Abstract
During exercise, oxygen and nutrient rich blood must be delivered to the active skeletal muscle, heart, skin, and brain through the complex and highly regulated integration of central and peripheral hemodynamic factors. Indeed, even minor alterations in blood flow to these organs have profound consequences on exercise capacity by modifying the development of fatigue. Therefore, the fine-tuning of blood flow is critical for optimal physical performance. At the level of the peripheral circulation, blood flow is regulated by a balance between the mechanisms responsible for vasodilation and vasoconstriction. Once thought of as toxic by-products of in vivo chemistry, free radicals are now recognized as important signaling molecules that exert potent vasoactive responses that are dependent upon the underlying balance between oxidation-reduction reactions or redox balance. Under normal healthy conditions with low levels of oxidative stress, free radicals promote vasodilation, which is attenuated with exogenous antioxidant administration. Conversely, with advancing age and disease where background oxidative stress is elevated, an exercise-induced increase in free radicals can further shift the redox balance to a pro-oxidant state, impairing vasodilation and attenuating blood flow. Under these conditions, exogenous antioxidants improve vasodilatory capacity and augment blood flow by restoring an “optimal” redox balance. Interestingly, while the active skeletal muscle, heart, skin, and brain all have unique functions during exercise, the mechanisms by which free radicals contribute to the regulation of blood flow is remarkably preserved across each of these varied target organs.
Keywords: Redox Balance, Oxidative Stress, Hyperemia, Antioxidant, Vascular Function, Vasodilation, Skeletal Muscle
Introduction
Despite the continual discovery of potent vasoactive molecules such as nitric oxide (NO), prostaglandins, endothelium derived hyperpolarizing factor (EDHF), adenosine triphosphate (ATP) [1–4], the identification of the mechanisms essential to the regulation of blood flow during exercise in humans has proven to be a difficult and arduous task [5]. Indeed, concurrent inhibition of multiple vasodilatory pathways during exercise has yielded significant, albeit modest, changes in blood flow [6–8]. Additionally, limitations in the ability to target specific enzymatic isoforms and pharmacologically dissect vasodilatory pathways during exercise in humans may be contributing to these disparate findings [9–12]. Based on these findings it may be concluded that either the underlying mechanism of exercise hyperemia has yet to be identified or adequately inhibited during in-vivo human experimentation or that the regulation of blood flow during exercise involves the complex interaction of numerous compensatory and redundant pathways that control the balance between vasodilation and vasoconstriction in the human vasculature.
Much attention has been focused on elucidating specific mechanisms regulating blood flow, with the ultimate goal of identifying a “master regulator”. When considering that blood flow is determined by the integration of central (cardiac output, mean arterial pressure) and peripheral hemodynamics (vascular conductance or resistance) and the multitude of factors that regulate these processes during exercise, it is unlikely that a single molecule will encompass the properties of a master regulator. Indeed, thus far, this notion is supported by many elegant investigations utilizing pharmacological dissection of specific vasodilatory pathways [6–8, 13–18]. However, when considering the overall goal of increased blood flow during exercise, to match oxygen delivery and oxygen demand, it becomes evident that even minor modifications in blood flow in the intact human may have significant consequences in terms of physical performance [19]. The impact of these alterations is likely to be most relevant during high intensity aerobic exercise or during exercise under conditions of compromised vascular function such as with aging and disease.
Active skeletal muscle demands a large increase in blood flow during exercise, increasing by 100-fold compared to resting values [20, 21]. As such, the skeletal muscle vascular bed is arguably the most important vascular bed in terms of the regulation of blood flow during exercise. The regulation of blood flow to this “sleeping giant” is important to maintain adequate oxygen and nutrient delivery to meet the energetic demands of the skeletal muscle during exercise [22], as inadequate oxygen delivery compromises exercise performance and accelerates the development of fatigue [23]. Importantly, minor changes in oxygen delivery have profound consequences in human performance, especially in conditions where components of the oxygen cascade are operating at near maximal capacity or have been altered by disuse, aging, or disease [24–28]. However, blood flow to other vital organs, including the brain and the heart, is of critical importance and small alterations in oxygen and nutrient delivery can have a profound impact on function and performance. Additionally, the skin, second only to the active skeletal muscle in regards to vasodilatory capacity, participates in critical thermoregulatory processes that are directly linked to exercise performance. Clearly, the regulation of blood flow during exercise requires a coordinated and integrated approach to adequately deliver oxygen and nutrient rich blood throughout the body.
The ubiquitous nature and potential vasoactive properties of free radicals is becoming increasingly appreciated. Indeed, NO, first identified 35 years ago as endothelium-derived relaxing factor by Furchgott and Zawadski [1] and later determined to be NO by Ignarro et al. [2], is actually a reactive nitrogen species (RNS) or free radical. NO certainly has potent vasodilatory properties and has, in fact, been a strong contender for the title of “master regulator” of blood flow. However, although still commonly discussed, the source and complex role of NO in vascular function and blood flow regulation is not the focus of this review and the reader is directed to several excellent reviews on this topic [29–31]). Instead, this review will focus on the regulation (or modification) of blood flow during exercise by other RNS and reactive oxygen species (ROS) such as peroxynitrite (ONOO−), superoxide (O2−), hydroxyl radical (OH−), and lipid radicals that promote oxidative stress. Ultimately this depends on several factors including the free radical species, the interaction of the free radical with vasodilators (or vasoconstrictors), possible direct effects of the radical on the vasculature, and the underlying redox balance. Here, the interaction of these factors will be addressed as they relate to the regulation of blood flow during exercise in health, aging, and disease as it appears that the underlying redox status dictates how an exercise-induced increase in free radicals promote or impair vasodilation (Figure 1).
Figure 1. A conceptual schematic of the proposed critical link between redox balance and the regulation of blood flow by free radicals during exercise.
Shifting the underlying redox status such that an imbalance is created may determine whether free radicals promote or impair vasodilation in the vasculature. Optimal vasodilation is defined as the precise matching of oxygen delivery and oxygen demand coupled with the appropriate pressor response to adequately perfuse the active tissue.
When information regarding the exercise associated regulation of blood flow is limited, free radical regulation of basal blood flow will be reviewed. Furthermore, to gain additional mechanistic insight into the possible regulatory roles of free radicals during exercise, investigations evaluating the control of isolated blood vessels will be discussed. Additionally, this review will extend beyond blood flow control in skeletal muscle as important insight may be gained from understanding how free radicals contribute to blood flow regulation in the vasculature of the heart, skin, and brain. As a complete overview of the factors that regulate blood flow during exercise is beyond the scope to this review, the reader is referred to the many excellent reviews on this topic [32–37].
Sources of free radicals
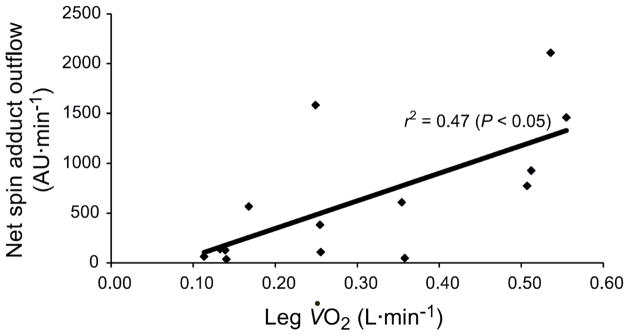
Over the last 30 years, our understanding and appreciation of free radical biology has changed a great deal. Indeed, these highly reactive molecules, characterized by an unpaired electron, were originally viewed as toxic by-products of in vivo chemistry, but are now considered critical regulators of cell signaling and play essential roles in facilitating the damage and adaptations that accompany exercise [38–45]. Importantly, the production of free radicals is augmented during exercise in proportion to the severity of exercise intensity. Seminal work by Davies et al., [46] provided the first electron paramagnetic resonance (EPR) spectroscopic evidence of increased free radical production during exhaustive exercise in animals [46]. These findings have been translated to humans in a series of innovative studies by Bailey and colleagues [47, 48] utilizing isolated knee extension exercise and ex-vivo spin trapping of blood and skeletal muscle (Figure 2). The production of free radicals is clearly augmented during exercise; however, the source(s) of these free radicals remains equivocal.
Figure 2. The relationship between net PBN (α-phenyl-tert-butylnitrone) spin-adduct outflow (venous – arterial difference), a direct measure of free radical outflow, and single-leg oxygen uptake during dynamic single-leg knee extension exercise performed at 25 and 70% of work rate maximum.
Data were collected in a heterogeneous group of 7 healthy men (48 ± 25 yr). Each exercise intensity was continued for 3 min to achieve steady-state pulmonary VO2. Modified from [43].
