Abstract
Immunosuppression is a major complication of alcoholism that contributes to increased rates of opportunistic infections and sepsis in alcoholics. The NLRP3 inflammasome, a multi-protein intracellular pattern recognition receptor complex that facilitates the cleavage and secretion of the pro-inflammatory cytokines IL-1β and IL-18, can be inhibited by ethanol and we sought to better understand the mechanism through which this occurs and whether chemically similar molecules exert comparable effects. We show that ethanol can specifically inhibit activation of the NLRP3 inflammasome, resulting in attenuated IL-1β and caspase-1 cleavage and secretion, as well as diminished ASC speck formation, without affecting potassium efflux, in a mouse macrophage cell line (J774), mouse bone marrow derived dendritic cells, mouse neutrophils, and human PBMCs. The inhibitory effects on the Nlrp3 inflammasome were independent of GABAA receptor activation or NMDA receptor inhibition, but was associated with decreased oxidant production. Ethanol treatment markedly decreased cellular tyrosine phosphorylation, while administration of the tyrosine phosphatase inhibitor sodium orthovanadate prior to ethanol restored tyrosine phosphorylation and IL-1β secretion subsequent to ATP stimulation. Furthermore, sodium orthovanadate-induced phosphorylation of ASC Y144, necessary and sufficient for Nlrp3 inflammasome activation, and secretion of phosphorylated ASC, were inhibited by ethanol. Finally, multiple alcohol-containing organic compounds exerted inhibitory effects on the Nlrp3 inflammasome, whereas 2-methylbutane (isopentane), the analogous alkane of the potent inhibitor isoamyl alcohol (isopentanol), did not. Our results demonstrate that ethanol antagonizes the NLRP3 inflammasome at an apical event in its activation through the stimulation of protein tyrosine phosphatases, an effect shared by other short-chain alcohols.
Keywords: inflammasome, IL-1β, ethanol, inflammation
Introduction
Inflammasomes are a family of large multi-protein intracellular pattern recognition receptors (PRRs) that respond to a wide variety of exogenous pathogen associated molecular patterns (PAMPs) and endogenous danger associated molecular patterns (DAMPs), facilitating the secretion of the pro-inflammatory cytokines, IL-1β and IL-18, as well as a form of inflammatory cell death known as pyroptosis (1). Unlike many innate immune pathways, stimulation of a functional inflammasome requires two steps. During priming (step 1), activation of the transcription factor NF-κB, downstream of the stimulation of many PRRs, leads to the production of several components of the inflammasome and the secretion of the pro-inflammatory cytokine TNF (2). Activation of the inflammasome (step 2) requires the exposure of cells to a separate set of PAMPs and DAMPs, which work through unique signaling pathways leading to the oligomerization of one of several different Nucleotide Oligomerization Domain (NOD)-Like Receptor (NLR) proteins, the adaptor protein Apoptosis-associated Speck-like protein containing a CARD (ASC), and pro-caspase-1 into an organized inflammasome complex (3). This oligomerization is mediated by homotypic PYRIN-PYRIN domain binding between NLRs and ASC, and CARD-CARD interactions between ASC and pro-caspase-1, resulting in the formation of a discrete ASC speck within stimulated cells (4). These ASC specks form rapidly and irreversibly within activated cells and are a platform for efficient pro-IL-1β and pro-IL-18 cleavage. While the activity of all inflammasomes is thought to be enhanced by the incorporation of ASC into their complexes, NLRP1 and NLRC4 contain their own CARD domains and can interact directly with pro-caspase-1 independent of ASC (5). This assembly allows for the conversion of pro-caspase-1 into an active caspase-1 enzyme, which cleaves pro-IL-1β and pro-IL-18 into their mature, secreted forms. These cytokines then function to promote vasodilation, attract and stimulate neutrophils, induce fever, and activate the acute phase response within an organism (6). Some consider the secretion of IL-1β and IL-18 to be a third step in the process of inflammasome activation. Both IL-1β and IL-18 are leaderless proteins, which still do not have a well-defined mode of release (1, 7). The final outcome of inflammasome formation, pyroptotic cell death, is believed to amplify the immune response while depleting pathogens of their host leukocyte niche (8).
The NLRP3 inflammasome is capable of responding to a particularly diverse set of PAMPs and DAMPs, including ATP, nigericin, alum, asbestos, silica, and cholesterol crystals (9–13). These agonists activate the inflammasome through disparate pathways, such as membrane pore formation and lysosomal rupture, eventually converging on K+ efflux and ASC phosphorylation and multimerization (14, 15). Reactive oxygen species (ROS) production has also been implicated to participate causally in the activation of NLRP3 (16–20). Expressed predominantly by macrophages, but also by several other leukocyte and non-leukocyte cell types, the NLRP3 inflammasome plays a major function in immune homeostasis (21). Beyond its protective roles in response to pathogens, over-activation of the NLRP3 inflammasome has been implicated in the pathogenesis of an array of diseases such as atherosclerosis, diabetes, gout, and multiple sclerosis (22–24). Similarly, gain of function mutations in NLRP3 lead to the set of debilitating diseases known as Cryopyrin-Associated Periodic Syndrome (CAPS) (25). Although many inhibitors of step 1 are known, until recently few compounds capable of directly inhibiting step 2 have been discovered.
Alcohol use disorders were estimated to be the third most common non-genetic cause of mortality in the U.S. in the year 2000 (26). Alcohol abuse predisposes individuals to opportunistic infections and organ damage, which are the two most prominent alcohol-related medical complications (26). The pattern of drinking differentially affects the consequences of alcohol abuse. Binge alcohol consumption suppresses host innate immune defense, while chronic alcohol consumption suppresses innate and adaptive immune systems, yet activates chronic inflammation (27). Ethanol is a known inhibitor of NF-κB activation and its consumption is associated with decreased circulating levels of TNF and IL-1β (28, 29). Recently, ethanol, but not its metabolite acetaldehyde, was found to also be capable of inhibiting step 2 for the NLRP3 and AIM2 inflammasomes (22). The methods through which ethanol exerts its immunosuppressive effects are still unclear, yet given the central role that inflammasomes play in the immune response, it is possible that direct inhibition of step 2 could be an important target of alcohol induced immunosuppression.
Ethanol is known to have a wide range of effects when administered to cells. At high doses, it can alter membrane fluidity and can diffuse across the plasma membrane to interact with cytosolic proteins (30). Some known intracellular effects of acute ethanol administration include tyrosine phosphatase and adenylyl cyclase activation (31, 32). At lower doses, ethanol is thought to interact with a variety of cell surface receptors, particularly neurotransmitter receptors, in an agonistic or antagonistic manner (33, 34). During chronic exposure to ethanol, gene expression can be altered, potentially contributing to the differences that chronic alcoholism and binge drinking exert on immune function (35). Furthermore, the effects of ethanol exposure can be cell type specific. Ethanol has been shown to induce inflammation and inflammatory cytokine release in the brain (via astrocytes, neurons, and microglia) and liver (via Kupffer cells), alongside its paradoxical tendency to induce immune suppression of leukocytes in the rest of the body (22, 28, 29, 36–40).
The goal of this study was to further elucidate the mechanism of ethanol’s inhibition of the NLRP3 inflammasome, primarily using the J774 mouse macrophage and THP-1 human monocyte cell lines and a protocol resembling binge drinking in humans. Experiments were designed to assess ethanol’s ability to directly inhibit step 2, rather than its already well-defined capacity to prevent NF-κB and step 1 activation. By identifying pathways involved in ethanol’s blockade of this key innate immune complex, we hope to better understand and determine potential sites of therapeutic intervention in ethanol mediated immunosuppression and also to identify potential targets for future NLRP3 inflammasome inhibitors.
Materials and Methods
Reagents
LPS, isoamyl alcohol, 2-methylbutane, picrotoxin, 3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid, sodium orthovanadate, and monoclonal anti-mouse β-actin antibodies were all purchased from Sigma-Aldrich (St. Louis, MO). Anti-mouse ASC and caspase-1 antibodies were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX), anti-phosphotyrosine antibodies from Cell Signaling (Danvers, MA), anti-mouse and human IL-1β antibodies from R&D Systems (Minneapolis, MN), and anti-human caspase-1 antibodies from BIOMOL International LP (Plymouth Meeting, PA). Recombinant mature mouse IL-1β was also purchased from R&D Systems. Biotin conjugated anti- mouse and rabbit secondary antibodies were from GE Healthcare UK Limited (Little Chalfont, Buckinghamshire, UK) and anti-goat IgG from Jackson ImmunoResearch (West Grove, PA). For inflammasome stimulation, ATP, nigericin, alum Imject, apoSAA, poly (dA:dT), and anthrax lethal factor and protective antigen were purchased from Amersham Biosciences (Piscatawy, NJ), Invivogen (San Diego, CA), Thermo Scientific (Waltham, MA), PeproTech Inc. (Rocky Hill, NJ), Invivogen, and BEI Resources (Manassas, VA), respectively. Muscimol was acquired from MP Biomedicals (Santa Ana, CA), and ethanol from Pharmco AAPER (Brookfield, CT). ASC (Tyr144) phospho-specific antibody was purchased from ECM Biosciences (Versailles, KY).
Cell culture
J774 cells purchased from American Type Culture Collection (ATCC, Manassas, VA) were maintained in DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 1% L-Glutamine (Gibco), and 1x Primocin (Invivogen, San Diego, CA). Cells were not used beyond passage 20 to reduce variability between the experiments and although cells at a lower passage demonstrated more robust cytokine secretion, the pattern of responsiveness remained the same. EYFP and ASC-EYFP stably transfected THP-1 cells (41) were maintained in RPMI medium (Gibco) supplemented with 10% FBS (Gibco), 1% L-Glutamine (Gibco), 1% Pen/Strep (Gibco), and 5μM β-ME (Sigma).
