Abstract
Background
Patients with pollen allergies are frequently poly-sensitized. Pollen contain epitopes that are conserved across multiple species.
Objective
Demostrate that cross-reactive T-cells which recognize conserved epitopes show higher levels of expansion than T-cells recognizing monospecific epitopes, due to more frequent stimulation.
Method
RNA was sequenced from nine pollens and the reads were assembled de-novo into >50,000 transcripts. T-cell epitopes from Timothy Grass (Phl p) were examined for conservation in these transcripts and this was correlated with their ability to induce T-cell responses. T-cells were expanded in vitro with Phl p-derived peptides and tested for cross-reactivity to pollen extracts in ELISPOT assays.
Results
We found that antigenic proteins are more conserved than non-immunogenic proteins in Phl p pollen. Additionally, Phl p epitopes that were highly conserved across pollens elicited more T-cell responses in grass allergic donors than less conserved ones. Moreover, conservation of a Phl p peptide at the transcriptomic level correlated with the ability of that peptide to trigger T-cells that were cross-reactive with other non-Phl p pollen extracts.
Conclusion
We found a correlation between conservation of peptides in plant pollens and their T-cell immunogenicity within Phl p as well as their ability to induce cross-reactive T-cell responses. T-cells recognizing conserved epitopes may be more prominent because they can be stimulated by a broader range of pollens and thereby drive poly-sensitization in allergic donors. We propose that conserved peptides could potentially be used in diagnostic or immunomodulatory approaches that address the issue of poly-sensitization and target multiple pollen allergies.
Keywords: T-cell, Epitope, Timothy Grass Allergy, Pollen Allergy, cross-reactivity, RNA sequencing, sequence conservation, transcriptome
Introduction
Patients with pollen allergies are often poly-sensitized as evidenced by positive RAST- and/or skin prick tests to multiple pollen allergens. The relatively low frequency of mono-sensitizations can be explained by the presence of cross-reactive IgE epitopes conserved across multiple pollens, that result in immune reactivity to homologous regions in allergens to which the patient was not originally sensitized1. In the context of immunotherapy, the high degree of poly-sensitization in subjects suggests that a single allergen administered in therapeutic mode could be sufficient to induce tolerance. In fact, several investigators have suggested that immunotherapy with a single grass species such as Phl p is sufficient to also treat allergies to other grass pollens due to observed cross-reactivity at the IgE level2,3. On the other hand, it is firmly established that allergen-specific T-cells play an important role in allergic inflammation4 and that induction of antigen specific T regulatory cells (Tregs)5 or elimination of allergen-specific T helper type 2 cells (Th2) might be a prerequisite for the induction of specific tolerance6. Yet, evaluation of cross-reactivity at the T-cell level has been less documented.
A recent study using tetramer co-staining of 6 different MHC:epitope complexes found limited cross-reactivity of these epitopes with homologues in other Pooidea grasses, and concluded that multiple-grass-pollen-species immunotherapy is likely to be more beneficial than single species immunotherapy7. While that study was limited to epitopes from two major allergens (Phl p 1 and 5), we have recently shown that a large fraction of Phl p-specific T-cells target epitopes contained in Timothy Grass T-cell antigens (TGTAs) that are unrelated to the known Phl p allergens that are also major targets of T-cell responses but were initially identified based on their high IgE reactivity8. Based on these data, we reasoned that a broader evaluation of T-cell cross-reactivity including more epitopes and also those from TGTAs would be of interest, particularly because clinical studies have shown a good degree of success for single-species immunotherapy9,10 contrary to what might be expected based on the data presented in the tetramer co-staining study for a selected set of epitopes. To gain a comprehensive picture of conservation between different grass-, weed-, and tree-pollens, we sequenced the transcriptome of nine allergenic pollen species and determined how the conservation between Phl p T-cell epitopes and other pollen transcriptomes related to their immunogenicity and their ability to elicit cross-reactive T-cell immune responses.
Methods
Patient population
Study participants were recruited as previously described11. We drew from a cohort of 55 patients between the ages of 19 and 62, 28 of our patients were female. We only included participants with Timothy grass allergy who developed a skin reaction of a wheal of ≥ 5 mm in diameter following skin prick test or Phl p -specific IgE levels of ≥0.35 kU/L as determined by radioallergosorbent test (RAST) and had a history of seasonal grass pollen allergic symptoms were included in the study. We did not control for other allergies and many patients were poly-sensitized. We included patient samples that were collected both in and out of season. Further details about the age, gender and allergy status of the patients used in our studies can be found in Supplementary Table 1. The patient PBMCs used in Figure 3 were further pre-screened based on their reactivity to Phl p peptides after Timothy Grass culture.
