Abstract
Age-related memory decline has been proposed to result partially from impairments in memory consolidation over sleep. However, such decline may reflect a shift toward selective processing of positive information with age rather than impaired sleep-related mechanisms. In the current study, young and older adults viewed negative and neutral pictures or positive and neutral pictures and underwent a recognition test after sleep or wake. Subjective emotional reactivity and affect were also measured. Compared to waking, sleep preserved valence ratings and memory for positive but not negative pictures in older adults and negative but not positive pictures in young adults. In older adults, memory for positive pictures was associated with slow wave sleep. Furthermore, slow wave sleep predicted positive affect in older adults but was inversely related to positive affect in young adults. These relationships were strongest for older adults with high memory for positive pictures and young adults with high memory for negative pictures. Collectively, these results indicate preserved but selective sleep-dependent memory processing with healthy aging that may be biased to enhance emotional well-being.
Keywords: memory, aging, sleep, affect, emotional bias, consolidation
1. Introduction
Memory decline is a well-known feature of aging, even in healthy individuals (Harada et al., 2013; Koen and Yonelinas, 2014). Sleep actively consolidates memories, preserving them for long-term retrieval and integrating them into current knowledge networks (Born and Wilhelm, 2012; Stickgold, 2005). Such processing is thought to comprise interactions among the thalamus, hippocampus, and neocortex, and evidence has linked the memory benefit of sleep to electroencephalographic waveforms reflecting these interactions, namely slow waves, sleep spindles, and sharp wave ripples (Buzsáki, 1996; Rasch and Born, 2013). Aging is accompanied by declines in many sleep properties, including efficiency, time spent in rapid eye movement (REM) sleep and slow wave sleep (SWS), slow wave activity, and sleep spindles (Carrier et al., 2001; Nicolas et al., 2001; Ohayon et al., 2004). Thus, it has been proposed that deficits in sleep underlie age-related memory decline (Fogel et al., 2012; Mander et al., 2013).
An intriguing alternative explanation is suggested by socioemotional selectivity theory (SST; Carstensen, 2006; Carstensen et al., 1999). This theory posits that the perception of future time determines the relative importance placed on different types of goals. Young adults, who perceive their futures to be relatively open-ended, prioritize knowledge acquisition and novel experiences. Older adults, who perceive their futures to be relatively limited, prioritize emotional regulation and well-being. Indeed, despite the potentially adverse changes that accompany aging, the ratio of positive to negative affect has been shown to increase with age (Carstensen et al., 2000, 2011).
Apparent memory decline with healthy aging may, at least in part, reflect this shift in priorities rather than impaired sleep-related mechanisms. According to this hypothesis, young adults would consolidate memories over sleep that support knowledge acquisition without regard for emotional well-being and may even prioritize negative information given its potential importance for survival. Indeed, sleep benefits negative memories in young adults (Baran et al., 2012; Nishida et al., 2009; Payne et al., 2008; Wagner et al., 2006). In older adults, however, sleep-dependent consolidation may still be functional but may be more selective, prioritizing positive memories, consistent with the goal of emotional well-being. However, the influence of sleep on emotional memory consolidation in older adults has not been investigated.
Sleep may also influence emotional reactivity, or the emotional response, associated with previously learned items. Reactivity is typically measured along independent dimensions of valence (how negative, neutral, or positive) and arousal (how calm or exciting) and has been assessed using subjective ratings and physiological measures including skin conductance, heart rate, facial electromyography, neural event-related potentials, and amygdala fMRI activity (Baran et al., 2012; Cunningham et al., 2014; Groch et al., 2013; Pace-Schott et al., 2011; van der Helm et al., 2011; Werner et al., 2015). It is unclear whether sleep preserves or reduces reactivity, as there is evidence of both. The influence of sleep on emotional reactivity has not been investigated in older adults. If sleep prioritizes consolidation of positive memories in this age group, then it may also selectively process reactivity associated with positive memories in older individuals.
To test these possibilities, we examined the effect of sleep on negative and positive memories and reactivity (measured here as subjective ratings of valence and arousal) in young and older adults. Age-related reductions in sleep yield the hypothesis that sleep-dependent consolidation declines with age, regardless of emotional valence. However, SST yields the alternative hypothesis that sleep prioritizes positive information in older adults. In this case, we expect such prioritization to be related to enhanced emotional well-being with aging. We thus investigated relationships among sleep, memory, and affect. Examining sleep-related changes in emotional processing in healthy aging is especially important given that affective disorders, which are associated with sleep abnormalities, are a known risk factor for age-related complications such as dementia (da Silva et al., 2013).
2. Materials and methods
2.1 Participants
Fifty-one older adults (40 female, 11 male; 27 Sleep group, 24 Wake group) between 50 and 80 years (mean = 64.08, SD = 8.15) and 81 young adults (49 female, 32 male; 53 Sleep group, 28 Wake group) between 18 and 30 years (mean = 20.39, SD = 2.19) participated in Experiment 1 (Exp. 1). Fifty-one older adults (38 female, 13 male; 26 Sleep group, 25 Wake group) between 50 and 80 years (mean = 65.26, SD = 8.16) and 89 young adults (63 female, 26 male; 53 Sleep group, 36 Wake group) between 18 and 30 years (mean = 20.26, SD = 1.18) participated in Experiment 2 (Exp. 2). Participants had normal or corrected-to-normal vision and no history of neurological disease, sleep disorders, head injury, or use of medications known to affect sleep or cognitive function. All participants were compensated with payment or course credit. Experimental procedures were approved by the University of Massachusetts, Amherst Institutional Review Board, and written informed consent was obtained before the experiment.
The target age ranges were chosen to maximize the effect of aging, while still taking recruitment into consideration. As sleep and memory start to decline in middle age, we chose the cutoff of 30 for young adults. A larger range was used for older adults to compensate for the challenge of recruiting this population. The higher numbers of young adults in the sleep groups reflect the fact that additional participants were run in order to measure polysomnography (PSG), which was added late in the data collection phase in these groups to follow up on behavior results. Participants with PSG did not significantly differ from those without PSG on total PSQI score, habitual sleep latency or duration, subjective sleep latency or duration for the experimental night, or our primary memory and reactivity outcome measures (p's≥0.08). These subgroups were thus combined. Nonetheless, primary analyses are presented both using the combined sleep groups as well as just the PSG subgroups to ensure this did not influence the pattern of results.
2.2. Materials
Ninety emotionally negative and 90 emotionally neutral pictures were used in Exp. 1. Ninety emotionally positive and 90 emotionally neutral pictures (the same as in Exp. 1) were used in Exp. 2. The majority of stimuli (110 in each experiment) were obtained from the International Affective Picture System (IAPS; Lang et al., 2005). The rest were from an in-house set and were chosen to match the IAPS pictures in content and emotionality (Baran et al., 2012). Based on normative data and previous studies in our lab, negative and positive pictures were moderate to high in arousal, and neutral pictures were low in arousal.
2.3. Procedure
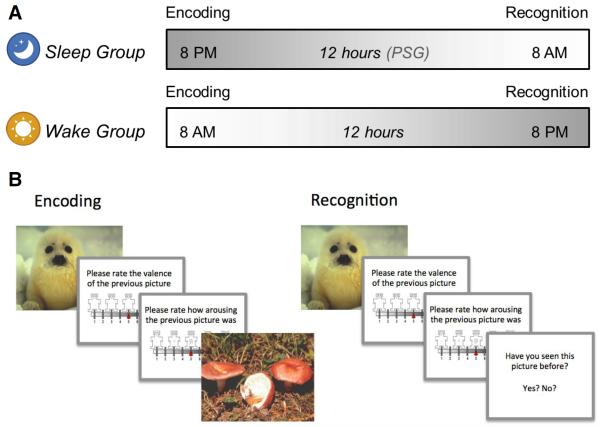
Each experiment consisted of two sessions (Fig. 1A). The first session (Encoding) took place either in the evening between 20:00 and 22:00 (sleep groups) or in the morning between 8:00 and 10:00 (wake groups), followed by the second session (Recognition) 12 hours later. The wake groups were asked not to nap or consume excessive amounts of caffeine between sessions, and all participants were asked not to consume alcoholic beverages. In the first session, all participants completed the Pittsburg Sleep Quality Index (Buysse et al., 1989), which measures subjective habitual sleep quality. Older adults were additionally administered the Mini-Mental State Examination (MMSE). All participants completed a sleep/wake diary and the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) at each session. The PANAS consists of 10 positive and 10 negative attributes that participants rated on a scale from 1 to 5 according to their current feelings, resulting in a possible score of 10–50 for each valence. Higher scores indicate higher affect.
