simple, compound, ID-shift compound) separately to compare with prior findings (Dickstein et al., 2007; Dickstein et al., 2004).
Variable selection for regression models
To determine which variables moderate the association between BD and deficits in reversal learning, we first compared the BD and HC groups with t-tests for continuous variables (e.g., age, CANTAB scores) and with chi-squared tests for categorical variables (e.g., gender, race) (Table 1). Variables with significant differences between groups were incorporated in our regression models, with two exceptions. We included gender as a variable of a priori interest given previous findings of gender differences in cognitive performance in BD samples, including differences in spatial working memory (Barrett et al., 2008; Carrus et al., 2010; Vaskinn et al., 2011). Furthermore, we had relatively few non-White participants, so we did not include race and simply re-ran the models without the non-White participants to assess the potentially confounding effects of race.
Table 1.
Demographics and CANTAB performance data for participants with BD and HC
| Participant s with BD |
HC Participant s |
BD vs. HC | ||||
|---|---|---|---|---|---|---|
| Characteristic | (N=75) | (N= 130) | ||||
|
Mea n |
SD | Mean | SD |
t (203 ) |
p | |
| Age (years) | 16.5 | 4.5 | 17.6 | 4.9 | 1.51 | 0.13 |
| FSIQ | 106.4 | 10. 9 |
111.4 | 11. 3 |
3.11 | 0.002 |
| N | % | N | % |
X2 (2) |
p | |
| Male | 45 | 60. 0 |
64 | 49. 2 |
2.22 | 0.14 |
| Female | 30 | 40. 0 |
66 | 50. 8 |
||
| White a | 64 | 88. 9 |
97 | 75. 8 |
5.04 | 0.03 |
| Non-white a | 8 | 11. 1 |
31 | 24. 2 |
||
| Performance Measure |
Mea n |
SD | Mean | SD |
t (203 ) |
p |
| ID/ED Errors | ||||||
| All Stages | 7.9 | 3.4 | 6.5 | 2.9 | 3.18 | 0.002 |
| Reversal-Stages | 5.1 | 2.3 | 3.9 | 1.4 | 4.54 | <0.00 1 |
| Non-Reversal-Stages | 2.8 | 2.2 | 2.6 | 2.4 | 0.75 | 0.45 |
| Simple-Reversal-Stage | 1.9 | 1.3 | 1.5 | 1.1 | 2.27 | 0.03 |
| Compound-Reversal-Stage | 1.8 | 1.3 | 1.3 | 0.7 | 3.69 | <0.00 1 |
| Intradimensional-Compound-Reversals | 1.4 | 0.9 | 1.2 | 0.5 | 2.66 | 0.01 |
| ID/ED Latencies | ||||||
| All Stages b | 106.1 | 51. 7 |
105.3 | 42. 7 |
0.13 | 0.90 |
| Reversal-Stages b | 35.2 | 24. 4 |
30.2 | 22. 0 |
1.51 | 0.13 |
| Non-Reversal-Stages b | 70.9 | 33. 7 |
75.1 | 28. 7 |
0.94 | 0.35 |
| SSP | ||||||
| Length | 6.0 | 1.4 | 7.1 | 1.4 | 5.00 | <0.00 1 |
| Errors | 13.6 | 6.3 | 13.5 | 7.0 | 0.17 | 0.86 |
| Usage Errors | 2.2 | 1.6 | 1.8 | 1.6 | 1.33 | 0.185 |
| SOC | ||||||
| Total Moves | 71.1 | 9.5 | 65.7 | 8.1 | 4.31 | <0.00 1 |
| Problems Solved in Minimum Moves | 7.7 | 2.2 | 9.1 | 2.0 | 4.44 | <0.00 1 |
| Total Initial Thinking Time (seconds) | 50.6 | 40. 1 |
70.2 | 48. 4 |
2.95 | 0.004 |
| Total Subsequent Thinking Time (seconds) |
7.5 | 10. 0 |
6.3 | 6.8 | 1.07 | 0.29 |
Three participants with BD and two HC did not report their race.
One BD participants' latencies were not recorded.
Legend: BD=Bipolar Disorder; FSIQ=Full-scale IQ; HC=Healthy comparison; ID/ED=Intradimensional/Extradimensional Shift; SD=standard deviation; SOC=Stockings-of-Cambridge; SSP=Spatial span.
Simple comparisons and extended regression analyses
After using t-tests to compare the two groups’ reversal and non-reversal ID/ED performance, we examined the effect of age, demographic, and neurocognitive variables on the relationship between diagnosis and ID/ED performance. For ID/ED Reversal-Stage Errors and ID/ED Non-Reversal-Stage Errors, we tested two predictive models via multiple linear regressions. The first, “base model” contained four predictors: Diagnosis, Age, the Diagnosis-by-Age interaction, and FSIQ, as in our prior work using a facial emotion recognition task (Wegbreit et al., 2015). The second, “extended model” contained seven predictors: the four base model predictors, plus Gender, SSP Span Length, and SOC Total Moves. We report analyses using SOC Total Moves because these models had the highest adjusted R-squared model fits, but results were unchanged when substituting other SOC variables. For all regression analyses, predictors were standardized to reduce multicollinearity (Cohen et al., 2003).