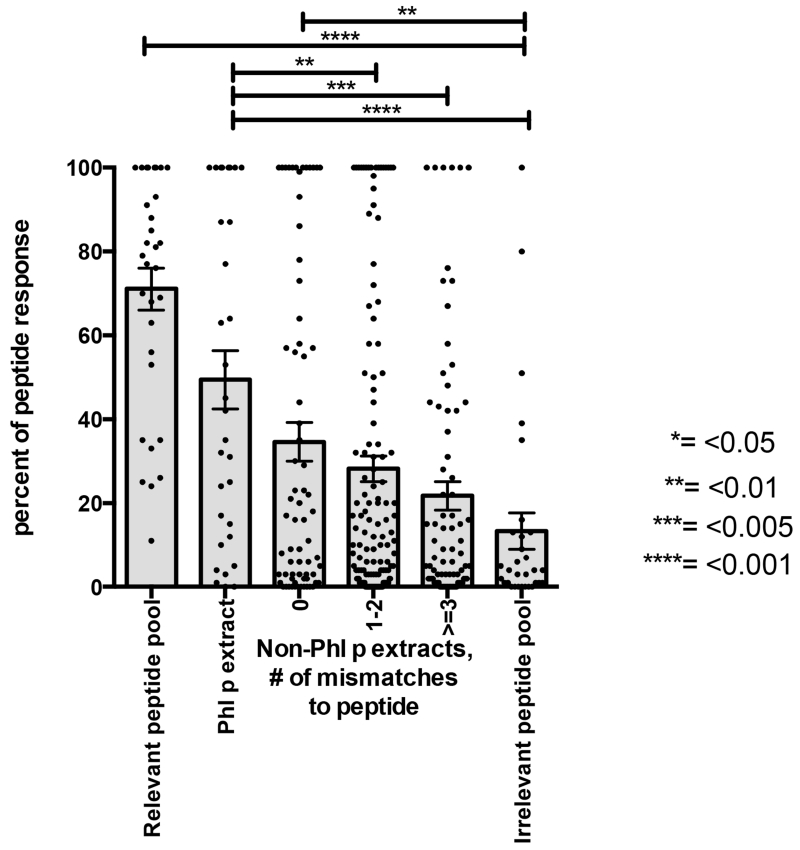
Figure 3. Conservation in transcriptome predicts peptide cross-reactivity.
For each peptide, Phl p allergic donors were selected that reacted to the peptide after expanding PBMCs in vitro with Phl p extract. PBMCs were stimulated with individual peptides for 14 days and IL-5 responses were measured by ELISPOT to i) the peptide itself, ii) Phl p extract, iii) the nine non-Phl p extracts for which transcriptomes were available, iv) peptide pools (P20 and P19) that did or did not contain the peptide, as relevant and irrelevant controls. T-cell cultures that did not induce a robust response above 200 SFC to the peptide itself were excluded (30% of cultures). Reponses to extracts and peptide pools are expressed as the relative fraction of the response to the peptide itself, and capped at 100%. Each grey bar represents the average response +/− SEM. Asterisks indicate p-values of statistical significance as indicated on the right according to one-tailed Mann-Whitney tests.
RNA sequencing and de-novo transcriptome assembly
Total RNA of pollen extract was isolated as previously described12. RNA was sequenced on a HiSeq 2500 sequencer. Replicate samples were run across all lanes of the sequencer to generate paired reads of 100bp in each direction. Prior to assembly, several preprocessing steps were performed: 1) Reads not passing Illumina filters were discarded, 2) Portions of reads matching to adapter/primer sequences were trimmed, 3) 3’ regions of reads following a low quality score (Q<20) were discarded, 4) Remaining reads less than 30 bp in length were discarded. These preprocessing steps were performed using a combination of FASTX-toolkit13 (0.0.13.2) and cutadapt14 (1.3). High quality reads were assembled into transcripts using Trinity15 (r2012-10-05), specifying ‘--min_kmer_cov 2’, to ensure each sequence was observed at least twice.
In vitro expansion of allergen-specific T-cells from peripheral blood mononuclear cells (PBMC)
PBMC were isolated from whole blood by density gradient centrifugation and cryopreserved as previously described8. For in vitro expansion, cells were thawed and cultured with RPMI 1640 (Omega Scientific) supplemented with 5% human AB serum (Cellgro) at a density of 2×106 cells per mL in 24-well plates (BD Biosciences) and stimulated with peptide (0.5 μg/mL). Cells were kept at 37 °C in 5% CO2, and additional IL-2 (10 U/mL; eBioscience) was added every 3 days after initial antigenic stimulation. On day 14, cells were harvested and screened for cytokine production by ELISPOT following restimulation with peptides or pollen extracts.
ELISPOT Assays
The production of IL-5 from in vitro expanded PBMCs in response to peptide pool or extract stimulation was measured by ELISPOT as described16. Briefly, 1×105 cells per well were incubated with peptide pool (5μg/mL) or extract (50 μg/ml, except for oak, which was tested at 25 μg/mL). After 22 hours, cells were removed, plates were washed and incubated with 2 μg/mL biotinylated anti-human IL-5 Ab (Mabtech) at 37 °C. After 2 hours, plates were washed, and avidin–peroxidase complex was added (Vector Laboratories) for 1 hour at room temperature. Peroxidase-conjugated spots were developed with 3-amino-9-ethylcarvazole solution (Sigma-Aldrich).
T-cell clones
PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured in complete RPMI 1640 supplemented with 2mM glutamine, 1% (vol/vol) nonessential amino acids, 1% (vol/vol) sodium pyruvate, penicillin (50 U/ml), streptomycin (50 μg/ml), and 5% human serum (Swiss Red Cross) at a density of 2×106 cells/ml in 24-well plates. Cells were stimulated with pools of peptides (0.5 μg/ml/peptide) and recombinant IL-2 (10 U/ml) was added on day 3 after initial antigenic stimulation. At day 11, cells were stained with Pacific Blue-labelled anti-ICOS mAb (C398.4A; BioLegend) and BV785-labelled anti-CD25 mAb (BC96; BioLegend). CFSElo ICOS+ CD25+ cells were sorted and cloned by limiting dilution, as previously described17. T cell clones were screened at day 20 after initial stimulation by culturing 3 × 104 T cells/well with autologous irradiated (95Gy) EBV-transformed B cells (2 × 104) in the absence or presence of allergen extracts (50 μg/ml) or peptides (0.5 μg/ml). Mycobacterium tubercolosis lysate (5 μg/mL) was used as negative control. Proliferation was measured on day 3 after 16 hours pulse with [3H]-thymidine (GE Healthcare).
