Abstract
Background
We sought to address how predictors and moderators of psychotherapy for bipolar depression – identified individually in prior analyses – can inform the development of a metric for prospectively classifying treatment outcome in intensive psychotherapy (IP) versus collaborative care (CC) adjunctive to pharmacotherapy in the Systematic Treatment Enhancement Program (STEP-BD) study.
Methods
We conducted post-hoc analyses on 135 STEP-BD participants using cluster analysis to identify subsets of participants with similar clinical profiles and investigated this combined metric as a moderator and predictor of response to IP. We used agglomerative hierarchical cluster analyses and k-means clustering to determine the content of the clinical profiles. Logistic regression and Cox proportional hazard models were used to evaluate whether the resulting clusters predicted or moderated likelihood of recovery or time until recovery.
Results
The cluster analysis yielded a two-cluster solution: 1) “less-recurrent/severe” and 2) “chronic/recurrent.” Rates of recovery in IP were similar for less-recurrent/severe and chronic/recurrent participants. Less-recurrent/severe patients were more likely than chronic/recurrent patients to achieve recovery in CC (p = .040, OR = 4.56). IP yielded a faster recovery for chronic/recurrent participants, whereas CC led to recovery sooner in the less-recurrent/severe cluster (p = .034, OR = 2.62).
Limitations
Cluster analyses require list-wise deletion of cases with missing data so we were unable to conduct analyses on all STEP-BD participants.
Conclusions
A well-powered, parametric approach can distinguish patients based on illness history and provide clinicians with symptom profiles of patients that confer differential prognosis in CC vs. IP.
Keywords: bipolar disorder, cluster analyses, psychotherapy
Introduction
Bipolar disorder, characterized by one or more periods of elevated mood, classically alternating with depressive episodes, is associated with high rates of disability (Calabrese et al., 2003). The foundation of treatment for bipolar disorder is usually pharmacotherapy. However, pharmacotherapy alone often fails to bring patients to full and sustained remission (Sachs et al., 2007). Therefore, pharmacotherapy is often paired with adjunctive psychotherapy to improve response, quality of life and prolong remission. Psychosocial treatments such as group or individual psychoeducation (Colom, 2010), cognitive behavioral therapy (CBT) (Thase et al., 2014), family focused therapy (FFT) (Miklowitz et al., 2000), interpersonal and social rhythm therapy (IPSRT) (Frank et al., 2000) and online adaptations (Lauder et al., 2015) combined with pharmacotherapy have been shown to improve medication adherence, acute mood symptoms, reduce functional impairment, and reduce likelihood of relapse (Miklowitz et al., 2006).
Although combination treatment yields improvements for many patients, there remains great variability in clinical response. Whereas mania is often relatively well controlled by pharmacotherapy (Post et al., 2014), bipolar depression is more often chronic and difficult to treat (Kohler et al., 2014). Bipolar disorder is also complicated by high rates of co-morbidity with anxiety or related disorders (Freeman et al., 2002), substance and alcohol use (Post and Kalivas, 2013), obesity (Liu et al., 2013), and medical problems (McElroy and Keck, 2014). In addition, many patients do not seek treatment in the initial stages of illness, bringing a chronic history of mood recurrences into treatment (Fagiolini et al., 2013).
Many of these variables have been shown to directly affect the potency of psychosocial treatments for bipolar depressive episodes. In prior studies, our group has investigated clinical predictors and moderators of response to adjunctive psychotherapy (Deckersbach et al., 2014; Peters et al., 2014a; Peters et al., 2015) using a large, cross-national randomized controlled trial that was part of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). In STEP-BD, acutely depressed individuals with bipolar disorder were randomized to one of three intensive (up to 30 sessions over 9 months) psychotherapies (CBT, IPSRT, or FFT) plus pharmacotherapy or to collaborative care (a 3-session psychoeducation intervention) plus pharmacotherapy (Miklowitz et al., 2007a). Thus, STEP-BD contains a large, nationally representative sample in a controlled trial of multiple psychotherapies. Our previous work has shown that repeated mood episodes and prolonged illness duration (Peters et al., 2014b), as well as medical co-comorbidity (Peters et al., 2015) are associated with overall treatment resistance, and that patients with co-morbid anxiety disorders and body mass index within a normal range respond better to intensive psychotherapy than those without comorbid anxiety disorders (Deckersbach et al., 2014).
Although these findings yielded valuable insights towards selection of intensive versus brief treatments, many patients with bipolar disorder exhibit several of the traits described above. From a treatment perspective, it is a challenge to know which of these selected findings should guide clinical decision-making. Cluster analysis provides a potentially elegant solution for combining these variables into well-powered and more clinically relevant metrics. Cluster analysis identifies subgroups of participants that are more similar on a set of variables than to individuals in other clusters. This analysis can help address the question of how numerous predictors and moderators of treatment – identified individually in prior analyses – can inform the development of a metric for prospectively classifying treatment outcome in bipolar disorder. Cluster analyses have also been shown to produce predictor/moderator sets with larger effect sizes than obtained with any of the individual variables of which the analytic solution is composed (Wallace et al., 2013). The primary aim of this study was to use a parametric method for creating a single combined variable from multiple individual clinical characteristics (Kraemer, 2013) and to evaluate whether the combined metric predicted or moderated response to intensive psychotherapy or collaborative care in the STEP-BD study.
