Abstract
IL-15 has been implicated as a key regulator of T and NK cell homeostasis in multiple systems; however, its specific role in maintaining peripheral T and NK cell populations relative to other gamma-chain (γc) cytokines has not been fully defined in primates. Here, we address this question by determining the effect of IL-15 inhibition with a rhesusized, anti-IL-15 mAb on T and NK cell dynamics in rhesus macaques. Strikingly, anti-IL-15 treatment resulted in rapid depletion of NK cells, and both CD4+ and CD8+ effector memory T cells (TEM) in blood and tissues, with little to no effect on naïve or central memory T cells. Importantly, whereas depletion of NK cells was nearly complete and maintained as long as anti-IL-15 treatment was given, TEM depletion was countered by the onset of massive TEM proliferation, which almost completely restored circulating TEM numbers. Tissue TEM, however, remained significantly reduced, and most TEM maintained very high turnover throughout anti-IL-15 treatment. In the presence of IL-15 inhibition, TEM became increasingly more sensitive to IL-7 stimulation in vivo, and transcriptional analysis of TEM in IL-15-inhibited monkeys revealed engagement of the JAK/STAT signaling pathway, suggesting alternative γc cytokine signaling may support TEM homeostasis in the absence of IL-15. Thus, IL-15 plays a major role in peripheral maintenance of NK cells and TEM. However, whereas most NK cell populations collapse in the absence of IL-15, TEM can be maintained in the face of IL-15 inhibition by the activity of other homeostatic regulators, most likely IL-7.
INTRODUCTION
Lymphocyte homeostasis and function are tightly regulated by the activities of the common gamma chain (γc) cytokines, in particular IL-2, IL-7 and IL-15. While these 3 cytokines share the γc receptor (CD132) their activity on various lymphocyte populations are often different, differences that are, in part, mediated by differential expression of the α-chain component of the receptor (1–7). For instance, IL-2 is mainly produced by CD4+ T cells, and to a lesser degree CD8+ T cells, NK cells and NKT cells, and it acts to generally potentiate the expansion of activated T and NK cells (8, 9). However, IL-2 has a unique primary role in regulating immune tolerance by promoting the production and maintenance of CD4+ CD25+ Foxp3+ T regulatory (Treg) cells, which constitutively express IL-2Rα (CD25). Indeed, this was demonstrated in early models of IL-2−/− and IL-2Rα−/−/IL-2Rβ−/− knockout (KO) mice, which manifested rapid lethal autoimmune diseases likely resulting from the failure of Treg development and homeostasis (10). IL-7 is produced by non-hematopoietic cells such as stromal and epithelial cells and is important for thymocyte development and peripheral T cell homeostasis. IL-7 signals via the receptor IL-7R, which is a heterodimer consisting of the IL-7Rα (CD127) and CD132. IL-7 is particularly important for promoting the proliferative expansion and survival of naïve T cells (TN) and central memory T cells (TCM), which maintain high levels of CD127 expression (11). IL-15, on the other hand, has been shown to regulate the homeostasis and activation of many cell types throughout the body, including memory T cells, NK cells, invariant NKT cells, γδ T cells and intestinal intraepithelial lymphocytes (12–22). As such, IL-15Rα−/− and IL-15−/− KO mice typically manifest severe deficiencies in these cell subsets (23). IL-15 signals via interaction with heterotrimeric receptor complex composed of IL-15Rα (CD215), IL-2/15Rβ (CD122) and CD132 (16, 24–27). IL-15Rα is expressed on antigen-presenting cells (macrophages, monocytes and dendritic cells) and binding of IL-15 to IL-15Rα enables trans-presentation to a responding cell expressing CD122 and CD132 (28). Because biologically active IL-15 has been shown to exist in a soluble form in complex with IL-15Rα (29), it has been questioned whether IL-15Rα is part of the receptor for IL-15 or part of a heterodimeric cytokine that interacts with the CD122/CD132 receptor. In either scenario, the IL-15Rα protein is thought to be a critical determinant of IL-15 specificity and function.
Although each of these γc cytokines has unique characteristics, their in vivo activity often manifests considerable overlap. For instance, IL-2 and IL-15 share the same β receptor (CD122) and are both involved in the initial amplification of antigen-specific T cell responses, and the regulation of memory T cell development, differentiation, and maintenance (30–32). In addition, both IL-2 and IL-15 induce the activation and proliferation of NK cells and enhance NK cell cytolytic activity by inducing the up-regulation of effector molecules such as perforin and granzyme B (33–35). Similarly, IL-7 and IL-15 both seem to play major, albeit non-exclusive, roles in maintaining peripheral TM homeostasis, supporting both TM proliferation and survival (31). Thus, the specific non-redundant roles these γc cytokines play in controlling various lymphocyte population dynamics in vivo are not completely characterized, a lack of understanding that complicates efforts to rationally develop therapeutic strategies based on their specific biologic activities to enhance immune responses to cancer or microbial agents, to promote immune reconstitution after conditions of lymphopenia (HIV infection, chemotherapy, aging), or to counter pathologic immune responses in the various autoimmune/inflammatory disorders (rheumatoid arthritis, celiac disease, inflammatory bowel disease, multiple sclerosis and type 1 diabetes) linked to dysregulation of these cytokines (36–40).
Due to its activity on NK cells and antigen-specific cytotoxic T cells, IL-15 is in clinical trials for the treatment of metastatic malignancies (41). Previous studies have shown that IL-15 can increase the production of long-lived antigen-specific TM (32, 42, 43), and can also induce the migration and redistribution of TM from circulation into tissues (44, 45). In nonhuman primates (NHP), provision of exogenous IL-15 typically induces an initial brief period of lymphopenia followed by lymphocytosis (45–47). Lymphocytosis is associated with the expansion of NK cells and TM (41, 44). However, the TM compartment is quite heterogeneous and comprises the TCM subset, which is responsible for anamnestic T cell responses and primarily recirculates between secondary lymphoid tissues, and the effector-differentiated memory subsets – transitional memory (TTrM) and TEM - which can also migrate to extra-lymphoid effector sites (48). In NHP, TEM and TTrM are very responsive to IL-15 in vivo, and studies from our lab and others have shown that exogenous IL-15 administration can dramatically increase the proliferative fraction and absolute numbers of TEM and TTrM in peripheral blood (44, 46, 47). In contrast, TN and TCM are less responsive to IL-15 administration, and it is still unclear whether IL-15 plays a direct role in vivo in regulating their homeostasis. Most of these studies have focused on CD8+ T cells, and in general, IL-15 has been more closely associated with regulation of CD8+ TM than with CD4+ TM. However, CD4+ TEM and TTrM are also highly responsive to IL-15 in vivo, suggesting that this discrepancy is largely attributable to the fact that the highly IL-15-responsive TEM subset comprises a much larger fraction of total circulating TM for CD8+ than for CD4+ lineage cells (44).
In this study, we sought to determine the specific, non-redundant role(s) of IL-15 in the regulation of CD4+ and CD8+ T and NK cell population dynamics in NHP. As indicated above, most previous studies addressing IL-15 activity in NHP have assessed the effect of therapeutic administration of IL-15, which results in unnaturally high levels of cytokine immediately after administration. Since such pharmacologic (supra-physiologic) IL-15 levels may not accurately reflect physiologic cytokine function in vivo, we have taken the alternative approach of determining the impact of inhibiting IL-15 activity in vivo with a newly developed rhesusized anti-IL-15 monoclonal antibody (mAb) on T cell and NK cell homeostasis in rhesus macaques (RM). We demonstrate that this rhesusized anti-IL-15 can be repeatedly administered to RM and is highly effective at long-term inhibition of IL-15 activity in vivo. We further demonstrate that in vivo inhibition of IL-15 activity resulted in a near complete depletion of NK cells and a significant decrease in the numbers of circulating CD4+ and CD8+ TEM with negligible effects on the TCM or TN subsets. Strikingly, however, TEM, but not NK cell numbers, rebounded by proliferative expansion, and in the absence of IL-15 signaling, TEM became increasingly more sensitive to IL-7 signaling. These data suggest that whereas IL-15 signaling is required for NK cell homeostasis, TEM can be maintained by other cytokines, most likely IL-7, when IL-15 signaling is not available.