Results
Determining Sequence Conservation among a Diverse Selection of Pollen Species
To address the potential impact of sequence conservation and T-cell cross-reactivity on allergic responses, we selected nine pollen species that represent the three major groups of pollen allergens (grasses, weeds and trees). Specifically, we included four grasses (Sweet vernal (Ant o), Rye (Lol p), Kentucky blue (Poa p) and Bermuda (Cyn d)), three trees (Ash (Fra e), Olive (Ole e) and Oak (Que a)), and two weeds (Western ragweed (Amb p) and English plantain (Pla I)). Since no pollen transcriptomic data was available for any of these, we isolated and sequenced pollen RNA, and assembled the reads de novo into transcripts resulting in over 50k transcripts for each pollen. Supplemental Table 2 provides an overview of the read counts and assembly statistics.
As a quality control, we examined for each pollen transcriptome whether the known IgE reactive allergens listed by the International Union of Immunological Societies (IUIS) were re-identified by our analysis. A total of 26 allergens are listed by IUIS (minimum length of 50 residues), covering all pollen species we sequenced (except for Sweet Vernal Grass). For 23 of these allergens, we identified transcripts that had >90% sequence identity for >50% of the length of previously described allergens (Table 1). For two of the three remaining allergens (Poa p 5 and Que a 1), isoforms of the IUIS allergens were listed in Allergome18 that met these criteria. Thus, our transcriptomic analysis re-identified isoforms of all but one (Amb p 5) of the known allergens from the pollen species we sequenced.
Table 1. Recovery of known allergen sequences in transcriptomic analysis.
All allergens from the 9 pollen species we sequenced for which there was a >50 amino acid residue protein sequence available from IUIS are listed. The ‘match in transcriptome’ column is set to ‘yes’ if there was a match with >90% sequence identity over >50% of the protein sequence. No sequences of sufficient length were available in IUIS for Sweet vernal grass (Ant o).
| Allergen | Match in transcriptome |
Allergen | Match in transcriptome |
|---|---|---|---|
| Bermuda Grass | Oak | ||
| Cyn d 1 | yes | Que a 1 | no |
| Cyn d 7 | yes | Ash | |
| Cyn d 12 | yes | Fra e 1 | yes |
| Cyn d 15 | yes | Olive | |
| Cyn d 23 | yes | Ole e 1 | yes |
| Cyn d 24 | yes | Ole e 2 | yes |
| Rye grass | Ole e 3 | yes | |
| Lol p 1 | yes | Ole e 6 | yes |
| Lol p 2 | yes | Ole e 8 | yes |
| Lol p 3 | yes | Ole e 9 | yes |
| Lol p 4 | yes | Ole e 10 | yes |
| Lol p 5 | yes | Ole e 11 | yes |
| Lol p 11 | yes | English Plantain | |
| Kentucky blue grass | Pla l 1 | yes | |
| Poa p 1 | yes | Western Ragweed | |
| Poa p 5 | no | Amb p 5 | no |
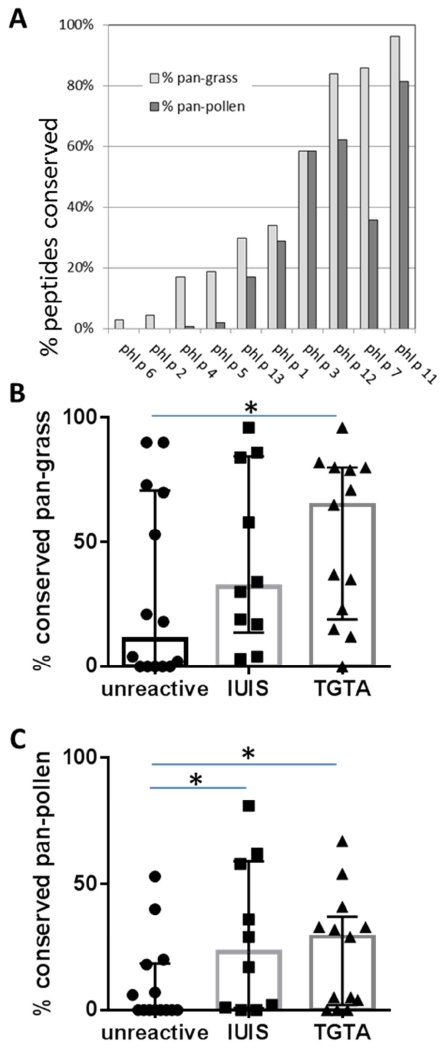
Next we wanted to determine the degree of conservation of the ten IgE reactive Phl p allergens listed by IUIS. We assessed the conservation of 15-mer peptides within each protein, as this constitutes the typical length of a MHC-class II restricted T-cell epitope. Peptides were considered conserved if a transcript encoding a homolog with two or fewer residue mismatches was found. Figure 1A plots the percent of peptides that are conserved in all the grasses we sequenced (pan-grass) or even in all pollens (pan-pollen) for each of the IUIS allergens. A broad spectrum of conservation was detected. Less than 20% of the Phl p 2, 4, 5 and 6 derived peptides were conserved across grasses. By contrast Phl p 7, 11 and 12 peptides were highly homologous with more than 60% of the peptides conserved across all grasses. Phl p 1, 3 and 13 exhibited intermediate conservation with 30%, 34% and 58% of their peptides conserved in pan-grasses, respectively. A similarly broad range of conservation was observed pan-pollen, with the Phl p 11 and 12 showing above 60% conservation.
Figure 1. Conservation of TG proteins across other pollens.
