Abstract
Ultrafine particles are airborne particulates of less than 100 nm in aerodynamic diameter. Examples of ultrafine particles are diesel exhaust particles, products of cooking, heating and wood burning in indoor environments, and more recently, products generated through the use of nanotechnology. Studies have shown that ambient ultrafine particles have detrimental effects on both the cardiovascular and respiratory systems, including a higher incidence of atherosclerosis and the exacerbation rate of asthma. Ultrafine particles have been found to alter in vitro and in vivo responses of the immune system to allergens and may also play a role in allergen sensitization. The inflammatory properties of ultrafine particles may be mediated by a number of different mechanisms, including the ability to produce reactive oxygen species, leading to the generation of pro-inflammatory cytokines and airway inflammation. In addition, because of their small size, ultrafine particles also have unique distribution characteristics in the respiratory tree and circulation and may be able to alter cellular function in ways that circumvent normal signaling pathways. Additionally, ultrafine particles can penetrate intracellularly and potentially cause DNA damage. The recent advances in nanotechnology, while opening up new opportunities for the advancement of technology and medicine, could also lead to unforeseen adverse health effects in exposed humans. Further research is needed to clarify the safety of nanoscale particles, as well as the elucidation of the possible beneficial use of these particulates to treat disease.
Keywords: Ambient ultrafine particles, engineered nanoparticles, particle deposition and distribution, allergic inflammation, asthma, lung inflammation, oxidative stress, impact on human health
Graphical Abstract
Introduction
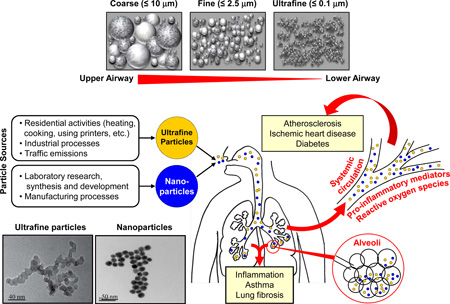
Compared with our understanding of the health effects of particulate matter with aerodynamic diameter of < 10 µm (PM10, coarse PM) and < 2.5 µm (PM2.5, fine PM), there is a considerable knowledge gap about the impact of particles < 100 nm on human health. Increasing evidence from air pollution and nanosafety research suggests these sub-micron scale particles have physicochemical properties significantly different from that of larger sized PM and may therefore exert adverse health effects, including promoting asthma exacerbation and allergic sensitization to common allergens, through different mechanisms (Table 1). 1,2 Currently these particles are classified into two major categories based on their sources. Ultrafine particles (UFP) refer to the particles that are incidentally generated in the environment, often as by-products of fossil fuel combustion, condensation of semi-volatile substances or industrial emissions, whereas nanoparticles (NP) are manufactured through controlled engineering processes.1 While there are many differences in the physicochemical composition of UFP and NP, one common feature is their extremely small size; this allows these particles to have unique characteristics that may cause harmful health effects to humans (Box 1, Table 2). 1
Table 1.
Comparison of PM10, PM2.5 and UFP
| Characteristics | PM10 | PM2.5 | UFP |
|---|---|---|---|
| Aerodynamic diameter (µm) | 2.5 – 10 | 2.5 – 0.1 | < 0.1 |
| Deposition in alveolar space | No | No | Yes |
| Surface area/mass ratio | + | ++ | +++ |
| Organic carbon content | + | ++ | +++ |
| Elemental carbon content | +++ | ++ | + |
| Metal content | +++ | ++ | + |
| Exposure metrics a, b | Mass | Mass | Particle number or surface area |
| Central monitoring sites a, b | Yes | Yes | None |
| National Ambient Air Quality Standards (NAAQS) set by the US EPA |
150 mg/m3 (24 hrs) (Not to be exceeded more than once per year on average over a 3-year period) |
35 mg/m3 (24 hrs) (98th percentile, averaged over 3 years) |
None |
Sub-micron particles have relatively little mass and are affected to a greater degree by forces other than gravity (e.g. thermal, radiation, and electrical forces, and particle concentration) so they are not efficiently collected by traditional particulate samplers that rely on gravitational or inertial forces for particle collection.
Instruments to measure airborne ultrafine particles operate on the principles of thermo-phoretics, diffusion charging or condensation, with results reported in units of particle number concentration, particle volume concentration, or particle surface area per volume of air sampled rather than by mass concentration as in the case of PM10 and PM2.5.
Box 1. Unique features of UFP and NP.
UFP: incidentally generated in the environment, aerodynamic diameter < 0.1 µm
NP: manufactured via controlled engineering processes, at least one dimension < 0.1 µm
Both particles can effectively deposit in alveolar space via diffusion
Both have high surface area/mass ratio
Large surface area allows UFP to carry a relatively large load of hazardous cargo
Both physical and chemical properties determine the health effects of UFP and NP
UFP and select engineered NP can induce oxidative stress, airway inflammation and toxicity
Particles can be transported by lung dendritic cells to draining lymph nodes or translocate to distant organs via blood stream and may have adverse systemic health effects in many organs
Table 2.
Comparison of ENM and ambient UFP
| Particle Type | ENM | UFP |
|---|---|---|
| Sources | Engineered (controlled synthesis) |
Incidental (combustion) |
| Morphology | Regular (sphere, tube, cube, rod, wire, plate) |
Irregular |
| Homogeneity | Yes | No |
| Organic Chemical Content | Low | High |
| Metal Impurity | Varies | High |
| ROS Generation | Varies | Yes |
| Exposure Route | Inhalation, skin, ingestion, injection | Inhalation |
| Adverse Health Effects | Unknown | Yes |
In 2013 the Health Effects Institute Review Panel concluded, based on the database available at that time, that there was no evidence that the adverse health effects of UFP were dramatically different from those of PM2.5. However, epidemiological and clinical trial studies published in 2014 and 2015 question this conclusion (see below for further discussion). 3–9 Moreover, experimental evidence suggests that UFP may be more dangerous than PM10 and PM2.5 due to their chemical composition, small size, large surface area/mass ratio, capability of generating reactive oxygen species (ROS), high retention rate, and deep penetration in the respiratory system. 10, 11
Several key facts indicate a critical need to address the adverse health effects of ambient UFP. First, while PM10 and PM2.5 can be easily removed by phagocytosis, the extremely small size of UFP enables them to evade such host defense and deposit in the lung with high rate of retention. Thus, for the same volume of air inhaled, the actual dose and regional impacts of UFP in the lung may be significantly greater than that of PM2.5. Moreover, the size of UFP allows them to translocate to other organs through the systemic circulation, leading to toxicological mechanisms that are very different from those of PM2.5. Second, the large surface area enables UFP to carry large quantities of adsorbed hazardous materials on a per mass basis including organic chemicals and metals that can generate ROS and oxidative stress. Oxidant injury plays an important role in UFP-induced adverse health effects including exacerbation and promotion of asthma, chronic obstructive pulmonary disease (COPD), and atherosclerosis. 11–14 Third, unlike PM2.5, UFP are not homogeneously distributed in the atmosphere, but rather localized in hot-spots of exposure (e.g., near roads with busy traffic). This has resulted in a lack of extensive UFP monitoring networks and limited epidemiological studies, a situation which is unlikely to change until regulatory agencies decide to track these particles as criteria pollutants. Fourth, the composition of semi-volatile organic compounds on the UFP surface may vary dynamically depending on the source and molecular size, challenging efforts to draw simple conclusions about their health effects. Fifth, while the health effects of PM10 and PM2.5 are determined based on PM mass, the “weightless” nature of UFP requires other exposure metrics, i.e. particle number and surface area. Unfortunately, epidemiological studies using these metrics are currently limited. Finally, although improved engine and fuel technologies have significantly reduced the emission of particulate soot, UFP can still be formed from vapor condensation and they can even be smaller than the emission particles, with more chemically reactive surface functional groups. 15–18
In contrast to UFP, NP are intentionally created with specific size, shape, surface characteristics, and functionality that are required for their applications (Table 2). Nanotechnology, especially the commercial production and usage of engineered nanomaterials (ENM), is a rapidly developing industry that increasingly affects our lives due to potential exposure to >1300 nanotechnology-based consumer products which include at least one nanocomponent (Table 3). 1, 19–29 Therefore, the extensive usage and environmental/occupational exposure to ENM have raised significant concerns regarding their safety profiles, especially for ENM in powder form, which can be inhaled during production, transfer, packaging, and processing.