Method
Study Design
STEP-BD was a multi-site, longitudinal study funded by the National Institute of Mental Health that examined course of illness, treatment effectiveness, and outcomes for individuals with bipolar disorder. The institutional review boards of the respective study sites approved the study protocol. The study incorporated several clinical trials that evaluated the efficacy of various treatment programs for bipolar disorder including antidepressants, mood stabilizing medications, atypical antipsychotics, and psychosocial interventions. With 4,361 participants enrolled across 21 sites, STEP-BD remains the largest study conducted in bipolar disorder. For a detailed description of the study methodology, see Sachs et al., 2003 (Sachs et al., 2003).
Among these nested clinical trials was a randomized, controlled trial of intensive psychotherapy versus collaborative care for acute bipolar depression (Miklowitz et al., 2007b). Participants randomly assigned to the intensive psychotherapy group received up to 9 months (30 sessions) of adjunctive pharmacotherapy together with manualized treatment with either CBT, IPSRT, or FFT. Participants randomly assigned to collaborative care received up to 6 weeks (3 sessions) of psychosocial treatment with adjunctive pharmacotherapy. All four psychotherapies incorporated relapse prevention planning, psychoeducation, and illness management techniques. Collaborative care, largely based in psychoeducation, consisted of an assortment of the most common psychosocial treatments for bipolar disorder (Miklowitz and Otto, 2007).
The three intensive psychotherapies incorporated psychoeducation, but were designed as enhanced interventions with particular treatment targets. CBT centered on restructuring cognitive distortions, challenging negative thought processes, problem solving, and activity planning (Lam et al., 2005). IPSRT aimed to stabilize the social rhythms that commonly precede mood episodes and interpersonal problems such as grief, relationship struggles, role transitions, and role disputes (Frank et al., 2005; Frank et al., 2008). FFT focused on educating family members about bipolar disorder as well as fostering improved communication and problem solving strategies between family members and patients (Miklowitz, 2008).
Participants
Eligible participants were 18 years or older and met DSM-IV criteria for bipolar I or II disorder. Diagnoses were confirmed by the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) with information corroborated from the Affective Disorders Evaluation (ADE) (Sachs et al., 2003). All eligible participants also met MINI criteria for an acute depressive episode at the time of randomization and were treated or willing to initiate treatment with a mood stabilizing medication. Participants were not receiving current psychotherapy outside of the study and, if so, were willing to either discontinue their non-study psychotherapy or reduce to 1 session or fewer per month. Participants were excluded from the study if they met criteria for a DSM-IV current mixed episode or depression not otherwise specified. Further description of inclusion and exclusion criteria can be found in Miklowitz et al., 2007 (Miklowitz et al., 2007b).
Measures
Primary Outcome Measures
Recovery
Mood symptoms (e.g., depression, mania) were assessed at each treatment visit using the Clinical Monitoring Form (CMF) (Sachs et al., 2002). Intraclass interrater reliability coefficients (referenced to gold standard ratings for CMF depression and mania items) ranged from 0.83 to 0.99 (Sachs et al., 2003). Designations of “recovery” or “non-recovery” were based upon the presence or absence of DSM-IV criteria for depression or mania/hypomania (Sachs et al., 2007). Participants were considered “recovered” if they experienced ≤ 2 moderate mood symptoms for ≥ 8 consecutive weeks. Participants were considered “non-recovered” if they did not meet these criteria. The number of days from randomization until achieving recovered/non-recovered status was also recorded for each participant, with a maximum of 365 days in the study.
Variables for Cluster Analysis
Six variables, yielding significant predictor or moderator effects in our previous studies, were used as input for the cluster analysis. These variables were: illness duration, number of manic episodes, number of depressive episodes, lifetime anxiety, medical burden and body mass index (more details provided below). The cluster analysis did not include other factors shown to relate to treatment outcome in other studies (e.g. substance use, trauma, personality disorders) as these were not significantly associated with outcome in the STEP-BD psychotherapy trial. Due to the analytic properties required of cluster analysis, participants included in the present investigation were required to have complete data for all of the variables listed below (i.e. no missing data on any one variable).
Course of Illness
The Affective Disorders Evaluation (ADE) was used to assess course of illness and onset of bipolar disorder. Three resulting variables were included: 1) Illness Duration (continuous), 2) Number of Manic Episodes, and 3) Number of Depressive Episodes. Episodes of depression and mania were reported separately as categorical variables and the effects of these variables were thus measured separately. Subcategories of number of episodes were defined as 1-9, 10-20, or >20 lifetime episodes each for depression and mania.
Lifetime Anxiety
A lifetime anxiety disorder was operationally defined as the presence of any current or past anxiety disorder as assessed by the Mini-International Neuropsychiatric Interview.