Figure 1.
Experimental design and procedure. A, Encoding took place either in the evening (sleep groups) or the morning (wake groups), followed by Recognition 12 hrs later. Sleep groups underwent PSG overnight. B, During Encoding participants viewed 60 pictures (targets) and rated the valence and arousal of each on 9-point Likert-type scales. During Recognition, participants viewed 180 pictures, a mixture of target and novel foil pictures, and rated each one on valence and arousal. Participants indicated whether or not they recognized the picture by responding yes/no.
During Encoding, participants viewed 60 target stimuli – either 30 negative and 30 neutral (Exp. 1) or 30 positive and 30 neutral (Exp. 2) – in pseudorandom order (Fig. 1B). Each picture appeared on the computer screen for 1000 ms, and the inter-stimulus interval was set to 1500 ms. After each picture, participants were first prompted to rate its valence on a nine-item self-assessment manikin (SAM) valence scale (1=negative, 5=neutral, 9=positive), and then prompted to rate its arousability on a nine-item SAM arousal scale (1=no arousal, 9=highly arousing). These ratings were used as measures of emotional reactivity (valence reactivity and arousal reactivity, respectively). Ratings were entered using numbers on a keyboard without any time limit. Participants were not informed that their memory for the pictures would be tested later.
During Recognition, participants were shown 180 pictures: the same 60 targets intermixed with 120 novel pictures (foils; 60 neutral and 60 negative (Exp. 1) or positive (Exp. 2)). Pictures were displayed for 1000 ms, and participants again rated each on valence and arousal. They were then prompted to indicate whether they had seen each picture before by pressing “y” for yes and “n” for no.
2.4 Polysomnography
Polysomnography (sleep groups only) was recorded in participants' homes using the Aura PSG ambulatory system (Grass Technologies) for 23 older adults in each experiment (technical difficulty prevented successful recording for the remaining 3 in each experiment), 25 young adults in Exp. 1, and 19 young adults in Exp. 2. Electrodes applied after completion of the Encoding Phase included two EOG (right and left ocular canthi), two chin EMG, and six cortical EEG leads (O1, O2, C3, C4, F3, F4), with all channels referenced to the contralateral mastoid. Recordings were obtained and scored according to the specifications provided by the American Academy of Sleep Medicine.
2.5 Data analysis
Participants' individual valence ratings of the pictures were used to categorize stimuli for analyses (St. Jacques et al., 2009). Targets were categorized based on ratings during the Encoding Phase, and foils were categorized based on ratings during the Recognition Phase. Negative (Exp. 1 only), neutral, and positive (Exp. 2 only) pictures were defined as those rated 1–3, 4–6, and 7–9, respectively. Hence, the analyzed picture sets were unique for each participant. Participants were excluded from analyses if they had fewer than 10 negative or neutral targets (n=6). The average number of pictures used in analyses was equivalent between the sleep and wake groups in each experiment (Table 1). Hit Rate (HR) was defined as the percentage of target pictures correctly identified as previously seen; False Alarm Rate (FAR) was defined as the percentage of foil pictures incorrectly identified as previously seen. Corrected recognition (CR) was calculated by subtracting the FAR from the HR. Memory discrimination (d') was also calculated based on HR and FAR. Given that primary measures of emotional reactivity (ΔValence and ΔArousal, described below) represented target pictures only, HR was chosen as the primary measure of memory, whereas FAR, CR, and d' were included as secondary measures (Baran et al., 2012). Changes in valence (ΔValence) and arousal (ΔArousal) ratings were calculated for target pictures (shown at both sessions) as follows: ΔValence = session 2 valence rating – session 1 valence rating; ΔArousal = session 2 arousal rating – session 1 arousal rating. A positive ΔValence score for a negative picture indicates a decrease of the initial negative reaction (toward neutrality). A positive ΔValence score for a positive picture indicates an increase of the initial positive reaction. A negative ΔArousal score for a negative or positive picture indicates a decrease in arousal from the first to the second session.
Table 1.
Target picture ratings in session 1 (mean (SEM))
| # Emo | # Neu | Val - Emo | Val - Neu | Aro - Emo | Aro - Neu | |
|---|---|---|---|---|---|---|
| OA 1 | ||||||
| Sleep | 22.85 (0.84) | 24.27 (1.08) | 1.70 (0.09) | 5.03 (0.03) | 7.15 (0.10) | 2.82 (0.19) |
| Wake | 25.00 (0.98) | 24.00 (1.80) | 1.83 (0.11) | 5.13 (0.03) | 7.19 (0.13) | 3.09 (0.23) |
| p-value | 0.102 | 0.893 | 0.354 | 0.041 | 0.791 | 0.368 |
| YA 1 | ||||||
| Sleep | 23.98 (0.54) | 29.47 (0.80) | 1.78 (0.07) | 5.02 (0.02) | 6.43 (0.24) | 1.93 (0.11) |
| Wake | 24.64 (0.70) | 27.96 (0.96) | 1.87 (0.13) | 5.03 (0.03) | 6.19 (0.30) | 2.28 (0.30) |
| p-value | 0.466 | 0.252 | 0.509 | 0.585 | 0.580 | 0.296 |
| OA 2 | ||||||
| Sleep | 29.31 (1.61) | 28.46 (1.72) | 7.86 (0.08) | 5.26 (0.03) | 6.33 (0.22) | 2.88 (0.23) |
| Wake | 28.96 (1.94) | 28.75 (1.94) | 7.79 (0.10) | 5.25 (0.03) | 6.34 (0.22) | 3.39 (0.30) |
| p-value | 0.890 | 0.911 | 0.586 | 0.682 | 0.977 | 0.189 |
| YA 2 | ||||||
| Sleep | 26.11 (1.02) | 30.00 (1.09) | 7.96 (0.05) | 5.14 (0.02) | 5.95 (0.21) | 2.53 (0.15) |
| Wake | 25.14 (0.83) | 31.64 (1.17) | 7.77 (0.07) | 5.15 (0.02) | 5.38 (0.26) | 2.57 (0.23) |
| p-value | 0.493 | 0.320 | 0.045* | 0.702 | 0.100 | 0.884 |
| p OA1 vs. YA1 | 0.570 | <0.001* | 0.760 | 0.026* | <0.001* | <0.001* |
| p OA1 vs. OA2 | <0.001* | 0.008* | <0.001*a | <0.001* | <0.001* | 0.462 |
| p YA1 vs. YA2 | 0.072 | 0.099* | <0.001*a | <0.001* | 0.017* | 0.004* |
| p OA2 vs. YA2 | 0.010* | 0.154 | 0.357 | <0.001* | 0.012* | 0.012* |
OA 1 = older adults in experiment 1, OA 2 = older adults in experiment 2, YA 1 = young adults in experiment 1, YA 2 = young adults in experiment 2, Emo = emotional (either positive or negative), Neu = neutral, Val = valence, Aro = arousal, p = p-values,
denotes statistical significance
comparison was made using the absolute difference from neutral (5) on the rating scale
The PANAS yielded a positive affect score and a negative affect score in each session. The affect ratio was calculated for each session by dividing the positive score by the negative score. Comparisons of means were conducted using Analyses of Variance (ANOVAs) and Student's independent t-tests. Post-hoc pairwise comparisons were conducted using Student's independent t-tests. Levene's test for homogeneity of variances was applied to all comparisons of means, and adjusted p-values are reported when applicable. Pearson's chi-square test was used to compare gender ratios between groups. Correlations (Pearson's r) were calculated in order to assess bivariate relationships between variables, and multivariate hierarchical regressions were conducted to assess interactions among more than two variables. Significant interactions were decomposed according to the guidelines of Aiken and West (1991), with fitted regression lines plotted at high (+1 SD) and low (−1 SD) levels of the moderating variable using estimates obtained from the final models. Slopes of these lines were then evaluated to determine if they were significantly different from zero. Significance levels were set to p < 0.05. A “trend-level” effect was defined as having a p value ≥ 0.05 and < 0.075. Univariate outliers were identified and removed using the Median Absolute Deviation method with a moderate cutoff criterion (median +/− 2.5 X MAD), as recommended in Leys et al. (2013). Multivariate outliers were identified and removed using Cook's Distance with a cutoff of 4/n.