As an exploratory analysis, we ran the base and extended models for errors on each reversal stage to investigate whether any effects were specific to a particular reversal stage. To investigate potential medication confounds, we conducted post-hoc analyses sequentially excluding participants by the medication class they were taking (e.g., lithium, antidepressants, etc.) (Henin et al., 2009; Pavuluri et al., 2006b). We also conducted additional separate posthoc analyses excluding all non-White participants and all non-euthymic participants with BD to preclude any race and mood state confounds, respectively (Wegbreit et al., 2015). Finally, because psychosis could influence clinical course and outcome, we conducted analyses excluding six adults with BD who had current or past psychotic features.
Results
Demographics
Overall, the BD and HC groups as a whole showed a significant difference in FSIQ but not age. For categorical variables, BD and HC groups showed a significantly different distribution of White vs. non-White participants, but their gender distribution did not significantly differ (Table 1). To compare with prior literature that splits youths and adults into groups, we tested for age-by-diagnosis interactions and found no significant effects (Table 2). We also report clinical variables, such as mood state, for BD youths and adults separately (Table 3). Of note, BD youths and adults did not show a significant difference in their approximate age of BD illness onset, t(70)=0.17, p=0.87.
Table 2.
Sample demographics for participants with BD and HC categorically split by age-group (pediatric vs. adult)
| Participants with BD | HC Participants | Diagnosis by Age Interaction |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BD Youths |
BD Adults | HC Youths |
HC Adults | |||||||
| Characterist ic |
(N=47) | (N=28) | (N=68) | (N=62) | ||||||
| Mea n |
SD | Mea n |
SD | Mea n |
SD | Mea n |
SD |
F(20 1) |
p | |
| Age in years | 13.8 | 3.0 | 21.1 | 2.4 | 13.7 | 2.9 | 21.9 | 2.3 | 1.30 | 0.2 6 |
| FSIQ | 106. 3 |
10. 7 |
106. 6 |
11. 3 |
111. 5 |
10. 9 |
111. 3 |
11. 9 |
0.03 | 0.8 7 |
| N | % | N | % | N | % | N | % |
G2(4) b |
p | |
| Female | 19 | 40. 4 |
11 | 39. 3 |
39 | 57. 4 |
27 | 43. 5 |
6.82 | 0.1 5 |
| Non-white a | 5 | 10. 9 |
3 | 11. 5 |
17 | 25. 4 |
14 | 23. 0 |
8.04 | 0.0 9 |
Three participants with BD and two HC did not report their race
Log-linear analyses for A×B×C contingency table (www.vassarstats.net/abc.html)
Legend: BD = Bipolar Disorder; FSIQ = Full-scale IQ; HC = Healthy comparison; SD = standard deviation;
Table 3.
Clinical characteristics of participants with BD categorically split by age-group (pediatric vs. adult)
| BD Youths | BD Adults | |||
|---|---|---|---|---|
| (N=47) | (N=28) | |||
| Clinical Variable | Mean | SD | Mean | SD |
| YMRSa | 7.1 | 4.9 | 4.4 | 2.7 |
| Depression Scoreb | 5.1 | 4.3 | 27.9 | 9.1 |
| Global Functioning Scorec | 60.7 | 13.3 | 65.8 | 11.8 |
| Illness Onset Aged | 9.9 | 3.7 | 9.8 | 3.7 |
| Mood Status | N | % | N | % |
| Euthymic | 37 | 78.7 | 24 | 85.7 |
| Hypomanic | 4 | 8.5 | 3 | 10.7 |
| Mixed | 1 | 2.1 | 0 | 0.0 |
| Depressed | 1 | 2.1 | 0 | 0.0 |
| Comorbid Conditions | N | % | N | % |
| Substance Abuse/Dependence | 1 | 2.1 | 11 | 39.3 |
| GAD | 4 | 8.5 | 4 | 14.3 |
| ADHD | 27 | 57.4 | 10 | 35.7 |
YMRS scores for two youths and one adult were not available.
CDRS-R for youths, HAM-D for adults; depression scores were not available from two youths and one adult.
CGAS for youths, GAF for adults; one adult's score was not available.
Illness onset ages for two youths and one adult were not available.
Legend: ADHD = Attention Deficit Hyperactivity Disorder; BD = Bipolar Disorder; GAD = Generalized Anxiety Disorder; SD = standard deviation; YMRS = Young Mania Rating Scale; CDRS-R = Children's Depression Scale-Revised; HAM-D = Hamilton Depression scale; CGAS = Children's Global Assessment Scale; GAF = Global Assessment of Functioning.
Between-group differences in CANTAB performance without added variables
In simple comparisons without added variables, participants with BD showed significantly more errors that HC on the ID/ED reversal stages (pooled across the three reversal stages). In contrast, the two groups did not show a significant difference in the number of errors made on the non-reversal stages of the ID/ED task (Table 1, Figure 1).
Figure 1.