Table 3.
Nanomaterials used in commercial products and their potential exposure route
| Type of NP | Products | Exposure Routs | References |
|---|---|---|---|
| Fumed silica | Food, pharmaceutics, rubber, plastics, paints, desiccants, and cosmetics |
Lung, gastrointestinal tract |
22, 23 |
| Silver | Filter, inks, food package, clothing, surgical masks, cosmetics, sprays |
Lung, gastrointestinal tract |
24, 25 |
| Carbon nanotubes |
Coating, film, microelectronics, composite materials, energy storage, biotechnology |
Lung, skin | 26 |
| Graphene and Graphene oxide |
Water purification, coating, battery electrode, medicine, transistors |
Lung, skin | 27 |
| Titanium dioxide | Sunscreen, food | Skin, gastrointestinal tract |
28 |
| Molybdenum disulfide |
Lubricant spray, petroleum refining |
Lung | 29 |
Although currently there is no definitive evidence to link NP exposure to any human disease, experimental data indicate that several types of ENM may be potentially hazardous. 1 The physicochemical characteristics of NP that may have health implications include particle size, shape, aspect ratio, composition, charge, surface reactivity, solubility, and ability to generate ROS. Similar to UFP, the nano-scale size can enhance NP translocation and deposition by interfering with their clearance. These features have the potential to induce cytotoxicity and inflammation, and activate an injury response pathway that includes calcium influx, mitochondrial depolarization, and plasma membrane damage. 30, 31
The objective of this paper is to provide an up-to-date report on the impact of UFP on human health and potential nanomaterial hazard. We will summarize the known health effects of UFP from cellular, animal, and human research data and discuss the potential mechanisms and exposure routes involved in the disease process focusing on the pro-inflammatory effects of UFP in the respiratory and immune systems. We will also review the adverse effects of ENM based on their unique physicochemical properties.
Ultrafine Particles (UFP)
Sources and generation
Ambient UFP originate from natural and anthropogenic activities and processes (Table 1). 32–35 Combustion-derived UFP characteristically have an elemental or organic carbon (OC) core carrying trace metals, sulfate, ammonium and volatile and semi-volatile components. 32, 34, 36, 37 Other components of combustion-derived UFP will depend on fuel type, burn conditions, and atmospheric conditions. There has been less research describing the composition of non-combustion sources of UFP, but environmental factors and human activities likely influence the composition of airborne UFP. 32, 38
Owing to the ubiquitous nature of their sources, the presence of UFP in outdoor and indoor air is not a recent or unusual occurrence. Monitoring particles in the ultrafine size range has focused on specific sources (roadways, combustion, appliances) and has required sampling equipment that addresses the unique behavior of UFP. However, there is currently no standardized UFP measurement method or reporting and there are no federal standards for UFP levels (Table 1).
Exposure assessment and environmental levels
Exposure assessment studies have used different particle metrics and have provided important, but limited characterization of UFP levels and types in ambient air, and recently in residential and office locations.
Ambient
Ambient levels of airborne UFP are challenging to characterize geographically or over time as number concentrations decrease sharply downwind from sources, and UFP shift in size from nucleation to accumulation mode with time and distance from their emission source through agglomeration and condensation. For combustion sources, the fuel, combustion conditions, and pollution controls will alter the particle numbers and size distribution. Occupational exposures will be particularly high during high temperature operations (e.g., welding, smelting), high-speed manufacturing, and combustion processes, but we currently have limited information about UFP exposure in these settings. The introduction of catalytic converters on cars and trucks to reduce tailpipe emissions of hydrocarbons and carbon monoxide had the unintended consequence of shifting the bulk of the particle size distribution of exhaust PM to smaller diameters of 20–30 nanometers. 39 Particle mass decreases with catalytic conversion, but the number of particles in the UFP range increases and includes traces of the catalyst used. 39, 40 As fuels have had to conform to lower sulfur content requirements, UFP in exhaust emissions has declined for vehicles using low and ultra-low sulfur fuels. 32, 41 However, UFP that are formed during vapor condensation may still be quite significant. 42
Most studies of ambient UFP have focused on urban areas and roadways. Background UFP concentrations in cities and upwind of roadways are summarized in Table 4, 33, 34, 36, 43–48 Higher concentrations were associated with lower humidity, 43, 49 greater proportion of diesel vehicles, 36, 44, 46, 49–51 winter months, 44, 47 and when traffic accelerates after stopping. 44, 51 Not surprisingly, UFP concentrations decline with distance from the highway. 39, 46
Table 4.
Sources of UFP and their background concentrations in cities and upwind of roadways
| Natural sources34 | |
| Biological agents (viruses, microbes and fungal parts), combustions, geological processes (volcanic eruptions), and atmospheric transformations (gas to nuclei mode and condensate aerosols) | |
| Anthropogenic sources34 | |
| High temperature processes (welding, smelting), combustion (power generation, mobile sources, residential and commercial heating, cooking), and industrial processes | |
| Background concentrations in cities and upwind of roadways | |
| Range | 1 × 103 – 5 × 104 p/cc a, 33, 34, 36, 43–48 |
| Peak concentration b | 8 × 104 – 3.5 × 105 p/cc |
| Factors affecting UFP levels | Season 44,47, relative humidity 43,49, traffic volume 36, 49,51, vehicle type 44,46,50, and traffic flow pattern 44,51 |
particles/cm3;
within 20 meters of the roadway
Residential/Office
Many common indoor sources in residential and office settings generate UFP, and ultrafine particle concentrations rise during specific indoor activities (Table 5). 34, 52–56 Although the spectrum of consumer products generating UFP is broad, exposure and risk assessments are not available for most products. Most of our understanding of in-home or in-office exposures to UFP comes from extrapolating from studies on incidental UFP levels and emission sources, and from UFP emission testing that is performed for products marketed outside the United States. Afshari and colleagues conducted chamber studies to quantify UFP emissions from common household activities; their findings as well as others’ are summarized in Table 5. 52, 34 Currently our knowledge about the fate of these particles in ambient air or after inhalation is limited.
Table 5.
| Household activity | Peak UFP concentration (p/cc*) |
|---|---|
| Burning pure wax candles | 24 × 104 |
| Burning three cigarettes for 10 min | 21 × 104 |
| Frying meat in oil in a Teflon pan on an electric stove for 45 min |
15 × 104 |
| Spraying 20 gram of a pure citrus air freshener | 3 × 104 |
| Vacuuming for 50 min | 2.1 × 104 |
| Operating a propane camping stove | 7.9 × 104 |
| Operating an electric radiator | 22 × 104 |
| Operating an electric stove | 11 × 104 |
| Operating an electric air heater | 12 × 104 |
| Dry ironing cotton material | 0.055 × 104 |
| Operating a vented gas clothes dryer | 10 × 104 (6 × 1012/drying cycle) |
| Office activity | UFP concentration (p/cc*) |
| Printing (10-min print run)** | 108 – 1012** 106 – 1010*** |
p/cc: particles/cm3;
the total UFP emissions, normalized to a 10 minute print run, over an hour;
in a 30.6 m3 office with an air change rate of 0.68
Office printers have recently been recognized to generate substantial amount of indoor UFP. In fact, several European countries have set emission limits for office printers with categories that include volatile organic compounds, formaldehyde, dust, and ozone. As of 2013, the European “Blue Angel” program (http://www.blauer-engel.de/en) included a detailed testing methodology for particles (7–300 nm) and prescribed an emission limit of 3.5 × 1011 (p/cm3) per 10-minute print run. Horner and Steady presented a summary of test results based on an initial compilation of over 35 different printers from several manufacturers. 57 Despite controlled test conditions, and relatively constant particle losses to the chamber, the variation of the emission levels from the printers was substantial (Table 5). Future studies investigating the fate and potential adverse effects of inhaling printer-derived UFP requires consideration.