Early investigators proposed that skeletal muscle mitochondria were the primary source of free radical production during exercise [46]. It was estimated that during exercise 2–5% of oxygen consumed during oxidative phosphorylation was converted to O2− during the transfer of electrons through the complexes of the electron transport chain (ETC) [42, 49–52]. This hypothesis centered on the notion that during state 3 mitochondrial respiration, when electron flux through the ETC is maximized, the potential for electrons to bypass the respiratory complexes and aberrantly reduce oxygen, producing O2−, would also be maximized [53, 54]. Contrary to this concept, it has recently been documented that ROS production is actually lowest during state 3 respiration, as the oupling of electron transfer to ATP production is optimized [55, 56]. Thus, the early estimates that 2–5% of oxygen consumed is converted to ROS have been re-evaluated, and it is now more commonly accepted that as little as 0.15% of consumed oxygen is converted to ROS [57]. While the contribution of skeletal muscle mitochondria to ROS production during exercise is somewhat equivocal, several studies, utilizing mitochondrial targeted antioxidants, have provided compelling evidence that mitochondria-derived ROS contribute to vascular endothelial dysfunction with age in murine models [58, 59] and that even the thin endothelial cell layer may be a significant source of free radicals [60]. Therefore, the location of the mitochondria, skeletal muscle versus endothelial cells or vascular smooth muscle, may be a critical factor in deciphering the source of free radicals during exercise and how free radicals impact blood flow.
In addition to endothelial cell and smooth muscle mitochondria, the vasculature contains several enzymatic sources of free radicals including nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), xanthine oxidase (XO), and nitric oxide synthases (NOS) [43, 60–62] that, each, likely contribute to the exercise-induced increase in free radicals. Indeed, elevations in blood flow and shear stress, as well as biochemical changes in the metabolic milieu of the blood that occur with exercise increase the activity of these enzymes resulting in increased free radical production [63, 64]. Animal studies using specific enzymatic inhibitors (allopurinol, apocynin) reveal important roles of these enzymes in the exercise-induced production of free radicals [65].
Overall, multiple sources, both enzymatic and mitochondria-based, contribute to the exercise-induced increase in free radicals such as O2−, ONOO−, and OH−. Importantly, these free radicals possess inherent vasoactive properties or are capable of altering the bioavailability of other vasoactive molecules, such as NO. Under normal healthy conditions, acute exposure to elevated free radicals, as occurs with exercise, induces the upregulation of endogenous antioxidant enzyme systems that are capable of combating the potential deleterious effect of heightened free radicals [66]. However, when the production of free radicals outweighs the system’s ability to neutralize these reactive molecules, oxidative stress ensues. Oxidative stress, a condition defined by a redox imbalance in which pro-oxidant forces outweigh antioxidant capacity, has been linked to damage and dysfunction associated with normal aging and the development of cardiovascular and metabolic disease [67, 68]. As will become evident throughout this review, the underlying level of oxidative stress is a critical factor that directly impacts the physiological role of free radicals in the context of blood flow regulation.
Free Radical Regulation of Vascular Function
The regulation of vascular tone and skeletal muscle blood flow are inherently linked. However, a complex, and often underappreciated, paradox exists whereby the control of conduit artery diameter and skeletal muscle blood flow may occur independently. This differential regulation of macro- (conduit) and micro-vascular (resistance arteries/arterioles and capillaries) tone may be due to distinct mechanisms contributing to the regulatory processes, made possible by the fact that modest changes in conduit vessel diameter do not regulate blood flow to the active skeletal muscle. This concept is supported by several studies reporting a vasoactive role of free radicals in the regulation of conduit artery diameter (specifically, the brachial artery), without a simultaneous change in skeletal muscle blood flow [69, 70]. Similarly, NOS inhibition can attenuate movement induced hyperemia in the leg without altering femoral artery diameter [71]. While a complete discussion of the many factors that contribute to the regulation of vascular function and blood flow is beyond the scope of this review, this background information is important as free radicals and oxidative stress impact both macro- and microvascular function. With these concepts establishing a conceptual framework, this section will focus on the regulation of vascular tone by free radicals during exercise with a specific emphasis on the in vivo assessment of conduit artery vasodilatory capacity. Ancillary studies utilizing isolated blood vessels to directly examine mechanisms of free radical regulation of vascular tone will also be discussed.
A series of investigations by our group revealed interesting, and somewhat unexpected, findings regarding the regulation of vasomotor tone during exercise in healthy individuals with low oxidative stress. Utilizing an oral antioxidant cocktail of Vitamins C, E, and alpha lipoic acid, our group [69, 70] reported impaired exercise-induced brachial artery vasodilation following treatment with the supplemental antioxidants (Figure 3A), that attenuated circulating free radicals during exercise [70]. Similarly, following 6-weeks of single leg knee extension exercise training which lowered oxidative stress in older adults, training-induced improvements in resting blood pressure and brachial artery vasodilation (Figure 3B), assessed by flow mediated dilation evoked by both post cuff occlusion hyperemia and during progressive handgrip exercise, were abolished with concomitant antioxidant consumption [69, 72]. Similar findings in isolated rat arterioles confirm a critical role of ROS in flow induced vasodilation [73]. Overall, these findings reveal that in the absence of disease and underlying oxidative stress, free radicals are critically involved in the regulation of vascular tone and act as pro-oxidant vasodilators.
Figure 3. The impact of oral antioxidant administration on brachial artery diameter during progressive handgrip exercise in young (A) and old (B) subjects and old subjects post-training (C).
Young subjects exhibited a progressive linear increase in brachial artery diameter under control (placebo) conditions and in these subjects the administration of an oral antioxidant significantly blunted exercise-induced vasodilation. Old subjects, exhibited impaired vasodilation under control conditions that was restored following antioxidant administration. Following 6-weeks of single-leg knee extension training, old subjects demonstrated a training-induced improvement in vasodilation and in these subjects the administration of an oral antioxidant significantly blunted exercise-induced vasodilation. Data were collected in 8 young (26 ± 2 yr) and 8 older (71 ± 6 yr) healthy subjects. The oral antioxidant consisted of “over the counter” Vitamins C, E, and α-lipoic acid administered in 2 doses separated by 30 min, with the first dose ingested 2 hours before the graded handgrip exercise protocol. The first dose consisted of 300 mg of α-lipoic acid, 500 mg of Vitamin C, and 200 IU of Vitamin E, whereas the second included 300 mg of α-lipoic acid, 500 mg of Vitamin C, and 400 IU of Vitamin E. Modified from [69].
H2O2 and ONOO−, a non-radical species and a RNS, respectively, are two reactive species that have been identified as vasodilatory under certain conditions [43]. Following the generation of O2−, this highly reactive molecule can react directly with NO to form ONOO− or combine with O2− dismutase (SOD) resulting in molecular oxygen and H2O2 [74]. The rate of reaction for O2− with NO is two-fold greater than O2− with SOD, thus creating a biochemical competition between SOD and NO [75]. While the damaging potential of ONOO− outweighs that of H2O2, both possess vasodilatory properties. Depending on the chemical environment, these free radicals can play a causative role in oxidative stress and associated damage, or, conversely, contribute to vasodilation. For instance, peroxy-based radicals induced a dose-dependent decrease in blood pressure in healthy rodents that was abolished following antioxidant treatment [76], but have also been linked to lipid peroxidation, platelet aggregation, mitochondrial DNA damage, and endothelial dysfunction [77]. Furthermore, under physiological conditions, H2O2 acts as a vasodilator [78] and regulator of eNOS levels during exercise training; however, under pathophysiological conditions with high oxidative stress, H2O2 evokes a contractile or vasoconstrictor response in the vascular smooth muscle [79].
Contrary to the pro-oxidant vasodilatory role of free radicals observed in healthy individuals, under conditions of underlying oxidative stress that typically accompany aging and disease, free radicals contribute to impaired vascular function, thus perpetuating sustained peripheral vasoconstriction. As previously discussed, exercise is associated with elevated free radical production. Importantly, when exercise is performed with a background of oxidative stress or when the exercise-induced increase in free radicals surpasses the systems natural antioxidant defense mechanisms, vasodilatory capacity may be impaired. In these circumstances, oxidative stress can enhance endothelin-1 activity [80, 81], potentiate angiotensin II-mediated vasoconstriction [82], augment sympathetically mediated vascular tone [83], suppress cyclooxygenase-2 mediated vasodilation [84], and attenuate NO bioavailability [85, 86].
Clearly, free radicals have the potential to impact peripheral resistance, and therefore blood flow, as both vasoconstrictor and vasodilator pathways are altered by these highly reactive molecules. In healthy older men, the aforementioned oral antioxidant cocktail reduced free radicals and concomitantly restored brachial artery vasodilation during progressive handgrip exercise and following ischemic cuff occlusion [69, 72]. Interestingly, following a 6-week exercise training regimen, the free radical-induced improvements in endothelial function were ameliorated, suggesting an upregulation of endogenous antioxidant defenses that were capable of improving the redox balance in these individuals. Improved endothelial dependent dilation following infusion or oral administration of high doses of Vitamin C alone or combined with other antioxidants have been reported in older individuals [87, 88] and patients with hypertension [89], COPD [90], CAD [91], and diabetes [92]. These improvements in endothelial function with exogenous antioxidants likely occur as a result of quenching free radicals, thereby minimizing their reaction with NO or by improving NOS coupling through tetrahydrobiopterin (BH4), a critical co-factor required for the chemical stabilization of NOS. Indeed, BH4 alone has been reported to improve endothelial function [93].