(A) 648 peptides from IgE reactive Phl p allergens listed by IUIS were examined for their conservation in other pollen species. Peptides were considered conserved in another pollen if its transcriptome encoded the peptide or a variant with up to 2 amino acid substitutions. The percentage of peptides conserved in all 4 additional grass transcriptomes (‘pan-grass’) is indicated in light grey bars, and the percentage of peptides conserved in all ten pollens (‘pan-pollen’) in dark grey bars. Antigens are sorted based on pan-grass conservation from low to high. The pan-grass (panel B) and pan-pollen (panel C) conservation of IUIS allergens was then compared to proteins identified in Phl p pollen based on a transcriptomic and proteomic analysis. Median and quantile ranges are indicated by boxes and error bars. Phl p pollen proteins that were not recognized by either T-cell or B cell responses were less conserved than both IUIS allergens and Timothy Grass T-cell antigens (TGTAs). Asterisks indicated statistically significant differences (one-tailed Mann-Whitney test, p < 0.05).
Sequence Conservation Contributes to Immunogenicity
In order to test the hypothesis that the level of conservation of a protein across pollens can contribute to its immunogenicity, we examined proteins distinct from IUIS allergens that were recently identified in a transcriptomic and proteomic characterization of Phl p pollen. We assembled two sets of proteins. The first set contained 13 TGTAs (Timothy Grass T-cell antigens), i.e. all Phl p proteins distinct from the IUIS allergens for which we have detected T-cell responses in 20% or more of allergic patients in our original screen8. The second set of proteins consisted of 14 unreactive proteins that were identified in the same transcriptomic and proteomic analysis as the TGTAs, but for which no T-cell or antibody responses were detected in any allergic donor. Supplemental Figure 1 shows a breakdown of conservation for each of the proteins in the unreactive protein and TGTA protein sets analogous to Figure 1A. These data were further condensed in Figure 1B and 1C which compare the pan-grass and pan-pollen conservation of these protein sets to the IUIS IgE allergens. In both the pan-grass and pan-pollen comparisons, the unreactive proteins showed the lowest degree of conservation. The median pan-grass conservation of peptides from unreactive proteins was 11% compared to 32% for the IUIS allergens and 65% for the TGTAs. For the pan-pollen conservation the medians were 0%, 23% and 29% for unreactive, IUIS allergens and TGTAs, respectively. These differences were significant (p<0.05, one-tailed Mann-Whitney test) for both comparisons of unreactive proteins vs. TGTA and for the pan-pollen conservation comparison of unreactive proteins vs. IUIS allergens. The pan-grass conservation comparison of unreactive proteins vs. IUIS allergens showed the same trend but was below the significance threshold (p=0.087). In conclusion, the data presented in Figure 1 demonstrate that pollen proteins recognized by immune cells of allergic donors, either at the T-cell or IgE level are more conserved compared to other pollen proteins, and that this trend is more pronounced for T-cell allergens. This suggests that sequence conservation might be of particular relevance in the context of T-cell immunogenicity.
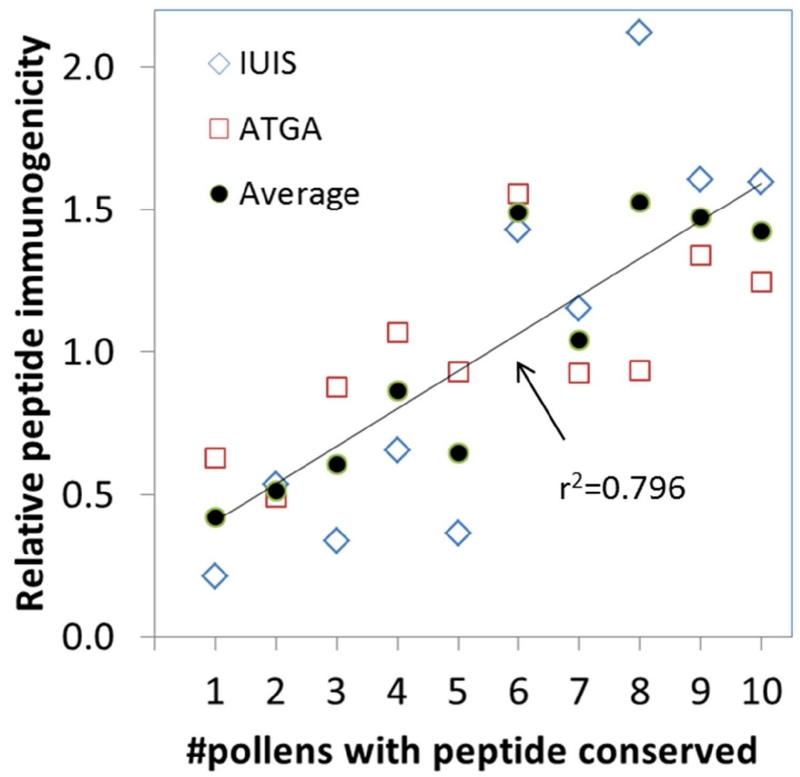
To determine if conservation of the individual peptides correlates with the immunogenicity of a peptide, we studied previously generated IL-5 responses in Phl p allergic donors. Originally, we tested 648 overlapping peptides from IUIS Phl p allergens16 in 25 allergic donors. For 85 donor:peptide pairs (patients used are designated IUIS in Supplementary Table 1), a significant IL-5 response was detected. Thus, the likelihood of an individual peptide to be immunogenic in an individual donor was 85 / (25 * 648) = 0.5%. In our follow up study testing additional Phl p proteins based on transcriptomic and proteomic analysis, we screened 822 peptides for immune recognition in 20 donors (referred to as ATGA in Supplementary Table 1) and identified 375 donor:peptide hits8, corresponding to a 2.3% likelihood for an individual peptide to be immunogenic in an individual donor. Given that the peptides in the second study were pre-selected based on predicted HLA binding affinities, a higher hit rate was expected. Next, we separated peptides into different sets based on the number of pollens they were conserved in. For each set we calculated the average frequency with which an individual peptide was immunogenic in an individual donor, and normalized these values to an average of 1.0 within each study to make them comparable (Figure 2). Indeed, peptides from both the IUIS allergens and the ATGAs, were more frequently immunogenic if they were more conserved. Peptides that were found conserved in only 1, 2 or 3 pollens were 49% less likely to be immunogenic than average, while peptides conserved in 8, 9 or 10 pollens were 47% more likely to be immunogenic than average. This correlation is highly significant (r2=0.796, p=0.00056). Thus, conservation of a Phl p peptide across multiple pollens is correlated with an increased likelihood of the peptide being recognized by T-cells in Phl p allergic individuals.