Sources and Exposure Assessment of Engineered Nanoparticles (NP)
With the emergence of nanotechnology, workplace exposures can occur throughout the life cycle of ENM from laboratory development, through production, sales, installation, use, disposal, or recycling. Occupational exposure assessments overall have lagged behind the rapid expansion of nanotechnology. Currently, only limited data are available on the concentration of air-borne nanomaterials in occupational settings, e.g. factories or laboratories. For example, in a silver nanoparticle (AgNP) manufacturing facility, airborne AgNP levels of 5–289 µg/m3 were detected in the injection room. 58 These measurements overlap with the recommended threshold limit value of 100 µg/m3 for AgNP inhalation by the American Conference of Industrial Hygienists. 59 As for carbon nanotube (CNT), Han et al. reported peak multiwall CNT (MWCNT)-containing airborne dust levels being as high as 400 µg/m3 in a production laboratory. 60 While numerous publications have described the challenges and knowledge gaps about safety issues of ENM including NP, 61–66 a 2008 survey of 40 companies in Europe working with nanomaterials found that most did not perform risk assessment. Moreover, for those that did, they did not consider use, waste disposal, or unintentional releases. 67 A few studies looking at workplace breathing zone concentrations in ENM manufacturers relied on various exposure metrics, e.g., gravimetric-based respirable or inhalable PM mass or elemental carbon (EC), as a more specific marker for nanotubes or fibers, 68 making characterization of occupational exposures across the nanomaterial lifecycle difficult. Federal Agencies in the US, e.g., the EPA, have produced risk assessments for certain common nanomaterials [e.g. Ag and titanium dioxide (TiO2) NP], but more research is needed in this area.
Biological Effects
Particle deposition, retention and distribution in the lung and beyond
UFP and NP are in the respirable size range and have a physicochemical composition that enables their penetration into airways, parenchyma and alveolar airspace in the lung. The extremely small size and large surface area per unit mass of UFP and NP are two of the major determinants for their potential adverse health effects during particle transport, deposition and cellular perturbation. In general, deposition of UFP or NP in the lung is accomplished almost exclusively by diffusion where the thermodynamic diameter (and not the aerodynamic diameter), is mainly responsible for efficient deposition in the alveolar airspace (Box 1). The sub-micron size of UFP enables them to travel to and deposit in the alveolar region with much higher efficiency due to their strong diffusion capability. 69 In addition, the small size allows UFP to evade their clearance from the area, leading to long-term particle retention. Kawanaka et al. found that UFP contributed as much as 23–30% of the alveolar deposition of polycyclic aromatic hydrocarbons (PAHs) coming from roadside sources whereas the contribution of UFP to the total PM mass was only 2.3%; this also suggests that the large surface area of inhaled UFP allows them to deliver a significantly greater amount of hazardous chemicals to the region where they may cause sub-acute and chronic inflammation (Box 1). 70 The surface characteristics of nanoscale particles facilitates the formation of a protein or lipid corona in biological media due to the binding of proteins or detergents, which may alter their cellular uptake and induced biological responses. 71 A sizeable number of UFP can be deposited in the alveolar airspace, where pulmonary surfactant aides their retention on lung epithelium. 72 In the case of poorly soluble iridium-192 NP, 70–80% of NP are translocated rapidly to the interstitium and hence do not remain in the alveolar airspace. 73
The link between UFP-induced oxidative stress and inflammation
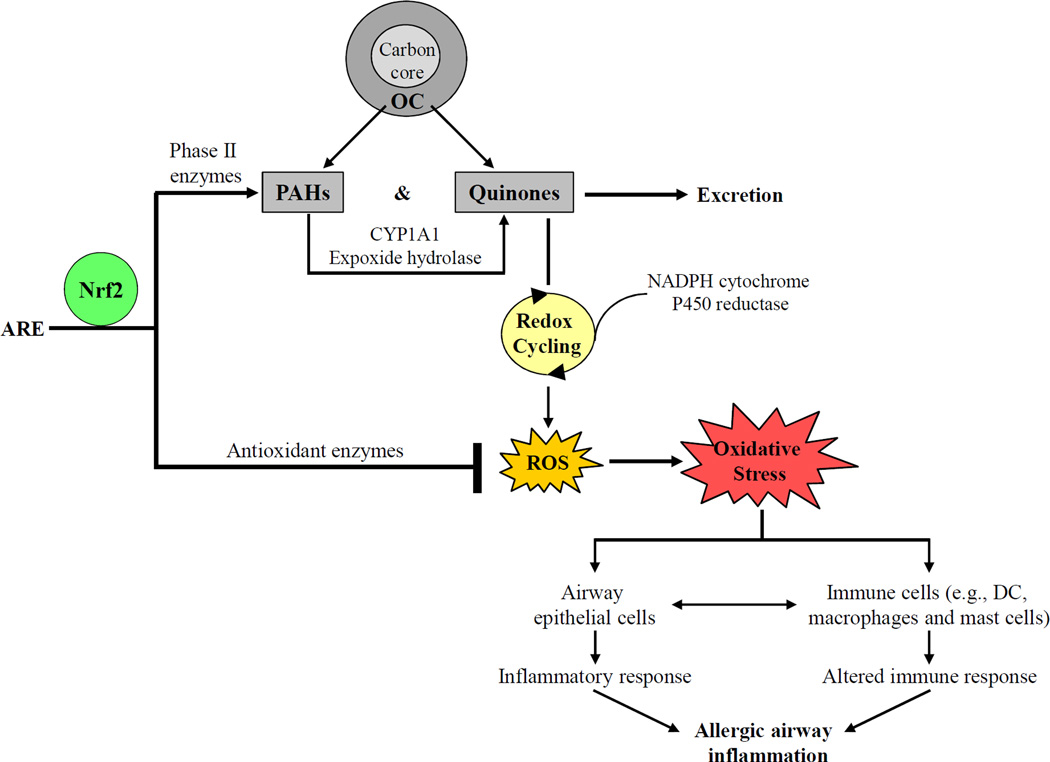
Experimental evidence from studies on the traffic-related UFP indicates that ROS produced by the OC and PAHs on the particle surface plays a key role in the injurious effects of UFP. Redox-active organic chemicals (e.g., PAHs and quinones) are the major contributors to UFP-generated ROS. 11, 74, 75 PAHs can be converted to quinones via biotransformation through reactions involving enzymes such as cytochrome P450 1A1, expoxide hydrolase, and dihydrodiol dehydrogenase. One electron reductions of redox-cycling quinones by NADPH cytochrome P450 reductase forms semiquinones, which can be re-cycled back to the original quinones with concomitant generation of superoxide and other types of ROS (Figure 1). 11, 75
Figure 1.
Generation of oxidative stress by ambient UFP and its role in allergic airway inflammation. UFP carry a large amount of OC including PAHs and quinones. Once inside cell PAHs can be converted to quinones via metabolism catalyzed by CYP1A1 and epoxide hydrolase. Quinones on the UFP surface undergo redox cycling between semi-quinones and original quinones through one-electron reduction by NADPH cytochrome P450 reductase, resulting in the generation of ROS. Nrf2 defends cells against oxidative injuries by binding to the antioxidant response element (ARE), together with other transcription factors, in the promoters of antioxidant and phase II enzymes, leading to the activation of effective protective mechanisms. When the Nrf2-mediated pathway is functional, activated antioxidant and phase II enzyme metabolize UFP-associated chemicals and remove excessive ROS. However, if antioxidant defense fails, ROS accumulation will escalate to cellular oxidative stress, which may induce inflammatory response and alter cellular functions in the respiratory (e.g., airway epithelial cells) and immune system (e.g., DC, macrophages and mast cells). The resulting allergic airway inflammation can be further amplified by the interactions between airway epithelial and immune cells.