Based on the aforementioned studies it is clear that, under certain conditions, reducing oxidative stress with exogenous antioxidants improves redox balance and vascular function. However, large scale interventional studies often fail to report improvements in health related outcome measures following oral antioxidant consumption [94]. One reason for this dichotomy between well-controlled mechanistic studies and large interventional investigations may be rooted in the inability of large scale studies to adequately target the individually quite variable levels of oxidative stress with non-specific oral antioxidants. An alternative and relatively new approach to combat oxidative stress is to directly target mitochondria-derived ROS production [95–97]. Based on their chemical structure, mitochondria targeted antioxidants directly attenuate free radicals produced by mitochondria. Several animal studies report complete restoration of endothelial function following treatment with mitochondrial targeted antioxidants [58, 59]. While early findings are promising, additional research is required to determine if these mitochondria specific antioxidants exhibit any impact on measures of exercise-induced vascular function. Additionally, under conditions of minimal background oxidative stress, where endogenous antioxidants are capable of remediating the deleterious effect of free radicals, the impact of these mitochondria targeted antioxidants may prove detrimental.
In summary, free radicals are important regulators of vascular tone and under normal, healthy, conditions with low oxidative stress and redox balance and contribute to vasodilation during exercise and following tissue ischemia. However, when the redox balance is shifted in favor of oxidative stress, which typically occurs in aging and disease, free radicals are associated with impaired vascular function. Interestingly, likely due to augmented endogenous antioxidants, exercise training corrects derangements in vascular function caused by augmented oxidative stress. Moreover, when exogenous antioxidants are administered post aerobic exercise training, the exercise-induced improvements in vascular function, as measured by conduit artery vasodilation, are further negated, supporting a vasodilatory role of free radicals in conditions of low oxidative stress.
Free Radical Regulation of Skeletal Muscle Blood Flow
At a given blood pressure, blood flow to the active vascular bed is inversely related to the tone of the resistance vessels (arterioles) in the arterial tree, as described by Ohm’s law (Flow = Pressure /Resistance). As discussed in the previous section, it should be note that the resistance vessels of the microvasculature and not the larger conduit vessels dictate blood flow. According to Ohm’s law blood pressure is clearly a critical determinant of blood flow. However, blood pressure is dictated by relative exercise intensity [98]. Thus, when comparing a trained athlete and an untrained individual at the same relative exercise intensity, the higher blood flow of the well-trained athlete is primarily regulated by reductions in vascular resistance [98]. Moreover, in accordance with Poiseuille’s Law, changes in vessel diameter are magnified to the fourth power. Indeed, the ability of the resistance vasculature to vasodilate in response to the release of autocrine and paracrine factors (e.g. NO, prostaglandins, EDHF, and ATP) plays a critical role in regulating skeletal muscle blood flow, especially during exercise when sympathetic nervous system activity is augmented [99–101]. This section focuses on the role free radicals play in the regulation of skeletal muscle blood flow by the regulation of resistance vessel tone.
Mechanistic investigations examining the regulation of blood flow by free radicals have, generally, been constrained to small muscle mass exercise paradigms, typically handgrip, knee extension, or plantar flexion exercise. Unlike whole-body dynamic exercise, such as running and cycling, skeletal muscle perfusion during these small muscle mass exercises is not limited by central hemodynamics [20, 21]. Thus, high levels of skeletal muscle perfusion can be attained and a reductionist approach adopted to elucidate mechanisms contributing to the regulation of skeletal muscle blood flow. In young healthy individuals, the impact of free radicals on blood flow regulation during handgrip exercise appears to be minimal to non-existent. Several investigations by our group and others observed no change in forearm blood flow during dynamic handgrip exercise in healthy young adults following the inhibition of free radical accumulation/production with oral antioxidant administration [69, 70]. However, during dynamic handgrip exercise in hyperoxic conditions (100% oxygen) those individuals that exhibited the greatest hyperoxia-induced attenuation in forearm blood flow experienced a restoration of blood flow following Vitamin C infusion [102]. Additionally, following sustained isometric handgrip exercise, Vitamin C augmented post contraction hyperemia by 50% [103]. Of note, unlike the majority of the studies that report no impact of ROS on blood flow regulation in healthy young individuals, both of these studies induced a condition of elevated ROS production (hyperoxia and ischemia) that was subsequently ameliorated with Vitamin C. Compared to arm exercise, typically handgrip, which has been relatively well-studied, there is a paucity of information regarding free radical regulation of blood flow during leg exercise in healthy young individuals.
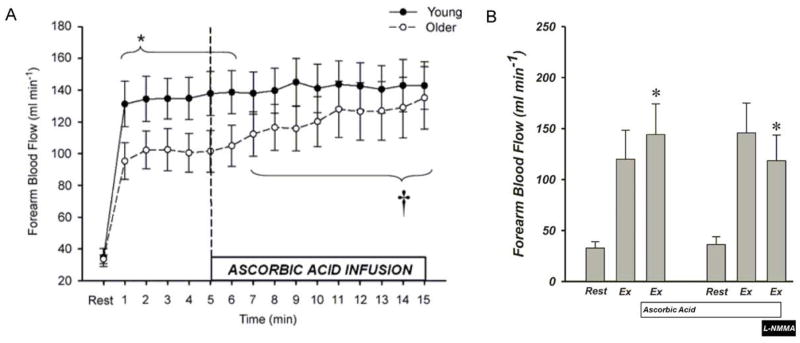
While the impact of free radicals on skeletal muscle blood flow appears to be minimal in healthy young individuals under conditions of normal redox balance, free radicals seem to be important in the control of hyperemia during dynamic small muscle mass exercise in populations exhibiting underlying evidence of oxidative stress. Specifically, during steady-state dynamic handgrip exercise in older individuals, Vitamin C infusion restored blood flow through an NO-dependent mechanism (Figure 4) [104, 105]. In a recent follow-up study, it was documented that the oral administration of Vitamin C similarly restored forearm blood flow in older individuals through local vasodilatory pathways and not a global sympatholytic effect of Vitamin C [106]. Interestingly, oxygen consumption was also improved following Vitamin C supplementation. Taken together, these findings provide convincing evidence that attenuating the level of free radicals augments blood flow in older individuals, a population associated with oxidative stress. The concomitant increases in blood flow and oxygen uptake may translate to improved exercise performance, ultimately leading to increased physical activity and improved peripheral vascular function.
Figure 4. The Ascorbic acid-induced improvement in forearm blood flow during handgrip exercise in older subjects.
(A) Young (n = 14, 22 ± 1 yr) and old (n = 14, 65 ± 2 yr) subjects performed rhythmic dynamic handgrip exercise at 10% of MVC (20 contractions per min). Older subjects exhibited attenuated forearm blood flow during the first 6 min of handgrip exercise (* P = 0.06 – 0.09 for minutes 1–6). Ascorbic acid infusion commenced at min 5 of handgrip exercise. Forearm blood flow gradually increased in the old, but not the young subjects, during ascorbic acid infusion († P < 0.05 vs. steady-state exercise within older group for minutes 7–15). Modified from Kirby et al. 2009. (B) A follow-up study, performed by the same group, utilized the same handgrip paradigm with the addition of intra-arterial L-NMMA (NG-monomethyl-L-arginine) infusion to inhibit endothelial nitric oxide synthase activity in the old (n = 14, 64 ± 3 yr) subjects. Inhibition of endothelial nitric oxide synthase abolished the ascorbic acid-induced improvement in forearm blood flow. * P < 0.05 vs. within-trial steady-state exercise condition. Modified from [73].
In contrast to the aforementioned studies in the forearm, we recently reported no impact of oral antioxidants on leg blood flow or leg vascular conductance during isolated knee extension exercise in healthy older adults [107]. However, in age-matched patients with chronic obstructive pulmonary disease (COPD), the oral antioxidants did improve leg blood flow, leg vascular conductance, and leg oxygen consumption [107]. Interestingly, COPD is associated with elevated oxidative stress, above and beyond that reported for normal healthy aging, as evidenced by attenuated antioxidant capacity and elevated markers of inflammation, which were selectively improved by the antioxidants only in the patients with COPD. This suggests that the healthy age-matched controls may not have been exposed to chronic elevations in oxidative stress. In a separate series of studies conducted under basal conditions, Vitamin C infusion improved leg blood flow in older men and postmenopausal women [108, 109]. These changes in leg blood flow were correlated with oxidized low density lipoprotein, a marker of oxidative stress, providing further support for the concept that an elevated level of background oxidative stress may be required to observe improved skeletal muscle blood flow following antioxidant administration.
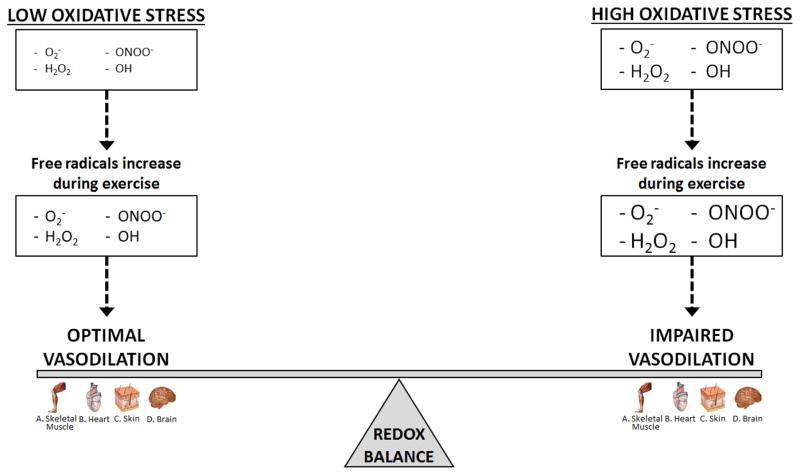
Overall, the contribution of free radicals to the regulation of skeletal muscle blood flow to the arms, and, although less well studied, to some extent the legs, appears to be minimal in healthy individuals under normal conditions. However, due to the paucity of research examining the role of free radicals in the regulation of leg blood flow in young healthy individuals, additional investigations are required to determine if free radicals critically contribute to the hyperemic and vasodilatory response of the leg in this population. In contrast, under conditions of elevated oxidative stress including aging and disease, free radicals are important in the control of blood flow during exercise in both the arms and the legs (Figure 5), as exogenous administration of antioxidants lessens free radical levels and restores skeletal muscle blood flow. Unfortunately, due to limited methodological approaches, a quantitative assessment of the necessary fall in free radicals to negatively impact cellular signaling in conditions of elevated oxidative stress is unavailable in the current literature.