3. Results
3.1 Sleep benefits negative memories and reactivity in young but not older adults
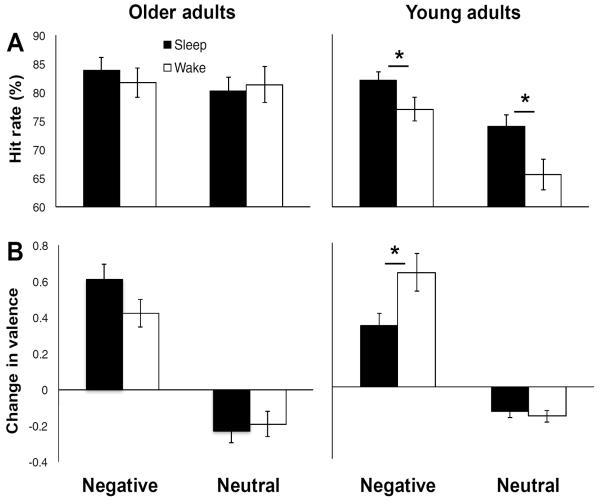
Given the known benefit of sleep on negative memories and reactivity in young adults (Baran et al., 2012; Nishida et al., 2009; Payne et al., 2008; Wagner et al., 2006), we first sought to examine whether this benefit is preserved in older individuals (Experiment 1). Note that portions of data from young adults have been reported previously (Baran et al., 2012). They were reanalyzed and are reported here to serve as a comparison to older adults. Five older adults (1 Sleep group, 4 Wake group) were excluded from these analyses for having fewer than 10 pictures rated as negative.
Memory variables (HR, FAR, CR, and d') were compared using two-factor mixed ANOVAs with a between-subjects factor Group (sleep or wake) and a within-subjects factor Valence (negative or neutral). Participants who were outliers on HR were excluded from these analyses (young adult (YA) Wake: n=1; older adult (OA) Sleep: n=2; OA Wake: n=1). In young adults (Sleep group: n=53; Wake group: n=27), there were significant main effects of group (F(1,78)=7.992, p=0.006; higher in sleep group) and valence (F(1,78)=35.018, p<0.001; higher for negative pictures) and no significant interaction (F(1,78)=1.087, p=0.30) on HR (Fig. 2A). The same pattern of results was obtained for CR (group: F(1,78)=13.671, p<0.001; valence: F(1,78)=26.666, p<0.001; interaction: F(1,78)=2.111, p=0.150) and d' (group: F(1,74)=12.448, p=0.001; valence: F(1,74)=19.651, p<0.001; interaction: F(1,74)=.85, p=0.360; calculation not possible for participants who scored 100% on HR and/or 0% on FAR (n=4)). For FAR, there was a trend-level effect of group (F(1,78)=3.897, p=0.052; higher in wake group), no significant effect of valence (F(1,78)=1.398, p=0.241), and no significant interaction (F(1,78)=0.663, p=0.418; Table 2).
Figure 2.
Influence of sleep and wake on negative and neutral memory and valence in older adults and young adults. A, Average percent of correctly recognized target pictures (hit rate). B, Average inter-session change in valence ratings. Valence was rated on a 9-point Likert-type scale: 1=most negative, 5=neutral, 9=most positive. A positive change in valence indicates ratings became less negative. Error bars represent standard errors of means. Asterisks indicate significant (p<0.05) differences. Graphs on the right are adapted from Baran et al. (2012).
Table 2.
Recognition memory performance (mean (SEM))
| Hit Rate (%) | False Alarm Rate (%) | Corrected Recognition (%) | d Prime | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Group | Emotional | Neutral | Emotional | Neutral | Emotional | Neutral | Emotional | Neutral |
| OA 1 | ||||||||
| Sleep | 83.91 (2.22) | 80.20 (2.44) | 17.41 (2.68) | 13.94 (2.92) | 66.50 (3.20) | 66.27 (3.71) | 1.94 (0.12) | 2.04 (0.14) |
| Wake | 81.65 (2.54) | 81.35 (3.19) | 18.76 (2.44) | 13.98 (1.95) | 62.63 (2.81) | 67.52 (3.33) | 1.95 (0.12) | 2.06 (0.13) |
| p | 0.854 | 0.841 | 0.751 | 0.633 | ||||
| YA 1 | ||||||||
| Sleep | 82.07 (1.43) | 74.12 (1.88) | 16.47 (1.41) | 14.70 (1.09) | 65.60 (1.78) | 59.42 (2.08) | 2.05 (0.08) | 1.80 (0.08) |
| Wake | 77.02 (2.05) | 65.67 (2.66) | 19.42 (1.47) | 19.10 (1.50) | 57.60 (2.57) | 46.57 (2.57) | 1.69 (0.09) | 1.35 (0.09) |
| p | 0.006* | 0.052 | <0.001* | 0.001* | ||||
| OA 2 | ||||||||
| Sleep | 78.13 (2.62) | 74.21 (2.92) | 12.08 (1.62) | 9.79 (1.34) | 66.00 (2.77) | 64.29 (3.13) | 2.14 (0.12) | 2.08 (0.13) |
| Wake | 67.79 (3.57) | 67.83 (2.85) | 14.04 (1.80) | 11.67 (1.59) | 53.79 (4.47) | 56.04 (3.09) | 1.65 (0.16) | 1.75 (0.11) |
| p | 0.033* | 0.348 | 0.023* | 0.022* | ||||
| YA 2 | ||||||||
| Sleep | 83.17 (1.71) | 69.77 (2.15) | 10.68 (1.95) | 6.84 (1.33) | 72.63 (2.47) | 63.26 (2.42) | 2.30 (0.12) | 2.23 (0.11) |
| Wake | 79.69 (2.38) | 68.05 (3.07) | 11.48 (1.44) | 7.02 (0.64) | 68.21 (2.83) | 61.04 (3.07) | 2.16 (0.12) | 2.08 (0.11) |
| p | 0.376 | 0.814 | 0.343 | 0.616 | ||||
OA 1 = older adults in experiment 1, YA 1 = young adults in experiment 1, OA 2 = older adults in experiment 2, YA 2 = young adults in experiment 2, p-values correspond to the main effect of group (sleep vs. wake) in 2×2 ANOVAs,
denotes statistical significance
If only participants who had PSG were included in the Sleep group (n=25), which resulted in better matched sample sizes, then results remained unchanged for HR (group: F(1,50)=6.348, p=0.015; valence: F(1,50)=23.446, p<0.001; interaction: F(1,50)=0.640, p=0.428), CR (group: F(1,50)=24.633, p<0.001; valence: F(1,50)=14.662, p<0.001; interaction: F(1,50)=1.729, p=0.195), and d' (group: F(1,49)=28.429, p<0.001; valence: F(1,49)=10.098, p=0.003; interaction: F(1,49)=1.129, p=0.293), but FAR was significantly higher in the wake group (F(1,50)=17.596, p<0.001) with no significant effect of valence (F(1,50)=2.676, p=0.108) or significant interaction (F(1,50)=1.660, p=0.204). These results suggest that sleep benefits memory for negative and neutral pictures in young adults.
In older adults (Sleep group: n=23; Wake group: n=19), there were no significant effects for HR (group: F(1,40)=0.034, p=0.854; valence: F(1,40)=0.864, p=0.358; interaction: F(1,40)=0.626, p=0.433; Fig. 2A), CR (group: F(1,39)=0.103, p=0.751; valence: F(1,39)=0.886, p=0.352; interaction: F(1,39)=1.074, p=0.306; calculation not possible for participants with no negative-rated foils (n=1)), or d' (group: F(1,32)=0.232, p=0.633; valence: F(1,32)=0.148, p=0.703; interaction: F(1,32)=0.455, p=0.505; calculation not possible for participants who scored 100% on HR and/or 0% on FAR (n=7)). For FAR, there was a significant main effect of valence (F(1,39)=8.525, p=0.006), no significant main effect of group (F(1,39)=0.041, p=0.841), and no significant interaction (F(1,39)=0.214, p=0.646; Table 2). These results suggest that sleep may not benefit memory for negative or neutral pictures in older adults.