The key regulator to protect cells against the damaging effects of ROS is nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor that mediates the majority of antioxidant and detoxification enzymes. 76 ROS accumulation as a result of either overproduction or inadequate antioxidant defense leads to oxidative stress. 12, 76 Several pro-inflammatory signaling pathways (e.g., MAPK and NFκB) are redox-sensitive. 14, 77, 78 Therefore, failure of cells to restore redox homeostasis can activate these pathways and induce airway inflammation (Figure 1). Interestingly, younger age appears to enhance susceptibility to oxidant effects of UFP exposure. For example, inhalation of combustion-derived flame-generated ultrafine soot particles caused more severe glutathione depletion and weakened induction of detoxification enzymes in neonate rats compared to the adult animals. 79
One controlled human exposure study concluded that the particle size fraction (coarse, fine and UFP) was not significantly associated with their cardiopulmonary health outcomes. 80 However, this lack of size fraction-dependent effects was likely due to the use of different dosimetry metrics, i.e. coarse and fine PM exposure was based on mass, whereas UFP exposure was based on particle number. 80 Based on the mass concentration, Li et al. demonstrated that ambient UFP had higher PAH content and greater oxidant potential, and were much more prone towards introducing cellular injury compared to PM10 and PM2.5 that were simultaneously collected at the same site. 81 Other studies also reported stronger pro-oxidative and pro-inflammatory effects of UFP. For instance, a study comparing different sizes of PM from urban and rural areas revealed that regardless of the collection site, the finest PM fractions were stronger in inducing the biomarkers of PAH exposure, oxidative stress, and inflammation in human airway epithelial cells. 82 Using ultrafine carbon black and ferric sulfate as a model UFP from combustion sources, Weissenberg et al. showed that particle-induced intracellular, rather than extracellular, oxidative stress was required for Akt and ERK1/2 activation. 83
In addition to the direct involvement of PM-induced oxidative stress, there are other mechanisms responsible for the adverse effects of UFP. Ambient UFP-induced increase of oxidized glutathione can lead to modifications of nitric oxide synthase and decreased nitric oxide production by human endothelial cells. 84 Ultrafine carbon particles can also down-regulate cytochrome P450 1B1 expression in bronchial epithelial cells, monocytes, and sputum macrophages from healthy non-smokers and COPD patients. 85 In the case of traffic-related UFP, this may lead to increased availability of organic compounds in the lung. Finally, the extremely small size alone has been found to be more potent in interfering with the immune response. 86, 87 For example, polystyrene particles of all sizes (coarse, fine and ultrafine) could enhance ovalbumin (OVA)-induced allergic airway inflammation (i.e. eosinophil influx in the lung and OVA-specific-IgE production); however, the strongest effect was observed in the animals exposed to ultrafine particles.87
Engineered NP
Similar to UFP, the size of ENM ranges from 1 to 100 nm in at least one dimension (Box 1). 1 However, they are inherently different from UFP in many aspects (Table 2). Evidence from extensive cellular and animal studies suggests that the hazardous potential of ENMs are determined by their physicochemical properties, including morphology, size, charge, dissolution, aspect ratio, surface coating and reactivity, redox-active properties, and aggregation. 88 While NPs can form agglomerates in the respiratory tract or in biological fluids, some NP fractions can remain and still exhibit “nano” properties even after several days, and potentially exert toxic effects in the lung. Ryman-Rasmussen et al. showed that 14 days after inhalation exposure, MWCNTs were still present as single tubes in the subpleural region in mice, along with subpleural fibrosis. 89 Wang et al. demonstrated that citrate-coated 110-nm AgNPs remained as singlet particles in the mouse lung 21 days after exposure and were associated with chronic lung inflammation. 90 The dosimetry for cellular and animal studies has been calculated based on real-life exposures to AgNPs and MWCNTs in manufacturing facilities. These calculations are developed based on the premise that same surface area dose (mass/surface area) for the lungs in humans and animals will generate similar responses. For example, lung exposure dose (mass/surface area) for animal experiments (0.1–2 mg/kg) using nano-Ag is comparable to the monthly lung deposition level in a human worker potentially exposed to 289 µg/m3 AgNPs in the injection room. Similarly, the in vitro dose range (12.5 ∼ 100 µg/mL) is also comparable to that used in the animal experiment based on surface area dose calculations. 90
To date, many studies have linked NP physicochemical properties to their toxicological outcomes. TiO2 NP, the most abundantly produced nanomaterials that can be found in many commercial products, can cause oxidative stress-mediated acute lung inflammation. 91, 92 Oberdorster et al. showed that on a mass-dose basis, ultrafine TiO2 is more toxic than fine TiO2 particles. However, when the particle doses were expressed as particle surface area, the responses of ultrafine and fine TiO2 particles fell on the same dose-response curve, suggesting that surface area is an important property for ENM’s toxic potential. 93 The crystal structure (e.g., anatase vs. rutile form) and photoactivation properties of TiO2 NP also play important roles in their capability of generating ROS and inducing cytotoxicity. 94,95 ZnO NP have received significant attention because of their use in sunscreens, electronics, optics, and photonics. 96 Pulmonary exposure to ZnO NP generated as a by-product of welding could lead to transient acute lung inflammation, a disease called metal fume fever. 97, 98 Xia et al. showed that the toxicity of ZnO was dependent on particle dissolution and shedding of toxic Zn ions. 99
CNTs are long aspect ratio nanomaterials that have wide applications. 100, 101 Studies have shown that their dispersal state, hydrophobicity, and purity could affect the pro-fibrogenic cellular responses, correlating with the extent of pulmonary fibrosis. 102, 103 Other long aspect ratio ENM also had similar effects. Ji et al. demonstrated that at lengths ≥ 200 nm and aspect ratios ≥ 22, cerium dioxide nanorods induced progressive pro-inflammatory response and cytotoxicity. The relatively low “critical” length and aspect ratio were associated with small nanorod/nanowire diameters (6–10 nm), which facilitate the formation of stacking bundles that pierce the lysosomal membrane, causing the release of cathepsin B, NLRP3 inflammasome activation, and the production of pro-inflammatory cytokine IL-1β. 104 Additional research is needed to understand the interactions occurring at the nano-bio interface between ENM and biological systems.