Mean errors for all reversal and all non-reversal stages of the Intradimensional/Extradimensional shift task for participants with bipolar disorder (BD) and healthy controls (HC)
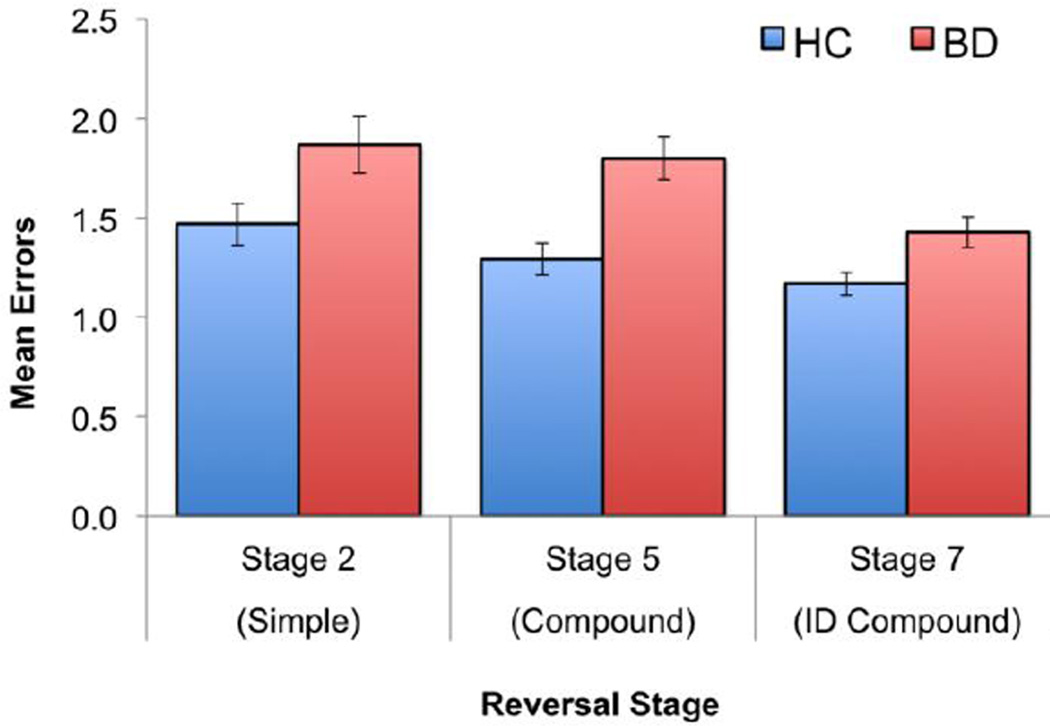
To test for specific differences across reversal stages, we conducted an omnibus repeated-measures ANOVA with reversal stage as the within-subjects variable and diagnosis as the between-subjects variable. Participants with BD showed significantly more errors overall than HC [F(1,203)=20.64, p<0.001], the reversal stages showed significant differences in difficulty across participants [F(2,203)=7.64, p<0.001], but diagnosis did not interact with reversal stage [F(2,203)=0.85, p=0.43]. Nevertheless, to compare to previous findings of significant differences between BD and HC on simple reversal stages (Dickstein et al., 2007; Dickstein et al., 2004), we evaluated between-group differences at each reversal stage and found that participants with BD showed more errors on each reversal stage than HC (Table 1, Figure 2).
Figure 2.
Mean errors for each reversal stage of the Intradimensional/Extradimensional shift task for participants with bipolar disorder (BD) and healthy controls (HC)
On the SSP task, participants with BD showed significantly shorter spatial span lengths than HC, but the same number of errors and usage errors. On the SOC task, participants with BD needed significantly more moves to complete the task compared to HC and solved significantly fewer problems in the minimum number of moves. On average, participants with BD spent significantly less time thinking before starting each SOC problem than HC, but spent the same amount of time on each problem as HC after starting (Table 1). Thus, participants with BD showed both reduced executive functioning and spatial span relative to their healthy peers.
Reversal-Stage Errors: Base and Extended Models
We examined whether age, FSIQ, or a diagnosis-by-age interaction influenced the effect of diagnosis on ID/ED reversal-stage errors, as in our previous work in facial emotion recognition (Wegbreit et al., 2015). This “base model” showed a significant effect of diagnosis and no significant effects for age, diagnosis-by-age interaction, or FSIQ (Table 4).
Table 4.
Regression analyses of reversal-stage vs. non-reversal-stage errors for individuals with BD or HC participants.