Figure 5. A schematic of the critical link between redox balance by the exercise-induced increase in free radicals that likely plays a role in the regulation of blood flow to active skeletal muscle, heart, brain, and skin.
Under conditions of high oxidative stress and redox imbalance free radicals generated during exercise impair vasodilation and contribute to attenuated blood flow in the active skeletal muscle, heart, brain, and skin. Under conditions of low oxidative stress and optimal redox balance free radicals generated during exercise promote vasodilation and contribute to augmented blood flow in the active skeletal muscle, heart, brain, and skin. Optimal vasodilation is defined as the precise matching of oxygen delivery and oxygen demand coupled with the appropriate pressor response to adequately perfuse the active tissue.
Free Radical Regulation of Coronary Blood Flow
During exercise, the increased demand for nutrient and oxygen rich blood throughout the body is fundamentally achieved by the augmented work of the heart, resulting in a 5 to 6 fold increase in cardiac output and myocardial metabolic demand during maximal aerobic exercise [98]. The myocardium relies almost exclusively on the aerobic oxidation of substrates to meet the energetic demands of contraction [110], which increases due to the rise in heart rate and stroke volume with exercise intensity [111]. Therefore, the regulation of blood flow through the coronary vasculature to the myocardium determines not only the performance of the heart, but also the entire body, as inadequate myocardial blood flow will detrimentally impact the ability of the heart to reach maximal pumping capacity which may occur with cardiovascular disease. Our understanding of how free radicals contribute to the regulation of coronary blood flow during exercise is severely limited. The lack of information on this topic stems from the difficulty in attaining such measures during exercise, especially in healthy humans. However, a recent investigation by Hays et al. [112] reported that coronary vasodilation during ischemic handgrip exercise in healthy humans is primarily NO-mediated (Figure 6). Although not directly examined in this study, factors that attenuate NO-bioavailability and alter redox balance, including free radicals, would be expected to impair coronary vasodilation, mimicking the attenuated vasodilation exhibited by patients with coronary artery disease during ischemic handgrip exercise. Despite this recent finding, there is still a lack of information regarding the regulation of the coronary vasculature during dynamic exercise in healthy populations. However, both in vivo and in vitro investigations in clinically relevant conditions provide evidence that free radicals are important regulators of coronary blood flow and vascular tone.
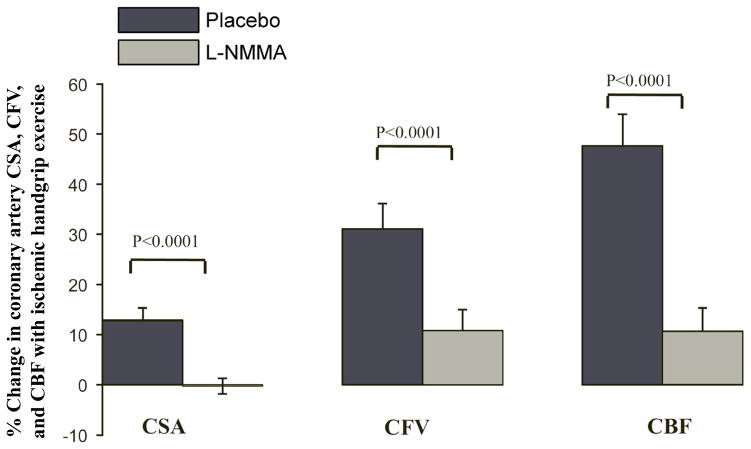
Figure 6. Contribution of nitric oxide (NO) to coronary artery cross-sectional area (CSA), flow velocity (CFV), and blood flow (CBF) during ischemic handgrip exercise in healthy adults.
Healthy coronary arteries respond to endothelial-dependent stressors with an increase in CSA, CFV, and CBF. Intravenous infusion of L-NMMA significantly blunted these responses indicating a significant NO contribution (n = 10, 31 ± 9 yr). Modified from [142].
Coronary blood flow and vascular tone can be assessed during cardiac catheterization using routine angiographic techniques or with sophisticated imaging methods. In individuals reporting atypical chest pain but no evidence of coronary artery disease, intracoronary infusion of reduced glutathione improved coronary endothelial function by suppressing acetylcholine (ACh)-induced vasoconstriction [113]. In a similar study, intracoronary Vitamin C infusion abolished ACh-induced vasoconstriction in spastic, but not control, coronary arteries [114]. Additionally, in smokers, Vitamin C administered either orally or by intracoronary infusion restored coronary microcirculatory function and coronary flow reserve, respectively [115, 116]. Finally, Vitamin C effectively abolished the reduction in coronary blood flow induced by elevated free radical production during hyperoxia (100% oxygen) in patients with ischemic heart disease [117]. Although there is a dearth of information regarding normal healthy hearts under normal redox balance conditions, taken together, the available in vivo assessments reveal an integral role of free radicals in the regulation of the coronary vasculature and the decrements in coronary endothelial function and coronary blood flow that accompany oxidative stress and disease.
Interestingly, in vitro several studies utilizing isolated human coronary resistance vessels report an important vasodilatory role of H2O2 in the microvasculature of the myocardium [118, 119]. Specifically, H2O2, derived from vascular endothelial mitochondrial respiration, evoked significant flow induced dilation in isolated coronary arteries [118]. Treatment of these arterioles with catalase to minimize H2O2 resulted in attenuated flow induced dilation. One caveat, however, of these studies [118, 119], is that the coronary vessels were obtained from patients with coronary artery disease; therefore, these findings may not be generalizable to the normal or healthy regulation of coronary function, as oxidative stress was likely elevated in these individuals.
In summary, both in vivo and in vitro investigations provide evidence of a regulatory role of free radicals in the coronary vasculature (Figure 5). Lowering free radical levels with oral or infused antioxidants appears to improve in vivo function of the coronary vessels when oxidative stress is present. A vasodilatory role of H2O2 has also been reported in isolated arteries obtained from patients with coronary artery disease; however, the role of free radicals in regulation of healthy human coronary vessels function is largely unknown and requires future research.
Free Radical Regulation of Skin Blood Flow
The vasodilatory capacity of the cutaneous circulation is second only to skeletal muscle. During whole body passive heating, it has been estimated that blood flow to the skin may reach between 7 and 8 L/min, a substantial portion of the total cardiac output [120]. During exercise, blood flow to the skin is increased in order to dissipate heat by sweating, as core temperature rises. During exercise in the heat, when core temperature is increased and the temperature gradient between the skin and core falls, the demand for skin blood flow places a tremendous burden on the cardiovascular system, which can significantly impact exercise performance [121].
The regulation of skin blood flow is unique as it is reflexively controlled by two branches of the sympathetic nervous system [122]. During the initial rise in core temperature, the onset of cutaneous vasodilation is mediated by withdrawal of adrenergic vasoconstriction and is further augmented by active vasodilation regulated by the sympathetic co-transmission of ACh and an unknown neurotransmitter [123, 124]. One benefit of the cutaneous circulation, from a research perspective, is that it is easily accessible, thus allowing for the simultaneous interrogation of multiple experimental sites. Combining pharmacological interventions with microdialysis and laser Doppler flowmetry, investigators have been able to perform well-controlled and mechanistic studies aimed at identifying the factors regulating skin blood flow.