Figure 2. Peptide conservation correlates with immunogenicity.
Panels of peptides from TG were previously tested for the ability to induce IL-5 responses in PBMCs from allergic patients after in vitro culture with TG extract in two separate cohorts. Peptides from each study were separated into sets based on the number of pollen species they were conserved in (x-axis). For each set, the average frequency of T-cell responses was calculated and normalized to 1.0 for each study(y-axis). Data for peptides derived from IUIS allergens are shown as blue diamonds, while peptides derived from TGTA antigens are shown as red boxes, and averages of the two are shown as black circles. The line depicts a linear correlation for the averaged data, which is highly significant with r2=0.796.
Sequence Conservation Predicts Allergic T-cell Responses
We hypothesized that the more frequent recognition of conserved peptides may be a result of selective expansion of cross-reactive T-cells by repeated stimulation with various allergen sources. Cross-reactive T-cells that recognize epitopes contained in multiple pollens may be stimulated more frequently than those recognizing epitopes exclusively found in a single source, which could result in dominance of the allergic response to conserved epitopes. To test this hypothesis, we expanded PBMCs from 19 Phl p reactive donors with individual epitopes derived from TGTA or IUIS allergens (these patients are designated as CR in Supplementary Table 1 and the sequence of the of the peptides used is described in Supplemental Table 3). After 14 days, IL-5 and IFN-γ release was measured to the epitope itself, Phl p extract and extracts from other pollen. T-cells from Phlp p allergic individuals released much more IL-5 than IFN-y (Supplementary Figure 2). To account for variation between patients, responses to extracts and peptide pools were expressed as the relative fraction epitope response (Fig 3A). When we correlated cross-reactive responses to different extracts with transcriptomic conservation of the epitope in those extracts, a clear hierarchy was observed. Non-Phl p extracts in which the Phl p-epitope is completely conserved (0 mismatches) showed the highest response, followed by non-Phl p extracts with 1-2 mismatches, and the lowest responses were observed for non-Phl p extracts with 3 or more mismatches. The exact same hierarchy was observed when analyzing peptides from IUIS allergens and TGTA peptides separately (Supplemental Figure 3). Thus, Phl p epitopes found conserved in other pollen transcriptomes were indeed more likely to induce T-cell responses that could cross-recognize different pollen species.
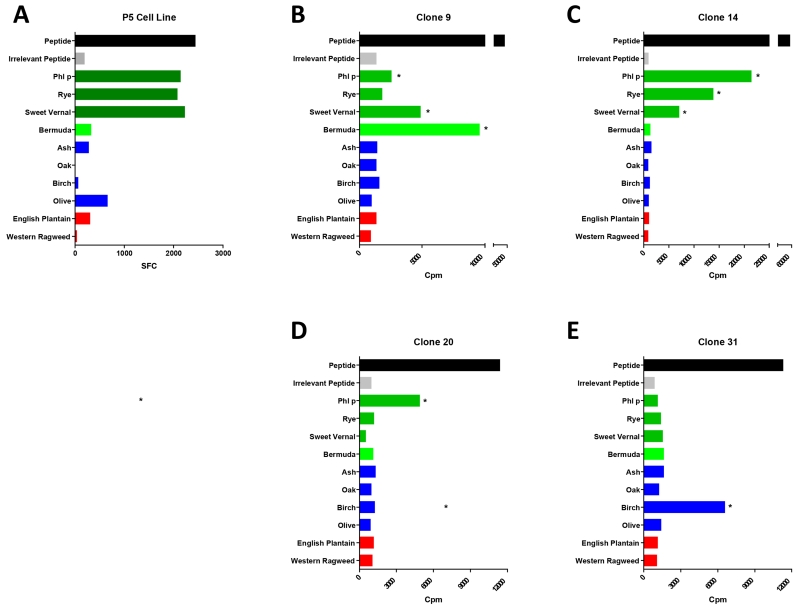
To examine how our results with polyclonal T cell cultures were reflected on a single cell level, we generated T cell clones from Phl p allergic patient PBMCs (see methods). Clones were in vitro expanded with the respective peptide used for T cell clone generation (Figure 3) and then restimulated with the relevant peptide, an irrelevant control peptide and extracts as previously described17. In Figure 4A we show the mean spot forming cells/ per million (SFC) for polyclonal T-cell line generated by culturing the PBMCs with peptide P5. In Figure 4B-E we show clones generated using the same peptide with the same patients PBMCs. Additional clones with another peptide (P7) are shown in Supplemental Figure 4. Several of the T-cell clones generated were cross-reactive (Figure 4B, C), but with different cross-reactivity patterns. We also found some monospecific T-cell clones that only reacted with Phl p or only Birch pollen (Figure 4D). The overall cross-reactivity pattern of clones for peptide P5 and P7 are summarized in Table 2, and compared to the T cell culture cross-reactivity. We find that the diverse specificity of the clones when averaged starts to mimic the reactivity pattern of the T cell culture, with all strongly cross-reactive extracts (>30% in the T cell culture) being represented with cross-reactivity also at the clonal level. These data suggest that the cross-reactivity pattern observed at the polyclonal level are the result of a heterogeneous population of clones with varying cross-reactivity patterns.