| Reversal-Stage Errors |
Non-Reversal-Stage Errors |
|||||
|---|---|---|---|---|---|---|
| Base-Model Effects | β | t(201) | p | β | t(201) | p |
| Diagnosis | 0.29 | 4.17 | <0.001 | 0.00 | 0.03 | 0.98 |
| Age | − 0.06 |
−0.86 | 0.39 | 0.01 | 0.18 | 0.86 |
| Diagnosis-by-Age | − 0.08 |
−1.22 | 0.22 | 0.02 | 0.33 | 0.75 |
| FSIQ | − 0.02 |
−0.32 | 0.75 | − 0.25 |
−3.58 | <0.001 |
|
Extended-Model Effects |
β | t(198) | p | β | t(198) | p |
| Diagnosis | 0.23 | 3.14 | 0.002 | − 0.03 |
−0.41 | 0.69 |
| Age | 0.04 | 0.48 | 0.63 | 0.05 | 0.67 | 0.50 |
| Diagnosis-by-Age | − 0.09 |
−1.31 | 0.19 | 0.02 | 0.25 | 0.80 |
| FSIQ | 0.04 | 0.54 | 0.59 | − 0.22 |
−2.91 | 0.004 |
| Gender | − 0.02 |
−0.22 | 0.82 | − 0.03 |
−0.41 | 0.69 |
| SSP | − 0.15 |
−1.88 | 0.06 | − 0.14 |
−1.61 | 0.11 |
| SOC Total Moves | 0.11 | 1.38 | 0.17 | − 0.01 |
−0.17 | 0.86 |
Legend: BD=Bipolar Disorder; FSIQ=Full-scale IQ; HC=Healthy comparison; SOC=Stockings-of-Cambridge; SSP=Spatial span.
Next, we ran the extended model, adding gender, spatial span, and SOC Total Moves, and found that the significant effect of diagnosis on reversal-stage errors remained even after controlling for these extra variables. No other statistically significant effects were found (Table 4).
Non-Reversal-Stage Errors: Base and Extended Models
For non-reversal-stage errors, we tested whether controlling for other variables could reveal a significant difference between diagnostic groups, as this finding could indicate a general ID/ED task impairment. However, the base model showed a non-significant diagnosis effect, no significant age effect, and no significant diagnosis-by-age interaction. Moreover, participants with lower FSIQs, regardless of diagnosis, made significantly more non-reversal-stage errors than those with higher FSIQs (Table 4).
The extended model for non-reversal stages also revealed only a significant effect of FSIQ, and the other effects, including the diagnosis effect, remained non-significant (Table 4). Thus, estimated general intelligence was the best predictor of performance on non-reversal task stages and diagnosis had no predictive power. This finding, along with our analysis of ID/ED reversal-stage errors, suggests a reversal-learning specific deficit in BD on the ID/ED task.
Follow-up analyses: Errors for specific reversal stages
In analyses for each reversal stage, diagnosis showed a significant effect for the base models for compound reversals and a marginal effect for simple reversals (Table S1). For the extended models, compound reversals showed significant effects of diagnosis and FSIQ, whereas the other stages did not show significant effects for any variable.
Post-hoc analyses of potential confounds: medication, psychiatric comorbidity, mood state, race, and psychotic features
In post-hoc analyses, we excluded participants affected by confounding variables and re-ran our models. To test for medication effects, we serially excluded participants with BD who were taking each class of medication (e.g., participants taking lithium, participants taking atypical neuroleptics, etc.) and re-ran our analyses. Next, we serially excluded participants with BD who met criteria for abuse/dependence for any substance (N=12) (Table 3). Then, we serially excluded BD participants with comorbid GAD (N=8), comorbid ADHD (N=37), and those without ADHD comorbidity (N=38; i.e., comparing BD+ADHD vs. HC) (Table 3). Although none of our participants met criteria for any psychotic disorder, six adults with BD reported a few current or past psychotic features, so we excluded these participants and re-ran the analyses. Across all of these analyses, the findings from our base and extended models were maintained. For reversal-stage errors, we found significant main effects of diagnosis, no significant diagnosis-by-age interactions, and few other significant effects (Table S2). For non-reversal-stage errors, we only found significant main effects of FSIQ, but no significant effects for diagnosis or any other variable (Table S3). Thus, medications, substances, and psychiatric comorbidities are highly unlikely to have influenced our results.
To examine mood state effects, we excluded 14 non-euthymic participants with BD, with non-euthymia defined as YMRS>12 (youths/adults); and/or CDRS-R>40 (youths) or HAMD>7 (adults) (Zimmerman et al., 2013), and we re-ran our base and extended models with the remaining 61 participants with BD. To be conservative, patients with missing mood ratings data were treated as non-euthymic (Table 3). For reversal-stage errors, we still found significant diagnosis effects while controlling for all other variables. Intriguingly, we also found significant effects of spatial span and SOC Total Moves that were independent of diagnosis (Table S2). These results underscore the notion that these tasks measure processes related to the reversal-learning task, but that BD also has an independent effect. For non-reversal-stage errors, we again found FSIQ effects, no significant effect of diagnosis, and no other significant results (Table S3). Thus, reversal-learning deficits occurred for euthymic participants with BD as well as symptomatic participants and cannot be explained by the BD participants’ mood states.
Finally, while we had no a priori hypotheses about the effect of participants’ race on CANTAB tasks, we re-ran our analyses excluding the 44 non-White BD and HC participants in our sample, because the groups significantly differed in their racial composition (Table 1). When restricting our sample to White participants (N=161), we still found significant effects of diagnosis for errors on reversal stages and no other significant effects (Table S2) and found significant effects of FSIQ for non-reversal stages and no other significant effects (Table S3). Thus, any racial difference between groups is unlikely to have influenced our results.
Post-hoc analyses
To test whether BD participants’ mood states influenced their CANTAB performance independently of euthymic status (i.e., dimensionally), we examined correlations between CANTAB performance and Depression, YMRS, and global functioning scores. We found no significant relationships between any clinical variables and CANTAB performance (Table S4), further suggesting that BD participants’ mood states did not influence their neuropsychological performance.