Medical Status and BMI
Participants reported the presence or absence of several medical conditions at baseline. These could include preexisting conditions or illnesses that may have been a result of previous treatments for bipolar disorder (e.g., Stevens-Johnson syndrome). These included sleep apnea, diabetes, cardiovascular problems, thyroid disease, cancer, hepatitis, multiple sclerosis, seizures, headaches, migraines, head trauma with loss of consciousness, other loss of consciousness, peptic ulcers, diastolic murmur, allergies, asthma, eczema, and Raynaud's phenomenon. We created an index of cumulative medical burden by summing across categories. This resulted in a continuous variable – total number of medical conditions endorsed. Participants’ weight and height were measured during their baseline visit. Body mass index (BMI), a ratio of height and weight, was calculated by dividing a participant's body weight by the square of their height.
Data Analytic Approach
In order to identify subsets of participants with similar clinical profiles, we first conducted agglomerative hierarchical cluster analyses using Ward's method of minimum variance with a squared Euclidean distance measure(Ward Jr, 1963). Ward's method is distinct from other methods because it uses an analysis of variance approach to evaluate the distances between clusters. The following variables were included in the cluster analysis based on prior findings that these variables predicted or moderated treatment outcome: illness duration, number of depressive episodes, number of manic episodes, lifetime anxiety, BMI, and total number of medical conditions. The best-distinguished cluster solution was determined from inspection of the dendogram, a tree diagram frequently used to illustrate the arrangement of the clusters produced by hierarchical clustering. After identifying two distinct clusters through inspection of the dendogram, k-means clustering, with 2 as the input for k, was used to determine the content of the distinct symptom profiles. One-way analysis of variance or chi-square tests were then used to compare clusters on relevant demographic and clinical characteristics (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Sample and Clusters
| Overall (n = 135) | Less-recurrent/severe (n = 73) | Chronic/recurrent (n = 62) | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Age** | 38.71 (11.82) | 32.04 (9.87) | 46.56 (8.58) |
| Age at Onset** | 20.70 (9.69) | 24.11 (9.93) | 16.69 (7.73) |
| Illness Duration** | 18.01 (12.62) | 7.93 (5.49) | 29.87 (7.07) |
| Depressive Severitya | 7.52 (2.35) | 7.36 (2.55) | 7.72 (2.10) |
| Manic Severityb | 1.18 (1.12) | 1.11 (1.21) | 1.27 (1.02) |
| Number of Sessions | 9.29 (10.35) | 7.97 (9.80) | 10.85 (10.84) |
| Global Functioning | 57.64 (9.41) | 58.07 (9.15) | 57.15 (9.76) |
| # Medical Co-morbidities | 2.21 (1.65) | 2.04 (1.55) | 2.42 (1.74) |
| # Psychiatric Co-morbidities | 1.85 (1.10) | 1.82 (1.12) | 1.88 (1.09) |
| # Anxiety Disordersc | 1.35 (1.35) | 1.27 (1.30) | 1.45 (1.42) |
| n (%) | n (%) | n (%) | |
|---|---|---|---|
| Female Sex* | 83 (62) | 51 (70) | 32 (52) |
| Education >1 yr college | 99 (73) | 54 (75) | 45 (74) |
| Married | 46 (34) | 24 (33) | 22 (36) |
| Diagnosis | |||
| Bipolar I | 84 (62) | 46 (63) | 38 (61) |
| Bipolar II | 51 (38) | 27 (37) | 24 (39) |
| Lifetime Anxiety Disorder | 91 (67) | 47 (64) | |
| Sleep Status (% normal)d | 54 (46) | 27 (43) | |
| Baseline Medications | |||
| Mood Stabilizers | 89 (66) | 45 (63) | 37 (59) |
| Antidepressants* | 73 (54) | 26 (36) | 36 (58) |
| Atypical Antipsychotics | 100 (74) | 21 (29) | 14 (23) |
| Anxiolytics | 35 (26) | 17 (24) | 18 (29) |
| Anticonvulsants | 77 (57) | 39 (53) | 38 (61) |
p < .05
p <.01
Depressive severity rated by the Clinical Monitoring Form (Sachs et al., 2002)
Manic severity rated by the Clinical Monitoring Form (Sachs et al., 2002)
Refers to total number of lifetime (current or past) anxiety disorders
Sleep status refers to being a short (<6 hours/night), normal (6 – 8 hours/night), or long (>8 hours/night) sleeper in the week prior to the baseline visit
Please see page 9 for a full description of statistics
To evaluate whether resulting clusters predicted or moderated likelihood of recovery or time until recovery, we conducted logistic regression and Cox proportional hazard (survival) models. All analyses were by intention to treat. Patients were included until their final assessment point, with a maximum of 365 days in the study (M = 168.88, SD = 104.55). The proportionality of risk assumption was met for all survival analyses. For both outcomes (recovery and time to recovery), cluster group was entered in the first block as a predictor (dummy coded with 0 = ‘less-recurrent/severe’ as the reference group), controlling for initial treatment condition (intensive therapy versus collaborative care). To test moderation, a treatment interaction term (cluster x treatment condition) was added to the second step of the models. Odds ratios are reported as the measure of effect size. For binary logistical regressions (and consistent with our previous studies (Deckersbach et al., 2014; Peters et al., 2014a; Peters et al., 2015)), we computed Number Needed to Treat (NNT) as an additional measure of treatment effects (Kraemer and Kupfer, 2006). In this study, NNT can be interpreted as the number of patients one would expect to treat with intensive psychotherapy to have one more responder (or one less non-responder) than if the same number were treated with collaborative care. NNT's could not be calculated for models predicting time to recovery; odds ratios were reported.