Student's independent t-tests were used to compare picture ratings between groups. Participants were excluded from all valence and arousal analyses if they were deemed not to have understood the rating scales (they rated neutral pictures as more arousing than negative ones or did not use the correct keys), were outliers on ratings at baseline, or were outliers on the intersession change in these ratings (YA Sleep group: n=15; YA Wake group: n=12; OA Sleep group: n=6; OA Wake group: n=6). In young adults (Sleep group: n=38; Wake group: n=16), there were no significant differences between sleep and wake groups for baseline ratings of valence or arousal (Table 1). For negative pictures, valence ratings increased (became less negative) significantly more (t=−2.652, p=0.011; Fig. 2B) and arousal ratings decreased significantly more (t=3.344, p=0.002; Fig. S1A) in the wake group. These differences remained significant when only Sleep group participants who had PSG were included (n=25; change in valence: t=−2.897, p=0.006; change in arousal: t=3.471, p=0.001). For neutral pictures, there was no significant difference between groups in intersession change in ratings when the full Sleep group was used (valence: t=0.251, p=0.992; arousal: t=0.744, p=0.466) or when only the PSG subgroup was used (p's>0.50). These results suggest that sleep may preserve valence and arousal reactivity for negative pictures in young adults.
In older adults (Sleep group: n=20; Wake group: n=15), there were no significant differences between groups for baseline ratings of valence or arousal, except the valence of neutral pictures was rated as slightly more positive in the Wake group (Table 1). There were no significant differences between groups for intersession change in valence (negative: t=1.769, p=0.086; neutral: t=−0.343, p=0.734; Fig. 2B) or arousal (negative: t=0.207, p=0.838; neutral: t=−0.065, p=0.949; Fig. S1A), suggesting sleep does not influence valence or arousal reactivity for negative pictures in older adults. Compared to young adults, older adults rated both negative and neutral pictures higher in arousal (p's<0.001), possibly accounting for the overall high memory retention in the latter group.
Sleep physiology is presented in Table 3. Percent of time in each sleep stage was calculated by dividing the time spent in each stage by the total sleep time. Young adults spent significantly less time in NREM2 sleep (t=−3.602, p=0.001) and more time in REM sleep (t=1.922, p=0.061; trend level) than older adults (Student's independent t-tests). Due to the significant effects of sleep group on behavior variables in young adults, correlation analyses (Pearson's r) were conducted to assess relationships between sleep physiology and HR, ΔValence, and ΔArousal in this age group. With regard to negative pictures, we focused on REM sleep and SWS, as both have been implicated in emotional processing. As SWS is important for memory consolidation more generally, we also examined relationships between SWS and memory for neutral pictures. Relationships were first assessed with % time spent in these stages across the entire night. Change in valence was significantly positively related to SWS (r=0.433, p=0.035), inconsistent with the relative preservation of valence reactivity in the sleep compared to wake group but consistent with the general direction of change in both groups. No other relationships were significant (p's>0.20).
Table 3.
Sleep characteristics (mean (SEM))
| TST (min) | SE (%) | RL (min) | NREM1 (%) | NREM2 (%) | SWS (%) | REM (%) | |
|---|---|---|---|---|---|---|---|
| OA 1 | 436.56 (8.41) | 93.51 (1.01) | 96.17 (11.41) | 9.25 (0.76) | 53.13 (1.37) | 17.10 (0.94) | 20.54 (1.34) |
| YA 1 | 420.23 (13.21) | 92.84 (1.24) | 104.48 (10.57) | 10.97 (1.30) | 44.63 (1.90) | 19.49 (0.95) | 24.91 (1.82) |
| OA 2 | 400.13 (16.74) | 91.60 (2.04) | 83.50 (12.67) | 6.96 (0.75) | 53.29 (1.59) | 17.48 (1.45) | 22.44 (1.58) |
| YA 2 | 407.23 (17.79) | 94.67 (1.24) | 106.39 (10.79) | 4.88 (1.13) | 52.19 (1.25) | 23.89 (1.44) | 19.04 (1.70) |
| p 1 | 0.312 | 0.683 | 0.595 | 0.266 | 0.001* | 0.080 | 0.061 |
| p 2 | 0.061 | 0.407 | 0.461 | 0.037* | 0.939 | 0.826 | 0.363 |
| p 3 | 0.552 | 0.316 | 0.901 | 0.001* | 0.003* | 0.012* | 0.026* |
| p 4 | 0.774 | 0.237 | 0.187 | 0.122 | 0.600 | 0.003* | 0.151 |
OA 1=older adults in experiment 1, OA 2=older adults in experiment 2, YA 1 =young adults in experiment 1, YA 2 = young adults in experiment 2, TST=total sleep time, SE=sleep efficiency, RL=REM latency, p 1=p-value when comparing OA 1 and YA 1, p 2=p-value when comparing OA 1 and OA 2, p 3=p-value when comparing YA 1 and YA 2, p 4=p-value when comparing OA 2 and YA 2,
denotes statistical significance
We next conducted exploratory analyses to investigate whether sleep during different parts of the night predicted behavioral outcomes. This approach is relevant for studies comparing older and young adults given that the distribution of sleep stages across the night changes with age (Lombardo et al., 1998; Sonni and Spencer, 2015). Quartiles (total sleep time divided by 4) were chosen as the unit of division due to their near correspondence to sleep cycle length (Sonni and Spencer, 2015) and to allow for comparison with past studies. Emotional processing may occur primarily during the second half of the night when REM sleep is more abundant and cortisol levels are rising. The first substantial amount of REM sleep occurs in the 3rd quarter of the night, and third-quarter REM sleep (REM3; Werner et al., 2015), or what would correspond to REM3 in a split-night paradigm (Wagner et al., 2001), has been implicated in preservation of emotional valence and emotional memory, respectively. Rapid eye movement sleep in the 4th quarter (REM4) has been implicated in preservation of emotional arousal (Werner et al., 2015), suggesting the possibility that arousal is processed when cortisol levels are highest during sleep. Thus, we examined relationships with REM3 and REM4 (% time). Change in valence was significantly negatively related to REM3 (r=−0.408, p=0.043), consistent with the relative preservation of valence reactivity in the sleep compared to wake group. Other relationships were not significant (p's>0.250).
Slow wave sleep occurs predominately in the first half of the night. It has been proposed that recent memories are strengthened in the first cycle of SWS and become integrated with older memories later during sleep (Spencer, 2013). Indeed, hippocampal replay, thought to be the neural instantiation of consolidation, is most prevalent in the first 30 min of sleep (Kudrimoti et al., 1999), and SWS in the first quartile has been related to visuospatial memory improvement in young adults (Spencer and Sonni, 2015). Thus, effects on memory may be strongest for SWS at the beginning of the night. We examined relationships with percent time in SWS in the first (SWS1) and second (SWS2) quartiles. No relationships were significant (p's>0.160), with the exception of a negative correlation between SWS2 and HR for neutral pictures (r=−0.450, p=0.024).
For comparison, relationships found to be significant in young adults were examined in older adults. Change in valence was positively related to SWS in older adults (r=0.486, p=0.022), again consistent with the general direction of change seen in both sleep and wake groups and thus not suggestive of sleep-specific processing. The others were not significant (p's>0.40). Overall, results thus far suggest that sleep benefits memory and preserves valence and arousal reactivity for negative pictures in young but not older adults and that REM sleep (particularly in the third quarter of the night) may be involved in preserving valence reactivity.