In contrast, illness duration did correlate with overall ID/ED errors, but not with reversal-stage errors (Table S4). To investigate this further, we ran versions of our model within the BD participant group with “age” replaced with “illness duration”. Illness duration did not significantly predict reversal-stage or non-reversal stage errors (Table S5), further supporting the notion that these reversal-learning deficits are independent of age.
Finally, to address potential concerns about the difference in FSIQ between the BD and HC groups confounding our results, we implemented a stepwise regression model and forced FSIQ into the model as the first step, before diagnosis. Even in this model, we found that diagnosis explained variance in reversal learning errors that FSIQ could not explain. In contrast, FSIQ was able to explain variance in the non-reversal-learning errors that diagnosis could not (see Table S6).
Discussion
Our study is the first to take a developmental approach to investigate impaired cognitive flexibility in youths and young adults with BD. Our primary findings were: (1) participants with BD made significantly more errors than HC on reversal stages, but not on non-reversal stages; (2) participants with BD showed reduced executive functioning and working memory capacity vs. HC; (3) however, we found a specific reversal-learning deficit for participants with BD even when controlling for working memory, executive functioning, and general intelligence. We did not find our hypothesized diagnosis-by-age interaction in reversal learning. Thus, the age-independent effect on reversal learning contrasts with our prior finding of a developmentally salient impairment in facial emotion recognition in BD youths (Wegbreit et al., 2015). Moreover, our effects generalized across genders, contrasting with prior gender-specific differences in cognitive performance in BD (Barrett et al., 2008; Carrus et al., 2010; Vaskinn et al., 2011). We found no influence of mood state, medication status, comorbid conditions, substance abuse/dependence, race, or psychotic features. Together, these findings suggest that among participants with childhood-onset BD, cognitive flexibility deficits may be integral to the pathophysiology of BD itself, both across development and independent of other cognitive and emotional issues in BD.
Our results showing age-independent reversal learning deficits are interesting in the context of prior work on reversal learning in BD. Specifically, in several studies, BD youths showed impaired cognitive flexibility not just vs. HC but also vs. youth with anxiety, major depression, and severe mood dysregulation (Adleman et al., 2011; Dickstein et al., 2010a; Dickstein et al., 2010b; Dickstein et al., 2007; Dickstein et al., 2004; Gorrindo et al., 2005). However, studies among BD adults are less consistent, as some report differences between BD adults and HC adults (Clark et al., 2001, 2002; Kozicky et al., 2013; Linke et al., 2013; Linke et al., 2012; McKirdy et al., 2009), but others do not (Roiser et al., 2009; Rubinsztein et al., 2000; Sweeney et al., 2000). This inconsistency may exist, in part, because adults in prior studies were in their 30s–50s, whereas our adults were considerably younger. Thus, the cognitive flexibility impairments that our adults exhibited could abate as they age further. However, impaired cognitive flexibility could be phenotypically inherent to childhood-onset BD. Specifically, people with childhood-onset BD could have impaired cognitive flexibility across the lifespan, whereas those with adult-onset BD do not. Answering this important question will require longitudinal studies that enroll participants with childhood-onset and adult-onset BD and study cognitive flexibility longitudinally.
No prior studies of cognitive flexibility in BD have evaluated the effects of age dimensionally in accordance the continuous nature of brain development found in longitudinal studies across the transition from childhood to adolescence (Giedd and Rapoport, 2010). Despite our dimensional approach to age, we did not find any age effect on reversal learning or any diagnosis-by-age interaction, unlike prior developmental work in facial emotion recognition in BD (Wegbreit et al., 2015). As such, individuals with childhood-onset BD may show longer-lasting deficits in reversal learning than in facial emotion recognition, consistent with the posterior-to-anterior development of the human brain from childhood young adulthood, with perceptual regions maturing years before prefrontal regions involved in cognitive flexibility (Giedd and Rapoport, 2010). Thus, cognitive flexibility training may be appropriate as a specific treatment target for individuals with childhood-onset BD throughout their entire childhood and adolescence, but such training may benefit individuals even if not started until young adulthood. Nevertheless, intervening earlier once a child is diagnosed with BD would be preferable, considering the well-studied links between reduced executive functioning and poor psychosocial outcomes in BD (Andreou and Bozikas, 2013; Buoli et al., 2014; Mora et al., 2013; Pavuluri et al., 2006a; Pavuluri et al., 2009; Peters et al., 2014).
Our results suggest that impaired cognitive flexibility in childhood-onset BD is independent from other executive functioning deficits and from general intelligence, potentially making it a good target for an intervention tailored to individuals with BD (Dickstein et al., 2015b). We found significant deficits for each reversal stage, unlike previous studies that only found effects for some reversal stages (Dickstein et al., 2007; Dickstein et al., 2004). This finding suggests that participants with BD show a rather general deficit in reversal learning relative to HC. Nevertheless, the ID/ED task is not the most sensitive measure of reinforcement learning, as other tasks can separate reward-based vs. punishment-based learning (Linke et al., 2011; van der Schaaf et al., 2011). These more sensitive reversal tasks could delineate the contributions of various brain regions (e.g. sub-regions of the PFC) to each aspect of reversal learning (Linke et al., 2011; Mitchell et al., 2009; van der Schaaf et al., 2011) and help us to better understand the behavioral and neural mechanisms underlying cognitive flexibility impairments in BD.