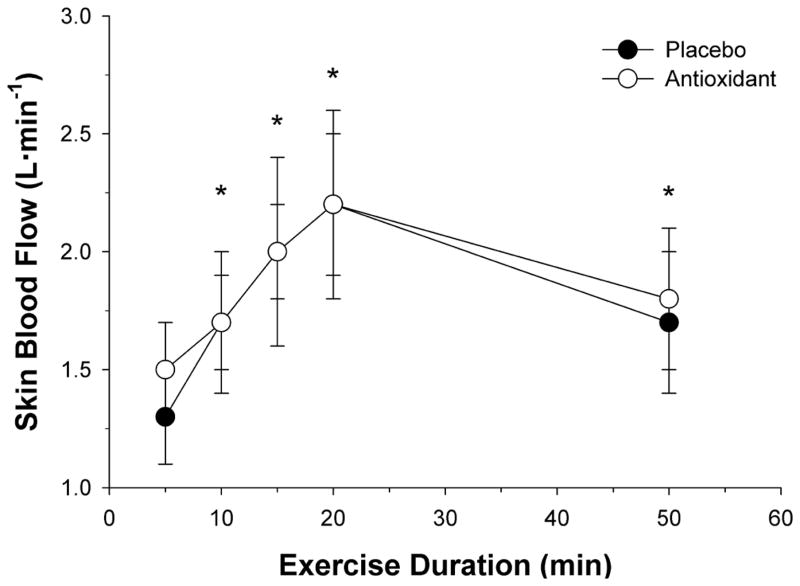
Numerous vasodilator pathways are involved in the regulation of skin blood flow [readers are referred to several excellent reviews regarding this topic [33, 125, 126]]. Importantly, in young healthy individuals, up to 40% of the total vasodilatory response appears to be NO-dependent [127, 128]. Therefore, factors that attenuate NO bioavailability, including free radicals, are expected to negatively impact cutaneous vasodilation, and vice versa, factors that increase NO bioavailability are expected to increase skin blood flow. Unfortunately, the number of studies that have systematically investigated the role of free radicals during exercise is surprisingly low; therefore, much of our current understanding is based on whole body and local heating protocols, with only a few exceptions. Although not a direct assessment of free radicals on skin blood flow, exercise trained athletes, characterized by an elevated maximal oxygen consumption, exhibited increased antioxidant capacity as well as superior cutaneous microvascular function compared to sedentary individuals, as measured by stimulated peripheral skin blood flow [129]. While the capacity for augmented cutaneous vasodilation is present in well-trained athletes with elevated endogenous antioxidant levels, further increasing antioxidant capacity via exogenous supplementation may not result in additional improvements in skin blood flow during exercise. Indeed, following 7 days of polyphenol antioxidant supplementation, well-trained endurance athletes did not exhibit improved skin blood flow, thermoregulatory capacity, or exercise performance during prolonged exercise in the heat (Figure 7) [130]. Based on these findings, exercise training-induced increases in endogenous antioxidant capacity and associated increases in cutaneous microvascular function yield improved skin blood flow that is not further augmented with exogenous antioxidants. Furthermore, attenuating the normal increase in free radicals, specifically H2O2, may actually have a negative impact on skin blood flow. Indeed, during local heating, inhibition of H2O2 production, reduced a portion of the vasodilatory response, suggesting that free radicals, may in part, participate in the normal skin blood flow response [131, 132].
Figure 7. The impact of polyphenol antioxidant supplementation on estimated skin blood flow (SkBF) during prolonged cycling exercise in the heat.
Twelve healthy well-trained male cyclists (27± 5yr) cycled in the heat (31.5°C, 55% relative humidity) following 7-days of placebo or polyphenol antioxidant supplementation. Exercise intensity started at 40% of VO2max and increased by 10% of VO2max every 5 min until min 20. At min 20, exercise intensity was adjusted to 5% above lactate threshold and maintained until min 50. Polyphenol antioxidant supplementation had no effect of SkBF during exercise in the heat. * P < 0.05 compared to previous value. Modified from [160].
The capacity for cutaneous vasodilation in aged and diseased individuals is markedly lower than healthy age-matched controls, and several studies indicate that elevated oxidative stress is an important factor in this attenuated response. Indeed, impaired cutaneous microvascular function leading to attenuated vasodilation has been linked to attenuated NO bioavailability associated with elevated ROS, as decreasing O2− through the local administration of tempol, a SOD mimetic, restored cutaneous vasodilation in young smokers and patients with chronic kidney disease [133]. Moreover, inhibition of NADPH oxidase (apocynin) restored cutaneous vasodilation in the chronic kidney disease patients, suggesting an important role of this free radical producing enzymatic complex in the production of O2− [134]. Additionally, acute Vitamin C supplementation, alone or in combination with arginase, improved reflex cutaneous vasodilation during whole body heating in both older [135] and hypertensive [136] individuals. The mechanisms underlying the Vitamin C induced improvements in cutaneous vasodilation may be due to a direct effect of quenching ROS or by improving eNOS coupling through the chemical stabilization of BH4, an essential cofactor for eNOS. Importantly, BH4 independently improves cutaneous vasodilation through an NO-dependent mechanism in older subjects [137] and those with hypercholesterolemia [138]. Therefore, elevations in NO bioavailability, accomplished through direct or indirect means, result in enhanced cutaneous vasodilation in conditions of elevated oxidative stress.
Despite its unique regulation, the cutaneous circulation appears to mimic other vascular beds in regards to the regulation of blood flow by role of free radicals (Figure 5). Specifically, under normal healthy conditions, attempts to augment skin blood flow with exogenous antioxidants have been unsuccessful and a vasodilatory role of free radicals, specifically H2O2, has been reported. However, under conditions of known oxidative stress including aging, kidney disease, hypertension, and smoking, antioxidant mediated-improvements in NO bioavailability augmented skin blood flow during whole body and local heating. The role of free radicals in the regulation of skin blood flow during exercise is currently unknown and certainly worthy of future investigation.
Free Radical Regulation of Cerebral Blood flow
Traditionally, cerebral blood flow was thought to remain relatively constant across a range of blood pressures (60 to 150 mmHg) and be unaffected by exercise [35, 139–141]. However, this concept has been challenged as exercise-induced increases in cerebral blood flow during moderate intensity (< 60% of maximal oxygen consumption) exercise have been reported [142–147]. During higher intensity exercise (> 60% of maximal oxygen consumption), cerebral blood flow returns towards baseline as exercise-induced hyperventilation reduces the partial pressure of CO2 in the arterial blood, resulting in cerebral vasoconstriction and indicating a potential mismatch between neuronal metabolism and activity and cerebral blood flow [148–150]. Methodological limitations in conjunction with technological advances in cerebral imaging likely contribute to the equivocal findings regarding the regulation of cerebral blood flow during exercise. However, despite these limitations, clear and fundamental differences exist in regards to cerebral and skeletal muscle hyperemia during exercise, with the latter exhibiting a linear increase in blood flow with exercise intensity in order match to perfusion and oxygen demand. Although a complete discussion of the factors regulating cerebral blood flow is beyond the scope of this review and the reader is referred to several excellent reviews for additional information [35, 151, 152]. This section of this review will highlight the existing knowledge regarding the contribution of free radicals to cerebral blood flow regulation during exercise. Additionally, insight collected from non-exercise related investigations will be presented to advance our understanding of the regulation of the cerebral vasculature by free radicals.
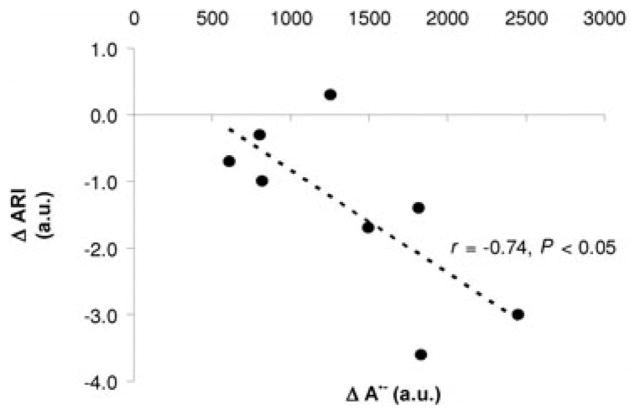
Similar to skeletal muscle, augmented free radical outflow from the cerebral vasculature has been directly documented using EPR spectroscopy. Following prolonged (9-hour) exposure to hypoxia, elevated free radical outflow was observed, as measured in blood obtained from the internal jugular vein [153]. In a separate study, intense exercise, evoking a systemic increase in oxidative-nitrosative stress, was associated with impaired cerebral autoregulation (Figure 8) [154]. Dynamic cerebral autoregulation refers to the inherent ability of cerebral blood vessels to maintain blood flow in response to rapid changes in arterial blood pressure via rapid counter-regulatory changes in the vascular resistance of cerebral arteries [155, 156]. Taken together, these studies, performed in healthy young physically active individuals, provide evidence that the free radical output across the cerebral vasculature is increased during exercise and that free radicals may, therefore, contribute to the regulation of cerebral blood flow.
Figure 8. Exercise-induced changes in ascorbate radical (A•−) production and associated changes in cerebral autoregulation index (ARD).
Eight physically active healthy men (35±7 yrs) exercised (semi-recumbent cycling) to exhaustion. Changes from rest to exhaustion for A•− were negatively correlated with ARI, suggesting a link between exercise-induced increases in free radicals and impaired dynamic cerebral autoregulation. Modified from [184].
Although not specific to exercise, free radicals, at low concentrations, appear to have a vasodilatory role in the cerebral vasculature and act as important mediators and modulators of cell signaling [157]. Specifically, at low concentrations, O2−, H2O2, and ONOO− have vasodilatory properties [158–164], however, at high concentrations, these free radicals contribute to the oxidative stress that underlies cerebral vascular disease and injury [158, 165, 166].