3.2 Sleep benefits positive memories and valence reactivity in older but not young adults
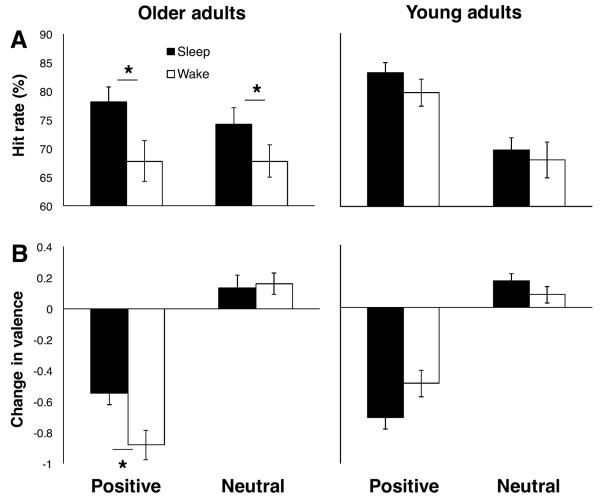
We next considered whether a sleep benefit exists for positive memories in older adults (Experiment 2). New groups of older (mean=65.26 yrs, SD=8.16 yrs) and young (mean=20.26 yrs, SD=1.18 yrs) adults underwent the same procedure except positive instead of negative pictures were used in conjunction with the same neutral stimulus set. One older adult (Wake group) and one young adult (Sleep group) were excluded from these analyses for having fewer than 10 pictures rated as positive.
Participants who were outliers on HR were excluded from memory analyses (YA Wake group: n=2; OA Sleep group: n=2). In young adults (Sleep: n=52, Wake: n=34), a two-factor mixed ANOVA with a between-subjects factor Group (sleep or wake) and a within-subjects factor Valence (negative or neutral) yielded a significant main effect of valence (F(1,84)=72.756, p<0.001; higher for positive pictures), no significant effect of group (F(1,84)=0.793, p=0.376), and no significant interaction (F(1,84)=0.364, p=0.548) on HR (Fig. 3A). The same pattern of results was obtained for CR (valence: F(1,82)=26.423, p<0.001; group: F(1,82)=0.909, p=0.343; interaction: F(1,82)=0.470, p=0.495; calculation not possible for those who rated no foil pictures as positive (n=2)) and, to a lesser extent, d' (valence: F(1,61)=3.892, p=0.053; group: F(1,61)=0.255, p=0.616; interaction: F(1,62)=0.099, p=0.754; calculation not possible for those scoring 100% on HR and/or 0% on FAR (n=21)). False alarm rate was higher for positive pictures (main effect of valence: F(1,82)=25.299, p<0.001) but did not differ between groups (F(1,82)=0.056, p=0.814), and there was no significant interaction (F(1,82)=0.145, p=0.704; Table 2).
Figure 3.
Influence of sleep and wake on positive and neutral memory and valence for older adults and young adults. A, Average percent of correctly recognized target pictures (hit rate). B, Average inter-session change in valence ratings (see Fig. 2 caption). Error bars represent standard errors of means. Asterisks indicate significant (p<0.05) differences.
If only participants who had PSG were included in the Sleep group (n=19), results were unchanged for HR (valence: F(151)=30.011, p<0.001; group: F(1,51)=2.276, p=0.138; interaction: F(1,50)=1.074, p=0.305), CR (valence: F(1,51)=9.603, p=0.003; group: F(1,51)=1.402, p=0.242; interaction: F(1,51)=0.181, p=0.672), and FAR (valence: F(1,51)=11.351, p=0.001; group: F(1,51)=0.016, p=0.899; interaction: F(1,51)=0.911, p=0.344), and there were no significant effects for d' (valence: F(1,38)=0.317, p=0.577; group: F(1,38)=1.520, p=0.225; interaction: F(1,38)=0.234, p=0.631; calculation not possible for those scoring 100% on HR and/or 0% on FAR (n=13)). As an additional control, we repeated analyses for HR, CR and d' using a randomly generated subset of participants from the sleep group to match the sample size of the wake group (n's=34). In each case, the main effect of valence was significant (p's<0.05), whereas the effect of group and the interaction were not (p's>0.170). These results suggest that sleep may not benefit memory of positive pictures in young adults, in line with previous research (Kaestner et al., 2013). They furthermore suggest that a sleep benefit for neutral pictures depends on the valence of the emotional pictures with which they are learned (a benefit was observed in Experiment 1 when they were learned together with negative pictures but not here when they were learned together with positive pictures).
In older adults (Sleep: n=24, Wake: n=24), there was a significant main effect of group (F(1,46)=4.841, p=0.033; higher in sleep group), no significant effect of valence (F(1,46)=1.009, p=0.320), and no significant interaction (F(1,46)=1.053, p=0.310) for HR (Fig. 3A). The same pattern of results was observed for CR (group: F(1,46)=5.526, p=0.023, valence: F(1,46)=0.016, p=0.900, interaction: F(1,46)=0.857, p=0.359) and d' (group: F(1,44)=5.622, p=0.022; valence: F(1,44)=0.243, p=0.624; interaction: F(1,44)=0.461, p=0.461; calculation not possible for participants who scored 100% on HR and/or 0% on FAR (n=2)). False alarm rate was higher for positive pictures (F(1,46)=0.023, p=0.023) but did not differ between groups (F(1,46)=0.899, p=0.348), and there was no significant interaction (F(1,46)=0.002, p=0.967; Table 2). These results suggest that sleep benefits memory for positive pictures in older adults and, as in young adults, suggests a benefit on neutral pictures depending on the valence of the emotional pictures with which they are learned.
Student's independent t-tests were used to compare picture ratings between groups. Participants were excluded from all valence and arousal analyses if they were deemed not to have understood the rating scales (they rated neutral pictures as more arousing than negative ones or did not use the correct keys), were outliers on ratings at baseline, or were outliers on the intersession change in these ratings (YA Sleep group: n=3; YA Wake group: n=10; OA Sleep group: n=6; OA Wake group: n=6). In young adults (Sleep group: n=49; Wake group: n=26), there were no significant differences between sleep and wake groups for baseline ratings of valence or arousal, except the sleep group rated positive pictures as slightly more positive than the wake group (Table 1). Change in valence did not differ significantly between groups (positive: t=−1.762, p=0.082; neutral: t=1.231, p=0.222; Fig. 3B). There was a significant difference between groups in change in arousal for positive pictures (t=−2.695, p=0.009; Fig. S1B), with arousal increasing in the wake group and decreasing in the sleep group. Change in arousal for neutral pictures was not significantly different between groups (t=−1.205, p=0.232).
If only participants with PSG were included in the sleep group (n=19), there was a significant difference between groups for change in valence (t=−2.122, p=0.040, greater reduction in sleep group) and change in arousal (t=−3.037, p=0.004; decreasing in sleep group, increasing in wake group) for positive pictures. Changes were not significantly different for neutral pictures (valence: t=1.750, p=0.087; arousal: t=−1.064, p=0.293). When groups were matched for sample size (using a randomly generated subset from the sleep group), neither the change in valence nor the change in arousal for positive pictures was significant (p=0.194 and p=0.206, respectively). Thus, in young adults, effects of sleep on reactivity to positive pictures seem less clear than effects for negative pictures in Experiment 1. However, interestingly, they may suggest opposing effects of sleep (or wake in the case of arousal) on reactivity for positive compared to negative pictures. Further work is needed to investigate this possibility.
In older adults (Sleep group: n=20; Wake group: n=18), there were no significant group differences in valence or arousal ratings at baseline (Table 1). For positive pictures, valence ratings declined significantly more in the wake group (t=2.794, p=0.008; Fig. 3B), and there was no significant difference in change in arousal ratings (t=−0.429, p=0.671; Fig. S1B). For neutral pictures, there was no significant difference in change in valence (t=−0.279, p=0.782) or arousal (t=−0.417, p=0.680). These results suggest that sleep may preserve valence reactivity for positive pictures in older adults. As in Experiment 1, older adults rated both positive and neutral pictures higher in arousal than did young adults (p's<.017).