For experiments in which cell supernatants were examined by ELISA, cells were plated at 2.5×105 cells/well in 250μl of media in a 48-well plate and allowed to grow overnight. The following day, the media was removed, fresh media was added and cells were treated as indicated within the figure legends for each experiment. Cell supernatants were harvested at the end of each experiment, spun down at 3,300×g for 10 minutes to pellet cellular debris, transferred to new tubes, and frozen at −20°C until analysis.
For experiments analyzed through western blotting, J774 cells were plated at 3×106 cells/well in 2ml of media in 6-well plates and allowed to grow overnight. The next day, the media was removed, cells were washed twice in warm PBS and placed in 1ml of serum free media. The cells were treated as indicated within each experiment’s figure legend. Supernatants were spun down at 3,300×g, transferred to new tubes, and frozen at −20°C. The cells were then washed twice with PBS and lysed in RIPA buffer (50mM Tris pH 8, 150mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) containing 1mM sodium orthovanadate (Sigma-Aldrich), 1x protease cocktail inhibitor (Sigma-Aldrich), and 1x PMSF (Sigma-Aldrich) on ice for 10 minutes with scraping. Cellular debris was removed through centrifugation at 3,300×g for 10 minutes, the lysates were transferred to new tubes, and frozen at −20°C. Before running on a gel, protein concentrations in the lysates were quantified by a detergent compatible protein assay. For western blots probed with an anti-ASC (Y144) phosphor-specific antibody, cells were lysed directly in Laemmli sample buffer containing β-ME due to the insolubility of ASC specks. In all experiments using J774 cells the previously determined dose of LPS for half maximal IL-1β secretion, upon stimulation, was used (37ng/ml).
For the visualization of ASC specks, ASC-EYFP and EYFP THP-1 cells were plated at 2.5×105 cells/well in 1ml of media in 12-well plates and differentiated with PMA (100ng/ml) (Sigma-Aldrich) for 24 hours. The cells were then washed with PBS and placed in 1ml of fresh media for an additional 48 hours. The THP-1 cells were then treated as indicated and imaged under bright field and fluorescence microscopy using an Eclipse TS100 microscope and DS-QiMc digital camera (Nikon, Melville, NY). Five images were taken per group, along with their corresponding bright field images, and the number of ASC specks per visual field per cell was counted.
Isolation of human PBMCs
Heparinized blood was diluted 1:1 in PBS, and 4ml of diluted blood was layered over 3ml of lymphocyte separation medium (LSM) (MP Biomedicals), and centrifuged at 400×g for 15 minutes at room temperature. The top layer of plasma was aspirated and discarded, leaving 2–3mm above the buffy coat, and the buffy coat and half the lower LSM layer was aspirated, mixed with PBS and centrifuged at 200×g for 10 minutes at room temperature. The cells were washed once in PBS, resuspended in DMEM plated at 1×106 cells/ml for measurement of IL-1β secretion by ELISA, and 3×106 cells/ml for protein detection by western blot.
Generation of bone marrow derived dendritic cells (BMDCs) and neutrophil isolation
Bone marrow from C57BL/6 mice was flushed from the femurs and tibiae and cultured on 24-well plates at 1 × 106 cells/well (1 ml/well) in RPMI-1640 containing 10% serum and 10% conditioned media from X63-GMCSF myeloma cells transfected with murine GM-CSF cDNA (kindly provided by Dr. Brent Berwin, Dartmouth College). Media was replaced on days 2 and 4 and the adherent and lightly-adherent BMDCs, predominantly CD11b+CD11c+ by FACS, were collected on day 6 at plated at 1×106 cells/ml. Neutrophils were isolated from the bone barrow of C57BL/6 mice as previously described (42).
Measurement of total protein, IL-1β, and TNF
Total protein levels from cell lysates were measured using the Lowry assay according to manufacturer’s protocols (Bio-Rad). IL-1β and TNF ELISAs were conducted according to manufacturer’s protocols (BD Biosciences, San Jose, CA).
Chloroform-methanol protein precipitation from cell supernatants
Supernatants from cells cultured in 6-well plates in serum free media were divided into Eppendorf tubes with 500μl of media per tube. An equal volume of methanol to supernatant (500μl) and ¼ volume of chloroform (125μl) was added, and the samples were vortexed for 20 seconds. Samples were then spun down at 20,000×g for 10 minutes at room temperature and the upper phase of the mixture was removed, keeping the intermediate protein phase intact. To this, 500μl of methanol was added, the samples were vortexed for 20 seconds, and spun down at 20,000×g for 5 minutes at room temperature. The liquid phase was removed and the pellet was dried at 55°C for 1–5 minutes. The pellet was then resuspended in Laemmli sample buffer containing β-ME, duplicate samples were pooled and were vortexed an additional 20 seconds.
Western blots
Cell culture supernatants were based on equivalent volumes, whereas cell lysate samples were normalized to total protein, and all samples were prepared by diluting in β-ME sample buffer, and heating at 100°C for 5 minutes. Samples were then loaded onto SDS-PAGE gels, run in 1x TGS running buffer, and transferred to nitrocellulose (ASC, phosphotyrosine, and p-ASC) or PVDF (IL-1β and caspase-1) membranes. Blots were blocked in 5% milk/TBST (IL-1β, ASC, caspase-1, p-ASC, and β-actin), or 5% BSA/TBST (phosphotyrosine) for 2 hours at room temperature, and placed in primary antibody overnight at 4°C. Blots were then washed three times in TBST for 10 minutes and placed in biotin-conjugated secondary antibody for 2 hours at room temperature. The blots were again washed and incubated in ECL reagent (Thermo Scientific) for 5 minutes before exposing to X-ray film and developing.
Ponceau staining
J774 cells were treated as indicated and lysates and supernatants were prepared as previously described and separated by SDS-PAGE. Proteins within the gel were transferred onto nitrocellulose and washed with TBS for 5 minutes. The membrane was then placed in Ponceau stain (0.1% Ponceau S (Sigma-Aldrich) in 5% acetic acid) for 5 minutes, and washed with water tree times for 5 minutes each.
Inductively coupled plasma optical emission spectroscopy (ICP-OES) measurements for intracellular potassium and sodium
ICP-OES protocols were adapted from those previously described (22, 43). Briefly, J774 cells were plated at 3×106 cells/well in 2ml of media in 6-well plates, were allowed to grow overnight and were then treated as indicated. Following stimulation, the supernatants were removed and cells were washed twice with sterile saline (0.9% NaCl), and lysed in 30% HNO3 overnight. The samples were then diluted in Milli-Q water to a final concentration of 3% HNO3 for potassium (K) measurement, and were diluted tenfold further in 3% HNO3 for sodium (Na) measurement. Analyses were performed on a PerkinElmer Optima 7000DV ICP optical emission spectrometer (OES) with a cyclonic spray chamber, concentric nebulizer, CCD array detector, a PerkinElmer S10 autosampler, and WinLab32 software (PerkinElmer, Shelton, CT). The axial detection mode was utilized for measurement of K and Na in all standards and samples. Concentrated K and Na standard solutions for ICP (1000 mg/L, TraceCERT®) were both obtained from Fluka Analytical (Buchs, Switzerland). The concentrated yttrium (Y) standard solution for atomic spectroscopy (1000 mg/L) was obtained from PerkinElmer. Triplicate external calibration was performed using combined K and Na standards in the range of 0 – 20 ppm (mg/L) for K and 0 – 10 ppm for Na. Yttrium (0.1 ppm) was used as an internal standard in all calibration standards and samples. The standards were prepared in 3% HNO3 in Milli-Q water to matrix match with the samples. Concentrations were quantified based on the emission wavelengths at 766.478 nm, 589.600 nm, and 371.029 nm for K, Na, and Y, respectively. All samples were measured in triplicate and averaged.
Cell health measurements
Cell death and cytotoxicity were measured using the CytoTox96 Non-Radioactive Cytotoxicity Assay according to manufacturer’s protocols (Promega, Madison, WI). Cell viability, cytotoxicity, and apoptosis were also measured using the ApoTox-Glo assay according to manufacturer’s protocols (Promega). Briefly, J774 cells were plated at 2×104 cells/well in 100μl of media in a clear bottomed, opaque walled 96-well plate overnight in phenol red and serum free media. The next day, the cells were treated as indicated, mixed with Viability/Cytotoxicity Reagent and incubated for 30 minutes before reading on a Synergy HTX Multi-Mode plate reader (BioTek, Winooski, VT) using the program Gen5 1.1 at 400Ex/505Em (viability) and 485Ex/520Em (cytotoxicity). Immediately following, cells were mixed with Caspase-Glo 3/7 Reagent, mixed, incubated for 2 hours, and apoptosis was measured by luminescence.
Measurement of intracellular ROS
Intracellular ROS was measured by DCF-DA assay as previously described (17). Briefly, J774 cells were plated at 1.5×105 cells/well in a clear and bottom black-walled tissue culture plate overnight. The next day, the cells were washed and treated as indicated with LPS in serum and phenol red free media for 5 hours. The media was then removed and replaced with fresh serum and phenol red free media containing 2.5mM probenecid (Tocris Bioscience, Bristol, UK) and with and without 10μM DCF-DA (Molecular Probes, Eugene, OR) for 40 minutes at room temperature. The cells were subsequently washed in HBSS containing 2.5mM probenecid for 5 minutes at 37°C. The cells were then placed in serum-free and phenol red-free media, read on a plate reader at 485Ex/520Em for 2 minutes, treated as indicated with ATP (1mM) and ethanol (0.38–3%), and read on the plate reader for 30 minutes with one read per minute. The baseline read for each well and the average fluorescence of the group that was not treated with DCF-DA were subtracted from each subsequent reading and the areas under the curves were calculated using GraphPad Prism 6.