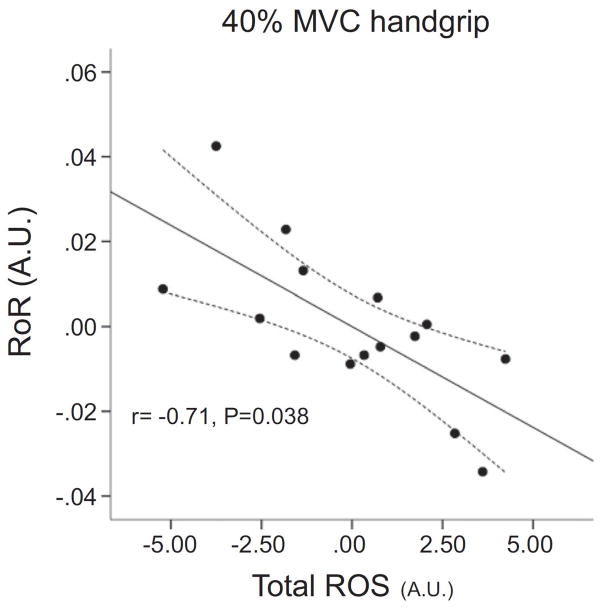
Under conditions of redox imbalance supporting evidence for an inhibitory role of free radicals in the regulation of cerebral blood flow has been obtained from the assessment of cerebral autoregulation in humans and the direct evaluation of cerebral artery function in animal models of cerebrovascular dysfunction. During handgrip exercise, Type 2 Diabetics experienced impaired dynamic cerebral autoregulation that was correlated to markers of oxidative stress (Figure 9) [167]. Interestingly, this impaired cerebral autoregulation was only present during exercise and may confer an elevated risk of an adverse cerebral event in these patients, especially when coupled with exaggerated sympathetically mediated vasoconstriction and impaired vasodilator capacity during exercise. In young African Americans, a population characterized by increased incidence of cardiovascular disease, acute flavonal antioxidant consumption augmented the cerebral vasodilatory response following a hypercapnic stimulus [168]. The mechanisms contributing to this improved response were not directly assessed. However, the authors speculated that a reduction in NADPH oxidase activity leading to lower O2− production may have contributed to the improvement. Utilizing obese Zucker rats, cerebrovascular dysfunction has been linked to oxidative stress, as pretreatment with SOD restored all dilator responses assessed in the basilar artery [169]. Additionally, reducing angiotensin-II mediated oxidative stress in older mice with treatment of angiotensin-converting enzyme 2 (ACE2) restored vasodilatory capacity [170].
Figure 9. Partial correlation plot in patients with Type Two Diabetes with and without hypertension demonstrating an inverse relationship between total reactive oxygen species (ROS) and rate of cerebral autoregulation (RoR) during 40% MVC handgrip exercise.
Partial correlation was used to account for the potential confounding influence of lipids, body mass index, age, duration of the disease, fasting glucose, and HbA1c. Modified from [197].
In summary, exercise is associated with elevated free radical outflow from the cerebral vasculature. The direct impact of this exercise-induced increase in free radicals on cerebral blood flow regulation is largely unknown, but has been linked to impaired cerebral autoregulation following exhaustive exercise. In contrast, under basal conditions, low levels of free radicals are associated with vasodilation; however, under conditions of oxidative stress, free radicals evoke cerebrovascular dysfunction as measured by impaired cerebral autoregulation in humans and cerebral vascular dysfunction in animals that can be restored with antioxidant interventions (Figure 5).
Summary
Adequate delivery of blood flow to the active skeletal muscle, heart, skin, and brain is of critical importance during exercise, as minor alterations in oxygen and nutrient delivery can accelerate the development of fatigue and impair exercise performance. Importantly, free radicals possess vasoactive properties that contribute to the regulation of blood flow to these organs. The magnitude of this regulatory potential is highly dependent on the underlying redox state. In normal, healthy, conditions of low oxidative stress, when endogenous antioxidant defenses are capable of appropriately combating the exercise-induced increase in free radicals thus avoiding excessive oxidation and maintaining redox balance, free radicals promote optimal vasodilation and hyperemia. Interestingly, efforts to further attenuate free radical production or accumulation with exogenous antioxidants in normal, healthy conditions shifts the redox balance to a reduced state and negatively impacts vasodilatory capacity. Importantly, in contrast, when the redox balance is disturbed in favor of oxidative stress, as typically occurs with aging and disease, the exercise-induced elevation in free radicals impairs blood flow and vasodilatory capacity. Therefore, attempts to mitigate the negative impact of free radicals in older and diseased individuals through antioxidants typically improve vasodilation and blood flow during exercise. Figure 10 depicts our working hypothesis regarding the impact of alterations in redox balance and free radical regulation of blood flow during exercise. Based on the accumulated evidence, this working hypothesis appears to be remarkably preserved across skeletal muscle, heart, skin and brain (Figure 5). Overall, free radicals have important hemodynamic regulatory potential that extends to several vascular beds during exercise and, importantly, it is the underlying redox state that appears to dictate the vascular impact of these highly reactive molecules.
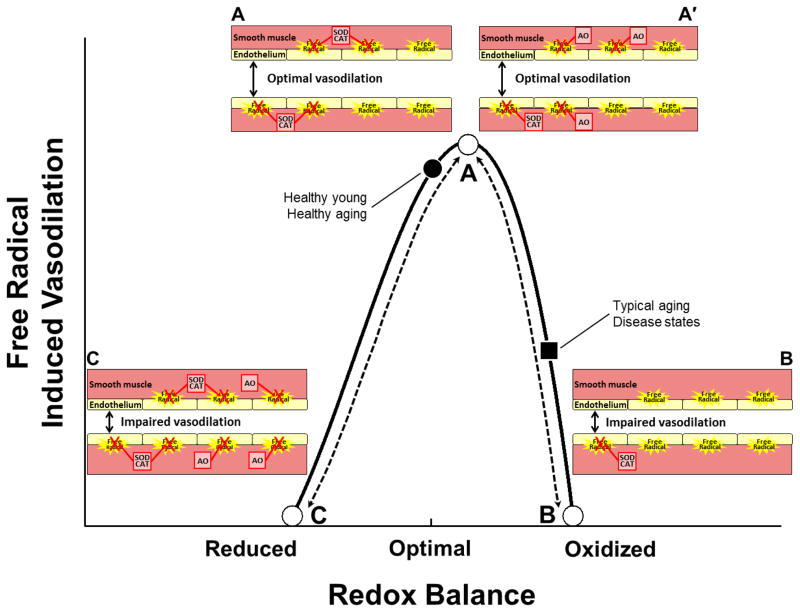
Figure 10. Working hypothesis: Impact of alterations in redox balance on free radical-induced vasodilation.
The impact of exercise-induced increases in free radicals on vasodilation is dependent upon the underlying redox status. In young and healthy aging conditions (●) free radicals produced during exercise contribute to optimal vasodilation (A). Under such conditions endogenous antioxidant enzymatic systems (including, but not limited to CAT [catalase] and SOD [superoxide dismutase]) are able to appropriately quench free radical production, thus avoiding excessive oxidation. In normal aging and disease states (■) the underlying redox balance is shifted towards a pro-oxidized state and free radicals produced during exercise contribute to impaired vasodilation (B). Interestingly, in normal aging and disease states (■) augmenting exogenous antioxidants shifts the redox balance and restores optimal vasodilation (A′). In contrast, augmenting exogenous antioxidants in young individuals creates a reduced redox state leading to impaired vasodilation (C) as the normal vasodilatory role of the free radicals produced during exercise is blocked. A similar hypothesis has been proposed for the link between redox balance and muscle force production [171].
Highlights.