Figure 4. T cell clone cross-reactivity.
Donor 1583 was cultured with peptide P5 and then restimulated with that same (relevant) peptide, an irrelevant peptide control and peptides from the 4 grasses (green), 4 trees (blue) and 2 weeds (red). A) Elispot data from the P5 cultured cell line. B)-E) Proliferation of four representative T-cell clones derived from the same patient.
Table 2. Comparison of T-cell Clonal Data with Data from T-cell culture.
The reactivity from 11 clones for Peptide 5 and 3 clones from peptide 7 was averaged and compared to the percent cross-reactivity generated using the T-cell culture methods
| Peptide 5 | Peptide 7 | |||
|---|---|---|---|---|
| Clone | T cell culture | Clone | T cell culture | |
| Peptide | 100% | 100% | 100% | 100% |
| Irr | 0% | 8% | 0% | 22% |
| Phl p | 64% | 88% | 33% | 30% |
| Lol p | 18% | 85% | 100% | 62% |
| Poa p | 55% | 91% | 100% | 45% |
| Cyn d | 45% | 14% | 0% | 16% |
| Fra e | 0% | 12% | 0% | 27% |
| Que a | 0% | 0% | 0% | 0% |
| Bet v | 9% | 3% | 0% | 3% |
| Ole e | 0% | 27% | 0% | 3% |
| Amb p | 0% | 2% | 0% | 5% |
| Pla l | 0% | 13% | 0% | 13% |
Discussion
The present study has generated the largest panel to date of pollen transcriptomes to perform an unbiased analysis of the impact of sequence conservation on shaping allergens-specific T-cell responses. We show that within and beyond the dominant IgE allergens, there is a substantial fraction of peptides and antigens that are highly conserved across pollens, and that this conservation is positively correlated with their likelihood to elicit an immune response. Notably, we could predict the likelihood of Phl p peptides to induce a cross-reactive T-cell immune response to other pollens based on their degree of sequence conservation in other pollens.
Based on these observations, we hypothesize that cross-reaction at the level of T-cell responses might play a key role in poly-sensitization to different allergens. Specifically, as outlined in the model presented in Figure 5, we hypothesize that cross-reactive T-cells that are elicited by allergen exposure will 1) be boosted and selectively expanded by exposure to additional allergen containing the conserved epitope, 2) generate help for any B cell specific for an allergen cross-reactive at the T-cell level through a classic “antigen bridge” linked T-cell:B cell help mechanism, regardless of whether the IgE response is cross-reactive or not.
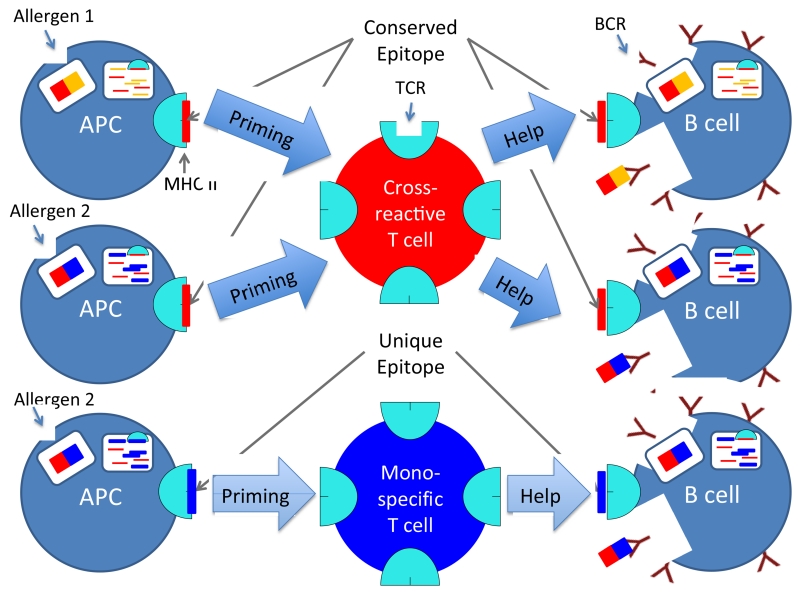
Figure 5. Schematic representation of allergen cross-reactive vs. mono-specific T-cell epitope recognition.
Allergen 1 and 2 contain a conserved T-cell epitope. Cross-reactive T-cells that respond to this epitope will therefore be primed more frequently (cross-reactive T-cell in red, top) than T-cells that are specific for an epitope that is unique to a single allergen (allergen mono-specific T-cell in blue, bottom). Consequently, cross-reactive T-cells can provide T-cell help to B cells that are specific to different allergens, and promote antibody production / isotype switching to IgE. Importantly, both B-cells that produce cross-reactive IgE and B-cells that produce mono-specific IgE can receive help from cross-reactive T-cells as long as they process and present a conserved T-cell epitope.