Results
Study Sample
Demographic and clinical characteristics for the total included sample that had all available measures (135 of 293 in the original sample), stratified by clusters, are presented in Table 1. Participants in the selected subsample resembled the full sample on the majority of demographic and clinical characteristics with a few exceptions. Participants in this subsample were on average 3 years younger (p = .003), had slightly poorer global functioning ratings (M = 57.64 vs. 54.96, p = .014), slightly higher depression ratings (M = 7.52 vs. 6.99, p = .046), more comorbid conditions (M = 1.85 vs. 1.58, p = .043), and fewer years of education (p = .009).
Psychosocial Treatment Outcome
Adjunctive intensive psychotherapy was shown to increase odds and hasten time to recovery compared to collaborative care in the full STEP-BD randomized cohort (N = 293; (Miklowitz et al., 2007a). In the original sample, 64% of participants achieved recovery in intensive psychotherapy versus 52% in collaborative care, corresponding to an NNT of 8.33 (medium – small). The effect of treatment condition on recovery, b = .16, SE = .36, p = .650, and time to recovery, b = .27, SE = .22, p = .219, was no longer significant in the smaller subsample of participants included in this analysis (N=135, NNT = 25 [small]). To determine whether this change in outcome was due to sample reduction or bias on behalf of the selected subsample, we tested whether a dummy coded variable (included selected subsample = 1, excluded = 0) interacted with treatment condition. Subgroup membership did not interact with treatment condition to predict recovery rates (p = .194) or time until recovery (p = .886). Thus, there was no evidence that the included sample was fundamentally less treatment responsive than the full sample.
Cluster Analysis
The cluster analysis yielded a two-cluster solution (see e-component file for dendogram). Comparisons between the two resultant clusters indicated that Cluster 1 (n = 73; Table 1) was characterized by younger age, later age at onset, shorter illness duration, fewer manic episodes, and fewer depressive episodes compared to Cluster 2 (n = 62). Clusters did not differ on BMI, medical burden, or proportion of individuals with lifetime anxiety. Given these clinical characteristics clusters were termed “less-recurrent/severe” (Cluster 1) and “chronic/recurrent” (Cluster 2).
Do Clusters Predict or Moderate Treatment Outcome?
Results of the modeling sequence are reported in Table 2.
Table 2.
Effects of Less-Recurrent/Severe and Chronic/Recurrent Clusters on Recovery Rates and Time to Recovery
| b | Wald | OR | P | 95% CI | |
|---|---|---|---|---|---|
| Logistic Regression: Predicting Recovery | |||||
| Treatment Groupa | −.60 | 1.34 | .55 | .247 | .20 – 1.51 |
| Clusterb | −1.10 | 3.94 | .33 | .047 | .11 - .98 |
| Clusterb × Treatment Groupa | 1.52 | 4.23 | 4.57 | .040 | 1.07 – 19.45 |
| Cox Regression: Predicting Time Until Recovery | |||||
| Treatment Groupa | −.22 | .58 | .80 | .448 | .45 – 1.42 |
| Clusterb | −1.01 | 8.24 | .37 | .004 | .18 - .72 |
| Clusterb × Treatment Groupa | .97 | 4.51 | 2.62 | .034 | 1.08 – 6.39 |
Treatment Group = intensive psychotherapy (1) vs. collaborative care (0)
Cluster = dummy coded with less-recurrent/severe cluster as the reference group
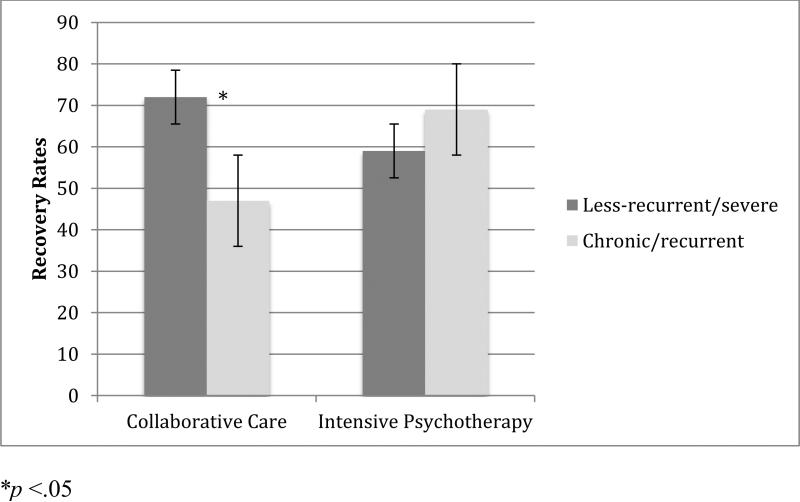
The clusters significantly predicted overall recovery rates; individuals in the chronic/recurrent cluster (Cluster 2) were less likely to achieve recovery than the individuals in the less-recurrent/severe cluster (Cluster 1; p = .047, OR = .33). In addition, clusters significantly moderated recovery rates (p = .040, OR = 4.56). The less-recurrent/severe group was more likely than the chronic/recurrent group to recover in collaborative care. The less-recurrent/severe and chronic/recurrent groups showed comparable rates of recovery in intensive psychotherapy (Figure 1). In collaborative care, 72% (n = 21 out of 29) of less-recurrent/severe participants achieved recovery, whereas 47% (n = 14 out of 30) of chronic/recurrent participants recovered. This corresponded to an NNT of 4 (medium significant effect, 95% CI: 2.0-62.7). In intensive psychotherapy, 59% (n = 26 out of 44) of less-recurrent/severe and 69% (n = 22 out of 32) of chronic/recurrent participants recovered. This corresponded to an NNT of 10 (small effect).