Statistical calculations
All experiments were repeated at least twice and representative results are presented. Results were analyzed by one-way ANOVA and Tukey’s post hoc test using GraphPad Prism 6 for Windows (GraphPad). A p value <0.05 was considered statistically significant.
Results
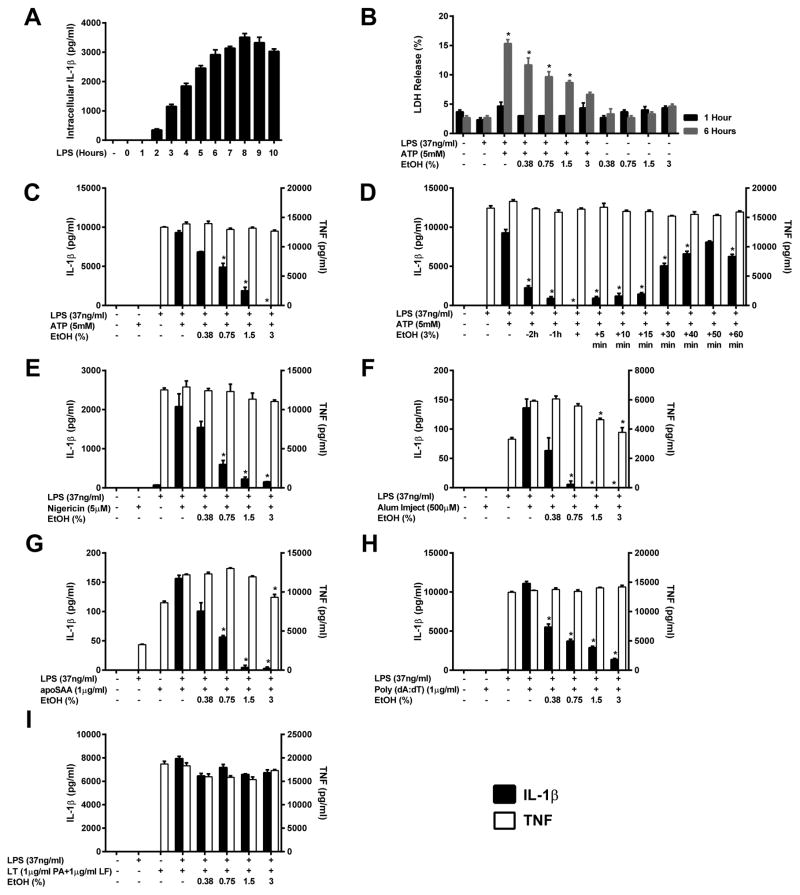
Ethanol rapidly inhibits IL-1β release induced by NLRP3 inflammasome agonists
While ethanol has been implicated to inhibit the production of pro-inflammatory cytokines when administered at the time of cell stimulation, to rule out the possibility that ethanol inhibits the inflammasome simply by preventing adequate priming prior to inflammasome activation, J774 cells were treated with LPS for 0–10 hours to find the time point at which intracellular pro-IL-1β reaches its highest concentration, and used this time point of 8 hours to prime J774 cells with LPS before treating them with ethanol and most inflammasome agonists (Figure 1A). To test whether ethanol is capable of inhibiting IL-1β secretion subsequent to Nlrp3 inflammasome stimulation and to evaluate upon which pathways leading to inflammasome activation ethanol can act, J774 cells were primed with LPS and treated with the agonists: ATP, nigericin, Alum, and apoSAA with and without increasing doses of ethanol (0.38–3%). Ethanol significantly and dose dependently inhibited the secretion of IL-1β relative to cells treated with LPS and a step 2 agonist alone (Figure 1C, E–G). In addition, TNF production was unaffected by ethanol, suggesting that NF-κB activation and priming of the inflammasome were not influenced by the alcohol at this time point. Therefore, it is likely that ethanol acts on either step 2 (activation) or step 3 (secretion) to prevent IL-1β secretion from the NLRP3 inflammasome. Moreover, since ethanol is capable of preventing IL-1β secretion in response to each Nlrp3 inflammasome agonist without altering TNF production, this implies that ethanol’s actions are likely on downstream events in inflammasome formation, beyond where the pathways initiated by each agonist converge.
Figure 1. Ethanol rapidly inhibits IL-1β release induced by NLRP3 inflammasome agonists.
IL-1β ELISA on lysates from J774 cells treated with LPS (37ng/ml) from 0–8 h and lysed in NP-40 lysis buffer (A). LDH release from J774 cells primed with or without LPS for 8 h and treated with ethanol and ATP for 1 or 6 hours (B). IL-1β and TNF ELISAs from the supernatants of J774 cells treated with LPS for 8 h and ethanol and ATP for 1 h (C), LPS for 8 h and ATP for 1 h along with ethanol (3%) at the indicated times before and after ATP addition (D), LPS for 8 h, and ethanol and nigericin for 1 h (E), LPS for 3 h, ethanol for 3 h and alum for 18 h (F), LPS for 3 h, ethanol for 3 h and apoSAA for 18 h (G), LPS for 8 h followed by ethanol and poly (dA:dT) for 18 h (H), or LPS for 8 h followed by ethanol and LT for 4 h (I). *<0.0001 by a Tukey’s post hoc test following a one-way ANOVA relative to the untreated (1 or 6 hours) (B), LPS+ATP (C and D), LPS+nigericin (E), LPS+ alum (F), LPS+apoSAA (G), LPS+poly (dA:dT) (H) or LPS+LT (I) treated groups. EtOH = ethanol, Alum = aluminum hydroxide, LT = anthrax lethal toxin, ns = not significant. All studies were performed in triplicate.
Ethanol inhibits AIM2 inflammasome activation
Like the NLRP3 inflammasome, the AIM2 inflammasome is an intracellular multi-protein pattern recognition receptor that requires the adaptor protein ASC to activate capsape-1 and promote IL-1β and IL-18 cleavage (44). To test whether ethanol is capable of preventing the formation of this inflammasome, J774 cells were primed with LPS and treated with ethanol and poly (dA:dT) simultaneously (Figure 1H). Parallel to the Nlrp3 inflammasome, ethanol was able to dose dependently inhibit poly (dA:dT) induced AIM2 inflammasome activation and subsequent IL-1β secretion without influencing TNF release.
Ethanol does not inhibit IL-1β secretion subsequent to NLRP1b inflammasome activation
To determine whether ethanol’s inhibition might be ASC dependent, LPS primed J774 cells were treated with the Nlrp1b agonist anthrax lethal toxin (LT), a combination of anthrax lethal factor (LF 1μg/ml) and protective antigen (PA 1μg/ml), both with and without increasing doses of ethanol (0.38–3%). In contrast to Nlrp3 agonists, Nlrp1b inflammasome stimulation could not be inhibited by the administration of ethanol (Figure 1I). As Nlrp1b inflammasome activation is enhanced by, but not dependent on, the adaptor protein ASC, these results support ASC speck formation as a target of ethanol’s inhibition.
Kinetics of ethanol inhibition
Elucidating at which time points ethanol is capable of blocking inflammasome stimulation is important for understanding the mechanisms through which this chemical might be acting. To observe the kinetics of ethanol inhibition, ethanol (3%) was administered to LPS primed cells 2 and 1 hours before and simultaneously with ATP stimulation, as well as at several times points following ATP addition. Ethanol treatment at each time point before ATP addition and up to 15 minutes after stimulation was found to inhibit Nlrp3 inflammasome activation, and consistent with previous experiments, ethanol treatment had no impact on macrophage priming and TNF production (Figure 1D). These results indicate that the effects of ethanol are immediate and unlikely to be due to slower cell signaling processes such as alterations in gene expression.
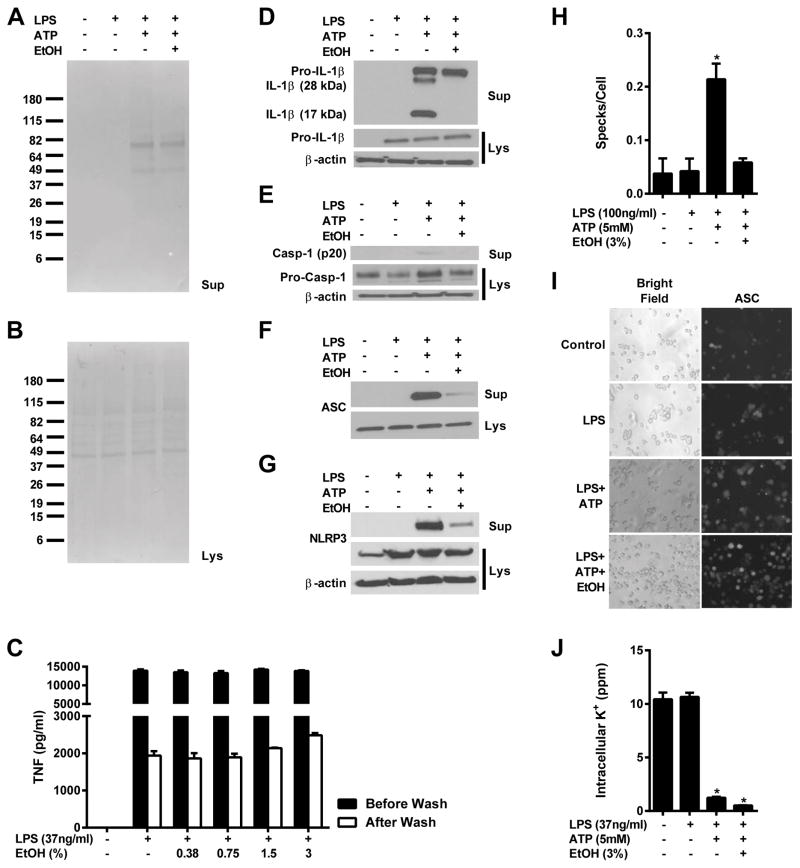
Ethanol cannot prevent ATP-induced protein secretion
J774 cells were treated as indicated and a Ponceau stain was performed on both cell lysates and precipitated supernatants (Figure 2A and B). Even amounts of protein were detected in the lysates of each group; however, secreted proteins could only be detected in those treated with ATP. There was no discernible inhibition of general protein secretion with the addition of ethanol, indicating that the absent mature IL-1β, caspase-1, and ASC secretion observed after treatment with ethanol is due to direct inhibition of the Nlrp3 inflammasome.