MATERIALS AND METHODS
Animals
A total of 41 purpose-bred RM (Macaca mulatta) of Indian genetic background and free of Macacine herpesvirus 1, D type simian retrovirus, simian T-lymphotrophic virus type 1, and SIV infection were used in this study. A group of 30 RM were administered the rhesus recombinant anti-IL-15 mAb, clone M111 (n=17) or rhesus recombinant IgG control antibody (n=13), i.v. once every two weeks at 20mg/Kg on day 0 and 10mg/Kg on days 14 and 21. The neutralizing anti-IL-15 antibody was constructed by grafting the complementarity determining regions of mouse anti-human IL-15 mAb, M111 (American Type Culture Collection) into rhesus variable region frameworks. MAb was expressed in Chinese hamster ovary cells as full-length Ig with rhesus IgG1 and kappa constant regions. Two RM were dosed with an immunologically silenced form of the same antibody (anti–IL-15 LALA) which contained the L235A, L236A mutation in the heavy chain constant region. RM dosed with the anti-IL-15 LALA mAb displayed similar phenotypes to the monkeys treated with the non-mutated anti-IL-15 mAb for all parameters assayed, and were therefore grouped as anti-IL-15 mAb-treated RM. BrdU (Sigma-Aldrich) was prepared as previously described (49), and administered i.v. in three separate doses of 30mg/Kg body weight over a 24-hour period prior to terminal necropsy at ≥35 days post-first anti-IL-15 treatment. A separate group of 11 RM received 6 biweekly doses of rhesus recombinant anti-IL-15 mAb (n=6) or rhesus recombinant IgG control (n=5) at 20mg/Kg on day 0, followed by 10mg/Kg on days 14, 28, 42, 56 and 77, concurrently, with subcutaneous administration of rhesus recombinant IL-7 at 30μg/Kg on days 35 and 42. All RM were housed at the Oregon National Primate Research Center in accordance with standards of the Center’s Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of plasma IL-7 concentration
The concentration of IL-7 in the plasma was measured by ELISA using the IL-7 Quantikine HS kit (R & D Systems) according to manufacturer’s instructions.
Flow cytometric analysis and cell sorting
Whole blood and mononuclear cells isolated from lymph nodes (LN), bronchoalveolar lavage (BAL), bone marrow, spleen, kidney, lung, liver, tonsil, and vaginal and intestinal mucosa were obtained and stained for flow cytometric analysis as described previously (49, 50). Polychromatic (8–12 parameter) flow cytometric analysis was performed on an LSR II instrument using Pacific blue, AmCyan, FITC, PE, PE–Texas red, PE-Cy7, PerCP-Cy5.5, Allophycocyanin, Allophycocyanin-Cy7, and Alexa Fluor 700 as the available fluorescent parameters. Instrument set-up and data acquisition procedures were performed as previously described (51). List mode multiparameter data files were analyzed using FlowJo software. Delineation of naive and memory T cell subsets and criteria for setting + versus − markers for CCR5 and Ki-67 expression have been previously described (49, 50, 52–54). In brief, TN constitute a uniform cluster of cells with a CD28moderate, CCR7+, CCR5−, CD95low phenotype, which is clearly distinguishable from the phenotypically diverse memory population that is CD95high and may display one or more of the following non-naive phenotypic features: CD28−, CCR7−, or CCR5+. The TCM, TTrM, and TEM components of the memory subset in the blood were further delineated based on the following phenotypic criteria: TCM (CD28+, CCR7+ and CCR5−), TTrM (CD28+, CCR7+/− and CCR5+), and TEM (CD28−, CCR7− and CCR5dim). For delineating NK cells in blood, small lymphocytes were gated to obtain CD3−, CD8α+, NKG2a+ cells that were CD20− and CD14−. NK cell subsets were further delineated based on CD16 and CD56 expression as previously described (55). For each subset to be quantified, the percentages of the subset within the overall small lymphocyte populations were determined. For quantification of peripheral blood subsets, absolute small lymphocyte counts were obtained using an AcT5diff cell counter (Beckman Coulter) and from these values, absolute counts for the relevant subset were calculated based on the subset percentages within the light scatter–defined small lymphocyte population on the flow cytometer. Baseline values were determined as the average of values at days −14, −7 and 0. Results are presented as percentage of baseline, with baseline shown as 100%, or changes in proliferative fraction, indicated as the difference in the %Ki-67+ (Δ%Ki-67+) measured at the designated time points from baseline (0% = no change).
For analysis of pSTAT5 expression, whole blood (100μL) was added to polystyrene flow tubes and stained with fluorochrome-conjugated mAbs against CD3, CD4, CD8, CD28, CCR5, CCR7 and CD95, at room temperature (RT) for 30 minutes. Next, tubes were either left unstimulated or stimulated with increasing concentrations of IL-7 or IL-15 (ranging from 0.5 – 32ng/mL) for 15 min at 37°C/5% CO2. Detection of pSTAT5 was assessed with the BD Phosflow staining protocol according to the manufacturer’s instructions. Briefly, cells were fixed with the BD Phosflow Lyse/Fix buffer for 5 min at RT and permeabilized in ice-cold BD Phosflow perm buffer IV for 5 min at RT. After washing, cells were stained for intracellular markers with fluorochrome-conjugated anti-pSTAT5 and anti-Ki-67 for 45 min at RT. Cells were washed and flow cytometric analysis was performed on the LSR II flow cytometer.
In vitro cytokine-induced expansion assay
PBMCs were sort purified using a FACS Aria II (BD Biosciences) based on defined phenotypic markers as described above and plated in 48-well plates in 1mL of R10 media [RPMI (HyClone), 10% Fetal Bovine Serum (FBS), 100units/mL Penicillin, 10mg/mL Streptomycin (Sigma-Aldrich), 200μM L-glutamine (Sigma-Aldrich)] at a density of 150,000 to 300,000 cells/mL. IL-7 or IL-15 were added at a concentration of 50ng/mL to the cultures and incubated at 37°C/5% CO2 for 14 days alone or in the presence of 10% sort purified CD14+ monocytes. After 7 days, the culture was resuspended and 0.5mL was removed for phenotypic analysis by flow cytometry. An equal amount of fresh R10 was added back to the remaining culture and incubated at 37°C/5% CO2 for a further 7 days. On day 14, the entire culture was harvested for phenotypic analysis by flow cytometry.
Antibodies and cytokines
The following antibodies were used for flow cytometry: CD3 Alexa 700 (SP34-2 BD Biosciences), CD4 AmCyan (L200 BD Biosciences), CD8 PerCP-Cy5.5 (SKI eBiosciences), CD8 AmCyan (SKI BD Biosciences), CD28 PE-Texas Red (CD28.2 Beckman Coulter, BD Biosciences), CD95 PE (DX2 BD Biosciences, eBiosciences), CCR5 Allophycocyanin (3A9 BD Biosciences), Ki-67 FITC (B56 BD Biosciences), CD56 PerCP-Cy5.5 (MEM-188 Invitrogen), CD16 Pacific Blue (3G8 BD Biosciences, Biolegend), CD20 Allophycocyanin-Cy7 (L27 BD Biosciences), HLA-DR PE-Texas Red (TU36 Invitrogen, Immu357 Beckman Coulter), NKG2A PE (Z199 Beckman Coulter), CD14 FITC (M5E2 BD Biosciences, R&D Systems), STAT5 PE (47/Stat5 (pY6) BD Biosciences), BrdU FITC (B44 BD Biosciences), BrdU Allophycocyanin (B44 BD Biosciences). Anti-CCR7 (150503) was purchased as purified immunoglobulin from R&D Systems, conjugated to biotin using a Pierce Chemical Co. biotinylation kit, and visualized with streptavidin–Pacific Blue (Invitrogen). Rhesus recombinant anti-IL-15 and rhesus recombinant control IgG1 mAb were provided through the National Institutes of Health’s Nonhuman Primate Reagent Resource Program. Simian recombinant IL-7 was provided by Cytheris SA (Issy-Les-Moulineaux, France). Rhesus recombinant IL-15 was provided by Francois Villinger (Emory University) through the Resource for Nonhuman Primate Immune Reagents.
Immunohistochemistry
Immunohistochemistry was performed as previously described (56). Antibodies used in this study were mouse monoclonal anti-human IL-15 (Antibodies Online; clone BDI150), rabbit monoclonal anti-active caspase-3 (Cell Signaling Technologies; clone 5A1E), rabbit monoclonal anti-phosphorylated STAT5 (1:100; clone C11C5; Cell Signaling Technologies, Inc.), and rabbit monoclonal anti-human CD3 (clone SP7; Labvision/Thermo Fisher Scientific). All stained slides were scanned at high magnification (x200) using the ScanScope CS System (Aperio Technologies) yielding high-resolution data from the entire tissue section. Representative regions of interest (ROIs; 250 to 500 mm2) were identified and high-resolution images extracted from these whole-tissue scans. The percent area of the lymph node T cell zone and lamina propria (colon) that stained for each protein/cell type of interest were quantified using Photoshop CS5 using Fovea tools.