Figure 1.
Moderating Effect of Clusters on Recovery Rates to Intensive Psychotherapy versus Collaborative Care
We then compared response rates to collaborative care vs. intensive psychotherapy within each of the clusters. Within the chronic/recurrent group, the recovery rates in intensive psychotherapy compared to collaborative care corresponded to an NNT of 4.5 (medium effect), favoring intensive psychotherapy. In contrast, recovery rates in the less-recurrent/severe group showed a slight advantage of collaborative care, corresponding to an NNT of 7.7 (small to medium effect).
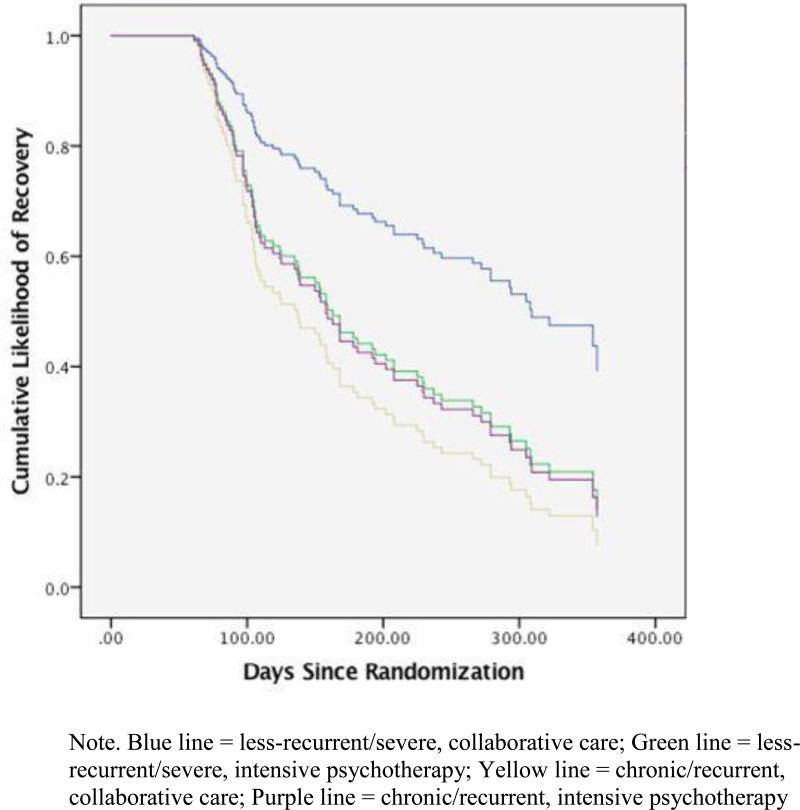
The clusters significantly predicted time to recovery; individuals in the chronic/recurrent cluster had a lower likelihood of recovery over time (p = .004, OR = .365). Clusters were also a significant moderator of time to recovery (p = .034, OR = 2.62). The less-recurrent/severe cluster had a higher probability of recovery over time in collaborative care relative to intensive psychotherapy. In contrast, the chronic/recurrent cluster had a higher probability of recovery over time in intensive psychotherapy than in collaborative care. Proportion of participants recovered over time according to cluster status and treatment group is illustrated in Figure 2.
Figure 2.