The specific pattern of the cross-reactive responses and our data in T-cell clones suggest that this is a MHC:T-cell dependent response and not a bystander effect. Benjaponpitak et al. observe a general increase of IL-4 cytokine production in response to a control allergen and tetanus toxoid during the escalation phase of an immunotherapy regimen. This they describe as evidence for a Th2 bystander effect19. If the effects in our culture were similarly non-specific we would have observed an increase cytokine release to all extracts tested in our culture and to the negative control pool. Instead we observe a pattern that shows a preferential increase in response to extracts that contain conserved sequences. Additionally our T-cell clone data shows that cells derived from one clone can produce cytokines when stimulated with several extracts pointing more towards true cross-reactivity rather than a bystander effect. However we observe some low level cross-reactivity against Cyn d and Fra e for in the T-cell cultures with peptide 7 that are not recapitulated on the clonal level. We can therefore not completely exclude that some of the reactivity we observe in our culture system is due to bystander activation.
Allergic cross-reactive responses are relevant in a variety of clinical settings extending beyond Pollen and Grass allergies. Patients sensitized to shrimp, for example, are very likely to also have positive skin prick test against other crustaceans and even other bi-valves20. In OAS (Oral Allergy Syndrome), patients become sensitized to pollen proteins via inhalation and then develop an IgE mediated allergic reaction against food antigens that are similar in structure21. Cross-reactivity of IgE antibodies is determined by structural homology of the epitope22, and the presence of cross-reactive IgE antibodies is typically reflective of clinical cross-sensitization23. The results of our present study suggest that allergen cross-reactivity at the T-cell level should also be explored in these settings.
In a more general context, the data we present here highlight how the interactions between the immune system and its environment are highly complex, and do not fit a simple paradigm where exposure to a single species of allergen elicits a species-specific response. Rather, our data suggest that T-cell responses are shaped by repeated exposure to related species that carry conserved and cross-reactive linear sequences. This phenomenon is not unique to allergies, and in this respect, it is noteworthy that a recent study which examined the level of sequence conservation associated with T-cell cross-reactivity in different Dengue viral strains, also derived a maximum of two substitutions as a threshold beyond which little or no cross-reactivity was observed 24. Furthermore, we have recently shown that inter-species conservation plays a key role in shaping the repertoire of human T-cell responses to epitopes conserved in different herpes virus such as CMV and EBV25, and in different species of the mycobacteria genus26.
It has to be stressed that the observed correlation between the transcriptomic conservation of epitopes and the observed cross-reactivity is a statistical phenomenon that is not deterministically predictive for an isolated sequence. T-cell lines expanded with individual Phl p peptides that are completely conserved in the transcriptome of other pollens nevertheless sometimes show no reactivity to those pollen extracts, while other peptides with many substitutions elicit high responses. This is expected, since other factors than the peptide sequence itself, notably abundance of the antigen in which it is embedded, can influence reactivity levels. Also, some substitutions are more likely to disrupt cross-reactivity than others, in particular non-conservative substitutions and those that target anchor residues for MHC binding and key residues for T-cell recognition. Although we can show a high polarization towards IL-5 secreting T-cells in allergic individuals, we have not shown that cross-reactivity can be observed with other Th2 cytokines. However we chose IL-5 because Schulten et al. previously showed that it is representative of multiple Th2 cytokines after extract and peptide pool stimulation8.
Additional limitations to our study have to be pointed out. First, donor samples were collected both in and out of allergen season, and the study was not designed or powered to distinguish if the exposure pattern at the time of sample collection could further impact the reactivity pattern. Future studies will have to test this potential impact of seasonality. Second, it is possible that some of the cross-reactivity observed is due to bystander activation. Given that the cross-reactivity pattern we observed at the clonal level mimicked that of the polyclonal T cell cultures, we do not think that bystander activation is a major effect in our system, but its contribution cannot be ruled out.
The fact that there is a large number of peptides in Phl p that are conserved across pollens and that are capable of inducing cross-reactive T-cell responses suggests that there is potential to design cocktails of peptides and antigens that could serve as diagnostic or immunotherapeutic reagents to simultaneously target multiple pollen allergens. It has previously been shown that immunotherapy with Phl p extract can induce cross-reactive responses to pollen extracts from other grass species3,2. Our work shows that different T-cell epitopes have different potential for eliciting cross-reactive responses, and that this potential is predictable based on sequence conservation of the peptides between different pollens. Thus, it should be possible to develop cocktails of peptides that specifically elicit cross-reactive immune responses, which could be utilized for pan-grass or even pan-pollen immunotherapy. Importantly, it is not necessary that these are the same peptides that elicit Th2 responses in allergic individuals, but it is also conceivable to develop SIT approaches using conserved peptides present across pollens that elicit bystander suppression of the allergic responses.
In terms of diagnostics, the use of extracts or recombinant allergens that are at least partially conserved across different pollen species means that patients for which there is a single clinically relevant allergen might still be classified as sensitized to multiple pollens because of cross-reactivity in skin-test or RAST test 27,28. It will be worth examining if the use of T-cell epitopes uniquely conserved in different pollen species could more clearly delineate patient sensitization patterns. This could be useful both to modify and improve the SIT preparations29, or to instruct patients what allergens to avoid.
In conclusion, we have assembled a large panel of pollen transcriptomes, and utilized these data to examine the interplay of sequence conservation and T-cell cross-reactivity. We thereby established thresholds of sequence conservation for T-cell epitopes that have potential immunological relevance, and found that many epitopes exist that are highly conserved across grass, tree and weed pollens. Our findings have potential relevance for the design of next-generation SIT treatments and development of pollen species specific allergy diagnostics.
Supplementary Material
Clinical Implications.
Our data suggest that conserved epitopes that elicit highly immunogenic T-cells could delineate patient sensitization patterns, may inform the design of therapeutics for poly-sensitized allergics and refine the understanding of mono-specific versus cross-reactive allergic responses.