Microarray analysis
For transcriptional analysis, sort-purified CD8+ TEM and CD8+ TCM from PBMC, obtained 28–49 days post-first anti-IL-15 or control IgG mAb administration, were resuspended in RLT lysis buffer (Qiagen) and stored at −80°C until use. RNA was isolated using RNeasy Micro Kits (Qiagen), and the quantity and quality of the RNA was confirmed using a NanoDrop 2000c (Thermo Fisher Scientific) and an Experion Electrophoresis System. Samples (50ng) were amplified using Illumina TotalPrep RNA amplification kits (Ambion). The microarray analysis was conducted using 750ng of biotinylated complementary RNA hybridized to HumanHT-12_V4 BeadChips (Illumina) at 58°C for 20h. The arrays were scanned using Illumina’s iSCAN and quantified using Genome Studio (Illumina). The analysis of the Genome Studio output data was conducted using the R and Bioconductor software packages. Quantile normalization was applied, followed by a log2 transformation performed using the Bioconductor LIMMA package (57). Outlier samples with abnormalities in gene expression based on hierarchical clustering methods and multidimensional scaling analysis as a dimensionality reduction method for the evaluation of similarities or dissimilarities between samples were identified and removed. The LIMMA package was used to fit a linear model to each probe and perform (moderated) t tests or F tests on the groups being compared. A Gene Set Enrichment Analysis (GSEA) (58) using 1000 permutations was performed on the genes differentially expressed between the groups compared in the subsets pre-ranked by the decreasing order of the absolute T-statistic. The canonical pathways of Ingenuity Pathway Analysis software (IPA, Ingenuity Systems) were used as the database to perform GSEA. This was followed by building modules of related pathways based on at least 25% gene overlap [Jaccard index (59) > 25%] between pathways using the enrichment map (60) strategy and representing the genes present in at least 25% of the pathways in the module. To control the expected proportions of false positives, the FDR for p-values was calculated using the Benjamini and Hochberg method implemented in LIMMA. The complete dataset is available at the Gene Expression Omnibus microarray repository (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=mbqtaouqztynzsz&acc=GSE76797), accession number GSE76797.
Statistics
To investigate if the administration of anti-IL-15 mAb blocks signaling in circulating lymphocytes, we estimated the EC50 (the dose where the response is the midpoint between the maximum and minimum) for each RM at given time points (days post-mAb) using the four-parameter logistic model often referred to as an Emax model, followed by repeated measures ANOVA to evaluate the effect of anti-IL-15 mAb with EC50 as a response variable and anti-IL-15 mAb-treated status as between group factor, and days post-antibody as within group factors. Longitudinal analysis of STAT5 phosphorylation, peripheral blood TN and TM (including TCM, TTrM and TEM subsets) counts and proliferation, peripheral blood NK cell counts and plasma IL-7 levels were evaluated using repeated-measures ANOVA with anti-IL-15 mAb and IgG control mAb-treated groups as between group factors and time points as within group factors; since in a typical experiment using repeated measures, two measurements taken at adjacent times are more highly correlated than two measurements taken several time points apart. Due to the limited sample size, a simpler covariance structure, first order auto-regressive (AR1), was used as correlation within each animal. Tukey-Kramer adjustment was used to control for multiple comparisons. Differences in the number of pSTAT5+ cells/mm2 in peripheral lymph nodes between pre-treatment and post-treatment were evaluated using Wilcoxon Signed rank tests. The difference in CD3+ caspase-3+ in colon and peripheral LN via immunostaining and BrdU incorporation in CD4+ and CD8+ T cell subsets between anti-IL-15 and IgG control mAb-treated groups was compared using Mann-Whitney U test. Data was analyzed using SAS 9.4. The p-values <0.05 were considered to be statistically significant.
RESULTS
Differential signaling of IL-15 and IL-7 on NK and T cell subsets in vitro
As a first step in characterizing the specific, non-redundant in vivo role(s) of IL-15 in controlling NHP T cell and NK cell population dynamics, we determined the extent to which RM CD4+ and CD8+ T cell and NK cell subsets, studied directly ex vivo in peripheral blood, could respond to IL-15 and IL-7. Since common γc cytokines mediate signal transduction via the JAK/STAT signaling pathway leading to the phosphorylation of the transcription factor STAT5 (61), we quantified the fraction of cells within each phenotypically defined subset that phosphorylated STAT5 (e.g., became pSTAT5+) within 15 min after exposure to increasing concentrations of these two γc cytokines. As shown in Figs. 1A and 1B, both IL-15 and IL-7 induced pSTAT5 in naïve and all memory T cell subsets; however, there were clear differences among these subsets with respect to their dose response to these cytokines, and the fraction of responding cells at the optimal dose. TN and TCM (both CD4+ and CD8+) were more responsive to IL-7 than to IL-15 with over 60% of cells in these subsets expressing pSTAT5 at 4ng of IL-7 vs. less than 12% of TN and TCM at the same dose of IL-15. In contrast, both CD4+ and CD8+ TEM showed increased responsiveness to IL-15 compared to IL-7 with pSTAT5 expression between 60–80% at 8ng, in contrast to less than 40% with the same dose of IL-7. This reduced response to IL-7 is likely due to the low expression of CD127 on TEM (62). Interestingly, CD4+ and CD8+ TTrM responded to IL-7 and IL-15 at similar levels, reflecting their intermediate phenotype between TCM precursors and effector-differentiated TEM [Supplementary Figure 1; (54)]. NK cells showed an even larger difference in responsiveness to IL-7 vs. IL-15. Although all circulating NK cell subsets [defined by CD16 and CD56; (55)] responded robustly to IL-15 with induction of pSTAT5 expression in >80% of cells at 16ng (Fig. 1C), only the CD56+ CD16− “regulatory” NK cell subset showed any response to IL-7 stimulation at the tested doses, and this response required a 4-fold higher dose of IL-7 than the response of the same cells to IL-15.
Figure 1.
Comparative analysis of IL-7 and IL-15 signaling in T cell and NK cell populations. (A) Representative histograms showing the induction of pSTAT5 in the indicated peripheral blood T cell subsets after ex vivo stimulation with 8ng/mL of IL-7 (blue), 8ng/mL of IL-15 (red) or no stimulation (gray). (B) Dose response curves showing the induction of pSTAT5 in the indicated peripheral blood T cell subsets after ex vivo stimulation with 0, 0.5, 1, 2, 4, 8, 16, 32 ng/mL of IL-7 (n=14) or IL-15 (n=14). Results (mean + SEM) are shown as change from baseline. (C) Induction of pSTAT5 in the indicated peripheral blood NK cell subsets after ex vivo stimulation with 0, 0.5, 1, 2, 4, 8, 16, 32 ng/mL of IL-7 (n=8) or IL-15 (n=8). Results (mean + SEM) are shown as change from baseline.