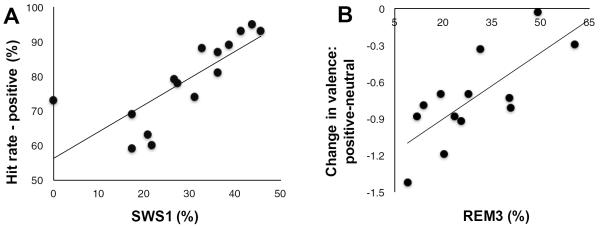
Most overnight sleep characteristics were equivalent in young and older individuals (Student's independent t-tests; Table 3). The exception was that young adults spent a significantly greater percent of time in SWS (t=3.108, p=0.003), consistent with previous reports (Ohayon et al, 2004). Due to the differences between sleep and wake groups for memory and change in valence in older adults and changes in valence and arousal in young adults, we ran correlation analyses between these variables and sleep physiology using the same sleep variables as in Experiment 1. Due to loss of data (stolen laptop), quartile analysis was possible for 19 older adults and 15 young adults. In older adults, SWS1 significantly predicted memory retention of positive pictures (r=0.78, p=0.001), and REM3 significantly predicted preservation of valence for positive relative to neutral pictures (r=0.746, p=0.003; Fig. 4A–B), consistent with better memory and less change in valence in the sleep compared to wake group. No other relationships were significant in this age group (p's>0.150) except a negative correlation between SWS2 and HR for neutral pictures (r=−0.616, p=0.008). Relationships found to be significant in older adults were not significant in young adults, nor were other relationships with change in valence or change in arousal (all p's>0.430). These results suggest early SWS may be important for consolidation of memory for positive pictures in older adults. As with young adults in Experiment 1, REM in the 3rd quarter of the night may be involved in preserving valence reactivity associated with memories consolidated during sleep.
Figure 4.
Relationships between sleep and positive memory and valence in older adults. A, Correlation between the percentage of the first quarter of the night spent in SWS (SWS1%) and hit rate for positive pictures in older adults. B, Correlation between the percentage of the third quarter of the night spent in REM sleep (REM3%) and the change in valence for positive relative to neutral pictures in older adults.
3.3 Comparisons between experiments
Results of these experiments suggest that sleep consolidates negative but not positive memories and valence reactivity in young adults and positive but not negative memories and valence reactivity in older adults. To determine whether the opposing patterns of results in the two experiments are statistically different, we performed follow-up ANOVAs. Hit rate was compared with Experiment (1 vs. 2), Age (older vs. young), and Group (sleep vs. wake) as between-subjects factors and Valence (emotional vs. neutral pictures) as a within-subjects factor. There was a trend for an Experiment X Age X Group interaction (F(1,248)=3.636, p=0.058), consistent with the opposing patterns of results stated above. Other significant effects were a main effect of Experiment (F(1,248)=8.872, p=0.003), with better memory in the negative picture groups than the positive picture groups; a main effect of Group (F(1,248)=8.482, p=0.004), with better memory over sleep than wake; an Experiment X Age interaction (F(1,248)=10.667, p=0.001); a main effect of Valence (F(1,248)=52.157, p<0.001), with emotional pictures remembered better than neutral; and a Valence X Age interaction (F(1,248)=25.434, p<0.001), indicating the difference between emotional and neutral pictures was larger in young than older adults. No other effects were significant (p's>0.20). Change in valence (absolute value) was compared for emotional pictures only using the same between-groups factors. There was a significant Experiment X Age X Group interaction (F(1,199)=16.331, p<0.001) and a significant main effect of Experiment (F(1,199)=5.946, p=0.016), with less change for negative than positive pictures. No other effects were significant (p's>0.20). These 3-way interactions support the interpretation that, compared to wake, sleep preserves negative relative to positive memories and valence in young adults and positive relative to negative memories and valence in older adults.
In control analyses, we separately compared older and young adult groups from the two experiments on several variables. Sleep and wake groups within both age groups did not differ significantly with regard to age or subjective sleep quality (p's>0.2; Table S1). The female to male gender ratio significantly differed only between the young adult wake groups (X2=4.700, p=0.030), making gender ratio an unlikely explanation for overall differences. Compared to positive pictures, both older and young adults rated negative pictures significantly stronger in valence (farther from neutral; p's<0.001) and higher in arousal (p's<0.05; Table 1). Both age groups rated neutral pictures as more positive when they were viewed with positive pictures compared to negative pictures (p's<0.001). As these discrepancies were present in both age groups, they are unlikely to account for the pattern of results seen here. Finally, though young adults differed significantly on the percent of time spent in different sleep stages (p's<0.03; Table 3), older adult groups were highly similar on all sleep variables, with the exceptions that percent time spent in NREM1 sleep (t=2.146, p=0.037) and total sleep time (t=1.944, p=0.061; trend level) were higher for participants in Experiment 1 where no benefit of sleep was observed. Thus, it is doubtful that differences in sleep duration, quality, or architecture could account for the overall pattern of findings.
3.4 Relationships among affect, sleep, and memory
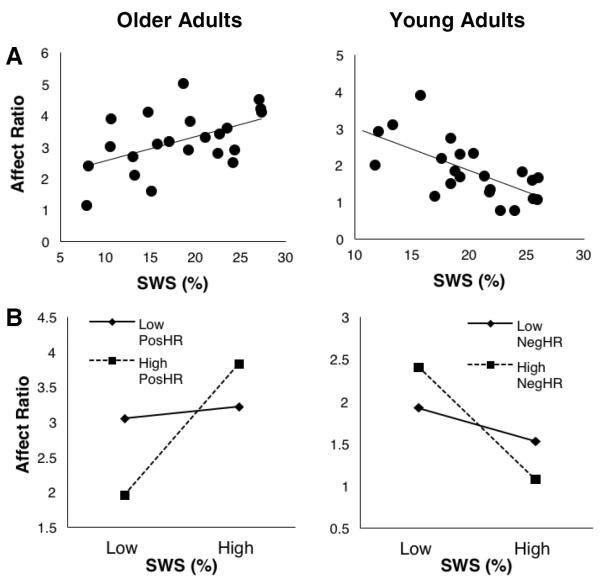
The ratio of positive to negative affect (assessed with PANAS) was compared between young and older adults in each experiment using Student's independent t-tests. Participants who were outliers on affect ratio in either session were excluded from these analyses. Affect was significantly higher in older adults than young adults in both experiments (p's<0.001; Fig. S2; see Table S1 for raw PANAS scores broken down by group and session). If sleep prioritizes consolidation of memories consistent with the goal of emotional well-being in older adults, then one might expect sleep-related processing to be positively related to emotional well-being in this age group. We thus examined relationships between sleep (%SWS and %REM) and affect measured the next morning in session 2 (Affect2), as well as between variables influenced by sleep group (HR and change in valence) and Affect2. Correlation analyses indicated that SWS was significantly positively related to Affect2 in older adults who viewed positive pictures, a group in which a benefit of sleep was observed (r=0.567, p=0.006; Fig. 5A), but not those who viewed negative pictures, in which a trend-level negative relationship was observed (r=−0.409, p=0.066). The former relationship remained significant when affect measured in session 1 (Affect1) was included as a control (r=0.484, p=0.026), suggesting SWS may have influenced next-day affect. The latter no longer approached significance when Affect1 was included (r=−0.353, p=0.139), suggesting SWS did not influence next-day affect but may have reflected more general or trait-like levels of affect. Affect2 was not significantly related to %REM, HR, or change in valence in either older adult group (p's>0.080).
Figure 5.
Relationships between sleep and affect in young and older adults. A, Relationships between the percent of time spent in SWS and the ratio of positive to negative affect in session 2. B, Interactions between percent of time spent in SWS and memory (hit rate) in predicting the session 2 ratio of positive to negative affect in older and young adults.
Since SWS may be associated with affect independently of memory-related processing, we examined the relationship between Affect2 and the interaction between SWS and HR. Multivariate regression analyses indicated that HR significantly moderated the relationship between SWS and Affect2 in those who viewed positive pictures (B=0.456, p=0.021, Table 4) but not negative pictures (B=0.959, p=0.128). Specifically, SWS significantly predicted Affect2 when there was a high level (+1SD) of memory for positive pictures (B=0.132, p=0.001) but not a low level (−1SD) of memory (B=0.012, p=0.704, Fig. 5B), suggesting that sleep-related processing of positive memories may promote positive affect in older adults.
Table 4.