UFP and NP in immune responses and models of allergic inflammation and asthma
Many animal model studies have documented the ability of inhaled UFP and NP to act as pro-allergic adjuvants, boosting the allergic immune response to inhaled allergens. 105–107 Because different UFP and dosing regimens were used, it is currently not possible to construct a unifying model for inhaled UFP’s enhancing effect on allergic inflammation. Ochs estimates that UFP could encounter 40 different cell types as they journey through the respiratory tract. 108 However, it is likely that the major cell types coming into contact with UFP are macrophages, epithelial cells, dendritic cells (DCs), and endothelial cells at the epithelial, interstitial and sub-interstitial layers, respectively. There are primarily three pathways for the fate of UFP following deposition in the lung: a) phagocytic clearance by alveolar/airway macrophages via the mucociliary escalator, b) uptake by lung resident DCs and transport to draining lymph nodes, 109 or c) translocation across the epithelial layer into the blood stream, pleural space, or distant organs. 89, 110 The pro-oxidant property of UFP plays an important role in this effect. Intranasally instilled ambient UFP with a high OC/PAH content and strong oxidant potential is a potent adjuvant for allergic sensitization to OVA in mice, leading to pronounced allergic inflammation in the lung and nose. 111 Moreover, inhalation of pro-oxidant UFP during OVA challenge further exacerbated this inflammation in previously sensitized animals. 112 Thus, ambient exposure to UFP can be considered a risk factor for both the development and exacerbation of asthma. Several studies used laboratory-generated ultrafine particles to represent a certain component of ambient UFP or the carbon core of traffic-derived PM. Using EC UFP (EC-UFP), Alessandrini et al. demonstrated that the adjuvant activity of inhaled EC-UFP on allergic lung inflammation was accompanied by local lipid peroxidation and NFκB activation. 113 Exposure of sensitized mice to EC-UFP prior to OVA challenge also led to the goblet cell metaplasia of Clara cells and overproduction of mucus and Clara cell protein. 114 These changes as well as the adjuvant activity of EC-UFP could be suppressed by antioxidant N-acetyl cysteine. 113, 114 In addition to their capability of up-regulating pro-inflammatory cytokines and chemokines through oxidative stress, UFP also alter the balance between pro- and anti-inflammatory lipid mediators. Exposure of OVA-sensitized mice to ultrafine carbon particles before OVA challenge enhanced allergic inflammation and lipid peroxidation in the lung and skewed lipid mediator balance towards a pro-inflammatory response with a significant increase of leukotriene B4. 115
Similar results have also been observed for NP and ENM. In rats some NP can interact and stimulate mast cells to secrete histamine, thereby modifying allergic responses in atopic models. 116 Inhalation of gold or TiO2 NP enhanced lung inflammation and airway hyper-reactivity (AHR) in a mouse model of isocyanate-induced asthma. 117 These effects may be due to direct activation of lung DC subset by inhaled NP. 118 Co-exposure to OVA and CNT synergistically enhanced airway fibrosis in mice, suggesting a possible role for ENM in airway remodeling. 119 A recent study concluded that intravenously administered CNT and graphene nanosheets induce T helper 2-immune responses via the IL-33/ST2 axis, since responses were partially attenuated in ST2-deficient mice. 120 In the case of NP, immune effects are influenced by particle size and shape. Intratracheal administration of agglomerated CNT results in granuloma formation 121, whereas dispersed CNT (e.g., coated by surfactant) results in more diffuse fibrosis. 110 Interestingly, CNT still accumulate in lung lymph nodes almost one year following aerosol exposure. 122 Consequently, it seems reasonable to conclude that pulmonary defense mechanisms are not able to handle the challenges posed by these new engineered nano-materials, and that more research of the immunological consequences of these biopersistant materials is urgently needed.
Although these studies suggest that inhaled UFP and NP will potentiate allergic lung inflammation other observations paint a more nuanced picture. For example, Rossi et al. showed that exposure to TiO2 NP over four weeks dramatically attenuated OVA-induced inflammation and AHR. 123 Additionally, certain fullerene-derived NP can suppress OVA-induced lung inflammation, probably by inhibiting mast cell activation. 124 Inhaled NP can induce local and systemic immunosuppression. For CNT this involves suppression of mitogen-driven antibody production and T cell proliferation in the spleen in a TGF-beta- and COX2-dependent manner. 125 Repeated inhalation of CNT suppressed the ability of macrophages to phagocytose and clear Listeria monocytogenes, which translated to enhanced lung inflammation. 126 Similar results were observed in a mouse model of Pseudomonas aeruginosa infection, although pathogen clearance was not affected. 41 These studies highlight the potential of UFP and NP to suppress immune response to infectious pathogens. Consequently, more research is needed to understand how UFP and NP composition and exposure conditions influence pro-inflammatory vs. anti-inflammatory and potentially immunosuppressive effects. Future studies also need to consider the potential for swallowed UFP and NP to impact the gut microbiome, given the increasing evidence that perturbations in intestinal microbes and their metabolism have a profound impact on asthma and allergies. 127–129
The Impact of Ambient UFP and NP on Human Health
The adverse cardiopulmonary effects of ultrafine particles have been demonstrated in epidemiologic association studies and an increasing number of controlled exposure human studies.
Epidemiologic association studies
An early study by Peters et al. reported that decreased peak expiratory flow (PEF) and increased respiratory symptoms of asthmatic subjects were associated with exposure to ambient fine and UF particles. 130 However, the effects of the 5-d mean of UFP number was larger than that of the mass of the fine particles and the effect of UFP number on PEF was stronger than that of PM10. 130 More recently, a case-control study from Chile found that increased outpatient visits due to respiratory illness were significantly correlated with increased levels of UFP generated from residential wood burning. 131 More evidence looking at the association between UFP and allergic diseases came from children's studies. A time-stratified, case-crossover study involving 74 children showed that the largest increase in the relative odds of pediatric asthma visits was associated with the 4-day mean concentration of ambient UFP, but not with accumulation mode PM, black carbon, and sulfur. 7 In addition, Song et al. reported that after a short-term exposure to ambient UFP, children with eczema had increased urinary level of 8-hydroxyl-2-deoxyguanosine, a major byproduct of oxidative DNA damage, compared to those without eczema. This increase was associated with the level of UFP and particles' PAH content. 132 The deleterious cardiovascular effects of UFP are continuously being reported by studies involving human subjects. Exposure to UFP is found to be associated with altered heart rate, heart rate variability, changes in microvascular function, and systemic inflammation. Two studies from Denmark demonstrated that exposure to UFP away from home was significantly inversely correlated with microvascular function and positively associated with systemic inflammation. 6, 8 There was no association between these changes and PM10 and PM2.5. Decreased lung function, i.e. forced expired volume in 1 sec (FEV1) and forced vital capacity, and elevated type 2 diabetes marker (HbA1c) were associated with the level of indoor UFP, but not PM2.5. 6, 8 A recently published 6-year (2001–2007) cohort study including more than 100,000 women in California reported that the mortality caused by ischemic heart disease (IHD) was significantly associated with UFP, their contents of EC and metals, and mobile source. Although similar association was also found between IHD mortality and PM2.5, statistical analysis showed that UFP mass and its constituents had a better fit and a lower p-value than those of PM2.5. 9 Whether the adverse cardiovascular effects of UFP are related to particles’ capability to penetrate into the systemic circulation is unclear.
Controlled human exposures
Chalupa et al. reported an inverse relationship between carbon UFP lung deposition and particle size; particle deposition was further increased in subjects with asthma. 133 EC-UFP could interfere with the distribution of blood leukocytes and the expression of adhesion molecules in healthy as well as asthmatic subjects, which may contribute to increased leukocyte retention in the alveolar bed. 134 Inhalation exposure to concentrated UFP collected in an area with busy traffic in Los Angeles, CA had acute adverse cardiopulmonary effects, including decreased arterial oxygen saturation and FEV1 in both healthy and asthmatic subjects. 135 Exposure to concentrated ambient UFP is also associated with increased production of fibrin degradation products (D-dimer) and IL-8 in bronchoalveolar lavage fluid from healthy subjects, suggesting mild prothrombotic and pro-inflammatory effects of these particles. 136 The potential long-term impact of inhaled ultrafine carbon particles on the course of inflammation in asthmatic patients was investigated in a double-blind randomized cross-over clinical pilot study. Using two different exposure protocols, Schaumann et al. reported that although UFP exposure had no acute effect on allergen-induced inflammation, the subgroup of subjects that inhaled UFP during the first exposure exhibited a surprising and significant increase in lung inflammation after either filtered air exposure or subsequent allergen challenge 28 days later. 4 The mechanisms for this apparent long-lasting effect of UFP are unclear.
UFP can also affect people with diabetes or metabolic syndrome. A single 2-hr inhalation of EC-UFP interfered with heart rate and heart rate variability in diabetic subjects, which could last for hours. 3 A randomized crossover study by Devlin et al. demonstrated that ambient UFP affected cardiac repolarization and heart rate variability, and induced markers of vascular inflammation and fibrinolysis in individuals that had metabolic syndrome and also carried glutathione S-transferase Mu1 null allele. Because these changes were mainly associated with particle number, it was concluded that UFP were responsible for these effects. 5 This suggests that defects in antioxidant defenses, whether genetic or acquired, can be considered a risk factor for adverse health effects of inhaled UFP. Future studies specifically defining susceptible cohorts of subjects are needed and will not only enhance our understanding of pathobiological mechanisms, but also lay the groundwork for rational preventative strategies.