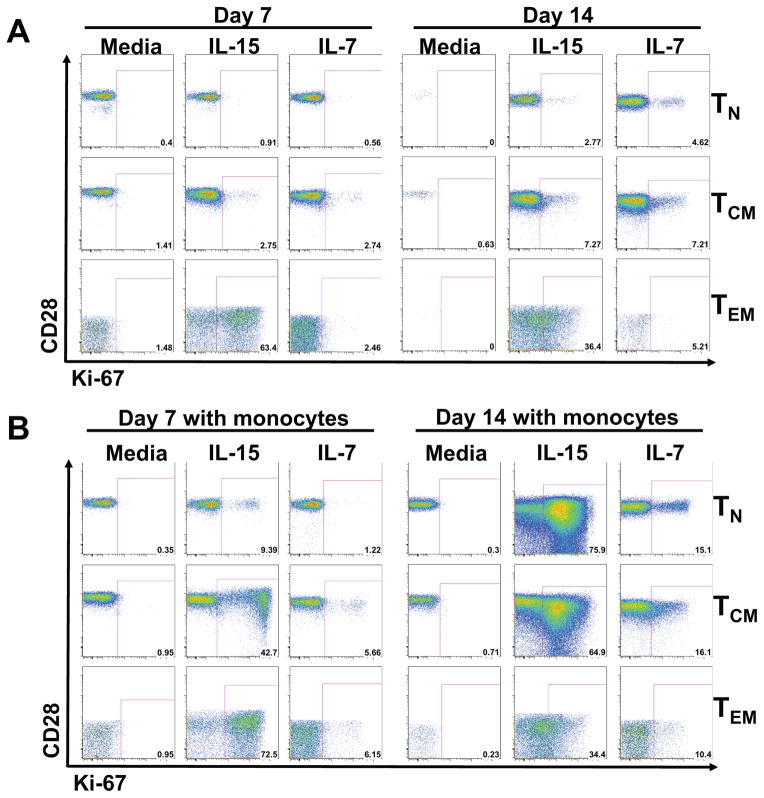
These results demonstrate that lymphocyte populations differ in their response to early signal transduction by IL-7 vs. IL-15, and suggest that IL-7 and IL-15 preferentially signal pre-effector and effector populations, respectively. We next sought to determine the degree to which this differential signaling translates to downstream gene expression and homeostatic regulation by examining the ability of these cytokines to support survival and proliferative expansion of sort-purified TN, TCM and TEM during 7 and 14 days of in vitro culture. Since the pattern of STAT5 phosphorylation to IL-7 and IL-15 among these differentiation-defined subsets was similar between the CD4+ and CD8+ lineages (Fig. 1A–B), we primarily focused on CD4+ T cells for these studies. In this in vitro CD4+ T cell model system, responses to IL-15 were generally more robust than to IL-7, but responsiveness to both cytokines was differentiation dependent. TEM were again found to be highly responsive to IL-15 compared to IL-7, with over 60% of IL-15-treated cells expressing the proliferation antigen Ki-67 by day 7 of culture, compared to background Ki-67 expression after IL-7 treatment (Fig. 2A). IL-7 and IL-15 induced similar, modest increases in Ki67 expression by CD4+ TCM, whereas IL-7 was more effective than IL-15 in inducing TN proliferation. Next, based on the concept that IL-15 might be more efficiently presented in trans, we cultured the same cells with IL-15 or IL-7 in the presence of sort-purified CD14+ monocytes (63, 64). In the presence of monocytes, we saw modestly enhanced responsiveness of TN, TCM, and TEM to IL-7, and profoundly increased responsiveness of TN and TCM to IL-15 (Fig. 2B). Interestingly the CD4+ TEM proliferative response to IL-15 was similar with or without monocytes, suggesting that trans-presentation or other monocyte-derived signals are not required for maximal TEM responsiveness. Similar IL-15 responsiveness was demonstrated for CD8+ TEM (Supplementary Figure 2A).
Figure 2.
Representative dot plots demonstrative of 8 separate experiments, showing induction of Ki-67 expression on CD4+ TN, TCM and TEM. CD4+ T cell subsets were purified from PBMC by multi-parameter cell sorting and cultured in media containing (A) recombinant IL-15 or recombinant IL-7 at 50ng/mL, alone or (B) in the presence of 10% highly purified CD14+ monocytes. After 7 and 14 days, cultures were analyzed for Ki-67 expression.
Collectively, these in vitro studies demonstrate that TN and TCM are more responsive to IL-7 than TEM, whereas TEM are more responsive to IL-15. However, IL-15 can induce robust proliferation in TN and TCM in the presence of monocytes, suggesting a more pleotropic role for this cytokine. Interestingly, culture of TN and TCM with IL-15 in the presence of monocytes induces up-regulation of CCR5 and down-regulation of CCR7 (Supplementary Figure 2B), consistent with induction of effector memory differentiation (50, 65). Thus, IL-15 may both maintain the homeostasis of pre-existing TEM and, under certain conditions, drive TEM differentiation from noneffector-differentiated precursors.
Anti-IL-15 specifically blocks IL-15 signal transduction in vivo
To investigate the role of IL-15 in T cell and NK cell homeostasis in vivo, we developed a “rhesusized” anti-IL-15 mAb that is suitable for repeated administration to RM. This mAb was based on the mouse, anti-human IL-15 mAb clone, M111, which is cross-reactive with RM IL-15 (66). Rhesusization was accomplished by exchanging the mouse amino acid sequences in the constant regions and variable binding surfaces for rhesus amino acid sequences, leaving variable region sequences responsible for IL-15 recognition unchanged (67, 68). We hypothesized that repeated administrations of this rhesusized M111 mAb would block IL-15 activity in vivo and result in changes to T and/or NK cell population dynamics that would reflect physiologic IL-15 function. To initially test this approach, we administered rhesus anti-IL-15 or a rhesus IgG control mAb to RM every other week for 6 weeks prior to necropsy, as shown in Fig. 3A. We assessed IL-15 signaling inhibition by performing pSTAT induction analysis with rhesus recombinant IL-15 on whole blood at various time points post-treatment. From day 1 through day 35 post-treatment, the ability of IL-15 to induce pSTAT5 expression in CD4+ and CD8+ T cells ex vivo in anti-IL-15-treated RM was significantly reduced (essentially abrogated) compared to baseline and to control IgG-treated RM (p<0.0001; Fig. 3B and Supplementary Figure 3A–B). To further assess the in vivo activity of our anti-IL-15 mAb treatment, we performed immunohistochemical analysis of LN sections from both control IgG mAb-treated and anti-IL-15 mAb-treated RM using the original mouse anti-IL-15 mAb M111. As expected, there was a substantial reduction in IL-15 immunoreactivity in LN of anti-IL-15-treated RM compared with control RM (Fig. 3C), demonstrating either absence of IL-15 post-treatment or blocking of immunostaining by the administered function-abrogating mAb (consistent with in situ neutralization). As a final confirmation of the in vivo effectiveness of our anti-IL-15 mAb, we determined whether anti-IL-15 treatment reduced JAK/STAT signaling in situ by measuring pSTAT5 expression in LN sections. As shown in Fig. 3D and Supplementary Figure 3C, we observed that RM treated with the anti-IL-15 mAb, but not the IgG control mAb, had a significant reduction in pSTAT5 detection post-treatment (untreated animals: p=0.38, anti-IL-15-treated RM: p<0.0001). This decline in pSTAT5 expression indicates a reduction in JAK/STAT signal transduction specific to IL-15. A complete inhibition of pSTAT5 in tissues was not expected as other γc cytokines such as IL-2 and IL-7, which are not inhibited by M111, also signal via the JAK/STAT pathway. Taken together, these results clearly demonstrate that the rhesusized anti-IL-15 mAb can effectively inhibit IL-15 signaling in vivo.
Figure 3.
Anti-IL-15 administration specifically blocks IL-15 signaling in RM. (A) Schematic representation of the anti-IL-15 treatment schedule used in this study. Healthy RM received 20mg/Kg of anti-IL-15 or IgG control antibody on day 0 and 10mg/Kg on days 14 and 28. 24 hours prior to necropsy, all RM received 3 doses of BrdU at 30mg/Kg. (B) Showing the change in pSTAT5 in peripheral blood TN, TCM, TTrM and TEM, after ex vivo stimulation with 8ng/mL of IL-15 in anti-IL-15 mAb-treated (n=9) or IgG control mAb-treated (n=5) RM. Results (mean + SEM) are shown as percentage of baseline and significance was assessed as described in materials and methods. (C) Representative images from immunohistochemical analysis performed on lymph node (LN) sections obtained from IgG control mAb-treated (top panel) and anti-IL-15 mAb-treated (bottom panel) RM using a mouse mAb which is cross-reactive with rhesus IL-15 (original magnification 200X). (D) Quantification of the number of pSTAT5+ cells/mm2 in peripheral LN of RM treated with anti-IL-15 mAb (n=15) or IgG control mAb (n=8). Significance was evaluated using Wilcoxon Signed rank tests.
IL-15 blockade results in near complete NK cell depletion
Having demonstrated the in vivo activity of the rhesusized anti-IL-15 mAb, we next examined the effect of this IL-15 blockade on peripheral lymphocyte population dynamics. As shown in Fig. 4A, compared to control mAb treatment, anti-IL-15 mAb administration resulted in a near complete loss of NK cells in the blood in the first 2 weeks of treatment. Importantly, the absolute numbers of total NK cells in anti-IL-15-treated RM were maintained at <5% of baseline through day 35. Since the cytotoxic CD16+ CD56− NK cell subset constitutes the most abundant population in the peripheral blood of RM (55), it is not surprising that the post-treatment dynamics of this subset closely followed the overall NK cell population with absolute counts declining to <14% of baseline by day 7, and <3% of baseline at day 35. The depletion of the other NK subsets was substantial but less complete. The CD16− CD56− NK subset declined to ~10% of baseline by day 14, but recovered to <30% of baseline by day 35, despite ongoing therapy. Interestingly, the largely regulatory CD16− CD56+ NK subset was least affected by IL-15 blockade, showing a maximal depletion to ~25% of baseline by day 28. We would note that CD16− CD56+ NK cells were the only NK subset to respond to IL-7 signaling in our in vitro assay (Fig. 1C), suggesting the possibility that IL-7 may compensate for IL-15 signaling inhibition to partially maintain CD16− CD56+ NK cell homeostasis (see below).