Multivariate Regression Analyses
| Session 2 Affect Ratio |
|||
|---|---|---|---|
| Predictor | Main Effects – B (S.E.) | Interaction – B (S.E.) | |
| YA 1 | |||
| (Constant) | 1.755 (0.114)*** | 1.731 (0.105)*** | |
| SWS | −0.099 (0.027)** | −0.098 (0.025)** | |
| HR Negative | −0.068 (1.494) | 0.117 (1.363) | |
| SWS X HR Negative | −0.668 (0.307)* | ||
| R2 | 0.433** | 0.556** | |
| ΔR2 | 0.123* | ||
| OA 2 | |||
| (Constant) | 3.084 (0.163)*** | 3.011 (0.146)*** | |
| SWS | 0.073 (0.024)** | 0.072 (0.021)** | |
| HR Positive | −0.040 (1.291) | −0.885 (1.180) | |
| SWS X HR Positive | 0.456 (0.180)* | ||
| R2 | 0.348* | 0.527** | |
| ΔR2 | 0.179* | ||
B=Unstandardized Regression Coefficient; S.E.=Standard Error; R2= Model Fit; ΔR2=Change in Model Fit, OA 2 = older adults in experiment 2, YA 1 = young adults in experiment 1, HR=Hit Rate
p<0.05,
p<0.01,
p<0.001
For comparison, these relationships were also evaluated in young adults. Slow wave sleep was inversely related to Affect2 in young adults who viewed negative pictures (r=−0.66, p=0.001, Fig. 5A), a group in which a benefit of sleep was observed, but not those who viewed positive pictures (r=0.010, p=0.972). The former relationship remained significant when Affect1 was included as a control (r=−0.471, p=0.036), suggesting that SWS may have influenced next-day affect. Memory (HR) did not significantly predict affect in either group of young adults (p's>0.450). However, multivariate regression analyses indicated that memory moderated the relationship between SWS and Affect2 in those who viewed negative pictures (B=−0.668, p=0.044, Table 4) but not positive pictures (B=−0.318, p=0.196). Specifically, more SWS was associated with lower affect in those who exhibited a high level (+1SD) of memory for negative pictures (B=−0.150, p<0.001) but not a low level (−1SD) of memory (B=−0.045, p=0.216, Fig. 5B), suggesting that negative memory-related processing during sleep may diminish affect in young adults.
Time spent in REM sleep was positively related to Affect2 in young adults who viewed negative pictures (r=0.530, p=0.014). However, this relationship was no longer significant when Affect1 was included as a control (r=0.056, p=0.816), nor was it significantly moderated by HR or change in valence (p's>0.50). Change in valence was not significantly related to session 2 affect in either young adult group (p's>0.10).
4. Discussion
These results suggest a valence-based dichotomy in the function of sleep on aspects of emotional processing with aging: negative but not positive emotional memories and reactivity are protected by sleep in young adults, while positive but not negative memories and reactivity (though only valence reactivity) receive this benefit in older adults. In other words, sleep promotes a shift in bias from negative material in young adults to positive material in older adults. Furthermore, sleep maintains positive affect in older adults while diminishing it in young adults. These results are aligned with the prediction of SST that sleep selectively consolidates positive memories in older adults in order to support emotional well-being. Though young adults might be expected to consolidate both negative and positive memories to support knowledge acquisition, a general negativity bias is evident across psychological domains and may reflect higher informative or adaptive value of negative stimuli (Baumeister et al., 2001).
Consolidation of positive memories in older adults was specifically related to SWS early in the night. Though emotional memory consolidation has predominately been linked to REM sleep, recent evidence has established the involvement of SWS in this process (Cairney et al., 2014; Groch et al., 2011; Hauner et al., 2013; Payne et al., 2015; Rolls et al., 2013). Slow wave sleep is widely implicated in declarative memory consolidation in general, with synchronized periods of high and low neuronal activity reflected in these waves providing the temporal structure for communication between the hippocampus and neocortex (Mölle and Born, 2011). Though REM sleep was not associated with memory per se, it was associated with valence reactivity to the stimuli. Specifically, REM sleep in the third quarter of the night was related to preserved valence in both young and older adults. This finding is consistent with a recent demonstration that third quarter REM sleep predicted preserved corrugator EMG response, an objective measure of valence reactivity (Werner et al., 2015). Previous studies have also linked REM sleep with preserved arousal reactivity (Lara-Carrasco et al., 2009; Pace-Schott et al., 2011; Werner et al., 2015). Though we did not find an association with a particular stage, sleep did preserve arousal reactivity compared to wake in young adults in Experiment 1. While hippocampal-medial prefrontal cortex (mPFC) interactions are implicated in consolidation of memory content during SWS, amygdala-mPFC and amygdala-hippocampal interactions during REM sleep may consolidate the affective tone or emotional salience of memories (Genzel et al., 2015). Notably, however, these findings are at odds with studies showing reduced reactivity over sleep (Cunningham et al., 2014; Pace-Schott et al., 2011; van der Helm et al., 2011), and our results in Experiment 2 are somewhat in line with such studies. More research is necessary to reconcile these contrasting results.
Importantly, with the exception of arousal reactivity, our results suggest these sleep-dependent mechanisms are preserved in older adults, though their expression is conditional on emotional salience of the learned material. Changing perspective of future time and goals with age may influence the emotional saliency of different types of information. Neuroimaging has revealed age-related, valence-dependent reversals in activity in both the ventromedial prefrontal cortex (vmPFC) and the amygdala during presentation of emotional items (Leclerc and Kensinger, 2010). These regions modulate hippocampal activity and may effectively “tag” emotionally relevant representations for later processing during sleep. Sleep increases functional connectivity among the vmPFC, hippocampus, and amygdala (Gais et al., 2007; Payne and Kensinger, 2011; Yoo et al., 2007). It has been proposed that the vmPFC integrates new learning from the hippocampus, amygdala, and ventral striatum during sleep in order to adjust the value and/or expectations associated with learning material and apply these adjustments to future experience (Niewenhuis and Takashima, 2011). Thus, we posit that processing during sleep is essential for both strengthening emotional bias for previously encountered stimuli and promoting emotional bias for novel stimuli encountered in the future. Additionally, in cases where the effect of sleep appears diminished or absent in older individuals, increasing emotional salience (i.e. positive emotionality) of the learning material should restore the benefit of sleep.
Whether emotional information benefits more or equally from sleep compared to neutral information is not entirely clear, given there is evidence of both (Cunningham et al., 2014; Lewis et al., 2011; Nishida et al., 2009; Payne et al., 2008). Interestingly, though we included the same neutral pictures in each experiment, we found memory for these items was differentially influenced by sleep according to the valence of the pictures with which they were learned. In older adults, recognition of neutral pictures benefited from sleep when learned with positive but not negative pictures. Conversely, in young adults, recognition of neutral pictures benefited from sleep when presented with negative but not positive pictures. The influence of the emotional pictures on neutral pictures may have taken place at encoding. Indeed, participants rated neutral pictures as more positive when viewed together with positive pictures. Alternatively (or additionally), due to their shared episodic context/elements, neutral representations may have benefited from neural reactivation of emotional items during sleep. At any rate, these findings suggest that emotional representations drive the beneficial effect of sleep, whereas neutral memories, when learned in close proximity, are strengthened as a byproduct. It is possible that such a “bleed-over” effect could explain the few instances in which a sleep benefit for positive memories has been observed in young adults, as positive items were encoded in the context of negative items in those studies (Atienza and Cantero, 2008; Sterpenich et al., 2007).
This finding is somewhat inconsistent with past studies reporting a selective benefit of sleep on emotional items, possibly due to differences in design and analysis. For example, Nishida et al. (2009) found a selective benefit when comparing difference scores between memory for pre- and post-sleep learning, but the selective benefit did not appear to be present when only considering pre-sleep learning, which would be more similar to our design. Hu et al. (2006) found a selective benefit for “know” judgments but not “remember” judgments, and we did not assess these judgments in our recognition task. Similarly, Payne et al. (2008) found a selective benefit for “general recognition” (when viewed items were later judged as “similar”) but not for “specific recognition” (when viewed items were later judged correctly as “same”). Thus, the “yes” responses in our task may be comparable to specific recognition or remember judgments in these past studies. Additionally, aspects of stimulus presentation, such as timing, and the similar content of emotional and neutral items in the current study may have been conducive to a carry-over effect as compared to other cases demonstrating a selective benefit of sleep (e.g., Wagner et al., 2001).