Free radicals regulate skeletal, coronary, skin, and cerebral blood flow
Exercise-induced increases in free radicals can promote or impair blood flow
Underlying redox balance determines the vasoactive response of free radicals
Evidence linking free radicals, redox balance, and blood flow is reviewed
Acknowledgments
Funding: This work was supported by the National Institutes of Health (PO1 HL-091830, R01 HL118313), the United States Veterans Administration (RR&D E6910-R, E1433-P, E1697-R, E9275-L), and the American Heart Association (0835209N, 1850039).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the Regulation of Human Skeletal Muscle Blood Flow and Oxygen Delivery: Role of Circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 5.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? The Journal of Physiology. 2007;583:855–860. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol. 2005;98:1251–1257. doi: 10.1152/japplphysiol.00966.2004. [DOI] [PubMed] [Google Scholar]
- 8.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of Neuronal Nitric Oxide Synthase on Human Coronary Artery Diameter and Blood Flow In Vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 10.Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal Nitric Oxide Synthase Regulates Basal Microvascular Tone in Humans In Vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- 11.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. The Journal of Physiology. 2013;591:2885–2896. doi: 10.1113/jphysiol.2013.251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. Journal of Applied Physiology. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-Mediated Vasoconstriction During Exercise With Advancing Age. J Gerontol A Biol Sci Med Sci. 2014:glu065. doi: 10.1093/gerona/glu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired Skeletal Muscle Blood Flow Control With Advancing Age in Humans: Attenuated ATP Release and Local Vasodilation During Erythrocyte Deoxygenation. Circ Res. 2012;111:220–230. doi: 10.1161/CIRCRESAHA.112.269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590:1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinity JD, Wray DW, Witman MAH, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regulatory Integrative Comp Physiol. 2013;305:R893–899. doi: 10.1152/ajpregu.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wray DW, Witman MAH, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol. 2011;300:H1101–1107. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011;301:H1302–1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyle EF. Physiological determinants of endurance exercise performance. Journal of Science and Medicine in Sport. 1999;2:181–189. doi: 10.1016/s1440-2440(99)80172-8. [DOI] [PubMed] [Google Scholar]
- 20.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- 22.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- 23.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. The Journal of Physiology. 2006;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams RP, Welch HG. Oxygen uptake, acid-base status, and performance with varied inspired oxygen fractions. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:863–8. doi: 10.1152/jappl.1980.49.5.863. [DOI] [PubMed] [Google Scholar]
- 25.Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- 26.Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- 27.Koskolou MD, McKenzie DC. Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol Occup Physiol. 1994;68:80–6. doi: 10.1007/BF00599246. [DOI] [PubMed] [Google Scholar]
- 28.Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol (1985) 1999;86:1048–53. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 29.Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- 30.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radical Biology and Medicine. 2004;36:707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab. 2008;33:151–61. doi: 10.1139/H07-148. [DOI] [PubMed] [Google Scholar]
- 32.Crecelius AR, Kirby BS, Dinenno FA. Intravascular ATP and the Regulation of Blood Flow and Oxygen Delivery in Humans. Exercise and Sport Sciences Reviews. 2015;43:5–13. doi: 10.1249/JES.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 33.Kenney WL, Johnson JM. Control of skin blood flow during exercise. Medicine and Science in Sports and Exercise. 1992;24:303–312. [PubMed] [Google Scholar]
- 34.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Comprehensive Physiology. John Wiley & Sons, Inc; 2010. Control of Blood Flow to Cardiac and Skeletal Muscle During Exercise. [Google Scholar]
- 35.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. 2009;107:1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 36.Segal SS. Regulation of Blood Flow in the Microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Aboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow. III. American Physiological Society; Bethesda, MD, USA: 1983. [Google Scholar]
- 38.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 39.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 40.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Experimental and Molecular Medicine. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 41.Finkel T. Oxygen radicals and signaling. Current Opinion in Cell Biology. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 42.Sachdev S, Davies KJA. Production, detection, and adaptive responses to free radicals in exercise. Free Radical Biology and Medicine. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Taniyama Y, Griendling KK. Reactive Oxygen Species in the Vasculature: Molecular and Cellular Mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 44.Powers SK, Jackson MJ. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Experimental Physiology. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 47.Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol. 2003;94:1714–1718. doi: 10.1152/japplphysiol.01024.2002. [DOI] [PubMed] [Google Scholar]
- 48.Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol. 2004;287:H1689–1699. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 49.Loschen G, Azzi A, Richter C, Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Letters. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 50.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barja G. Mitochondrial Oxygen Radical Generation and Leak: Sites of Production in States 4 and 3, Organ Specificity, and Relation to Aging and Longevity. Journal of Bioenergetics and Biomembranes. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 52.Muller FL, Liu Y, Van Remmen H. Complex III Releases Superoxide to Both Sides of the Inner Mitochondrial Membrane. Journal of Biological Chemistry. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 53.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 54.Kanter MM. Free radicals, exercise, and antioxidant supplementation. International journal of sport nutrition. 1994;4:205–220. doi: 10.1123/ijsn.4.3.205. [DOI] [PubMed] [Google Scholar]
- 55.Brand M, Orr A, Perevoshchikova I, Quinlan C. The role of mitochondrial function and cellular bioenergetics in ageing and disease. British Journal of Dermatology. 2013;169:1–8. doi: 10.1111/bjd.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinlan CL, Treberg JR, Perevoshchikova IV, Orr AL, Brand MD. Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Free Radical Biology and Medicine. 2012;53:1807–1817. doi: 10.1016/j.freeradbiomed.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of Superoxide Production from Different Sites in the Mitochondrial Electron Transport Chain. Journal of Biological Chemistry. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 58.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RAJ, Cochemé HM, Murphy MP, Dominiczak AF. Mitochondria-Targeted Antioxidant MitoQ10 Improves Endothelial Function and Attenuates Cardiac Hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 59.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. The Journal of Physiology. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct Evidence of Endothelial Oxidative Stress With Aging in Humans: Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-{kappa}B. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 61.Duarte JAR, Appell HJ, Carvalho F, Bastos ML, Soares JMC. Endothelium-Derived Oxidative Stress May Contribute to Exercise-Induced Muscle Damage. Int J Sports Med. 1993;14:440–443. doi: 10.1055/s-2007-1021207. [DOI] [PubMed] [Google Scholar]
- 62.Griendling KK, Sorescu D, Ushio-Fukai M. NAD (P) H oxidase role in cardiovascular biology and disease. Circulation research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 63.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329(Pt 3):653–7. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 65.Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of Free Radical Production in Exhaustive Exercise in Humans and Rats; Role of Xanthine Oxidase and Protection by Allopurinol. IUBMB Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Cabrera M-C, Domenech E, Viña J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radical Biology and Medicine. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 68.Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donato AJ, Uberoi A, Bailey DM, Walter Wray D, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298:H671–678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol. 2007;292:H1516–1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 71.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. The Journal of Physiology. 2012;590:1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci. 2009;116:433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 73.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. The Journal of Physiology. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 75.Huie RE, Padmaja S. The Reaction of no With Superoxide. Free Radical Research. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 76.Peluso I, Serafini M, Campolongo P, Palmery M. Effect on rat arterial blood pressure of chemically generated peroxyl radicals and protection by antioxidants. The Journal of Nutritional Biochemistry. 2004;15:323–327. doi: 10.1016/j.jnutbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Ballinger SW, Patterson C, Yan C-N, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen Peroxide and Peroxynitrite-Induced Mitochondrial DNA Damage and Dysfunction in Vascular Endothelial and Smooth Muscle Cells. Circulation Research. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 78.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. Journal of Hypertension. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 79.Lauer N, Suvorava T, Rüther U, Jacob R, Meyer W, Harrison DG, Kojda G. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. 2005;65:254–262. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 Vasoconstrictor Tone Increases With Age in Healthy Men But Can Be Reduced by Regular Aerobic Exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 81.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Groot AA, van Zwieten PA, Peters SLM. Involvement of Reactive Oxygen Species in Angiotensin II-Induced Vasoconstriction. Journal of Cardiovascular Pharmacology. 2004;43:154–159. doi: 10.1097/00005344-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 83.Thomas GD, Zhang W, Victor RG. Impaired Modulation of Sympathetic Vasoconstriction in Contracting Skeletal Muscle of Rats With Chronic Myocardial Infarctions: Role of Oxidative Stress. Circulation Research. 2001;88:816–823. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- 84.Gano LB, Donato AJ, Pasha HM, Hearon CM, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Lüscher TF. Reactive Oxygen Species Mediate Endothelium-Dependent Relaxations in Tetrahydrobiopterin-Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 86.Hwang J, Wang J, Morazzoni P, Hodis HN, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radical Biology and Medicine. 2003;34:1271–1282. doi: 10.1016/s0891-5849(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 87.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. The Journal of Physiology. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MAH, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute Reversal of Endothelial Dysfunction in the Elderly After Antioxidant Consumption. Hypertension. 2012;59:818–824. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-Related Reduction of NO Availability and Oxidative Stress in Humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 90.Ives SJ, Harris RA, Witman MAH, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular Dysfunction and Chronic Obstructive Pulmonary Disease: The Role of Redox Balance. Hypertension. 2014;63:459–467. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Vita JA. Ascorbic Acid Reverses Endothelial Vasomotor Dysfunction in Patients With Coronary Artery Disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 92.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 94.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14d–19d. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Murphy MP, Smith RAJ. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annual Review of Pharmacology and Toxicology. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 96.Smith RAJ, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. European Journal of Biochemistry. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 97.Szeto H. Mitochondria-targeted peptide antioxidants: Novel neuroprotective agents. The AAPS Journal. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowell LB. Human cardiovascular control. Oxford University Press; 1993. [Google Scholar]