Figure 2. Ethanol blocks IL-1β and caspase-1 cleavage, ASC speck formation and release, but not potassium efflux.
Ponceau stains (A and B), IL-1β (D), caspase-1 (E), ASC (F), and NLRP3 (G) western blots and from the lysates and supernatants of J774 cells treated with LPS (37ng/ml) for 8 h and ethanol (3%) and ATP (5mM) for 1 h. TNF ELISA from the supernatants of J774 cells treated with LPS for 4 h, washed and treated with ethanol for 1 h (C). ASC-EGFP transfected THP-1 cells treated with LPS (100ng/ml) for 4 h, and ethanol (3%) and ATP (5mM) for 1 h and imaged using bright field and fluorescence microscopy for total cell counts and ASC speck formation (H and I). ICP-OES measurement of intracellular potassium from J774 cells treated with LPS (37ng/ml) for 8 h and ethanol (3%) and ATP (5mM) for 1 h (J). *<0.0001 (J) or *<0.05 (I) by a Tukey’s post hoc test following a one-way ANOVA relative to the untreated groups. EtOH = ethanol. All studies were performed in triplicate.
To further rule out the inhibition of general protein secretion by ethanol as a cause for ethanol’s blockade of IL-1β secretion, J774 cells were treated with LPS for 4 hours to activate NF-κB and induce TNF secretion. These supernatants were collected, the cells were washed, and fresh supernatants with and without ethanol were added for an additional 1 hour. The concentration of TNF in the cell supernatants both before and after ethanol addition were measured to access whether ethanol can differentially inhibit TNF secretion from cells in which NF-κB is already stimulated (Figure 2C). There was no statistically significant difference in TNF secretion from cells treated with or without ethanol, indicating that ethanol is not capable of non-specifically blocking protein secretion.
Ethanol prevents the cleavage of pro-IL-1β and pro-caspase-1 into their mature forms
Since the decrease in IL-1β production observed by ELISA could be due to retention of cleaved IL-1β within the cell rather than inhibition of caspase-1 and inflammasome action, western blots were performed to determine whether mature IL-1β and caspase-1 could be found in the lysates or the supernatants of primed, ethanol treated macrophages stimulated with ATP. Upon inflammasome stimulation, active cleaved caspase-1 along with cleaved IL-1β and IL-18 is secreted (45, 46). Consistent with an absence of caspase-1 enzymatic activity, there was no cleaved IL-1β or caspase-1 visible in either the cell lysates or supernatants of ethanol treated J774 cells (Figure 2D–E). There was, however, abundant 17kDa mature and alternatively cleaved 28kDa IL-1β, and mature caspase-1 in cells treated with LPS and ATP alone. From LPS and ATP treated cells both with and without ethanol administration, pro-IL-1β and caspase-1 were detected in the supernatants, which could be due to ATP induced cell death and subsequent leakage of proteins from damaged cell membranes.
Treatment with ethanol prevents ASC and NLRP3 secretion and speck formation
The activity of functional inflammasomes results in the secretion of not only mature IL-1β, IL-18, and caspase-1 but also other inflammasome components, including NLRP3 and ASC (46, 47). To further validate that ethanol inhibits inflammasome activation, western blots of the lysates and supernatants of J774 cells were probed with anti-ASC or anti-NLRP3 antibodies. While constitutively expressed ASC and NLRP3 were detected in the lysates of every group (Figure 2F–G), there was significantly less ASC and NLRP3 identified in the supernatants of cells pre-treated with ethanol despite robust detection of these proteins in the supernatants of cells treated with LPS and ATP. These results indicate that ethanol is also capable of inhibiting the secretion of these inflammasome components.
Since ASC speck formation is a point of convergence for all NLRP3 inflammasome agonists and we have shown that ethanol cannot inhibit IL-1β secretion from the partially ASC-independent Nlrp1b inflammasome, we tested the ability of ethanol to prevent the formation of ASC specks. THP-1 cells stably transfected to express a fusion protein of ASC and YFP were treated with nothing, LPS, LPS and ATP, or LPS, ethanol and ATP and were observed by fluorescence microscopy to visualize the generation of ASC specks. Five images were taken per group along with corresponding bright field images to calculate the number of specks formed per total number of cells per image. EYFP stably transfected THP-1 cells were used as a negative control and consistently demonstrated no visible speck formation under fluorescence microscopy in response to any of the four treatments. Both untreated and LPS primed ASC-EYFP THP-1 cells exhibited low levels of speck formation (Figure 2H–I). Stimulation with ATP significantly increased the number of ASC specks present per cell per visual field, and this increase was nearly completely ameliorated by the administration of ethanol alongside ATP.
Ethanol does not block potassium efflux
Potassium efflux has been shown to be necessary for NLRP3 inflammasome activation to occur (43). To determine whether ethanol inhibits this step of inflammasome activation, J774 cells treated with nothing, LPS, LPS and ATP, or LPS, ATP and ethanol (3%) were dissolved in 30% HNO3 and intracellular potasium was measured by ICP-OES. Control and LPS treated cells contained similar amounts of intracellular potassium, which was significantly diminished in cells treated with LPS and ATP both with and without ethanol (Figure 2J). This indicates that ethanol does not inhibit the Nlrp3 inflammasome by blocking potassium efflux from stimulated cells, and is likely acting downstream of this critical point.
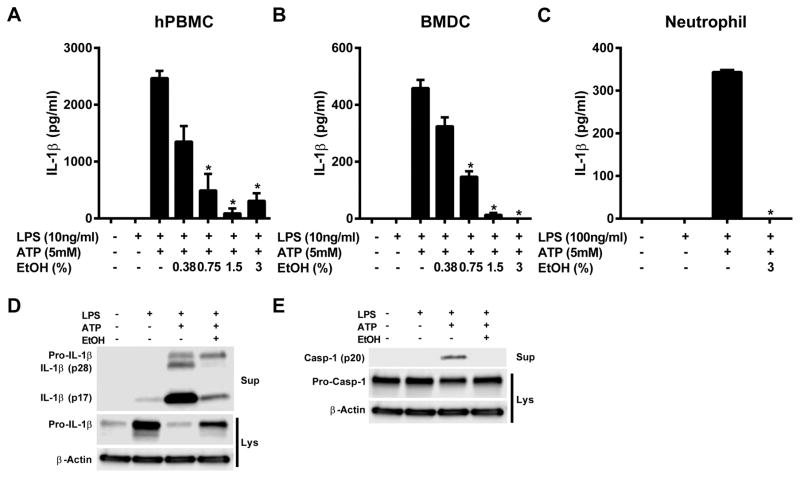
Ethanol blocks NLRP3 inflammasome activation in several cell types
Components of the NLRP3 inflammasome are present in multiple different cell types relevant to the immune response (21). To validate that ethanol can inhibit the inflammasome in a range of cell types, human peripheral blood mononuclear cells (hPBMCs), and murine bone marrow derived dendritic cells (BMDCs) and neutrophils were treated with LPS and ATP to stimulate the NLRP3 inflammasome both in the presence and absence of ethanol. As with J774 cells, ethanol treatment dose dependently inhibited IL-1β secretion from each cell type (Figure 3A–C). Additionally, western blots of the supernatants from hPBMCs demonstrated dramatically reduced cleaved IL-1β and absent mature caspase-1 when administered ethanol in addition to LPS and ATP (Figure 3D and E).
Figure 3. Ethanol blocks inflammasome activation in several cell types.
IL-1β ELISAs from the supernatants of human PBMCs (A), mouse BMDCs (B), and mouse neutrophils (C) primed with LPS for 4 h and treated with ethanol and ATP for 1 h. IL-1β (D) and caspase-1 (E) western blots from the supernatants and lysates of human PBMCs primed with LPS (10ng/ml) for 4 hours and treated with ethanol (3%) and ATP (5mM) for 1 h. *<0.0001 by a Tukey’s post hoc test following a one-way ANOVA relative to the LPS+ATP treated groups. EtOH = ethanol, BMDC = bone marrow derived dendritic cell, Sup = cell supernatants, Lys = cell lysates. All studies were performed in triplicate.