Slow-wave sleep predicted enhanced post-sleep emotional affect in older adults and poorer post-sleep affect in young adults. When controlling for pre-sleep affect, these relationships were only significant in the groups that received a memory benefit of sleep, namely older adults who saw positive pictures and young adults who saw negative pictures. Within these groups, memory performance moderated these relationships such that better memory was associated with a stronger relationship between SWS and affect. These results suggest that memory processing during sleep can influence emotional well-being. Disruption of SWS has been shown to improve depressive symptoms, with a reduction in slow wave activity correlated with improvement in negative affect (Cheng et al., 2015; Landsness et al., 2011). As depressed individuals show an exaggerated negativity bias (Gotlib and Joorman, 2010), interruption of negative memory processing may contribute to the beneficial effects of SWS restriction in this population. In contrast, with healthy aging, SWS may facilitate enhanced emotional well-being via selective preservation and integration of positive memories. Interactions among sleep, memory, and affect may converge in the vmPFC, since this area has been implicated in slow wave generation, emotional memory encoding and consolidation, and self-rated affect in healthy adults (Dang Vu et al., 2005; Niewenhuis and Takashima, 2011; Zald et al., 2002). Further research is needed to better understand these relationships. Critically, such research in healthy aging will provide a foundation of knowledge and yield insight regarding unhealthy aging, given that affective disorders are a risk factor for a variety of age-related dysfunctions including cardiovascular disease (Dhar and Barton, 2016), Type 2 diabetes (Rotella and Mannucci, 2013), and dementia (da Silva et al., 2013).
Limitations of this study should be considered. First, the between-subjects design may be problematic for comparisons across emotion conditions. A within-subjects design would be ideal, though it would need to be conducted in such a way as to minimize carry-over effects between stimulus categories. Another potential limitation is the representativeness of our older adult sample, given that their memory performance was equivalent, or in some cases superior, to that of young adults. However, this outcome can likely be explained by the higher arousal ratings in older compared to young adults, particularly in Experiment 1. Arousal is known to enhance encoding and subsequent memory, but it does not diminish the effect of sleep (Cunningham et al., 2014). Indeed, stronger encoding in older adults leads to a sleep benefit on memory (Sonni and Spencer, 2015). Thus, while an age difference (as well as the difference between negative and positive picture groups) in arousal and potentially encoding strength confounds comparisons of overall memory performance, it should not influence the interpretation of the role of sleep. Nevertheless, future studies that equate or systematically vary arousal and encoding strength would be informative.
Supplementary Material
Highlights.
Sleep preserves negative memories and reactivity in young but not older adults.
Sleep preserves positive memories and reactivity in older but not young adults.
Sleep-related memory processing predicts lower positive affect in young adults.
Sleep-related memory processing predicts higher positive affect in older adults.
Acknowledgements
This work was supported by NIH R01 AG040133 and an Honors Research Grant from Commonwealth Honors College. Thanks to Rahul Bussa for assistance collecting and entering data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement The authors declare no conflicts of interest. All procedures were approved by the Institutional Review Board of the University of Massachusetts, Amherst.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J. Sleep Res. 2008;17:285–94. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RMC. Processing of emotional reactivity and emotional memory over sleep. J. Neurosci. 2012;32:1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev. Gen. Psychol. 2001;5:323–370. [Google Scholar]
- Born J, Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cereb. Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Cairney SA, Durrant SJ, Power R, Lewis PA. Complementary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation. Cereb. Cortex. 2014 doi: 10.1093/cercor/bht349. doi: 10.1093/cercor/bht349. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: a theory of socioemotional selectivity. Am. Psychol. 1999;54:165–81. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J. Pers. Soc. Psychol. 2000;79:644–55. [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Brooks KP, Nesselroade JR. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychol. Aging. 2011;26:21–23. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Goldschmied J, Casement M, Kim HS, Hoffmann R, Armitage R, Deldin P. Reduction in delta activity predicted improved negative affect in Major Depressive Disorder. Psychiatry Res. 2015;228:715–8. doi: 10.1016/j.psychres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Crowell CR, Alger SE, Kensinger EA, Villano MA, Mattingly SM, Payne JD. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiol. Learn. Mem. 2014;114:155–64. doi: 10.1016/j.nlm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, Maquet P, Peigneux P. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- da Silva J, Goncalves-Pereira M, Xavier M, Mukaetova-Ladinska EB. Affective disorders and risk of developing dementia: Systematic review. Br. J. Psychiatry. 2013;202:177–186. doi: 10.1192/bjp.bp.111.101931. [DOI] [PubMed] [Google Scholar]
- Dhar AK, Barton DA. Depression and the link with cardiovascular disease. Front. Psychiatry. 2016 Mar 21; doi: 10.3389/fpsyt.2016.00033. doi: 10.3389/fpsyt.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav. Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Fogel S, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Latreille V, Gagnon JF, Doyon J, Carrier J. NREM sleep oscillations and brain plasticity in aging. Front. Neurol. 2012;3 doi: 10.3389/fneur.2012.00176. doi: 10.3389/fneur.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, Maquet P, Peigneux P. Sleep transforms the cerebral trace of declarative memories. Proc. Natl. Acad. Sci. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Spoormaker VI, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol. Learn. Mem. 2015;122:110–21. doi: 10.1016/j.nlm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joorman J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36:1342–50. doi: 10.1016/j.psyneuen.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurbiol. Learn. Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin. Geriatr. Med. 2013;29:737–52. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat. Neurosci. 2013;16:1553–5. doi: 10.1038/nn.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psych. Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J. Cogn. Neurosci. 2013;25:1597–1610. doi: 10.1162/jocn_a_00433. [DOI] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. The effects of healthy aging, amnestic cognitive impairment, and Alzheimer's disease on recollection and familiarity: a meta-analytic review. Neuropsychol. Rev. 2014;24:332–54. doi: 10.1007/s11065-014-9266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J. Psychiatr. Res. 2011;45:1019–26. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Univ. Florida; Gainesville, FL: 2005. Tech. Rep. A-6. [Google Scholar]
- Lara-Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. J. Sleep Res. 2009;18:178–87. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Soc. Neurosci. 2010;5:560–76. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Seifritz E, Rasch B. Sleep benefits emotional and neutral associative memories equally. Somnologie. 2016 doi: 10.1007/s11818-015-0034-4. [Google Scholar]
- Lewis PA, Cairney S, Manning L, Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia. 2011;49:2619–2629. doi: 10.1016/j.neuropsychologia.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013;49:764–766. [Google Scholar]
- Lombardo P, Formicola G, Gori S, Gneri C, Massetani R, Murri L, Salzarulo P. Slow wave sleep (SWS) distribution across night sleep episode in the elderly. Aging. 1998;10:445–448. doi: 10.1007/BF03340157. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog. Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Nicolas A, Petit D, Rompré S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin. Neurophysiol. 2001;112:521–7. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res. 2011;218:325–34. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, Propper RE, Stickgold R. Napping promotes inter-session habituation to emotional stimuli. Neurobiol. Learn. Mem. 2011;95:24–36. doi: 10.1016/j.nlm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. J. Cogn. Neurosci. 2011;23:1285–1297. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA, Wamsley EJ, Spreng RN, Alger SE, Gibler K, Schacter DL, Stickgold R. Napping and the selective consolidation of negative aspects of scenes. Emotion. 2015;15:176–186. doi: 10.1037/a0038683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psych. Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep's role in memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Makam M, Kroeger D, Colas D, de Lecea L, Heller HC. Sleep to forget: Interference of fear memories during sleep. Mol. Psychiatry. 2013;18:1166–70. doi: 10.1038/mp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: A meta-analysis of longitudinal studies. J. Clin. Psychiatry. 2013;74:31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- Sonni A, Spencer RM. Sleep protects memories from interference in older adults. Neurobiol. Aging. 2015;36:2272–81. doi: 10.1016/j.neurobiolaging.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC. Neurophysiological basis of sleep's function on memory and cognition. ISRN Physiol. 2013 Jan 1; doi: 10.1155/2013/619319. 619319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, Dang-Vu TT, Desseilles M, D'Argembeau A, Gais S, Rauchs G, Schabus M, Degueldre C, Luxen A, Collette F, Maquet P. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J.Cogn. Neurosci. 2000;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of functional magnetic resonance imaging data. Psych. Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr. Biol. 2011;21:2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Werner GG, Schabus M, Blechert J, Kolodyazhniy V, Wilhelm FH. Pre- to postsleep change in psychophysiological reactivity to emotional films: Late-night REM sleep is associated with attenuated emotional processing. Psychophysiology. 2015;52:813–25. doi: 10.1111/psyp.12404. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep – a prefrontal amygdala disconnect. Curr. Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc. Nat. Acad. Sci. 2002;99:2450–4. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.