- 99.Richardson RS. OXYGEN TRANSPORT AND UTILIZATION: AN INTEGRATION OF THE MUSCLE SYSTEMS. Advan Physiol Educ. 2003;27:183–191. doi: 10.1152/advan.00038.2003. [DOI] [PubMed] [Google Scholar]
- 100.Hearon CM, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. The Journal of Physiology. 2015 doi: 10.1113/JP270593. n/a n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional Sympatholysis During Muscular Activity: OBSERVATIONS ON INFLUENCE OF CAROTID SINUS ON OXYGEN UPTAKE. Circulation Research. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 102.Ranadive SM, Joyner MJ, Walker BG, Taylor JL, Casey DP. Effect of vitamin C on hyperoxia-induced vasoconstriction in exercising skeletal muscle. 2014;117:1207–1211. doi: 10.1152/japplphysiol.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caruana H, Marshall J. Effects of modest hyperoxia and oral vitamin C on exercise hyperaemia and reactive hyperaemia in healthy young men. European Journal of Applied Physiology. 2015:1–12. doi: 10.1007/s00421-015-3182-0. [DOI] [PubMed] [Google Scholar]
- 104.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:H1633–1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. The Journal of Physiology. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richards JC, Crecelius AR, Larson DG, Dinenno FA. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. 2015 doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossman MJ, Trinity JD, Garten RS, Ives SJ, Conklin JD, Barret-O’Keefe Z, Witman MAH, Bledsoe AD, Morgan DE, Runnels S, Reese VR, Zhao J, Amann M, Wray DW, Richardson RS. Oral Antioxidants Improve Leg Blood Flow During Exercise in Patients with Chronic Obstructive Pulmonary Disease. 2015 doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 109.Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. 2007;102:890–895. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- 110.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial Energetics and Efficiency: Current Status of the Noninvasive Approach. Circulation. 2007;115:918–927. doi: 10.1161/CIRCULATIONAHA.106.660639. [DOI] [PubMed] [Google Scholar]
- 111.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circulation Research. 1986;58:281–91. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 112.Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schär M, Gerstenblith G, Stuber M, Weiss RG. Coronary Vasomotor Responses to Isometric Handgrip Exercise are Primarily Mediated by Nitric Oxide: a Noninvasive MRI Test of Coronary Endothelial Function. American Journal of Physiology - Heart and Circulatory Physiology. 2015 doi: 10.1152/ajpheart.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kugiyama K, Ohgushi M, Motoyama T, Hirashima O, Soejima H, Misumi K, Yoshimura M, Ogawa H, Sugiyama S, Yasue H. Intracoronary Infusion of Reduced Glutathione Improves Endothelial Vasomotor Response to Acetylcholine in Human Coronary Circulation. Circulation. 1998;97:2299–2301. doi: 10.1161/01.cir.97.23.2299. [DOI] [PubMed] [Google Scholar]
- 114.Kugiyama K, Motoyama T, Hirashima O, Ohgushi M, Soejima H, Misumi K, Kawano H, Miyao Y, Yoshimura M, Ogawa H, Matsumura T, Sugiyama S, Yasue H. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. Journal of the American College of Cardiology. 1998;32:103–109. doi: 10.1016/s0735-1097(98)00185-5. [DOI] [PubMed] [Google Scholar]
- 115.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schäfers KP, Lüscher TF, Camici PG. Coronary Heart Disease in Smokers: Vitamin C Restores Coronary Microcirculatory Function. Circulation. 2000;102:1233–1238. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 116.Teramoto K, Daimon M, Hasegawa R, Toyoda T, Sekine T, Kawata T, Yoshida K, Komuro I. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. American Heart Journal. 2004;148:300–305. doi: 10.1016/j.ahj.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 117.McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. 2007;102:2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial Sources of H2O2 Generation Play a Key Role in Flow-Mediated Dilation in Human Coronary Resistance Arteries. Circulation Research. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 119.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for Hydrogen Peroxide in Flow-Induced Dilation of Human Coronary Arterioles. Circulation Research. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 120.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. 27:19–9. 673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- 121.Sawka MN, Cheuvront SN, Kenefick RW. High skin temperature and hypohydration impair aerobic performance. Experimental Physiology. 2012;97:327–332. doi: 10.1113/expphysiol.2011.061002. [DOI] [PubMed] [Google Scholar]
- 122.Grant R, Holling H. Further observations on the vascular responses of the human limb to body warming; evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci. 1938;3:273–85. [Google Scholar]
- 123.Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. The Journal of Physiology. 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kellogg DL, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous Active Vasodilation in Humans Is Mediated by Cholinergic Nerve Cotransmission. Circulation Research. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 125.Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. Journal of Applied Physiology. 2010;109:1221–1228. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. 2010;109:1538–1544. doi: 10.1152/japplphysiol.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 128.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 129.Franzoni F, Plantinga Y, Femia FR, Bartolomucci F, Gaudio C, Regoli F, Carpi A, Santoro G, Galetta F. Plasma antioxidant activity and cutaneous microvascular endothelial function in athletes and sedentary controls. Biomedicine & Pharmacotherapy. 2004;58:432–436. doi: 10.1016/j.biopha.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 130.Trinity JD, Pahnke MD, Trombold JR, Coyle EF. Impact of polyphenol antioxidants on cycling performance and cardiovascular function. Nutrients. 2014;6:1273–1292. doi: 10.3390/nu6031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brunt VE, Minson CT. Cutaneous thermal hyperemia: more than skin deep. 2011;111:5–7. doi: 10.1152/japplphysiol.00544.2011. [DOI] [PubMed] [Google Scholar]
- 132.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. 2011;111:20–26. doi: 10.1152/japplphysiol.01448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujii N, Brunt VE, Minson CT. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. 2014;306:H1507–H1511. doi: 10.1152/ajpheart.00886.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. 2014;306:F1499–F1506. doi: 10.1152/ajprenal.00058.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. 2006;291:H2965–H2970. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- 136.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. 2007;293:H1090–H1096. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 137.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. 2012;112:791–797. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. 2013;304:R164–R169. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Globus M, Melamed E, Keren A, Tzivoni D, Granot C, Lavy S, Stern S. Effect of Exercise on Cerebral Circulation. J Cereb Blood Flow Metab. 1983;3:287–290. doi: 10.1038/jcbfm.1983.43. [DOI] [PubMed] [Google Scholar]
- 140.Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiodtz G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245. [DOI] [PubMed] [Google Scholar]
- 141.Scheinberg P, Blackburn LI, Rich M, Saslaw M. Effects of vigorous physical exercise on cerebral circulation and metabolism. The American Journal of Medicine. 1954;16:549–554. doi: 10.1016/0002-9343(54)90371-x. [DOI] [PubMed] [Google Scholar]
- 142.Jorgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol. 1992;73:1825–1830. doi: 10.1152/jappl.1992.73.5.1825. [DOI] [PubMed] [Google Scholar]
- 143.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol. 1992;72:1123–1132. doi: 10.1152/jappl.1992.72.3.1123. [DOI] [PubMed] [Google Scholar]
- 144.Ogoh S, Ainslie PN, Miyamoto T. Onset responses of ventilation and cerebral blood flow to hypercapnia in humans: rest and exercise. J Appl Physiol. 2009;106:880–886. doi: 10.1152/japplphysiol.91292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. The Journal of Physiology. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- 147.Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans O, Secher NH, Raven PB. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1526–1531. doi: 10.1152/ajpheart.00979.2004. [DOI] [PubMed] [Google Scholar]
- 148.Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- 149.Moraine JJ, Lamotte M, Berré J, Niset G, Leduc A, Naeijel R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. European Journal of Applied Physiology and Occupational Physiology. 1993;67:35–38. doi: 10.1007/BF00377701. [DOI] [PubMed] [Google Scholar]
- 150.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. Journal of Clinical Investigation. 1948;27:484. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Querido J, Sheel AW. Regulation of Cerebral Blood Flow During Exercise. Sports Medicine. 2007;37:765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 152.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- 153.Bailey DM, Taudorf S, Berg RMG, Lundby C, McEneny J, Young IS, Evans KA, James PE, Shore A, Hullin DA, McCord JM, Pedersen BK, Moller K. Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness? Am J Physiol Regulatory Integrative Comp Physiol. 2009;297:R1283–1292. doi: 10.1152/ajpregu.00366.2009. [DOI] [PubMed] [Google Scholar]
- 154.Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE, Ogoh S, Ainslie PN, Lucchesi C, Rockenbauer A, Culcasi M, Pietri S. Exercise-induced oxidative nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood brain barrier leakage. Experimental Physiology. 2011;96:1196–1207. doi: 10.1113/expphysiol.2011.060178. [DOI] [PubMed] [Google Scholar]
- 155.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 156.Tzeng Y-C, Ainslie P. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. European Journal of Applied Physiology. 2014;114:545–559. doi: 10.1007/s00421-013-2667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends in Molecular Medicine. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 159.Iida Y, Katusic ZS. Mechanisms of Cerebral Arterial Relaxations to Hydrogen Peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- 160.Sobey CG, Heistad DD, Faraci FM. Mechanisms of Bradykinin-Induced Cerebral Vasodilatation in Rats: Evidence That Reactive Oxygen Species Activate K+ Channels. Stroke. 1997;28:2290–2295. doi: 10.1161/01.str.28.11.2290. [DOI] [PubMed] [Google Scholar]
- 161.Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension. 1990;16:162–9. doi: 10.1161/01.hyp.16.2.162. [DOI] [PubMed] [Google Scholar]
- 162.Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- 163.Yang Z-w, Zhang A, Altura BT, Altura BM. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: a potential new cerebral dilator mechanism. Brain Research Bulletin. 1998;47:257–263. doi: 10.1016/s0361-9230(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 164.You J, Golding EM, Bryan RM. Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. 2005;289:H1077–H1083. doi: 10.1152/ajpheart.01046.2004. [DOI] [PubMed] [Google Scholar]
- 165.Faraci FM. Oxidative Stress: The Curse That Underlies Cerebral Vascular Dysfunction? Stroke. 2005;36:186–188. doi: 10.1161/01.STR.0000153067.27288.8b. [DOI] [PubMed] [Google Scholar]
- 166.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 167.Vianna LC, Deo SH, Jensen AK, Holwerda SW, Zimmerman MC, Fadel PJ. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. 2015;308:H681–H687. doi: 10.1152/ajpheart.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hurr C, Harrison ML, Brothers RM. Acute flavanol consumption improves the cerebral vasodilatory capacity in college-aged African Americans. Experimental Physiology. 2015 doi: 10.1113/EP085269. n/an/a. [DOI] [PubMed] [Google Scholar]
- 169.Erdös B, Snipes JA, Miller AW, Busija DW. Cerebrovascular Dysfunction in Zucker Obese Rats Is Mediated by Oxidative Stress and Protein Kinase C. Diabetes. 2004;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 170.Silva RAP, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD. Impact of ACE2 Deficiency and Oxidative Stress on Cerebrovascular Function With Aging. Stroke. 2012;43:3358–3363. doi: 10.1161/STROKEAHA.112.667063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reid MB. Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don’t. Journal of Applied Physiology. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]