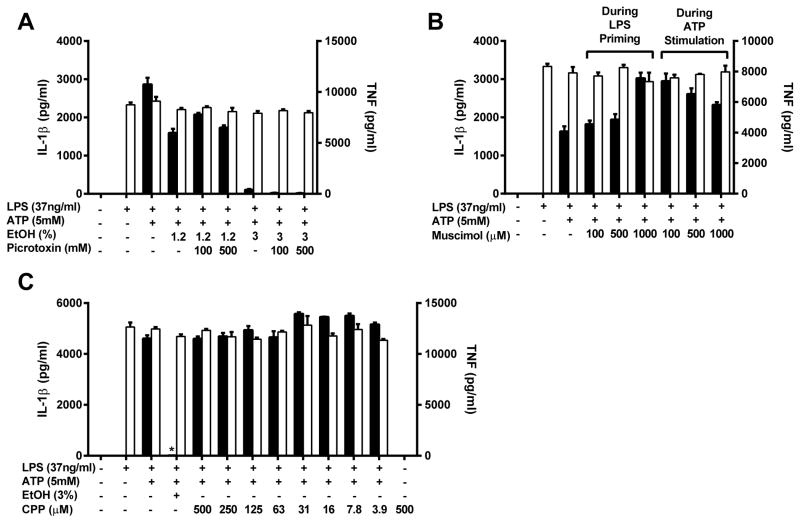
GABAA and NMDA receptors do not mediate the inhibitory effects of ethanol
Ethanol is an agonist for GABAA receptors, which when activated function as a chloride specific ion channel (48). GABAA receptors have been found on macrophages and their activation has been shown to have anti-inflammatory effects (49). To test whether activation of GABAA receptors on J774 cells is responsible for ethanol’s rapid immunosuppressive effects, groups were treated with the GABAA receptor antagonist picrotoxin (100 or 500mM) immediately before the addition of ethanol (1.2% or 3%) and ATP to prevent channel opening. Blockade of GABAA receptors had no impact on the inhibitory effects of ethanol either at its half maximal inhibitory dose of 1.2% or is fully inhibitory concentration of 3% (Figure 4A). This indicates that GABAA receptors are not necessary for ethanol to inhibit Nlrp3 inflammasome activation. To test whether GABAA receptor activation was sufficient for Nlrp3 inflammasome blockade, J774 cells were given the GABAA receptor agonist muscimol (100–1000μM), directly before LPS priming or ATP stimulation. Muscimol was unable to prevent IL-1β and TNF secretion when given before the step 1 agonist LPS or the step 2 agonist ATP (Figure 4B). From these data, we conclude that GABAA receptor activation is unable to inhibit either of the two steps needed for Nlrp3 inflammasome activation to occur.
Figure 4. GABAA and NMDA receptors do not mediate the inhibitory effects of ethanol.
IL-1β and TNF ELISAs from the supernatants of J774 cells treated with LPS for 8 h and picrotoxin, ethanol and ATP for 1 h (A), LPS for 8 hours followed by ethanol and ATP for 1 hour, with the addition of muscimol at the time of LPS or ethanol and ATP treatment (B), or LPS for 8 h and CPP, ethanol and ATP for 1 hour (C). *<0.0001 by a Tukey’s post hoc test following a one-way ANOVA relative to the LPS+ethanol (3 or 1.2%)+ATP treated group (A) or the LPS+ATP treated group (B and C). EtOH = ethanol, CPP = 3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid, ns = not significant. All studies were performed in triplicate.
NMDA receptors are ionotropic neurotransmitter receptors that open to form a non-specific cation channel and are antagonized by ethanol (34). Like GABAA receptors, they have also been found on leukocytes, and in microglial cells their activation results in TNF and IL-1β production (50). To determine whether NMDA receptor antagonism could block inflammasome activation, the antagonist 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP: 500-3.9μM) was administered to LPS primed J774 cells before stimulation with ATP. Antagonism of this neurotransmitter receptor did not prevent IL-1β secretion at any dose (Figure 4C). Therefore, we conclude that NMDA receptor inhibition does not block Nlrp3 inflammasome activation and is unlikely to be involved in the pathway of ethanol’s inhibition of this system.
Ethanol inhibits inflammasome activation-induced ROS production
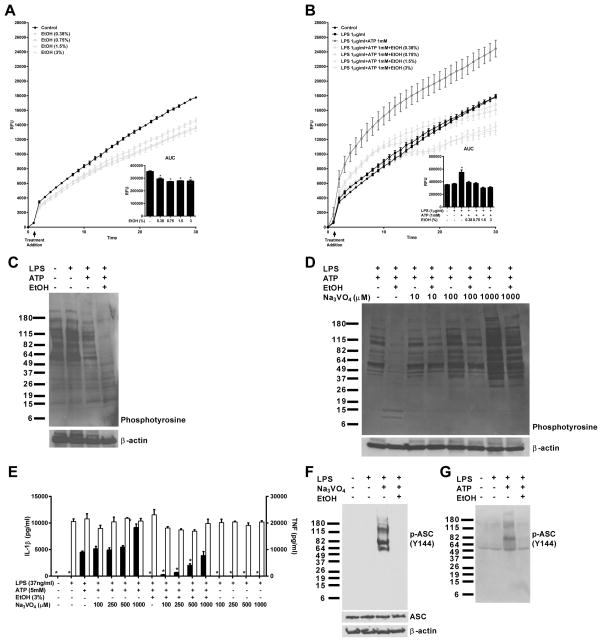
The generation of intracellular ROS has been proposed as part of the mechanism leading to NLRP3 inflammasome stimulation (17–20). To determine whether perturbations in ROS production induced by ethanol could play a role in its inhibitory effects on the NLRP3 inflammasome, J774 cells were primed with LPS (1μg/ml) and treated with ATP (1mM) and ethanol (0.38–3%), or were treated with ethanol alone (0.38–3%) and the formation of intracellular ROS over 30 minutes was measured by a DCF-DA assay (Figure 5A–B). Although it has been previously reported using astrocytes that ethanol acutely induces ROS (36), our results indicate that ethanol inhibited ROS production both alone or in the setting of LPS and ATP induced Nlrp3 inflammasome activation.
Figure 5. Ethanol mediated inhibition of IL-1β secretion involves inhibition of ROS production and is dependent on protein tyrosine phosphatase activity.
ROS production measured by a DCF-DA assay of J774 cells treated with ethanol for 30 minutes (A), LPS (1μg/ml) for 5 h and ethanol and ATP (1mM) for 30 minutes (B). A western blot probing for mouse phosphotyrosine residues in the lysates of J774 cells primed with LPS (37ng/ml) for 8 h and treated with ethanol (3%), and ATP (5mM) for 1 h (C), or treated with LPS (37ng/ml) for 8 h, pre-treated with Na3VO4 (10–1000μM) for 1 h prior to ethanol (3%) and ATP (5mM) for 1 h (D). An IL-1β and TNF ELISA from the supernatants of J774 cells primed with LPS for 8 h and pre-treated with Na3VO4 for 1 h prior to the addition of ethanol and ATP for 1 h (E). A western blot probing for p-ASC (Y144) and ASC in lysates from J774 cells treated with LPS (37ng/ml) for 7 h, Na3VO4 (1mM) for 1 h, and ethanol (3%) for 1 h (F). A western blot probing for p-ASC (Y144) in the supernatants of J774 cells treated with LPS (37ng/ml) for 8 h, and EtOH (3%) and ATP (5mM) for 1 h (G). *<0.0001 by a Tukey’s post hoc test following a one-way ANOVA relative to the LPS+ATP treated group. EtOH = ethanol, Na3VO4 = sodium orthovanadate, RFU = relative fluorescence units, AUC = area under the curve. All studies were performed in triplicate.
Ethanol decreases global tyrosine phosphorylation that is preventable by phosphatase inhibitors
Ethanol has been reported to activate tyrosine phosphatases, and the phosphorylation of ASC at Y144 (mouse) and Y146 (human) is critical for ASC speck and inflammasome formation to occur (14). To test whether ethanol has global effects on tyrosine phosphorylation in macrophages, we treated J774 cells as indicated and performed western blots on the lysates, probing for phosphotyrosine. As anticipated, ethanol treatment greatly reduced tyrosine phosphorylation relative to controls, while having no effect on β-actin levels (Figure 5C). There was an unexpected decrease in tyrosine phosphorylation in LPS and ATP treated cells, possibly due to the loss of select proteins secreted during inflammasome activation.
To determine whether ethanol-induced decreases in global tyrosine phosphorylation were dependent upon the activity of tyrosine phosphatases, sodium orthovanadate a potent tyrosine phosphatase inhibitor, was administered to LPS primed J774 cells 1 hour prior to treatment with ATP and ethanol. A western blot from lysates of these cells probing for phosphotyrosine residues demonstrated a dose dependent restoration of global phosphotyrosine levels with increasing concentrations of sodium orthovanadate (Figure 5D).
Phosphatase blockade ameliorates ethanol’s inhibition of IL-1β secretion
To evaluate whether ethanol’s Nlrp3 inhibitory activity might be a result of increased tyrosine phosphatase activity, J774 cells were pre-treated with sodium orthovanadate (100–1000μM) 1 hour prior to ethanol and ATP stimulation. All doses of sodium orthovanadate used reversed the effects of ethanol’s inhibition (Figure 5D). To ensure that any increases in IL-1β detected by ELISA would not be due to cell death and leakage of pro-IL-1β into the supernatants, or sodium orthovanadate dependent inflammasome stimulation, cells were primed with LPS and treated with the tyrosine phosphatase inhibitor at the same doses and for the same period of time as those receiving ethanol and ATP. In these groups, no IL-1β was detected in the supernatants, indicating that sodium orthovanadate’s reversal of ethanol’s inhibition was due to true restoration of IL-1β secretion.