Figure 4.
IL-15 blockade induces profound depletion of NK cells in blood and tissues. (A) Quantification of absolute NK cell counts, including CD16+ CD56−, CD16 −CD56+ and CD16− CD56− subsets in blood after anti-IL-15 mAb (n=17) or IgG control mAb (n=13) treatment. Significance was assessed as described in materials and methods. Results (mean + SEM) are shown as percentage of baseline. (B) Dot plots showing CD3− NKG2A+ NK cells in peripheral blood, spleen, liver, lung, kidney and tracheobronchial lymph node (LN TB) of two representative RM that received either anti-IL-15 mAb or IgG control mAb prior to necropsy. (C) Lymphocytes isolated from the indicated tissues were analyzed to determine frequencies of total NK cells (CD3−, CD8α+, NKG2A+) within the overall small lymphocyte population in anti-IL-15 mAb-treated (n=9) or IgG control mAb-treated (n=5) RM. Histograms show mean frequencies of the NK cells as a fraction of total lymphocytes in each tissue, error bars show SEM.
To more comprehensively assess the extent of NK cell depletion after anti-IL-15 treatment we isolated cells from peripheral lymphoid and extra-lymphoid tissues at necropsy of the anti-IL-15-treated vs. control-treated RM, and determined the fraction of NK cells (CD3− CD8α+ NKG2A+ lymphocytes) among total lymphocytes in each tissue. As shown in Fig. 4B–C, NK cells were dramatically reduced in anti-IL-15 mAb-treated RM compared with controls. Taken together, these data demonstrate that anti-IL-15 mAb administration can induce systemic depletion of NK cells in RM, with the implication that both circulating and tissue resident NK cells in RM are highly dependent on IL-15 signaling for their homeostatic stability.
IL-15 blockade results in selective perturbation of TEM homeostasis
As expected based on the above-described differential sensitivity of T cells to IL-15 signaling according to differentiation status, the impact of IL-15 blockade on T cell population dynamics varied by differentiation defined subset. Anti-IL-15 mAb treatment had a negligible effect on circulating CD4+ and CD8+ TN, as absolute counts and proliferative responses in anti-IL-15 mAb-treated RM were not significantly different from what was observed in control RM (Fig. 5A). A similar pattern was observed with the TCM subsets, as IL-15 blockade had no significant effect on CD4+ or CD8+ TCM absolute counts and CD8+ TCM proliferation, and resulted in only marginal enhancement of CD4+ TCM proliferation. However, IL-15 blockade had a profound effect on TEM dynamics, with anti-IL-15 administration resulting in a rapid reduction in CD4+ and CD8+ TEM absolute counts to ~30% of baseline by day 14 post-treatment (CD4+: p=0.0006, CD8+: p<0.0001). Strikingly, starting about day 14, this depletion was countered by a dramatic increase in the fraction of proliferating TTrM and TEM, such that absolute CD4+ and CD8+ TEM counts in blood were restored to pretreatment (CD4+), or near-pretreatment levels (CD8+) by day 35. While anti-IL-15 treatment significantly increased the proliferative fraction of CD4+ and CD8+ TTrM (CD4+: p<0.0001, CD8+: p<0.0001), absolute TTrM counts remained unchanged, possibly reflecting a proportion of TTrM differentiating to TEM. Indeed, we would note that the relative proportions of all memory T cell subsets in the blood prior to anti-IL-15 administration returned to baseline by day 35 post-treatment (Fig. 5B). Taken together, these data demonstrate a key, specific role for IL-15 signaling for TEM homeostasis, but also indicate that a significant proportion of TEM, both CD4+ and CD8+, can persist in the presence of IL-15 signaling inhibition (similar to the CD16− CD56+ NK cell subset), suggesting other regulatory molecules could play a role in modulating peripheral TEM regeneration and stability in the absence of IL-15.
Figure 5.
Comparison of T cell dynamics in blood of anti-IL-15-treated RM. (A) Absolute counts and proliferative fraction of CD4+ and CD8+ TM, including the TCM, TTrM, and TEM subsets in blood of RM treated with anti-IL-15 mAb (n=17) or IgG control mAb (n=13). Results (mean + SEM) are shown as percentage of baseline, or for percentage of Ki-67+, change (Δ) from baseline with significant p-values shown as: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Significance of difference between treatment groups was assessed as described in materials and methods. (B) Pie charts showing the relative proportions of CD4+ and CD8+ TCM, TTrM and TEM subsets as fraction of total memory T cells in the blood of anti-IL-15-treated RM (n=16) prior to treatment, and days 14 and 35 post-anti-IL-15 mAb treatment.
We next determined whether the robust increases in CD4+ and CD8+ TEM and TTrM proliferation observed in response to anti-IL15 mAb treatment was confined to cells in the blood or a reflection of cells proliferating in tissues prior to entering into peripheral circulation. To address this question we used in vivo BrdU labeling to label all cells in S phase around the time of peak proliferation (see Fig. 3A). BrdU incorporation offers a more accurate indication of the location and timing of cell proliferation (specifically delineating S-phase of the mitotic cycle) than Ki-67 expression, which is maintained for several days following cell division during which time cell migration might occur (50). We administered three doses of BrdU within 24 hours prior to necropsy to label cells synthesizing DNA in this 24-hour period. We observed that the frequencies of BrdU-labeled CD4+ and CD8+ TCM in all tissues were unchanged in anti-IL-15 mAb-treated RM compared to controls (Fig. 6A), substantiating our previous conclusion that anti-IL-15 treatment has negligible effects on steady state TCM homeostasis (Fig. 5A). There was also no difference between control mAb- and anti-IL-15-treated RM in the frequencies of BrdU-labeled CD8+ TTrM, although we did observe significant differences in the %BrdU+ CD4+ TTrM in the blood (p=0.018), spleen (p=0.044), lung (p=0.024) and liver (p=0.018) with anti-IL-15 mAb treatment. Strikingly, however, the TEM compartment saw the most dramatic increases in cell turnover in the presence of IL-15 signaling inhibition, which was apparent for both the CD4+ and CD8+ T cell lineages. Anti-IL-15-treated RM showed increased frequencies of BrdU+ CD8+ TEM within most peripheral LN (iliosacral: p=0.024 and tracheobronchial: p=0.018) as well as within the lung (p=0.045), whereas frequencies of BrdU+ CD4+ TEM were significantly higher in blood (p=0.044), lung (p=0.024), kidney (p=0.045) and liver (p=0.018) of anti-IL-15-treated animals. Interestingly, the frequencies of BrdU-labeled CD4+ and CD8+ TEM in the intestinal lamina propria (including the colon, ileum, jejunum) and vaginal mucosa were not significantly different between anti-IL-15 mAb- and IgG control mAb-treated RM.
Figure 6.
IL-15 blockade increases TEM turnover in vivo. (A) RM treated with anti-IL-15 mAb (n=7) or IgG1 control mAb (n=5) were intravenously administered three doses (30 mg/Kg) of BrdU 24 hours prior to necropsy. Lymphocytes were isolated from the indicated tissues and further analyzed for T cell markers and BrdU positivity. Results (mean + SEM) are shown as the percentage of BrdU+ cells (CD4+ or CD8+ TCM, TTrM and TEM) determined for each tissue. Significance was evaluated using Mann-Whitney U test (*p<0.05). (B–C) Quantification of the T cell population within the colon lamina propria (LP) as determined by (B) the percent area of the LP that stained CD3+ and (C) extent of apoptosis measured by caspase-3 positive within the colon and (D) lymph node (LN) via immunostaining in RM following anti-IL-15 mAb or IgG control mAb treatment. The significance in these parameters between groups was evaluated with Mann-Whitney U test. (E–F) Images show active caspase-3 expression (red, original magnification 200X) in the colon (E) and LN (F) of three representative RM treated with either the IgG control mAb (top images) or anti-IL-15 mAb (bottom images).
Collectively, this data suggests that in the presence of IL-15 blockade, a significant proportion of TEM initially undergo apoptosis due to abrupt withdrawal of IL-15 signaling, but subsequently are replaced by TEM cells generated by a homeostatic proliferative response that is IL-15-independent (e.g., occurs despite ongoing IL-15 blockade). Although this homeostatic response returns the numbers of circulating TEM back to baseline levels, it does not appear to restore the TEM compartment to normal as we observed a significant reduction in CD3+ T cell density in the colonic lamina propria (p<0.0001) in anti-IL-15 mAb-treated RM compared with control RM (Fig. 6B). In should be noted that the blood TEM compartment is very small compared to the tissue effector site TEM compartment represented by the colonic lamina propria, and thus the latter is a much more accurate indicator of the status of the overall TEM population than the former (which can be “reconstituted” by relatively few cells). In addition, we observed a significant increase in caspase-3 expression within lymphocytes of both colonic lamina propria (p=0.0087) and LN T cell zones (p = 0.02) (Fig. 6C–F), suggesting increased and sustained levels of T cell apoptosis. Thus, the compensatory mechanism that restores circulating TEM numbers in the absence of IL-15 results in a high turnover state that is unable to completely reestablish normal TEM homeostasis.