Moderating Effect of Clusters on Likelihood of Recovery over Time in Intensive Psychotherapy versus Collaborative Care
Note. Blue line = less-recurrent/severe, collaborative care; Green line = less-recurrent/severe, intensive psychotherapy; Yellow line = chronic/recurrent, collaborative care; Purple line = chronic/recurrent, intensive psychotherapy
Discussion
We utilized a novel approach to the analysis of treatment response prediction and moderation (Kraemer, 2013) to develop profiles of patients likely to benefit from more and less intensive psychotherapies for bipolar depression. Using this exploratory approach, we found that a combination of variables related to illness course (older age, earlier age at onset, longer illness duration, more manic episodes, and more depressive episodes) produced clear profiles of patients in an advanced/chronic stage of illness and those in an earlier illness course. Our findings indicate that individuals in later, more chronic illness stages are more resistant and slower to respond to psychotherapy overall. Rates of recovery in intensive psychotherapy were similar for less-recurrent/severe and chronic/recurrent participants, whereas those in the less-recurrent/severe phase were more likely than chronic/recurrent patients to achieve recovery in the briefer collaborative care. Intensive psychotherapy yields a faster recovery for participants in a chronic/recurrent stage of illness than for those in the less-recurrent/severe stage of illness, whereas collaborative care led to recovery sooner in the less-recurrent/severe cluster.
These findings have implications for clinical assessment. The metric driving rates and time to response is comprised of clinical characteristics that are easily obtained through routine clinical interviews and screening. Age at onset, illness duration, and chronicity of episodes are routinely reported by patients to mental health providers during initial or pre-treatment evaluations. Currently, standard clinical practice guidelines call for a detailed assessment of symptom severity (as evaluated by clinician interview and self-report) to dictate selection of the most appropriate treatment modalities and frequencies (Kendall et al., 2014). Our results suggest that illness history deserves considerable attention in a baseline evaluation as it affects patients’ likely responses to psychotherapy. Importantly, our findings suggest that illness course and history characteristics more strongly influence response to psychotherapy than current level of symptoms, diagnostic subgroup, anxiety (Deckersbach et al., 2014), medical illnesses, or weight problems (Peters et al., 2015).
Patients in the early stages of illness demonstrated higher rates of response to brief care than chronic patients. This finding would suggest that if given the option between a brief or intensive intervention for a patient in a less-recurrent/severe stage of illness, a brief intervention may not only be more efficacious, but also more cost-effective. For example, in the full trial, patients received a mean of 14.3 sessions of intensive psychotherapy and 2.2 sessions of collaborative care. Accordingly, collaborative care can be delivered for just 16% of the costs of intensive psychotherapy. Assuming an average session cost of $200, provision of collaborative care is, on average, $2,420 less expensive than intensive psychotherapy. In contrast, chronic/recurrent patients sustained a modestly higher likelihood of recovery over time in intensive psychotherapy, which may justify the higher cost of care.
The findings are broadly concordant with Berk and colleagues’ staging model (e.g., (Berk et al., 2014), which predicts that treatment response should be greater earlier in the course of illness. Using only total the number of episodes as a proxy of staging, those STEP-BD participants in later stages also had an overall poorer response, both in naturalistic treatment and in the adjunctive antidepressant trial (Magalhaes et al., 2012). It could be the case that the chronic/recurrent group is in the later stages of illness, where achieving recovery becomes more complex and difficult and intensive psychotherapy shows a modest advantage. Given that the chronic cluster was more likely to be taking an antidepressant, a greater functional burden of depression may contribute to the advantage of intensive psychotherapy in this group. Importantly though, we cannot determine whether the chronic cluster patients necessarily have had a more severe course of illness over time or whether the less-recurrent/severe cluster simply has yet to experience a recurrent course of bipolar disorder. Our previous findings (Peters et al., 2014b) and those from a study specifically of CBT (Scott et al., 2006) suggest that these benefits may asymptote after a certain number of episodes (e.g. between 12-20). These findings are also concordant with those of Reinares, who showed that early stage individuals were more likely to respond to psychoeducation than late stage individuals, manifested by a longer time to recurrence (Colom, 2010). It is possible that this is related to the cognitive progression that is seen in the disorder, intact cognition being necessary for cognitively based approaches (Rosa et al., 2014). Alternative to progressive staging models that tend to assume a unitary progression, it could also be the case that our findings represent two distinct forms of bipolar illness with different genetic underpinnings that present as generally mild or more severe phenotypes.
This study has several strengths, including leveraging a large, randomized controlled trial of psychotherapy for bipolar disorder with a well-powered, combined metric to evaluate predictors and moderators. Limitations include that cluster analyses require list-wise deletion of cases with missing data. Thus, we could not conduct our analyses on the full original sample enrolled in the STEP-BD psychotherapy trial. Furthermore, the subsample did not show the same significant difference in recovery rates between intensive psychotherapy and collaborative care as was observed in the full sample. In this respect, our analyses should be considered exploratory and warrant replication. Furthermore, the illness characteristics distinguished the resulting clusters (mood episodes, illness duration) were retrospectively reported and may have been subject to recall bias. Lastly, patients reported on their number of prior mood episodes, but did not specify the intervals of recurrence. Thus, we cannot fully disentangle whether clinical effects are driven by the sheer number of recurrences, their severity, or the speed with which patients cycle between episodes.