Limitations
In addition to its strengths, the current study has several limitations. First, to address our focus on developmental alterations in cognitive performance associated with childhood-onset BD, we harnessed cross-sectional data, rather than conducting a prospective longitudinal study. Specifically, our child BD and HC participants were enrolled in a cross-sectional study (K22MH074945). Our young adult BD and HC participants were enrolled in a cross-sectional study (R01MH087513), though the adult BD participants were originally enrolled and prospectively followed for child-onset BD by the longitudinal COBY study (R01MH059929). Thus, while we had a great deal of information about our participants, including age of BD illness onset and assessment for psychopathology, mood, and functioning at the time of CANTAB testing, we did not have prospective information for the child BD participants about number and duration of mood episodes prior to testing. Given that some, but not all studies suggest that clinical course influences cognitive performance, further work is needed using longitudinal studies assessing both psychopathology and cognitive performance (Cardoso et al., 2015; Dickstein et al., 2015a). Our lack of age effects or age-diagnosis interactions could also stem from cohort differences between our younger participants with BD and our older participants with BD. Thus, our youth participants with BD might show normalized reversal-learning performance if followed longitudinally. However, the youths and young adults in our study were very similar in many respects. Furthermore, with some exceptions (e.g., Roiser et al., 2009), studies of adults with BD have found reversal-learning deficits even into middle adulthood (i.e., 30s–50s) (Clark et al., 2001, 2002; Kozicky et al., 2013; Linke et al., 2013; Linke et al., 2012; McKirdy et al., 2009). Thus, deficits in cognitive flexibility may be an enduring trait in BD.
Second, we did not test for nonlinear age effects because our ID/ED task was not sensitive enough to valence. Cognitive flexibility in healthy individuals exhibits both linear and nonlinear age-related components across childhood, adolescence, and adulthood, with linear effects for valence-dependent reversal learning and nonlinear effects for valence-independent reversal learning (van der Schaaf et al., 2011). Thus, using a reversal-learning task with greater sensitivity to valence effects may be a productive future direction to better understand age-related cognitive flexibility deficits in BD.
Third, because this was not a treatment study, participants with BD remained on their outpatient medications, which could have influenced their performance on the CANTAB tasks (Henin et al., 2009; Pavuluri et al., 2006b). Nevertheless, medication treatment may normalize cognitive deficits rather than exacerbate them (Hafeman et al., 2012), and our post-hoc analyses found no influence of medication usage on reversal learning.
Finally, to obtain a representative sample, we included some participants with BD meeting criteria for other comorbid disorders, such as ADHD or GAD, or for substance abuse/dependence. However, analyses excluding these participants showed the same results. Moreover, prior studies have not shown comorbid ADHD or anxiety to have a significant influence on reversal learning in BD (Dickstein et al., 2010a; Dickstein et al., 2010b; Dickstein et al., 2007). Importantly, our participants’ reversal-learning deficits also showed no significant relationship with their mood symptoms, suggesting that these deficits are a trait of BD.
Conclusions
People with childhood-onset BD exhibit a specific deficit in reversal learning, which generalizes across genders and across the developmental transition from late childhood to early adulthood. Further research can build on these findings in two ways. Longitudinal studies following participants with childhood-onset BD could examine the course and functional significance of reversal-learning deficits. Furthermore, targeted cognitive remediation interventions could attempt to ameliorate this specific issue faced by youths and young adults with BD, as has been done for specific issues faced by individuals with schizophrenia or anxiety (Eldar et al., 2012; Wykes et al., 2011). Intervening earlier to reduce or even prevent cognitive issues in BD before they cause negative psychosocial consequences not only could reduce health care costs (Dickstein et al., 2015b), but also could improve outcomes for individuals with BD, their families, and society.
Supplementary Material
Highlights.