Ethanol inhibits the phosphorylation of ASC at Y144
To examine whether ethanol’s inhibition of global tyrosine phosphorylation extended to the phosphorylation of ASC at Y144, J774 cells were primed with LPS for 7 hours before being stimulated with 1mM sodium orthovanadate for 1 hour with and without ethanol treatment for an additional hour. A western blot from lysates of these cells was then performed, using an antibody specific to ASC phosphorylated on Y144. At this time point, 1mM sodium orthovanadate alone did not lead to IL-1β secretion (Figure 5D). However, there was a robust quantity of phosphorylated ASC, in the form of high molecular weight ASC specks, retained within the cell (Figure 5F). In cells treated with ethanol during the final hour of sodium orthovanadate stimulation, no phospho-specific ASC was detected (Figure 5F). Furthermore, large amounts of phospho-ASC could be detected in the supernatants of J774 cells primed with LPS and treated with ATP, although no intracellular phospho-ASC could be detected at this time point. The secretion of high molecular weight phosphorylated ASC specks in response to LPS and ATP stimulation was completely abrogated by co-treatment with ethanol (Figure 5G).
Organic compounds containing hydroxyl groups inhibit NLRP3 inflammasome activation and reduce global tyrosine phosphorylation
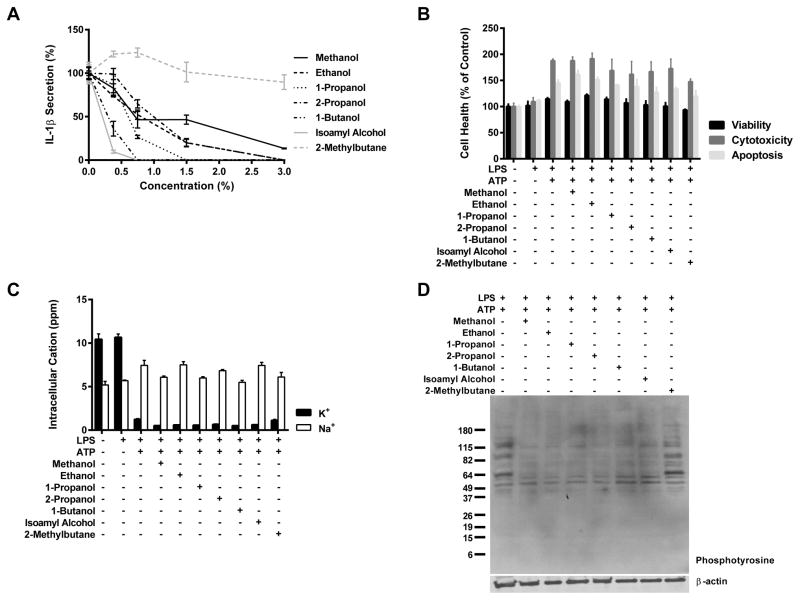
To test whether inhibition of the Nlrp3 inflammasome is specific to ethanol, we treated cells with the hydroxyl group containing organic compounds: methanol, 1-propanol, 2-propanol, 1-butanol, and isoamyl alcohol (isopentanol). We found that when administered to J774 cells these alcohols had inhibitory actions on Nlrp3 inflammasome stimulation parallel to those of ethanol and that this effect was more profound in alcohols with longer carbon chain lengths (Figure 6A). Importantly, 2-methylbutane (isopentane), the non-alcohol analog of 5-carbon isoamyl alcohol, lacked the ability to antagonize IL-1β secretion (Figure 6A). These data indicate that several types of alcohols are capable of inhibiting the Nlrp3 inflammasome and that this inhibition is dependent upon the presence of a hydroxyl group.
Figure 6. Organic compounds containing hydroxyl groups inhibit NLRP3 inflammasome activation and activate protein tyrosine phosphatases.
IL-1β ELISA from J774 cells primed with LPS (37ng/ml) for 8 h and treated with the indicated organic compound (0, 0.38, 0.75, 1.5, and 3%) and ATP (5mM) for 1 hour (A). The graph displays the percent of IL-1β accumulation when cells were in the presence of the organic alcohols relative to LPS+ATP treated groups. Cell health measured by ApoTox-Glo Assay of live J774 cells primed with LPS (37ng/ml) for 8 h and treated with the indicated organic compound at its calculated IC90 dose (when attained) and ATP (5mM) for 1 h (B). ICP-OES measurement of intracellular potassium from J774 cells treated with LPS (37ng/ml) for 8 h and the indicated organic compound at its calculated IC90 dose (when attained) and ATP (5mM) for 1 hour (D). All studies were performed in triplicate.
To rule out cell death as a cause for their capacity to inhibit the Nlrp3 inflammasome, an ApoTox-Glo assay was performed on J774 cells primed with LPS and treated with the calculated IC90 values (when attained) of the organic compounds alongside ATP addition (Figure 6B). While the combined stimulation of LPS and ATP increased the measured levels of cytotoxicity and apoptosis above those of untreated and LPS treated cells, there was no additional rise in either form of cell death subsequent to treatment with any of the organic compounds. In addition, there was no significant change in cell viability across any of the groups.
To validate that alcohols of various carbon chain lengths act on the Nlrp3 inflammasome in a similar mechanism as ethanol, lysates from J774 cells primed LPS and treated with the IC90 doses of the organic compounds and ATP were analyzed for intracellular potassium and global tyrosine phosphorylation by ICP-OES and western blotting. None of the tested compounds were capable of preventing potassium efflux from LPS and ATP treated cells, although levels of intracellular sodium remained constant across all groups (Figure 6C). Yet similar to ethanol, the other alcohols reduced the quantity of phosphotyrosine residues detected by western blot, while treatment with 2-methylbutane had no effect on tyrosine phosphorylation (Figure 6D).
Discussion
There has been a long noted association between alcohol abuse and susceptibility to opportunistic infections. Alcoholics have globally disturbed immune function, including decreased macrophage phagocytosis, disrupted T-cell signaling, diminished levels of circulating pro-inflammatory cytokines, and paradoxically, symptoms of chronic inflammation (28, 51–53). In these studies, we show that in the setting of an acute exposure to ethanol macrophages display markedly attenuated activation of the NLRP3 inflammasome and production of the pro-inflammatory cytokine IL-1β, thus providing a potential site of action for ethanol in the complex syndrome of alcohol induced immunosuppression. The doses of ethanol used in this experiment were high (0.38–3%, equivalent to 64–512mM). However, considering that concentrations of alcohol in the blood, brain, and upper gastrointestinal tract can reach 100, 200, and 3400mM, respectively, following binge drinking (22, 54, 55), and that none of our employed doses induced measureable cell death (Figure 1B), we feel that our chosen doses have physiologic relevance.
We have demonstrated that ethanol is capable of preventing IL-1β secretion after sufficient priming with the bacterially derived TLR4 agonist LPS without impacting TNF release (Figure 1). TNF production occurs as a result of NF-κB activation, therefore the lack of a decline in the production of TNF subsequent to ethanol treatment validates that the object of the alcohol’s inhibition in these experiments is likely (directly or indirectly) assembly of the Nlrp3 inflammasome itself, or the secretion of the leaderless protein.
The fact that ethanol can prevent IL-1β secretion occurring in response to a variety of step 2 agonists implies that its site of action is probably an apical event in Nlrp3 inflammasome stimulation in which each of the four pathways tested converge (Figure 1C, E–G). The endogenous DAMP, ATP, and the bacterially derived PAMP, nigericin, are both believed to activate the NLRP3 inflammasome by inducing pore formation in the plasma membrane. This occurs via activation of the P2×7 receptor and the pannexin 1 hemichannel by ATP and through the formation of pores in the plasma membrane by nigericin itself (9, 10). Aluminum hydroxide, a particulate commonly used as an adjuvant in vaccines, incites inflammasome activation through frustrated phagocytosis, lysosomal rupture, and leakage of cathepsins into the cytosol (11). ApoSAA is a recombinant protein containing an amino acid sequence that is a hybrid of the endogenous acute phase proteins human SAA1 and 2, and an N-terminal methionine. It is not yet completely understood how apoSAA stimulates the NLRP3 inflammasome, but due to the protein’s ability to form amyloid plaques disrupted phagocytosis is a possibility (56, 57). All of these pathways are dependent upon K+ efflux from activated cells, however as ethanol does not inhibit the release of cellular potassium induced by ATP (Figure 2J), we do not believe this to be a mechanism to explain the effects of ethanol on Nlrp3 inflammasome activation. The complete pathway for each of these stimuli is still unclear, but all lead to the formation of an ASC speck upon inflammasome activation (14). In addition to K+ efflux, this is one of the first well characterized points of convergence between the pathways of all known NLRP3 inflammasome step 2 agonists.
Also lending credibility to the hypothesis that ethanol inhibits apical events in Nlrp3 inflammasome activation is our finding that the alcohol can reduce IL-1β secretion when given up to 15 minutes after stimulation with ATP (Figure 1D). This implies that ethanol’s effects on macrophages are nearly immediate, making ethanol’s known ability to alter gene expression an unlikely candidate for its influence on Nlrp3 inflammasome activity.