In summary, by way of using a well-powered, combined parametric approach to prediction and moderation, a simple assessment of illness duration and mood episode history can help assess which patients are most likely to respond to intensive psychotherapy vs. brief psychoeducation. Though these findings remain exploratory, they replicate other studies of pharmacotherapy (Berk et al., 2011; Franchini et al., 1999; Ketter et al., 2006), that suggest that prior illness course in bipolar disorder is a critical factor in determining who is likely to achieve recovery. These findings suggest that past course of illness is an important consideration in pretreatment assessment for optimizing treatment response to psychosocial intervention.
Highlights.
Cluster analyses can identify subsets of individuals with similar clinical profiles
We identified “less-recurrent/severe” and “chronic/recurrent” clusters of bipolar patients
Chronic/recurrent patients are less likely and slower to respond to psychotherapy
Intensive psychotherapy leads to faster recovery for chronic/recurrent participants
Illness history warrants further attention in clinical assessment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, Conus P, Bechdolf A, Moylan S, Malhi GS. Bipolar disorders. 2014;Stage managing bipolar disorder.16:471–477. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]
- Berk M, Brnabic A, Dodd S, Kelin K, Tohen M, Malhi GS, Berk L, Conus P, McGorry PD. Does stage of illness impact treatment response in bipolar disorder? Empirical treatment data and their implication for the staging model and early intervention. Bipolar disorders. 2011;13:87–98. doi: 10.1111/j.1399-5618.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Hirschfeld RM, Reed M, Davies MA, Frye MA, Keck PE, Lewis L, McElroy SL, McNulty JP, Wagner KD. Impact of bipolar disorder on a U.S. community sample. The Journal of clinical psychiatry. 2003;64:425–432. doi: 10.4088/jcp.v64n0412. [DOI] [PubMed] [Google Scholar]
- Colom F, Reinares M, Pacchiarotti I, Popovic D, Mazzarini L, Martínez-Arán A, Torrent C, Rosa A, Palomino-Otiniano R, Franco C, Bonnin CM, Vieta E. Has number of previous episodes any effect on response to group psychoeducation in bipolar patients? A 5-year follow-up post hoc analysis. Acta Neuropsychiatrica. 2010;22:50–53. doi: 10.1111/j.1601-5215.2010.00450.x. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Peters AT, Sylvia L, Urdahl A, Magalhaes PV, Otto MW, Frank E, Miklowitz DJ, Berk M, Kinrys G, Nierenberg A. Do comorbid anxiety disorders moderate the effects of psychotherapy for bipolar disorder? Results from STEP-BD. The American journal of psychiatry. 2014;171:178–186. doi: 10.1176/appi.ajp.2013.13020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell'Osso MC, Pellegrini F, Rossi A. Prevalence, chronicity, burden and borders of bipolar disorder. Journal of affective disorders. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Franchini L, Zanardi R, Smeraldi E, Gasperini M. Early onset of lithium prophylaxis as a predictor of good long-term outcome. European archives of psychiatry and clinical neuroscience. 1999;249:227–230. doi: 10.1007/s004060050091. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DK, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski V, Houck P, Scott J, Thompson W, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, Grochocinski VJ, Houck PR, Kupfer DJ. The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder. 2008;165:1559–1565. doi: 10.1176/appi.ajp.2008.07121953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biological psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Freeman SA, McElroy SL. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology, and treatment issues. Journal of affective disorders. 2002;68:1–23. doi: 10.1016/s0165-0327(00)00299-8. [DOI] [PubMed] [Google Scholar]
- Kendall T, Morriss R, Mayo-Wilson E, Marcus E, Guideline Development Group of the National Institute for, H. Care E. Assessment and management of bipolar disorder: summary of updated NICE guidance. Bmj. 2014;349:g5673. doi: 10.1136/bmj.g5673. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Houston JP, Adams DH, Risser RC, Meyers AL, Williamson DJ, Tohen M. Differential efficacy of olanzapine and lithium in preventing manic or mixed recurrence in patients with bipolar I disorder based on number of previous manic or mixed episodes. The Journal of clinical psychiatry. 2006;67:95–101. doi: 10.4088/jcp.v67n0113. [DOI] [PubMed] [Google Scholar]
- Kohler S, Gaus S, Bschor T. The challenge of treatment in bipolar depression: evidence from clinical guidelines, treatment recommendations and complex treatment situations. Pharmacopsychiatry. 2014;47:53–59. doi: 10.1055/s-0033-1364004. [DOI] [PubMed] [Google Scholar]
- Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Statistics in medicine. 2013;32:1964–1973. doi: 10.1002/sim.5734. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biological psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Lam DH, Hayward P, Watkins ER, Wright K, Sham P. Relapse prevention in patients with bipolar disorder: cognitive therapy outcome after 2 years. The American journal of psychiatry. 2005;162:324–329. doi: 10.1176/appi.ajp.162.2.324. [DOI] [PubMed] [Google Scholar]