Tested reversal learning in individuals with childhood-onset bipolar disorder (BD)
Their ages (7–27 yrs.) spanned developmental transition from childhood to adulthood
BD group showed deficits in reversal learning compared to age-matched healthy peers
Deficits independent of age, intelligence, and other executive functioning issues
No confounding influences of gender, race, medications, comorbidity, or mood state
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Kayser R, Dickstein D, Blair RJR, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:1173–1185. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Bozikas VP. The predictive significance of neurocognitive factors for functional outcome in bipolar disorder. Curr Opin Psychiatry. 2013;26:54–59. doi: 10.1097/YCO.0b013e32835a2acf. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Michiel PT, Rock D. Spatial working memory and planning ability: contrasts between schizophrenia and bipolar I disorder. Cortex. 2005;41:753–763. doi: 10.1016/s0010-9452(08)70294-6. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Kelly C, Bell R, King DJ. Gender influences the detection of spatial working memory deficits in bipolar disorder. Bipolar Disord. 2008;10:647–654. doi: 10.1111/j.1399-5618.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Iyengar S, Kim E, Yen S, Hower H, Esposito-Smythers C, Goldstein T, Ryan N, Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Tseng WL, Olsavsky AK, Fromm SJ, Muhrer EJ, Rutenberg JG, Deveney CM, Adleman NE, Zarate CA, Pine DS, Leibenluft E. Fronto-limbic-striatal dysfunction in pediatric and adult patients with bipolar disorder: impact of face emotion and attentional demands. Psychol Med. 2013:1–13. doi: 10.1017/S003329171300202X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Caldiroli A, Caletti E, Zugno E, Altamura AC. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55:1561–1566. doi: 10.1016/j.comppsych.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition Limited. Manual version 5.0.0. Cambridge: Cambridge Cognition Limited; 2012. CANTAB Eclipse Test Administration Guide. [Google Scholar]
- Cardoso T, Bauer IE, Meyer TD, Kapczinski F, Soares JC. Neuroprogression and Cognitive Functioning in Bipolar Disorder: A Systematic Review. Current psychiatry reports. 2015;17:75. doi: 10.1007/s11920-015-0605-x. [DOI] [PubMed] [Google Scholar]
- Carrus D, Christodoulou T, Hadjulis M, Haldane M, Galea A, Koukopoulos A, Kumari V, Frangou S. Gender differences in immediate memory in bipolar disorder. Psychol Med. 2010;40:1349–1355. doi: 10.1017/S0033291709991644. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. The British journal of psychiatry : the journal of mental science. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LA. Applied multiple regression/correlations analysis for the behavioral sciences. 3rd. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Axelson D, Weissman AB, Yen S, Hunt JI, Goldstein BI, Goldstein TR, Liao F, Gill MK, Hower H, Frazier TW, Diler RS, Youngstrom EA, Fristad MA, Arnold LE, Findling RL, Horwitz SM, Kowatch RA, Ryan ND, Strober M, Birmaher B, Keller MB. Cognitive flexibility and performance in children and adolescents with threshold and sub-threshold bipolar disorder. Eur Child Adolesc Psychiatry. 2015a doi: 10.1007/s00787-015-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Cushman GK, Kim KL, Weissman AB, Wegbreit E. Cognitive remediation: potential novel brain-based treatment for bipolar disorder in children and adolescents. CNS Spectr. 2015b:1–9. doi: 10.1017/S109285291500036X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010a;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010b;12:707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E. Cognitive flexibility in phenotypes of pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Dusetzina SB, Farley JF, Weinberger M, Gaynes BN, Sleath B, Hansen RA. Treatment use and costs among privately insured youths with diagnoses of bipolar disorder. Psychiatric services (Washington, D.C.) 2012;63:1019–1025. doi: 10.1176/appi.ps.201100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, Pine DS, Bar-Haim Y. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of clinical psychiatry. 2006;67(Suppl 9):3–8. discussion 36–42. [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Hauser M, Galling B, Correll CU. Suicidal ideation and suicide attempts in children and adolescents with bipolar disorder: a systematic review of prevalence and incidence rates, correlates, and targeted interventions. Bipolar Disord. 2013;15:507–523. doi: 10.1111/bdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund JL, Vieweg BW. The Hamilton rating scale for depression. J Operational Psychiatry. 1979;10:149–165. [Google Scholar]
- Henin A, Mick E, Biederman J, Fried R, Hirshfeld-Becker DR, Micco JA, Miller KG, Rycyna CC, Wozniak J. Is psychopharmacologic treatment associated with neuropsychological deficits in bipolar youth? The Journal of clinical psychiatry. 2009;70:1178–1185. doi: 10.4088/JCP.08m04696. [DOI] [PubMed] [Google Scholar]
- Holma KM, Haukka J, Suominen K, Valtonen HM, Mantere O, Melartin TK, Sokero TP, Oquendo MA, Isometsa ET. Differences in incidence of suicide attempts between bipolar I and II disorders and major depressive disorder. Bipolar Disord. 2014 doi: 10.1111/bdi.12195. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Duketis E, Poustka L, Zepf FD, Poustka F, Bolte S. Bipolar disorder in children and adolescents in Germany: national trends in the rates of inpatients, 2000–2007. Bipolar Disord. 2010;12:155–163. doi: 10.1111/j.1399-5618.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- Jabben N, Arts B, van Os J, Krabbendam L. Neurocognitive functioning as intermediary phenotype and predictor of psychosocial functioning across the psychosis continuum: studies in schizophrenia and bipolar disorder. The Journal of clinical psychiatry. 2010;71:764–774. doi: 10.4088/JCP.08m04837yel. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Benson BE, Plate RC, Guyer AE, Detloff AM, Pine DS, Leibenluft E, Ernst M. Developmental effects of decision-making on sensitivity to reward: An fMRI study. Developmental Cognitive Neuroscience. 2012;2:437–447. doi: 10.1016/j.dcn.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2008;18:595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatr Pract. 2008;14(Suppl 2):31–38. doi: 10.1097/01.pra.0000320124.91799.2a. [DOI] [PubMed] [Google Scholar]
- Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. PharmacoEconomics. 2003;21:601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- Kozicky JM, Ha TH, Torres IJ, Bond DJ, Honer WG, Lam RW, Yatham LN. Relationship between frontostriatal morphology and executive function deficits in bipolar I disorder following a first manic episode: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) Bipolar Disord. 2013;15:657–668. doi: 10.1111/bdi.12103. [DOI] [PubMed] [Google Scholar]
- Kyte ZA, Carlson GA, Goodyer IM. Clinical and neuropsychological characteristics of child and adolescent bipolar disorder. Psychol Med. 2006;36:1197–1211. doi: 10.1017/S0033291706007446. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7:111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Post RM, Keck PE, Jr, Altshuler LL, Frye MA, Kupka RW, Nolen WA, Suppes T, McElroy SL, Grunze H, Denicoff K, Moravec MKM, Luckenbaugh D. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biol Psychiatry. 2013;74:908–916. doi: 10.1016/j.biopsych.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, Meyer-Lindenberg A, Wessa M. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169:316–325. doi: 10.1176/appi.ajp.2011.11050711. [DOI] [PubMed] [Google Scholar]
- Linke J, Sonnekes C, Wessa M. Sensitivity to positive and negative feedback in euthymic patients with bipolar I disorder: the last episode makes the difference. Bipolar Disord. 2011;13:638–650. doi: 10.1111/j.1399-5618.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med. 2009;39:1289–1293. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Cui L, Kattan G, Carlson GA, Youngstrom EA, Angst J. Mania with and without depression in a community sample of US adolescents. Arch Gen Psychiatry. 2012;69:943–951. doi: 10.1001/archgenpsychiatry.2012.38. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Ehrenreich H, Christensen EM, Kessing LV, Vinberg M. Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder: a double-blind, randomized, placebo-controlled phase 2 trial. The Journal of clinical psychiatry. 2014;75:1347–1355. doi: 10.4088/JCP.13m08839. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Luo Q, Avny SB, Kasprzycki T, Gupta K, Chen G, Finger EC, Blair RJ. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10827–10834. doi: 10.1523/JNEUROSCI.0963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2013;43:1187–1196. doi: 10.1017/S0033291712001948. [DOI] [PubMed] [Google Scholar]
- Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Moss M, Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: A clinical translation. Biol Psychiatry. 2006a;60:951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006b;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AT, Peckham AD, Stange JP, Sylvia LG, Hansen NS, Salcedo S, Rauch SL, Nierenberg AA, Dougherty DD, Deckersbach T. Correlates of real world executive dysfunction in bipolar I disorder. J Psychiatr Res. 2014;53:87–93. doi: 10.1016/j.jpsychires.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Freeman LN, Mokros HB. Children's Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21:979–984. [Google Scholar]
- Roiser JP, Cannon DM, Gandhi SK, Taylor Tavares J, Erickson K, Wood S, Klaver JM, Clark L, Zarate CA, Jr, Sahakian BJ, Drevets WC. Hot and cold cognition in unmedicated depressed subjects with bipolar disorder. Bipolar Disord. 2009;11:178–189. doi: 10.1111/j.1399-5618.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Birmaher B, Axelson DA, Iosif AM, Williamson DE, Gill MK, Goldstein BI, Strober MA, Hunt J, Goldstein TR, Esposito-Smythers C, Iyengar S, Ryan ND, Keller M. Negative life events in children and adolescents with bipolar disorder. The Journal of clinical psychiatry. 2009;70:1452–1460. doi: 10.4088/JCP.08m04948gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- Schorre BE, Vandvik IH. Global assessment of psychosocial functioning in child and adolescent psychiatry. A review of three unidimensional scales (CGAS, GAF, GAPD) Eur Child Adolesc Psychiatry. 2004;13:273–286. doi: 10.1007/s00787-004-0390-2. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- van der Schaaf ME, Warmerdam E, Crone EA, Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: relevance for dopamine's role in adolescent decision making. Dev Cogn Neurosci. 2011;1:578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskinn A, Sundet K, Simonsen C, Hellvin T, Melle I, Andreassen OA. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology. 2011;25:499–510. doi: 10.1037/a0022677. [DOI] [PubMed] [Google Scholar]
- Weathers J, Brotman MA, Deveney CM, Kim P, Zarate C, Jr, Fromm S, Pine D, Leibenluft E. A developmental study on the neural circuitry mediating response flexibility in bipolar disorder. Psychiatry Res. 2013;214:56–65. doi: 10.1016/j.pscychresns.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, Brotman MA, Zarate CA, Jr, Connolly ME, Fromm SJ, LeBourdais SB, Pine DS, Leibenluft E. A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. Am J Psychiatry. 2012;169:633–641. doi: 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 2005. [Google Scholar]
- Wegbreit E, Cushman GK, Puzia ME, Weissman AB, Kim KL, Laird AR, Dickstein DP. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926–935. doi: 10.1001/jamapsychiatry.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegbreit E, Weissman AB, Cushman GK, Puzia ME, Kim KL, Leibenluft E, Dickstein DP. Facial emotion recognition in childhood-onset bipolar I disorder: an evaluation of developmental differences between youths and adults. Bipolar Disord. 2015 doi: 10.1111/bdi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.