Conclusion
Although recent progress has been made in understanding the adverse effects of ambient UFP and NP and their potential mechanisms of action, there is still a critical knowledge gap in clearly identifying the impact of exposure to these nano-scale pollutants on human health. Because of their extremely small size, UFP and ENM have unique physicochemical properties that may affect their exposure, deposition and translocation in the body, and capability to cause different health issues. Increasing evidence strongly suggests that UFP and NP may cause adverse health outcomes in humans including those with asthma, likely through a number of similar mechanisms that have been demonstrated by experimental studies. Thus, it is imperative to further strengthen our research in the health effects of nano-scale pollutants so that preventive strategies and regulatory guidelines can be developed to reduce exposure and protect human health.
Acknowledgments
Supported by grant NSF DBI-1266377 (to A.N.), NIEHS U19ES019528 (to A.N.), and NIEHS P30 ES01247 (to S.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
"The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Defense, Department of Army, US Army Medical Department or the U.S. Federal Government"
References
- 1.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 2.Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 3.Vora R, Zareba W, Utell MJ, Pietropaoli AP, Chalupa D, Little EL, et al. Inhalation of ultrafine carbon particles alters heart rate and heart rate variability in people with type 2 diabetes. Part Fibre Toxicol. 2014;11:31. doi: 10.1186/s12989-014-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaumann F, Fromke C, Dijkstra D, Alessandrini F, Windt H, Karg E, et al. Effects of ultrafine particles on the allergic inflammation in the lung of asthmatics: results of a double-blinded randomized cross-over clinical pilot study. Part Fibre Toxicol. 2014;11:39. doi: 10.1186/s12989-014-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, et al. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci. 2014;140:61–72. doi: 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- 6.Olsen Y, Karottki DG, Jensen DM, Beko G, Kjeldsen BU, Clausen G, et al. Vascular and lung function related to ultrafine and fine particles exposure assessed by personal and indoor monitoring: a cross-sectional study. Environ Health. 2014;13:112. doi: 10.1186/1476-069X-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014;129:11–19. doi: 10.1016/j.envres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karottki DG, Beko G, Clausen G, Madsen AM, Andersen ZJ, Massling A, et al. Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle-aged subjects. Environ Int. 2014;73:372–381. doi: 10.1016/j.envint.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, et al. Associations of Mortality with Long-Term Exposures to Fine and Ultrafine Particles, Species and Sources: Results from the California Teachers Study Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Verma MK, Srivastava AK. Ultrafine particles in urban ambient air and their health perspectives. Rev Environ Health. 2013;28:117–128. doi: 10.1515/reveh-2013-0008. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breysse PN, Delfino RJ, Dominici F, Elder ACP, Frampton MW, Froines JR, et al. US EPA particulate matter research centers: summary of research results for 2005–2011. Air Qual Atmos Health. 2013;6:333–355. [Google Scholar]
- 13.Terzano C, Di Stefano F, Conti V, Graziani E, Petroianni A. Air pollution ultrafine particles: toxicity beyond the lung. Eur Rev Med Pharmacol Sci. 2010;14:809–821. [PubMed] [Google Scholar]
- 14.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank B, Schlogl R, Su DS. Diesel soot toxification. Environ Sci Technol. 2013;47:3026–3027. doi: 10.1021/es4003873. [DOI] [PubMed] [Google Scholar]
- 16.Frank B, Schuster ME, Schlogl R, Su DS. Emission of highly activated soot particulate--the other side of the coin with modern diesel engines. Angew Chem Int Ed Engl. 2013;52:2673–2677. doi: 10.1002/anie.201206093. [DOI] [PubMed] [Google Scholar]
- 17.Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, et al. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176:317–326. doi: 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins BJ, Kicic A, Ling KM, Mead-Hunter R, Larcombe AN. Biodiesel exhaust-induced cytotoxicity and proinflammatory mediator production in human airway epithelial cells. Environ Toxicol. 2014 doi: 10.1002/tox.22020. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Mao SS. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107:2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 20.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 21.Ivask A, Suarez E, Patel T, Boren D, Ji Z, Holden P, et al. Genome-Wide Bacterial Toxicity Screening Uncovers the Mechanisms of Toxicity of a Cationic Polystyrene Nanomaterial. Environ Sci Technol. 2011;46:2398–2405. doi: 10.1021/es203087m. [DOI] [PubMed] [Google Scholar]
- 22. https://www.ihs.com/products/silicates-chemical-economics-handbook.html.
- 23.Scribd I. Fumed Silica Market. 2010 [Google Scholar]
- 24.Glover RD, Miller JM, Hutchison JE. Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 2011;5:8950–8957. doi: 10.1021/nn2031319. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–6913. doi: 10.1021/nn102272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Duch MC, Mansukhani N, Ji Z, Liao YP, Wang M, et al. Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano. 2015;9:3032–3043. doi: 10.1021/nn507243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Mansukhani ND, Guiney LM, Ji Z, Chang CH, Wang M, et al. Differences in the Toxicological Potential of 2D versus Aggregated Molybdenum Disulfide in the Lung. Small. 2015;11:5079–5087. doi: 10.1002/smll.201500906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012;6:4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EPA US, editor. U.S.EPA Integrated Science Assessment for Particulate Matter (Final Report) 2009. Dec, [Google Scholar]
- 33.Oberdorster G, Gelein RM, Ferin J, Weiss B. Association of particulate air pollution and acute mortality: involvement of ultrafine particles? Inhal Toxicol. 1995;7:111–124. doi: 10.3109/08958379509014275. [DOI] [PubMed] [Google Scholar]
- 34.Murr LE, Garza KM. Natural and anthropogenic environmental nanoparticulates: their microstructural characterization and respiratory health implications. Atmos Environ. 2009;43:2683–2692. [Google Scholar]
- 35.England GC. Development of fine particulate emission factors and speciation profiles for oil and gas-fired combustion systems, New York State Energy Research and Development Authority Final Report. 2004 [Google Scholar]
- 36.Hughes LS, Cass GR, Gone J, Ames M, Olmez I. Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles area. Environ Sci Technol. 1998;32:1153–1161. [Google Scholar]
- 37.Pakkanen TA, Kerminen V, Korhonen CH, Hillamo RE, Aarnio P, Koskentalo ea. Urban and rural ultrafine (PM0.1) particles in the Helsinki area. Atmos Environ. 2001;35:4593–4607. [Google Scholar]
- 38.Chung A, Herner JD, Kleeman MJ. Detection of alkaline ultrafine atmospheric particles at Bakersfield, California. Environ Sci Technol. 2001;35:2184–2190. doi: 10.1021/es001879l. [DOI] [PubMed] [Google Scholar]
- 39.Wilson WE, Spider LL, Ellestad TG, Lamothe PJ, Dzubay TG, Stevens RKea. General Motors sulfate dispersion experiment: Summary of PA measurements. J Air Pollut Control Asoc. 1977;27:46–51. [Google Scholar]
- 40.Wiseman CL, Zereini F. Airborne particulate matter, platinum group elements and human health: a review of recent evidence. Sci Total Environ. 2009;407:2493–2500. doi: 10.1016/j.scitotenv.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 41.Walling BE, Kuang Z, Hao Y, Estrada D, Wood JD, Lian F, et al. Helical carbon nanotubes enhance the early immune response and inhibit macrophage-mediated phagocytosis of Pseudomonas aeruginosa. PLoS One. 2013;8:e80283. doi: 10.1371/journal.pone.0080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold F, Pirjola L, Ronkko T, Reichl U, Schlager H, Lahde T, et al. First online measurements of sulfuric acid gas in modern heavy-duty diesel engine exhaust: implications for nanoparticle formation. Environ Sci Technol. 2012;46:11227–11234. doi: 10.1021/es302432s. [DOI] [PubMed] [Google Scholar]