- Lauder S, Chester A, Castle D, Dodd S, Gliddon E, Berk L, Chamberlain J, Klein B, Gilbert M, Austin DW, Berk M. A randomized head to head trial of MoodSwings.net.au: an Internet based self-help program for bipolar disorder. J Affect Disord. 2015;171:13–21. doi: 10.1016/j.jad.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Liu CS, Carvalho AF, Mansur RB, McIntyre RS. Obesity and bipolar disorder: synergistic neurotoxic effects? Advances in therapy. 2013;30:987–1006. doi: 10.1007/s12325-013-0067-7. [DOI] [PubMed] [Google Scholar]
- Magalhaes PV, Dodd S, Nierenberg AA, Berk M. Cumulative morbidity and prognostic staging of illness in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Aust N Z J Psychiatry. 2012;46:1058–1067. doi: 10.1177/0004867412460593. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Keck PE., Jr. Metabolic syndrome in bipolar disorder: a review with a focus on bipolar depression. The Journal of clinical psychiatry. 2014;75:46–61. doi: 10.4088/JCP.13r08634. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ. Bipolar disorder: A family-focused treatment approach. 2nd ed. Guilford Press; New York: 2008. [Google Scholar]
- Miklowitz DJ, Otto MW. Psychosocial interventions for bipolar disorder: a review of literature and introduction of the systematic treatment enhancement program. Psychopharmacol Bull. 2007;40:116–131. [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, Thase ME, Calabrese JR, Marangell LB, Ostacher MJ, Patel J, Thomas MR, Araga M, Gonzalez JM, Wisniewski SR. Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial. The American journal of psychiatry. 2007a;164:1340–1347. doi: 10.1176/appi.ajp.2007.07020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Kogan JN, Nierenberg AA, Calabrese JR, Marangell LB, Gyulai L, Araga M, Gonzalez JM, Shirley ER, Thase ME, Sachs GS. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007b;64:19–26. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Wisniewski SR, Araga M, Frank E, Reilly-Harrington NA, Lembke A, Sachs GS. Psychotherapy, symptom outcomes, and role functioning over one year among patients with bipolar disorder. Psychiatr Serv. 2006;57:959–965. doi: 10.1176/ps.2006.57.7.959. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, Sachs-Ericsson N, Suddath R. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biological psychiatry. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. [DOI] [PubMed] [Google Scholar]
- Peters A, Sylvia LG, da Silva Magalhaes PV, Miklowitz DJ, Frank E, Otto MW, Hansen NS, Dougherty DD, Berk M, Nierenberg AA, Deckersbach T. Age at onset, course of illness and response to psychotherapy in bipolar disorder: results from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Psychological medicine. 2014a:1–13. doi: 10.1017/S0033291714000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sylvia LG, Magalhaes PV, Miklowitz DJ, Frank E, Otto MW, Hansen NS, Dougherty DD, Berk M, Nierenberg AA, Deckersbach T. Age at onset, course of illness and response to psychotherapy in bipolar disorder: results from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Psychol Med. 2014b;44:3455–3467. doi: 10.1017/S0033291714000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AT, Shesler LW, Sylvia L, da Silva Magalhaes PV, Miklowitz DJ, Otto MW, Frank E, Berk M, Dougherty DD, Nierenberg AA, Deckersbach T. Medical burden, body mass index and the outcome of psychosocial interventions for bipolar depression. Aust N Z J Psychiatry. 2015 doi: 10.1177/0004867415616694. [DOI] [PubMed] [Google Scholar]
- Post RM, Kalivas P. Bipolar disorder and substance misuse: pathological and therapeutic implications of their comorbidity and cross-sensitisation. The British journal of psychiatry : the journal of mental science. 2013;202:172–176. doi: 10.1192/bjp.bp.112.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Ostacher MJ, Singh V. Controversies in the psychopharmacology of bipolar disorder. The Journal of clinical psychiatry. 2014;75:e30. doi: 10.4088/JCP.13095tx2cj. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Magalhaes PV, Czepielewski L, Sulzbach MV, Goi PD, Vieta E, Gama CS, Kapczinski F. Clinical staging in bipolar disorder: focus on cognition and functioning. The Journal of clinical psychiatry. 2014;75:e450–456. doi: 10.4088/JCP.13m08625. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Guille C, McMurrich SL. A clinical monitoring form for mood disorders. Bipolar disorders. 2002;4:323–327. doi: 10.1034/j.1399-5618.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz DJ, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biological psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. The British journal of psychiatry : the journal of mental science. 2006;188:313–320. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–23. quiz 34-57. [PubMed] [Google Scholar]
- Thase ME, Kingdon D, Turkington D. The promise of cognitive behavior therapy for treatment of severe mental disorders: a review of recent developments. World psychiatry : official journal of the World Psychiatric Association. 2014;13:244–250. doi: 10.1002/wps.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, Frank E, Kraemer HC. A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA psychiatry. 2013;70:1241–1247. doi: 10.1001/jamapsychiatry.2013.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH., Jr Hierarchical grouping to optimize an objective function. Journal of the American statistical association. 1963;58:236–244. [Google Scholar]