IL-15 blockade disrupts pathways that regulate T cell activation, cell cycle entry and survival
To further characterize the impact of IL-15 signaling inhibition on TEM homeostasis, we performed transcriptomic profiling on sort-purified CD8+ TEM obtained at the approximate peak of TEM proliferation in the blood. These studies demonstrated that CD8+ TEM from anti-IL-15 mAb-treated RM showed significant differences with control IgG mAb-treated RM with respect to gene expression in several signal transduction pathways and transcriptional nodes that regulate proximal T cell activation, cell cycle entry and cell survival/cell death (Fig. 7A–B). With regards to T cell activation, components of TCR signaling (CD3D, CD3E), activation markers such as MHC molecules, and transcription factors downstream of TCR signaling (REL, CIITA) (69), were all up-regulated in CD8+ TEM from anti-IL-15 mAb-treated RM (Fig. 7A). Genes associated with cell cycle progression (ATM, E2F2, PIK3CB, PIK3CG, PIK3R4, PIK3C2A) were also up-regulated in these cells, while the cyclin-dependent kinase inhibitor, CDKN2B/p15, which inhibits cell cycle G1 progression, was down-regulated (Fig. 7B). In addition, genes associated with the mitochondrial respiration machinery (MRM) pathway, including NDUFV1, NDUFA2, UQCR1, UQCRB and ATP5C1, were significantly up-regulated in CD8+ TEM from the anti-IL-15 mAb-treated RM (Fig. 7C). This was further supported by the up-regulation of genes for enzymes with antioxidant activity, such as PRDX3 and GPX4, which protect cells from death by their effect on lipid peroxidation (70). All of these changes in TEM from anti-IL-15 mAb-treated RM are consistent with their increased proliferation observed in vivo (Fig. 5A). However, we also noted concomitant changes in gene pathways associated with T cell survival, in particular the upregulation of cells that promote cell death, including Bax, Fas, and Casp6 (p53 pathway) and Cabin1 and Mef2 (Nur77 signaling pathway) (71) (Supplementary Table 1). In addition, disruption of cell survival mechanisms was further shown by the down-regulation of BCL-2 (Fig. 7B). Collectively, these observations suggest that IL-15 signaling inhibition results in induction of TEM activation and proliferation, but at the same time, results in transcriptional changes that may limit the long-term TEM survival.
Figure 7.
Transcriptomic profiling on sort-purified CD8+ TEM and CD8+ TCM obtained from anti-IL-15 mAb-treated RM and compared with samples taken from RM treated with the IgG control mAb. GSEA was performed to identify pathways that are enriched at a p-value <0.05 and for grouping pathways into functional modules. The genes represented in the heatmaps are enriched in at least 50% of the gene sets of the module (if the number of gene sets in the module is less than or equal to 4), and genes enriched in at least 25% of the gene sets of the module (if the number of gene sets in the module is greater than 4). The color scale of the pathways represent the −log10(p-value) of the pathway (A, B, and D). (A) Module 1 enriched in CD8+ TEM is representative of T cell activation. (B) Module 2 enriched in CD8+ TEM is representative of cell cycle progression. (C) Representation of the mitochondrial respiration machinery pathway in CD8+ TEM up-regulated in the anti-IL-15 mAb-treated RM compared to IgG control mAb-treated RM. (D) Module enriched in CD8+ TCM is representative of up-regulation of pro-apoptotic signaling and down-regulation of Type I Interferon activity with blockade of IL-15.
Transcriptional profiling of CD8+ TCM also revealed differences in gene expression pathways between anti-IL-15 mAb-treated and IgG control mAb-treated RM (Fig. 7D); however, these differences involved pathways and genes distinct from those observed in TEM. In addition, the number of genes differentially expressed between anti-IL-15 mAb-treated RM vs. IgG- control mAb-treated RM in CD8+ TEM (2808 genes) was significantly greater than those differentially expressed in the CD8+ TCM (2225 genes) (p-value < 2.2e-16, using a Pearson’s chi-squared test for equality of proportions). Strikingly, IL-15 blockade did not induce changes in the expression of genes associated with the MRM pathway in TCM, suggesting no disruption to their steady state mitochondrial activity. However, as shown by the module enrichment in TCM, we did observe an up-regulation of pro-apoptotic genes (BID, APAF1, CASP8, CASP3) in this cell type in anti-IL-15 mAb-treated RM, possibly reflecting an increase in cell death in a fraction of TCM induced to differentiate in the absence of IL-15 signaling. Interestingly, we also observed the enrichment of the S1P1 pathway in TCM that regulates lymphocyte egress from the lymph node (72) (Supplemental Table 1). However, these differences did not translate to significant changes in TCM population dynamics in vivo, as absolute counts and proliferative fractions of CD8+ TCM were statistically indistinguishable between anti-IL-15 mAb-treated RMs and controls (Fig. 5A).
TEM become increasingly sensitive to IL-7 in response to IL-15 blockade
Collectively, gene expression profiling demonstrates the dramatic effect IL-15 blockade has on peripheral TEM, in comparison to the TCM. Somewhat paradoxically, in the absence of IL-15 signaling, circulating CD8+ TEM predominantly display the induction of genes involved with T cell activation, cell cycle progression and mitochondrial activity, raising the possibility that in the periphery, TEM are responding to another pro-proliferative cytokine. In this regard, we asked whether IL-7 might be supporting TEM homeostasis in the absence of IL-15. IL-7 is the most likely candidate to act as a compensatory cytokine for IL-15 because of its bioavailability in the blood and LN (73–75). Notably, IL-15 blockade did not change plasma levels of IL-7 (Fig. 8A) suggesting that anti-IL-15 mAb treatment did not increase overall production of IL-7. We then determined whether TEM proliferating in response to anti-IL-15 mAb treatment manifest increased sensitivity to IL-7. To address this question, we performed ex vivo pSTAT induction analysis on whole blood at various time points post-anti-IL-15 mAb treatment with 4ng of IL-7 (this dose was selected because it reflected the minimum dose that elicited a maximal response in most T cell subsets in our in vitro assay; Fig. 1B). Interestingly, we observed a significant increase in pSTAT5 expression in response to this dose of IL-7 in both CD4+ and CD8+ TEM from anti-IL-15 mAb-treated RM compared with controls (CD4+: p=0.0073, CD8+: p=0.0016) (Fig. 8B). Importantly, this increase in IL-7 responsiveness was only observed in the TEM subset, as the Δ pSTAT5 expression in TN, TCM or TTrM after IL-7 stimulation was statistically indistinguishable between both groups.
Figure 8.
TEM are more responsive to IL-7 in the presence of IL-15 signaling inhibition. (A) Plasma collected at indicated time points post-anti-IL-15 mAb (n=6) or IgG control mAb (n=4) treatment was analyzed for IL-7 levels by ELISA. Results (mean + SEM) are shown as percent change from baseline in IL-7 concentration (NS = not significant). (B) Showing change in pSTAT5 expression in peripheral blood TN, TCM, TTrM and TEM, after ex vivo stimulation with 4ng of IL-7 in RM treated with anti-IL-15 mAb (n=9) or IgG control mAb (n=5). Significance of difference in all parameters was assessed as described in materials and methods (*p<0.05).