Activation of inflammasomes leads to conventionally and alternatively cleaved 17 and 28kDa mature IL-1β. Consistent with a lack of Nlrp3 inflammasome activity, no cleaved IL-1β was detected in primed J774 cells given ethanol alongside ATP, despite abundant production from those cells given LPS and ATP alone. There was additionally no cleaved IL-1β present in the lysates of ethanol treated cells, supporting the hypothesis of inhibition of inflammasome activation rather than protein secretion, although it is possible that retained IL-1β might have been degraded before the lysates were collected and therefore went undetected (Figure 2D). Furthermore, no cleaved caspase-1 or secreted ASC and Nlrp3 could be identified in the supernatants of ethanol treated cells, which are other signs of inflammasome activity (Figure 2E–G). Taken together, these results support adequate Nlrp3 inflammasome stimulation in J774 cells following 8 hours of priming with LPS and 1 hour of stimulation with ATP, which is completely blocked when ethanol is administered alongside ATP. This blockade of mature IL-1β, caspase-1, and ASC secretion is unlikely to be due to inhibition of protein secretion (step 3) by ethanol, as we have shown by Ponceau stain that ethanol treatment does not influence general protein secretion in response to ATP and that ethanol is not capable of inhibiting TNF secretion from J774 cells in which NF-κB is already activated (Figure 2A–C).
The inhibitory effects of ethanol on LPS and ATP induced IL-1β secretion were also recapitulated in a variety of primary cell types, including mouse bone marrow derived dendritic cells (BMDCs), and neutrophils, and human PBMCs (Figure 3A–C). And, similar to J774 cells, ethanol treatment prevented the cleavage and secretion of both IL-1β and caspase-1 from human PBMCs (Figure 3D and E). These results mean that in both mouse and human cells, and in multiple cell types, ethanol is able to inhibit NLRP3 activation and IL-1β production.
In an attempt to test whether ethanol may act by preventing ASC speck formation, ethanol was given simultaneously with the addition of the Nlrp1b agonist anthrax LT. Unlike its complete inhibition of IL-1β secretion due to NLRP3 inflammasome activation, ethanol at our highest administered dose of 3% ethanol was incapable of inhibiting Nlrp1b reliant IL-1β secretion (Figure 1I). Nlrp1b inflammasomes are amplified by, but are not dependent on, ASC speck formation. Therefore, the absent responses to ethanol in these experiments could be due to a lack of a requirement for ASC speck formation by this inflammasome. If this is the case, this would indicate that a main point of action for ethanol is inhibition of the adaptor protein’s ability to mediate inflammasome assembly. As a more direct method of determining whether ethanol can prevent ASC speck formation, we used ASC-EYFP stably transfected THP-1 cells to visualize ASC speck formation in real time. In response to LPS and ATP, the quantity of specks visible per cell per visual field rose above baseline and this increase was completely prevented by ethanol treatment (Figure 2H and I). Consistent with ethanol’s inability to inhibit the Nlrp1b inflammasome, this demonstrates that ethanol does act to prevent NLRP3 inflammasome activation at the level of ASC speck formation.
We next attempted to determine how ethanol first transduces its signal into macrophages. The alcohol can serve as an agonist and antagonist to a number of neurotransmitter receptors, which leads to many of its effects on cerebral functioning (33, 34). Several of these receptors have recently been found to be expressed on leukocytes and their activation can skew immune responses towards pro- or anti-inflammatory states. Activation of GABAA receptors results in decreased production of TNF, IL-1β, and several other pro-inflammatory cytokines from macrophages and T-cells, and treatment of mice with GABAA agonists can improve disease status in models of multiple sclerosis and asthma (49, 58). In contrast, stimulation of NMDA receptors on microglial cells leads to increases in TNF and IL-1β secretion (50). Ethanol is both an agonist of GABAA receptors and an antagonist of NMDA receptors, making each of these proteins feasible targets for the alcohol in NLRP3 inflammasome suppression (33, 34). However, our results show that neither the stimulation of GABAA nor the antagonism of NMDA receptors is sufficient to inhibit IL-1β secretion and mimic the effects of ethanol (Figures 4A–C). These receptors were attractive targets since they are expressed on macrophages, have been shown to alter IL-1β production, and are modified by ethanol. However, there are many other receptors on macrophages, not already associated with inflammasome activity, with which ethanol may interact (59, 60). It is possible that one of these may be the relevant target of the alcohol to prevent inflammasome activation. It is equally likely that ethanol might diffuse across the plasma membrane and directly modify cytosolic proteins. Additional techniques beyond the scope of this thesis work will be required to provide meaningful insight into these interesting possibilities.
It has recently been shown that phosphorylation at Tyr146 (human) and Tyr144 (mouse) of ASC is necessary for speck formation to occur (14). Additionally, treatment of neurons with ethanol results in the activation of several tyrosine phosphatases (61, 62). Therefore, we measured total levels of tyrosine phosphorylation in the lysates of J774 cells stimulated to activate the Nlrp3 inflammasome, treated with and without ethanol, to determine whether ethanol might decrease tyrosine phosphorylation in macrophages as well. A 3% dose of ethanol was capable of markedly decreasing global tyrosine phosphorylation when measured via western blot (Figure 5C). In addition, pre-treatment with the phosphatase inhibitor sodium orthovanadate prevented this reduction in tyrosine phosphorylation and the ability of ethanol to prevent IL-1β secretion subsequent to ATP stimulation (Figure 5D and E). It is likely that ethanol is exerting its inhibitory effects on the NLRP3 inflammasome through the activation of a phosphatase that targets phosphotyrosine residues, yet the identities of the specific phosphatase(s) and the target proteins remain unknown.
Here we show that ethanol is able to dose dependently inhibit ROS production alone and in LPS and ATP treated cells (Figure 5A and B). As the generation of intracellular ROS is believed to be an important part of the mechanism leading to NLRP3 inflammasome activation, ethanol’s inhibition of ROS production in response to LPS and ATP could contribute to its inhibition of this inflammasome. ROS are also known to inhibit the activity of protein tyrosine phosphatases (63, 64). Therefore, it is possible that the decrease in global phospho-tyrosine residues that we have shown with ethanol treatment could be a result of decreased ROS mediated inhibition of protein tyrosine phosphatases, resulting in a relative increase in the activity of these phosphatases in the presence of ethanol. This mechanism of ethanol-induced increases in protein tyrosine phosphatase activity leading to decreased inflammasome activation is also supported by the ability of sodium orthovanadate to block ethanol-mediated inflammasome inhibition.
Sodium orthovanadate has been shown to induce ASC speck formation, IL-1β secretion, and pyroptosis in macrophages over the course of several hours (41). In agreement with this, treatment of LPS primed J774 cells with sodium orthovanadate for two hours resulted in robust quantities of phosphorylated ASC at Y144 in high molecular weight ASC specks, although no secreted IL-1β could be detected at this early time point (Figure 5E and F). Treatment of these cells with a 3% dose of ethanol during the final hour of sodium orthovanadate stimulation completely abrogated the phosphorylation of ASC at Y144 (Figure 5F) and co-treatment of LPS primed J774 cells with ethanol and ATP abolished LPS and ATP induced secretion of phosphorylated ASC specks (Figure 5G). This indicates that there is a critical balance between ASC phosphorylation and dephosphorylation that occurs during the course of inflammasome activation prior to its full formation and subsequent IL-1β secretion, which is in part controlled by the activity of protein tyrosine phosphatases. As proper functioning of the NLRP3 inflammasome requires the phosphorylation of ASC at this critical tyrosine residue, it is possible that ethanol might work by increasing the activity of phosphatases, inducing the dephosphorylation of ASC, and preventing its aggregation.
Alcohols of similar chain lengths can exert comparable effects on proteins (31). Here we show that several different organic compounds containing alcohol residues can mimic ethanol’s antagonism of the Nlrp3 inflammasome (Figure 6A). The parallel actions of these alcohols on the inflammasome could be due to similar chemical properties, or the fact that many of the binding pockets for ethanol within proteins can interact with similarly sized alcohols (65). As has been described in studies on other alcohol binding proteins, the potency of these short carbon chain alcohols increased with alongside carbon chain length (66). Notably, we show here that isoamyl alcohol possesses the ability to inhibit IL-1β secretion subsequent to Nlrp3 inflammasome stimulation while 2-methylbutane, containing the same carbon backbone but lacking an alcohol group, does not (Figure 6A). These short chain alcohols were shown to act in a similar mechanism as ethanol, since none were capable of inhibiting K+ efflux, but all induced global reductions in tyrosine phosphorylation (Figure 6C and D). Additionally, none of the organic compounds tested induced cell death at their IC90 doses (Figure 6B). These findings indicate that the hydroxyl group of a chemical is important in exerting an inhibitory effect on this system.
The knowledge that alcohol groups play a key role in inhibiting NLRP3 inflammasome formation provides insight into the mechanisms of ethanol’s action within cells and might prove useful in identifying other relevant molecules capable of inhibiting this vital PRR. The ketone metabolite β-hydroxybutyrate (BHB), a four carbon compound containing both a hydroxyl and carboxyl group, is capable of specifically inhibiting the NLRP3 inflammasome by preventing potassium efflux and ASC oligomerization (45). Fasting and exercise induce endogenous BHB production to support ATP generation during states of energy deficit, providing a possible explanation for the reduction in inflammation observed at these times (45). Similar to our results obtained from isoamyl alcohol and 2-methylbutane, BHB’s immunosuppressive activity on the NLRP3 inflammasome is absent in its alcohol free analog, butyrate (45). As we have shown that similarly sized short-chain alcohols exert comparable inhibition on the Nlrp3 inflammasome and decrease global tyrosine phosphorylation, it is possible that all of these small molecules are acting through the same pathway. Therefore, it is probable that still more endogenous metabolites containing hydroxyl groups could possess inhibitory activity against the NLRP3 inflammasome similar to that of ethanol. Moreover, understanding the mechanisms of alcoholism-associated immunosuppression could afford insight into novel treatments for the condition as well as the development of inhibitors of other chronic pro-inflammatory disease states.
Acknowledgments
This work was funded by National Institute of Health grants R01 HL107291, P30 GM103532, P20 GM103496, R01 HL076278, a UVM/State of Vermont Pre-Seed Fund Grant, and grants from the UVM Office of Undergraduate Research.