- 43.Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Number concentration and size of particles in urban air: effects on spirometric lung function in adult asthmatic subjects. Environ Health Perspect. 2001;109:319–323. doi: 10.1289/ehp.01109319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olvera HA, Lopez M, Guerrero V, Garcia H, Li WW. Ultrafine particle levels at an international port of entry between the US and Mexico: exposure implications for users, workers, and neighbors. J Expo Sci Environ Epidemiol. 2013;23:289–298. doi: 10.1038/jes.2012.119. [DOI] [PubMed] [Google Scholar]
- 45.Ruuskanen J, Tuch T, Ten Brink H, Peters A, Khlystov A, Mirme Aea. Concentrations of ultrafine, fine and PM 2.5 particles in three European cities. Atmos Environ. 2001;35:3729–3738. [Google Scholar]
- 46.Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C. Study of ultrafine particles near a major highway with heavy duty diesel traffic. Atmos Environ. 2002;36:4323–4335. [Google Scholar]
- 47.Noble CA, Mukerjee S, Gonzales M, Rodes CE, Lawless PA, Natarajan Sea. Continuous measurement of fine and ultrafine particulate matter, criteria pollutants and meteorological conditions in urban El Paso, Texas. Atmos Environ. 2003;37:827–840. [Google Scholar]
- 48.McAuley TR, Ferro A, Spengler JD, Hopke PH, Jaques PA. Spatial measurements of ultrafine particles using an engine exhaust particle sizer within a local community downwind of a major international trade bridge in Buffalo New York. Aersol Sci Technol. 2010;44:1096–1104. [Google Scholar]
- 49.Cheng YH, Huang CH, Huang HL, Tsai CJ. Concentrations of ultrafine particles at a highway toll collection booth and exposure implications for toll collectors. Sci Total Environ. 2010;409:364–369. doi: 10.1016/j.scitotenv.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Wahlin P, Palmgren F, Van Dingene R. Experimental studies of ultrafine particles in streets and the relationship to traffic. Atmos Environ. 2001;35(suppl 1):S63–S69. [Google Scholar]
- 51.Klems JP, Pennington MR, Zordan CA, Johnston MV. Ultrafine particles near a roadway intersection: origin and apportionment of fast changes in concentration. Environ Sci Technol. 2010;44:7903–7907. doi: 10.1021/es102009e. [DOI] [PubMed] [Google Scholar]
- 52.Afshari A, Matson U, Ekberg LE. Characterization of indoor sources of fine and ultrafine particles: a study conducted in a full-scale chamber. Indoor Air. 2005;15:141–150. doi: 10.1111/j.1600-0668.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 53.Dennekamp M, Howarth S, Dick CA, Cherrie JW, Donaldson K, Seaton A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup Environ Med. 2001;58:511–516. doi: 10.1136/oem.58.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waring MS, Siegal JA, Corsi RL. Ultrafine removal and generation by portable air cleaner. Atmos Environ. 2008;42:5003–5014. [Google Scholar]
- 55.Wallace L. Ultrafine particles from a vented gas clothes dryer. Atmos Environ. 2005;39:5777–5786. [Google Scholar]
- 56.Wallace L, Ott W. Personal exposure to ultrafine particles. J Expo Sci Environ Epidemiol. 2011;21:20–30. doi: 10.1038/jes.2009.59. [DOI] [PubMed] [Google Scholar]
- 57.Horner WE, Steady S. Ultrafine PM emissions from hardcopy devices measured per RAL UZ 171; Annual Conference of the American Association for Aerosol Research; Portland Oregon. 2013. [Google Scholar]
- 58.Lee JH, Ahn K, Kim SM, Jeon KS, Lee JS, Yu IJ. Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. J Nanopart Res. 2012:14. [Google Scholar]
- 59.ACGIH. Silver and Compounds: TLV® Chemical Substances. 7th 2001. [Google Scholar]
- 60.Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, et al. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20:741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- 61.Nowack B, Brouwer C, Geertsma RE, Heugens EH, Ross BL, Toufektsian MC, et al. Analysis of the occupational, consumer and environmental exposure to engineered nanomaterials used in 10 technology sectors. Nanotoxicology. 2013;7:1152–1156. doi: 10.3109/17435390.2012.711863. [DOI] [PubMed] [Google Scholar]
- 62.Bhatt I, Tripathi BN. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere. 2011;82:308–317. doi: 10.1016/j.chemosphere.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Hischier R, Walser T. Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps. Sci Total Environ. 2012;425:271–282. doi: 10.1016/j.scitotenv.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Brouwer D, van Duuren-Stuuren B, Berges M, Jankowska E, Bard D, Mark D. From workplace air measurements results toward estimates of exposure? Development of a strategy to assess exposure to manufactured nano-objects. J Nanopart Res. 2009;11:1867–1881. [Google Scholar]
- 65.Fiorino DJ. Voluntary Initiatives, regulation and nanotechnology oversight: Charting a path. Woodrow Wilson International Center for Scholars Project on Emerging Nanotechnologies. 2010 [Google Scholar]
- 66.NNI2011. National Nanotechnology Initiative Environmental, Health and Safety Research Strategy. National Science and Technology Council Committee on Technology. 2011 [Google Scholar]
- 67.Helland A, Scheringer M, Siegrist M, Kastenholz H, Wiek A, Scholz R. Risk assessment of engineered nanomaterials: a survey of industrial approaches. Environ Sci Technol. 2008;42:640–646. doi: 10.1021/es062807i. [DOI] [PubMed] [Google Scholar]
- 68.Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, et al. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10:53. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heal MR, Kumar P, Harrison RM. Particles, air quality, policy and health. Chem Soc Rev. 2012;41:6606–6630. doi: 10.1039/c2cs35076a. [DOI] [PubMed] [Google Scholar]
- 70.Kawanaka Y, Tsuchiya Y, Yun SJ, Sakamoto K. Size distributions of polycyclic aromatic hydrocarbons in the atmosphere and estimation of the contribution of ultrafine particles to their lung deposition. Environ Sci Technol. 2009;43:6851–6856. doi: 10.1021/es900033u. [DOI] [PubMed] [Google Scholar]
- 71.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175:1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 72.Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 73.Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, et al. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect. 2007;115:728–733. doi: 10.1289/ehp.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess. 2010;169:597–606. doi: 10.1007/s10661-009-1199-8. [DOI] [PubMed] [Google Scholar]
- 75.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 77.Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 78.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan JK, Kodani SD, Charrier JG, Morin D, Edwards PC, Anderson DS, et al. Age-specific effects on rat lung glutathione and antioxidant enzymes after inhaling ultrafine soot. Am J Respir Cell Mol Biol. 2013;48:114–124. doi: 10.1165/rcmb.2012-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samet JM, Graff D, Berntsen J, Ghio AJ, Huang YC, Devlin RB. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal Toxicol. 2007;19(Suppl 1):29–32. doi: 10.1080/08958370701492706. [DOI] [PubMed] [Google Scholar]
- 81.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Val S, Martinon L, Cachier H, Yahyaoui A, Marfaing H, Baeza-Squiban A. Role of size and composition of traffic and agricultural aerosols in the molecular responses triggered in airway epithelial cells. Inhal Toxicol. 2011;23:627–640. doi: 10.3109/08958378.2011.599445. [DOI] [PubMed] [Google Scholar]
- 83.Weissenberg A, Sydlik U, Peuschel H, Schroeder P, Schneider M, Schins RP, et al. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic Biol Med. 2010;49:597–605. doi: 10.1016/j.freeradbiomed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Du Y, Navab M, Shen M, Hill J, Pakbin P, Sioutas C, et al. Ambient ultrafine particles reduce endothelial nitric oxide production via S-glutathionylation of eNOS. Biochem Biophys Res Commun. 2013;436:462–466. doi: 10.1016/j.bbrc.2013.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eder C, Frankenberger M, Stanzel F, Seidel A, Schramm KW, Ziegler-Heitbrock L, et al. Ultrafine carbon particles down-regulate CYP1B1 expression in human monocytes. Part Fibre Toxicol. 2009;6:27. doi: 10.1186/1743-8977-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samuelsen M, Nygaard UC, Lovik M. Particle size determines activation of the innate immune system in the lung. Scand J Immunol. 2009;69:421–428. doi: 10.1111/j.1365-3083.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 87.Alberg T, Hansen JS, Lovik M, Nygaard UC. Particles influence allergic responses in mice--role of gender and particle size. J Toxicol Environ Health A. 2014;77:281–292. doi: 10.1080/15287394.2013.863746. [DOI] [PubMed] [Google Scholar]