To determine whether anti-IL-15 treatment increases IL-7 responsiveness in TEM in vivo, we designed an in vivo experiment in which anti-IL-15 mAb-treated or IgG control mAb-treated RM were treated with recombinant simian IL-7 (rsIL-7) (11, 62). RM received three doses of anti-IL-15 or IgG control mAb bi-weekly followed by two doses of rsIL-7 (30μg/Kg) one week apart and then a further three doses of anti-IL-15. To ensure a steady state of IL-15 neutralization at the time of IL-7 dosing, rsIL-7 was administered between the 3rd and 4th doses of anti-IL-15. As shown in Fig. 9A, rsIL-7 induced a 2-fold increase in the absolute numbers of CD4+ and CD8+ TCM in the blood, which was similar between anti-IL-15 mAb-treated RM and controls, further supporting our previous observations that IL-15 blockade has negligible effects on steady state TCM homeostasis (Fig. 5A and 6A). In contrast, there was a greater increase in both CD4+ and CD8+ TEM absolute counts in the blood of anti-IL-15 mAb-treated RM in response to IL-7, which was significant on days 10 (p=0.016), 12 (p=0.003) and 14 (p<0.001) for CD4+ and on days 10 (p=0.002) and 12 (p=0.0069) for CD8+. This increase in absolute TEM counts peaked by day 14, before declining to levels similar to controls by day 28. These data strongly support the concept that TEM become more responsive to IL-7 in vivo in the absence of IL-15 signaling. This increased sensitivity to IL-7 could help support the rapid TEM proliferative expansion observed after anti-IL-15 treatment. Consistent with this interpretation, the transcriptional analysis of peripheral blood CD8+ TEM described above also showed an up-regulation of genes associated with the JAK/STAT pathway in anti-IL-15-treated RM, highlighted by the up-regulation of STAT1 and SOCS3 (known to be downstream of IL-7 and other cytokines), suggesting engagement of this pathway (Fig. 9B). Altogether these data support the involvement of secondary γc receptor cytokine signaling in supporting TEM homeostasis in the presence of IL-15 signaling inhibition.
Figure 9.
The TEM response to IL-7 in vivo is increased with anti-IL-15 treatment. (A) RM were administered anti-IL-15 mAb (n=6) or IgG control mAb (n=5) biweekly over 8 weeks for a total of 5 doses. On days 35 and 42 post-treatment, both groups received 2 subcutaneous injections of IL-7 at 30ug/Kg. Results (mean + SEM) are shown as percentage of baseline absolute counts of the indicated T cell subsets. The significance in parameters between RM groups was evaluated using rANOVA with Tukey-Kramer adjustment (significant p-values shown as: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). (B) Transcriptomic profiling of sort purified CD8+ TEM showing genes associated with the JAK/STAT pathway (source gene set from MSigDB) at p<0.05 in anti-IL-15 mAb-treated RM compared with IgG control mAb-treated RM.
DISCUSSION
IL-2, IL-7 and IL-15 share a common γc receptor and mediate most of their immunoregulatory function through activation of the JAK/STAT signaling pathway. Because of these common features, the downstream activities of these cytokines often overlap, but nevertheless, each has its own biologic role. In this study, we sought to characterize the distinct role IL-15 plays in maintaining lymphocyte population dynamics in NHP. To achieve this we developed an RM model of in vivo IL-15 blockade that involved repeated administration of a rhesusized anti-IL-15 mAb. We hypothesized that blocking IL-15 signaling would result in substantial changes in lymphocyte population dynamics specific to IL-15 function. Similar to previous reports in humans and cynomolgus macaques, anti-IL-15 was well tolerated, with no adverse side effects observed with repeated dosing. We directly demonstrated that anti-IL-15 administration was effective at inhibiting IL-15 signaling in vivo.
A major consequence of IL-15 blockade was the systemic, nearly complete depletion of NK cells, demonstrating the essential role of IL-15 in the development and survival of most NK cells in RM. A recent report by Lebrec et al., suggests that IL-15 may not be required for NK cell homeostasis in humans, as a blocking monoclonal antibody directed against human IL-15 did not decrease circulating NK cell counts (66). While it is unclear why anti-IL-15 administration did not affect NK cell homeostasis in that study, our data confirms previous observations in mice and NHP that IL-15 blockade significantly diminishes NK cell populations in vivo (23, 34, 76–79). In our study, the CD16+ CD56− NK cell subset, which constitutes the major NK population in the blood of RM was severely depleted by IL-15 blockade. In contrast, the minor NK cell subset (CD16− CD56+) was less sensitive to IL-15 signaling inhibition. Considering CD16− CD56+ NK cells can induce pSTAT5 expression in response to both IL-7 and IL-15 in vitro, whereas CD16+ CD56− NK cells respond only to IL-15 (Fig. 1C), it is possible that this less efficient depletion of the CD16− CD56+ NK cell subset is due to the ability of IL-7 to compensate for IL-15 signaling inhibition, partially supporting their homeostatic maintenance.
The impact of IL-15 blockade on both CD4+ and CD8+ TEM was also both dramatic and specific, revealing a unique aspect of TEM homeostasis. In the absence of IL-15 signaling, absolute CD4+ and CD8+ TEM (but not TN, TCM or TTrM) counts in blood rapidly declined to less than 70% of baseline levels. However, starting between 7 and 14 days after treatment initiation, this initial TEM decline was countered by a burst of sustained proliferation in both TEM and TTrM that corresponded with an increase in TEM counts in blood back to baseline or near baseline levels. Although some anti-γc cytokine antibodies that display blocking effects in vitro sometimes become activating (pro-proliferative) when administered in vivo (80–85), we feel that this mechanism is unlikely in our study as the effects of anti-IL-15 included (1) the ex vivo inhibition of IL-15 signaling as measured by the induction of pSTAT5 expression, (2) the systemic depletion of NK cells and (3) the selective effects of anti-IL-15 mAb treatment on TTrM and TEM, but not TN or TCM populations. However, we did experimentally address the issue of whether TEM were indirectly induced to proliferate in response to IL-15/anti-immune complexes via an Fc-dependent mechanism. To test this we treated 2 RM with a variant of the rhesus anti-IL-15 mAb that had an inactivating LALA mutation in the Fc receptor-binding site (86). We found that this Fc inactivation had no effect on the in vivo activity of the anti-IL-15 mAb on T cell and NK cell dynamics (data not shown), and we thus concluded that the TEM proliferative response following anti-IL-15 mAb treatment was most likely not an immune complex mediated artifact, but rather a compensatory, IL-15-independent homeostatic process.
This conclusion is also supported by the observations that T cell densities in colonic lamina propria [an extra-lymphoid effector site with predominant populations of resident memory T cells with TEM or TTrM phenotypes; (48, 87)] remained significantly depleted well after normalization of absolute TEM counts in blood, and that high TEM proliferation was maintained for the duration of treatment (7 weeks), associated with increased levels of active caspase-3, consistent with apoptosis and a high turnover state (88), which wad widely (although not homogeneously) distributed in peripheral lymphoid and extra-lymphoid tissues. Transcriptional profiling of CD8+ TEM in the blood confirmed a predominant up-regulation of genes associated with T cell activation and cell cycle progression in anti-IL-15 mAb-treated RM. In particular, genes involved with mitochondrial respiration were up-regulated in CD8+ TEM, indicating that in the periphery, TEM can receive secondary signals that support proliferative expansion and an active metabolic state in the absence of IL-15. The cell substrate upon which this homeostatic mechanism depends remains an open question with 3 non-mutually exclusive possibilities. First, the homeostatic mechanism may simply operate on TEM cells that are normally maintained by IL-15, but can respond to other cytokines in the absence of IL-15. Although, in this scenario, we would posit the availability or activity of these putative non-IL-15 cytokines is suboptimal, accounting for the incomplete TEM reconstitution and failure to achieve (or approach) quiescence during the 7 weeks of observation. Second, since in mice there is clear heterogeneity in the IL-15 dependence of effector memory CD8+ T cell populations in various sites (89), it is possible the rebounding population in anti-IL-15 mAb-treated RM derives from the IL-15-independent subsets. In this scenario, the incomplete reconstitution observed in the IL-15-deficient RM might be due to an inability of these IL-15-independent subsets to effectively fill TEM niches usually occupied by IL-15-dependent cells. Finally, it is possible that the TEM regeneration that occurs in the absence of IL-15 derives from TTrM or even TCM precursors that are induced by non-IL-15 signals to both proliferate and differentiate towards TEM. As with possibility #2, the observed incomplete TEM reconstitution in this situation might be due to inability of these non-IL-15 regulators to induce full differentiation into the panoply of TEM cells needed to fill all TEM niches.
The next question to be addressed concerns the nature of homeostatic factor(s) responsible for stimulating the TEM proliferative expansion in the presence of IL-15 signaling inhibition. In this regard, it is interesting that treatment of monkeys with the JAK3 inhibitor CP-690550, which would inhibit all γc cytokines (90) results in depletion of NK cells and CD8+ memory T cells (particular TEM) similar to what we report here for anti-IL-15 mAb treatment; however, with JAK3 inhibition CD8+ TM rebound occurs only after drug cessation and there is no evidence of the TEM regenerative process described in this report (43). These differences suggest that the TEM regenerative process observed with anti-IL-15 depends upon the JAK3 signaling, and therefore might involve a non-IL-15 member or members of the γc cytokine family.