Acknowledgements
This work has been partially funded with Federal funds from the National Institutes of Allergy and Infectious Diseases under Grant No. U19AI100275 and from the European Research Council under Grant No. 323183 PREDICT, in addition to funds from ALK-Abello A/S (Horsholm, Denmark). The Institute for Research in Biomedicine and the Center of Medical Immunology are supported by the Helmut Horten Foundation.
Abbreviations
- PBMC
Peripheral blood mononuclear cell
- Phl p
Timothy Grass
- Th2
T helper type 2 cells
- RAST
radioallergosorbent test
- Ant o
Sweet vernal grass
- Lol p
Rye grass
- Poa p
Kentucky blue grass
- Cyn d
Bermuda grass
- Fra e
Ash
- Ole e
Olive
- Que a
Oak
- Amb p
Western ragweed
- Pla I
English plantain
- IUIS
International Union of Immunological Societies
- TGTAs
Timothy Grass T-cell antigens
- OAS
Oral Allergy Syndrome
- SIT
Specific Immunotherapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weber RW. Cross-reactivity of pollen allergens. Current allergy and asthma reports. 2004;4:401–408. doi: 10.1007/s11882-004-0091-4. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Cocera C, et al. Immunotherapy with a Phleum pratense allergen extract induces an immune response to a grass-mix allergen extract. Journal of investigational allergology & clinical immunology. 2010;20:13–19. [PubMed] [Google Scholar]
- 3.Hejl C, et al. Phleum pratense alone is sufficient for allergen-specific immunotherapy against allergy to Pooideae grass pollens. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2009;39:752–759. doi: 10.1111/j.1365-2222.2008.03195.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwok WW, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. The Journal of allergy and clinical immunology. 2010;125:1407–1409. e1401. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomares O, et al. Role of Treg in immune regulation of allergic diseases. European journal of immunology. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 6.Wambre E, et al. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. The Journal of allergy and clinical immunology. 2012;129:544–551. 551, e541–547. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archila LD, et al. Grass-specific CD4(+) T-cells exhibit varying degrees of cross-reactivity, implications for allergen-specific immunotherapy. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014;44:986–998. doi: 10.1111/cea.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulten V, et al. Previously undescribed grass pollen antigens are the major inducers of T helper 2 cytokine-producing T cells in allergic individuals. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3459–3464. doi: 10.1073/pnas.1300512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. Journal of evaluation in clinical practice. 2014;20:225–238. doi: 10.1111/jep.12112. [DOI] [PubMed] [Google Scholar]
- 10.Roberts G, Hurley C, Turcanu V, Lack G. Grass pollen immunotherapy as an effective therapy for childhood seasonal allergic asthma. The Journal of allergy and clinical immunology. 2006;117:263–268. doi: 10.1016/j.jaci.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 11.Schulten V, et al. Association between specific timothy grass antigens and changes in T1- and T2-cell responses following specific immunotherapy. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallner M, et al. Immunologic characterization of isoforms of Car b 1 and Que a 1, the major hornbeam and oak pollen allergens. Allergy. 2009;64:452–460. doi: 10.1111/j.1398-9995.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 13.Hannon G. FASTX-Toolkit. 2010. [Google Scholar]
- 14.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. [Google Scholar]
- 15.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature biotechnology. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oseroff C, et al. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. Journal of immunology. 2010;185:943–955. doi: 10.4049/jimmunol.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messi M, et al. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nature immunology. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 18.Allergome.
- 19.Benjaponpitak S, et al. The kinetics of change in cytokine production by CD4 T cells during conventional allergen immunotherapy. The Journal of allergy and clinical immunology. 1999;103:468–475. doi: 10.1016/s0091-6749(99)70473-2. [DOI] [PubMed] [Google Scholar]
- 20.Pedrosa M, Boyano-Martinez T, Garcia-Ara C, Quirce S. Shellfish Allergy: a Comprehensive Review. Clinical reviews in allergy & immunology. 2014 doi: 10.1007/s12016-014-8429-8. [DOI] [PubMed] [Google Scholar]
- 21.Kondo Y, Urisu A. Oral allergy syndrome. Allergology international: official journal of the Japanese Society of Allergology. 2009;58:485–491. doi: 10.2332/allergolint.09-RAI-0136. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Lopez JC, Rodriguez-Garcia MI, Alche JD. Analysis of the effects of polymorphism on pollen profilin structural functionality and the generation of conformational, T- and B-cell epitopes. PloS one. 2013;8:e76066. doi: 10.1371/journal.pone.0076066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aalberse RC, Akkerdaas J, van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56:478–490. doi: 10.1034/j.1398-9995.2001.056006478.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiskopf D, et al. Immunodominance Changes as a Function of the Infecting Dengue Virus Serotype and Primary versus Secondary Infection. Journal of virology. 2014;88:11383–11394. doi: 10.1128/JVI.01108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu C, et al. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV, HSV and EBV. PLoS pathogens. 2014;10:e1004008. doi: 10.1371/journal.ppat.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindestam Arlehamn CS, et al. Immunological consequences of intragenus conservation of Mycobacterium tuberculosis T-cell epitopes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E147–155. doi: 10.1073/pnas.1416537112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber RW. Patterns of pollen cross-allergenicity. The Journal of allergy and clinical immunology. 2003;112:229–239. doi: 10.1067/mai.2003.1683. quiz 240. [DOI] [PubMed] [Google Scholar]
- 28.Marcucci F, et al. Which allergen extract for grass pollen immunotherapy? An in vitro study. Immunological investigations. 2010;39:635–644. doi: 10.3109/08820131003796876. [DOI] [PubMed] [Google Scholar]
- 29.Stringari G, et al. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever. The Journal of allergy and clinical immunology. 2014;134:75–81. doi: 10.1016/j.jaci.2014.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.