- 88.Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, et al. Nanomaterial Toxicity Testing in the 21st Century: Use of a Predictive Toxicological Approach and High-Throughput Screening. Accounts of Chemical Research. 2012 doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao YP, et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small. 2014;10:385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 92.Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Stone V. Identification of the mechanisms that drive the toxicity of TiO(2 )particulates: the contribution of physicochemical characteristics. Part Fibre Toxicol. 2009;6:33. doi: 10.1186/1743-8977-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 94.Jin C, Tang Y, Yang FG, Li XL, Xu S, Fan XY, et al. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol Trace Elem Res. 2011;141:3–15. doi: 10.1007/s12011-010-8707-0. [DOI] [PubMed] [Google Scholar]
- 95.Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS One. 2012;7:e30321. doi: 10.1371/journal.pone.0030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang ZL. Zinc oxide nanostructures: growth, properties and applications. J Physics-Condensed Matter. 2004;16:R829–R858. [Google Scholar]
- 97.Osmond MJ, McCall MJ. Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology. 2010;4:15–41. doi: 10.3109/17435390903502028. [DOI] [PubMed] [Google Scholar]
- 98.Fine JM, Gordon T, Chen LC, Kinney P, Falcone G, Beckett WS. Metal fume fever: characterization of clinical and plasma IL-6 responses in controlled human exposures to zinc oxide fume at and below the threshold limit value. J Occup Environ Med. 1997;39:722–726. doi: 10.1097/00043764-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Xia T, Kovochich M, Liong M, Maedler L, Gilbert B, Shi H, et al. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Z, Robinson JT, Tabakman SM, Yang K, Dai H. Carbon materials for drug delivery & cancer therapy. Materials Today. 2011;14:316–323. [Google Scholar]
- 101.Avouris P, Chen Z, Perebeinos V. Carbon-based electronics. Nat Nanotechnol. 2007;2:605–615. doi: 10.1038/nnano.2007.300. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Xia T, Duch MC, Ji Z, Zhang H, Li R, et al. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett. 2012;12:3050–3061. doi: 10.1021/nl300895y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X, Xia T, Ntim SA, Ji Z, Lin S, Meng H, et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano. 2011;5:9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji Z, Wang X, Zhang H, Lin S, Meng H, Sun B, et al. Designed synthesis of CeO2 nanorods and nanowires for studying toxicological effects of high aspect ratio nanomaterials. ACS Nano. 2012;6:5366–5380. doi: 10.1021/nn3012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Haar C, Kool M, Hassing I, Bol M, Lambrecht BN, Pieters R. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol. 2008;121:1246–1254. doi: 10.1016/j.jaci.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 106.Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free Radic Biol Med. 2010;48:924–934. doi: 10.1016/j.freeradbiomed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 107.Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Lovik M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol Sci. 2009;109:113–123. doi: 10.1093/toxsci/kfp057. [DOI] [PubMed] [Google Scholar]
- 108.Ochs M, Weibel E. Fisherman's pulmonary diseases and disorders. New York: McGraw Hill; 2008. [Google Scholar]
- 109.Blank F, Stumbles PA, Seydoux E, Holt PG, Fink A, Rothen-Rutishauser B, et al. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am J Respir Cell Mol Biol. 2013;49:67–77. doi: 10.1165/rcmb.2012-0387OC. [DOI] [PubMed] [Google Scholar]
- 110.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 111.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117:1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, et al. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010;299:L374–L383. doi: 10.1152/ajplung.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alessandrini F, Beck-Speier I, Krappmann D, Weichenmeier I, Takenaka S, Karg E, et al. Role of oxidative stress in ultrafine particle-induced exacerbation of allergic lung inflammation. Am J Respir Crit Care Med. 2009;179:984–991. doi: 10.1164/rccm.200807-1061OC. [DOI] [PubMed] [Google Scholar]
- 114.Alessandrini F, Weichenmeier I, van Miert E, Takenaka S, Karg E, Blume C, et al. Effects of ultrafine particles-induced oxidative stress on Clara cells in allergic lung inflammation. Part Fibre Toxicol. 2010;7:11. doi: 10.1186/1743-8977-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beck-Speier I, Karg E, Behrendt H, Stoeger T, Alessandrini F. Ultrafine particles affect the balance of endogenous pro- and anti-inflammatory lipid mediators in the lung: in-vitro and in-vivo studies. Part Fibre Toxicol. 2012;9:27. doi: 10.1186/1743-8977-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen EY, Garnica M, Wang YC, Mintz AJ, Chen CS, Chin WC. A mixture of anatase and rutile TiO(2) nanoparticles induces histamine secretion in mast cells. Part Fibre Toxicol. 2012;9:2. doi: 10.1186/1743-8977-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hussain S, Vanoirbeek JA, Luyts K, De Vooght V, Verbeken E, Thomassen LC, et al. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37:299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- 118.Bezemer GF, Bauer SM, Oberdorster G, Breysse PN, Pieters RH, Georas SN, et al. Activation of pulmonary dendritic cells and Th2-type inflammatory responses on instillation of engineered, environmental diesel emission source or ambient air pollutant particles in vivo. J Innate Immun. 2011;3:150–166. doi: 10.1159/000321725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X, Podila R, Shannahan JH, Rao AM, Brown JM. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int J Nanomedicine. 2013;8:1733–1748. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 122.Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rossi EM, Pylkkanen L, Koivisto AJ, Nykasenoja H, Wolff H, Savolainen K, et al. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part Fibre Toxicol. 2010;7:35. doi: 10.1186/1743-8977-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Norton SK, Wijesinghe DS, Dellinger A, Sturgill J, Zhou Z, Barbour S, et al. Epoxyeicosatrienoic acids are involved in the C(70) fullerene derivative-induced control of allergic asthma. J Allergy Clin Immunol. 2012;130:761–769. doi: 10.1016/j.jaci.2012.04.023. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shvedova AA, Fabisiak JP, Kisin ER, Murray AR, Roberts JR, Tyurina YY, et al. Sequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivity. Am J Respir Cell Mol Biol. 2008;38:579–590. doi: 10.1165/rcmb.2007-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35. doi: 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Panzer AR, Lynch SV. Influence and effect of the human microbiome in allergy and asthma. Curr Opin Rheumatol. 2015;27:373–380. doi: 10.1097/BOR.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 129.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602. doi: 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- 131.Diaz-Robles LA, Fu JS, Vergara-Fernandez A, Etcharren P, Schiappacasse LN, Reed GD, et al. Health risks caused by short term exposure to ultrafine particles generated by residential wood combustion: a case study of Temuco, Chile. Environ Int. 2014;66:174–181. doi: 10.1016/j.envint.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 132.Song S, Paek D, Park C, Lee C, Lee JH, Yu SD. Exposure to ambient ultrafine particles and urinary 8-hydroxyl-2-deoxyguanosine in children with and without eczema. Sci Total Environ. 2013;458–460:408–413. doi: 10.1016/j.scitotenv.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 133.Chalupa DC, Morrow PE, Oberdorster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879–882. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frampton MW, Stewart JC, Oberdorster G, Morrow PE, Chalupa D, Pietropaoli AP, et al. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gong H, Jr, Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, et al. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol. 2008;20:533–545. doi: 10.1080/08958370801911340. [DOI] [PubMed] [Google Scholar]
- 136.Samet JM, Rappold A, Graff D, Cascio WE, Berntsen JH, Huang YC, et al. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med. 2009;179:1034–1042. doi: 10.1164/rccm.200807-1043OC. [DOI] [PubMed] [Google Scholar]