The use of the Eclipse TS100 microscope and DS-QiMc digital camera was granted to us by Dr. Jason Botten (University of Vermont).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JS, Kim KD, Na HS, Jeong SY, Park HR, Kim S, Chung J. Tumor necrosis factor-alpha and interleukin-1beta expression pathway induced by Streptococcus mutans in macrophage cell line RAW 264.7. Mol Oral Microbiol. 2012;27:149–159. doi: 10.1111/j.2041-1014.2012.00639.x. [DOI] [PubMed] [Google Scholar]
- 3.Narayanan KB, Park HH. Purification and analysis of the interactions of caspase-1 and ASC for assembly of the inflammasome. Appl Biochem Biotechnol. 2015;175:2883–2894. doi: 10.1007/s12010-014-1471-4. [DOI] [PubMed] [Google Scholar]
- 4.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J. 2013;449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 6.Keyel PA. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine. 2014;69:136–145. doi: 10.1016/j.cyto.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao PC, Chao LK, Chou JC, Dong WC, Lin CN, Lin CY, Chen A, Ka SM, Ho CL, Hua KF. Lipopolysaccharide/adenosine triphosphate-mediated signal transduction in the regulation of NLRP3 protein expression and caspase-1-mediated interleukin-1beta secretion. Inflamm Res. 2013;62:89–96. doi: 10.1007/s00011-012-0555-2. [DOI] [PubMed] [Google Scholar]
- 10.Perregaux D, Barberia J, Lanzetti AJ, Geoghegan KF, Carty TJ, Gabel CA. IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J Immunol. 1992;149:1294–1303. [PubMed] [Google Scholar]
- 11.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC, Lin WW. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. 2015 doi: 10.1189/jlb.3HI0814-371RR. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Sun B, Wang X, Ji Z, Wang M, Liao YP, Chang CH, Li R, Zhang H, Nel AE, Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 18.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 20.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 21.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 22.Nurmi K, Virkanen J, Rajamaki K, Niemi K, Kovanen PT, Eklund KK. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages--a novel anti-inflammatory action of alcohol. PLoS One. 2013;8:e78537. doi: 10.1371/journal.pone.0078537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gris D, Matsushima GK, Ting JP. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kone-Paut I, Piram M. Targeting interleukin-1beta in CAPS (cryopyrin-associated periodic) syndromes: what did we learn? Autoimmun Rev. 2012;12:77–80. doi: 10.1016/j.autrev.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 27.Dai Q, Pruett SB. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J Immunotoxicol. 2006;3:217–225. doi: 10.1080/15476910601080156. [DOI] [PubMed] [Google Scholar]
- 28.von Maltzan K, Tan W, Pruett SB. Investigation of the role of TNF-alpha converting enzyme (TACE) in the inhibition of cell surface and soluble TNF-alpha production by acute ethanol exposure. PLoS One. 2012;7:e29890. doi: 10.1371/journal.pone.0029890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afshar M, Richards S, Mann D, Cross A, Smith GB, Netzer G, Kovacs E, Hasday J. Acute immunomodulatory effects of binge alcohol ingestion. Alcohol. 2015;49:57–64. doi: 10.1016/j.alcohol.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonmez M, Ince HY, Yalcin O, Ajdzanovic V, Spasojevic I, Meiselman HJ, Baskurt OK. The effect of alcohols on red blood cell mechanical properties and membrane fluidity depends on their molecular size. PLoS One. 2013;8:e76579. doi: 10.1371/journal.pone.0076579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Zhang ZY. Reactivity of alcohols toward the phosphoenzyme intermediate in the protein-tyrosine phosphatase-catalyzed reaction: probing the transition state of the dephosphorylation step. Biochemistry. 1996;35:11797–11804. doi: 10.1021/bi960471r. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura M, Pearson S, Kadota Y, Gonzalez CE. Identification of ethanol responsive domains of adenylyl cyclase. Alcohol Clin Exp Res. 2006;30:1824–1832. doi: 10.1111/j.1530-0277.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 33.Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Johnson D, Borghese CM, Hanrahan JR, Johnston GA, Chebib M, Harris RA. GABAA receptors containing rho1 subunits contribute to in vivo effects of ethanol in mice. PLoS One. 2014;9:e85525. doi: 10.1371/journal.pone.0085525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes BA, Smothers CT, Woodward JJ. Dephosphorylation of GluN2B C-terminal tyrosine residues does not contribute to acute ethanol inhibition of recombinant NMDA receptors. Alcohol. 2013;47:181–186. doi: 10.1016/j.alcohol.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis BJ, Zahs A, Kovacs EJ. Epigenetic targets for reversing immune defects caused by alcohol exposure. Alcohol Res. 2013;35:97–113. doi: 10.35946/arcr.v35.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in ALD/AAH and NAFLD/NASH. Hepatology. 2016 doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso-Loeches S, Urena-Peralta J, Morillo-Bargues MJ, Gomez-Pinedo U, Guerri C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem Res. 2016;41:193–209. doi: 10.1007/s11064-015-1760-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Chu G, Yang Z, Sun Y, Zhou H, Li M, Shi J, Tian B, Zhang C, Meng X. Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology. 2015;334:104–110. doi: 10.1016/j.tox.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 41.Ghonime MG, Shamaa OR, Eldomany RA, Gavrilin MA, Wewers MD. Tyrosine phosphatase inhibition induces an ASC-dependent pyroptosis. Biochem Biophys Res Commun. 2012;425:384–389. doi: 10.1016/j.bbrc.2012.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nick JA, Young SK, Arndt PG, Lieber JG, Suratt BT, Poch KR, Avdi NJ, Malcolm KC, Taube C, Henson PM, Worthen GS. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase. J Immunol. 2002;169:5260–5269. doi: 10.4049/jimmunol.169.9.5260. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol. 2013;190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- 45.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 47.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmuller W, Latz E. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bright DP, Smart TG. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front Neural Circuits. 2013;7:193. doi: 10.3389/fncir.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Le Charpentier T, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loeffler JP, Kavelaars A, Verney C, Mantz J, Gressens P. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–549. doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- 51.Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cell Immunol. 2012;274:61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghare S, Patil M, Hote P, Suttles J, McClain C, Barve S, Joshi-Barve S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: potential mechanism of alcohol-induced immune suppression. Alcohol Clin Exp Res. 2011;35:1435–1444. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Reimers E, Santolaria-Fernandez F, Martin-Gonzalez MC, Fernandez-Rodriguez CM, Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol. 2014;20:14660–14671. doi: 10.3748/wjg.v20.i40.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell MC, Jr, Teigen EL, Ramchandani VA. Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcohol Clin Exp Res. 2014;38:1200–1204. doi: 10.1111/acer.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rae CD, Davidson JE, Maher AD, Rowlands BD, Kashem MA, Nasrallah FA, Rallapalli SK, Cook JM, Balcar VJ. Ethanol, not detectably metabolized in brain, significantly reduces brain metabolism, probably via action at specific GABA(A) receptors and has measureable metabolic effects at very low concentrations. J Neurochem. 2014;129:304–314. doi: 10.1111/jnc.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2×7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 57.Ather JL, Martin RA, Ckless K, Poynter ME. Inflammasome activity in non-microbial lung inflammation. J Environ Immunol Toxicol. 2014;1:108–117. [PMC free article] [PubMed] [Google Scholar]
- 58.Munroe ME, Businga TR, Kline JN, Bishop GA. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J Immunol. 2010;185:5586–5597. doi: 10.4049/jimmunol.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagre NN, Subbanna S, Shivakumar M, Psychoyos D, Basavarajappa BS. CB1-receptor knockout neonatal mice are protected against ethanol-induced impairments of DNMT1, DNMT3A, and DNA methylation. J Neurochem. 2015;132:429–442. doi: 10.1111/jnc.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez A, Yevenes GE, San Martin L, Burgos CF, Moraga-Cid G, Harvey RJ, Aguayo LG. Control of ethanol sensitivity of the glycine receptor alpha3 subunit by transmembrane 2, the intracellular splice cassette and C-terminal domains. J Pharmacol Exp Ther. 2015;353:80–90. doi: 10.1124/jpet.114.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. J Biol Chem. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- 62.Wu PH, Coultrap SJ, Browning MD, Proctor WR. Functional adaptation of the N-methyl-D-aspartate receptor to inhibition by ethanol is modulated by striatal-enriched protein tyrosine phosphatase and p38 mitogen-activated protein kinase. Mol Pharmacol. 2011;80:529–537. doi: 10.1124/mol.110.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basu Ball W, Kar S, Mukherjee M, Chande AG, Mukhopadhyaya R, Das PK. Uncoupling protein 2 negatively regulates mitochondrial reactive oxygen species generation and induces phosphatase-mediated anti-inflammatory response in experimental visceral leishmaniasis. J Immunol. 2011;187:1322–1332. doi: 10.4049/jimmunol.1004237. [DOI] [PubMed] [Google Scholar]
- 64.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 65.Hasanuzzaman M, Yoshimura M. Effects of straight chain alcohols on specific isoforms of adenylyl cyclase. Alcohol Clin Exp Res. 2010;34:743–749. doi: 10.1111/j.1530-0277.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 66.Carignan D, Desy O, Ghani K, Caruso M, de Campos-Lima PO. The size of the unbranched aliphatic chain determines the immunomodulatory potency of short and long chain n-alkanols. J Biol Chem. 2013;288:24948–24955. doi: 10.1074/jbc.M113.466334. [DOI] [PMC free article] [PubMed] [Google Scholar]