Although expression of the IL-7 receptor CD127 is maintained at low levels on TEM, the relative abundance of IL-7 in peripheral circulation as well as constitutive expression within lymphoid microenvironments suggests IL-7 as a primary candidate for this role. Initial analysis of plasma cytokine levels showed no changes in IL-7 or other immune-modulating cytokines such as TNFα, and IL-12 (data not shown). Although this indicated that anti-IL-15 mAb treatment did not increase IL-7 production, it did lead us to ask whether TEM might acquire increased sensitivity to endogenous IL-7 in the absence of IL-15 signaling. In line with this, we observed a significant increase in the fraction of both CD4+ and CD8+ TEM with pSTAT5 expression in response to IL-7 stimulation. Interestingly, this increase in IL-7 responsiveness was not observed in other T cell subsets (TN, TCM or TTrM). This effect was further demonstrated in vivo by administering IL-7 to monkeys that were being treated with anti-IL-15 vs. control mAbs. Notably, absolute numbers of CD4+ and CD8+ TEM, but not TCM, were significantly increased in response to IL-7 in anti-IL-15 mAb-treated RM compared to controls. While it’s important to note that we did not examine whether TEM were more responsive to other γc cytokines (i.e., IL-2), our data strongly supports the concept that TEM generated in the absence of IL-15 signaling become increasingly more responsive to JAK/STAT signaling via at least one other γc cytokine (IL-7). Transcriptional profiling also supported this conclusion by revealing an increased expression of the transcription factor, STAT1 in TEM. STAT1 is up-regulated during conditions of lymphopenia and plays a role in regulating the expansion and survival of CD8+ T cell responses, particularly after viral infection (91, 92). Since IL-7 can activate STAT1 and JAK1/STAT1 signaling has been linked with supporting T cell survival (93), it is possible that increases in STAT1 expression contributed to TEM becoming more responsive to IL-7 signaling in animals treated with anti-IL-15. Other non-mutually exclusive possibilities are that in the absence of IL-15, expanding and differentiating TTrM retain IL-7 sensitivity as they differentiate into TEM and/or that IL-15-independent TEM subsets have the ability to more efficiently respond to IL-7. Although elucidation of these mechanisms will require further study, the data presented here strongly support the concept that even when γc cytokines have primary roles in the homeostatic regulation of specific subsets, there is often (TEM; CD56+ NK cells), but not always (CD16+ NK cells), plasticity in this regulation such that other members of this cytokine family can provide “back-up” support for population maintenance.
As previously discussed, compensatory γc signaling does not appear to be sufficient to normalize peripheral TEM homeostasis, and this compensation is likely to become increasingly inadequate over the long-term. In this regard, IL-15 appears to be required for optimal development of TEM and effector T cells to new Ags, and for the emigration of these cells to tissue effector sites (44, 45). In keeping with this, we have observed that anti-IL-15 mAb administration during acute SIV infection severely diminishes the magnitude of SIV-specific T cell responses in mucosal compartments (i.e., BAL) but not in the peripheral blood (Okoye et al., manuscript in preparation). Thus, inadequate tissue homing may play a role in the reduced TEM in tissue effector sites such as the colonic lamina propria. The decrease in T cell density observed in the colonic lamina propria may also reflect the inability of TEM resident within such effector sites, or TTrM recently emigrating into these sites, to effectively compete with other lymphocyte populations (such as B cells, γδ T cells, intraepithelial lymphocytes, CD56+ NK cells) for IL-7 or other compensatory γc cytokines when IL-15 is not available. Such competition would decrease TEM population stability in tissues where IL-7 and/or other compensatory γc cytokines are limiting. Indeed, the precise role that IL-15 plays in regulating the migration and subsequent maintenance of TEM in such tissues still needs to be more clearly defined in primates.
In summary, this study unequivocally establishes a major in vivo role for IL-15 in the development and survival of NK cells in an NHP model. In addition, this study demonstrates the important role IL-15 plays in maintaining peripheral CD4+ and CD8+ TEM homeostasis, and the complex interaction of IL-15 with other γc cytokines in the regulation of the TEM compartment. IL-15 is overexpressed in several autoimmune disorders, where it is thought to drive the activation and proliferation of self-reactive memory T cells (94). Based on this, blockade of IL-15 activity is being evaluated as a therapeutic strategy for these diseases (95, 96). We would propose that in vivo IL-15 blockade of IL-15 in RM provides a useful model for exploring the implications of this therapeutic strategy. As shown here, anti-IL-15 therapy will likely not uniformly deplete T and NK cell effectors, but rather will change their balance, potentially resulting in unexpected, off-target immune reactions. These results suggest that further study will be necessary to fully characterize the therapeutic potential of anti-IL-15 mAbs in the treatment of autoimmune disorders.
Supplementary Material
Acknowledgments
We thank Cytheris SA for providing rsIL-7, as well as Francois Villinger (Emory University) and the Resource for Nonhuman Primate Immune Reagents for providing rhesus IL-15. Reagents were provided by the Nonhuman Primate Reagent Resource supported by the National Institute of Allergy and Infectious Diseases (Contract HHSN2722000130031C) and the National Institutes of Health (Grant OD010976). This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We thank S. Planer, J. Turner, P. Jewett, T. Swanson, M. Fischer and J. Dewane for expert animal husbandry. We also thank S. Shiigi, E. McDonald, A. Kiddle, N. Hamilton, C. Pexton, I. Axthelm, S. Hagen, Y. Fukazawa, R. Lum, C. Abana, H. Park, A. Townsend and L. Boshears for technical or administrative assistance.
Grant Support: This work was supported by the US National Institutes of Health (including grants 5R37AI054292, 5R01A1082529, 5U19AI067854, U42OD010426 and 8P51OD01109255).
Abbreviations used in this article
- RM
rhesus macaque
- TN
naïve T cell
- TM
memory T cell
- TCM
central memory T cell
- TTrM
transitional memory T cell
- TEM
effector memory T cell
- pSTAT5
phosphorylated STAT5
- NHP
nonhuman primates
- mAb
monoclonal antibody
Footnotes
Disclosures: All authors have no financial conflicts of interest.
References
- 1.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 2.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 4.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 5.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 6.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, Sugamura K. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int Immunol. 1995;7:115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 9.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 11.Okoye AA, Rohankhedkar M, Konfe AL, Abana CO, Reyes MD, Clock JA, Duell DM, Sylwester AW, Sammader P, Legasse AW, Park BS, Axthelm MK, Nikolich-Zugich J, Picker LJ. Effect of IL-7 Therapy on naive and memory T cell homeostasis in aged rhesus macaques. J Immunol. 2015;195:4292–4305. doi: 10.4049/jimmunol.1500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 13.Ohteki T. Critical role for IL-15 in innate immunity. Curr Mol Med. 2002;2:371–380. doi: 10.2174/1566524023362519. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier M, Ratthe C, Girard D. Mechanisms involved in interleukin-15-induced suppression of human neutrophil apoptosis: role of the anti-apoptotic Mcl-1 protein and several kinases including Janus kinase-2, p38 mitogen-activated protein kinase and extracellular signal-regulated kinases-1/2. FEBS Lett. 2002;532:164–170. doi: 10.1016/s0014-5793(02)03668-2. [DOI] [PubMed] [Google Scholar]
- 15.Ottonello L, Frumento G, Arduino N, Bertolotto M, Dapino P, Mancini M, Dallegri F. Differential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. Role of oxidants, Bax and caspase-3. J Leukoc Biol. 2002;72:125–132. [PubMed] [Google Scholar]
- 16.Kennedy MK, Park LS. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J Clin Immunol. 1996;16:134–143. doi: 10.1007/BF01540911. [DOI] [PubMed] [Google Scholar]
- 17.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 18.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 19.Musso T, Calosso L, Zucca M, Millesimo M, Puliti M, Bulfone-Paus S, Merlino C, Savoia D, Cavallo R, Ponzi AN, Badolato R. Interleukin-15 activates proinflammatory and antimicrobial functions in polymorphonuclear cells. Infect Immun. 1998;66:2640–2647. doi: 10.1128/iai.66.6.2640-2647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–2809. [PubMed] [Google Scholar]
- 21.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Suzuki T, Miyagi T, Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 22.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci USA. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 24.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 27.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 29.Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, Chertova E, Rosenberg SA, Felber BK, Pavlakis GN. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120:e1–8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, Sugimoto C, Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci USA. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 35.Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25:377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunol Rev. 2014;260:221–234. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broux B, Mizee MR, Vanheusden M, van der Pol S, van Horssen J, Van Wijmeersch B, Somers V, de Vries HE, Stinissen P, Hellings N. IL-15 amplifies the pathogenic properties of CD4+CD28- T cells in multiple sclerosis. J Immunol. 2015;194:2099–2109. doi: 10.4049/jimmunol.1401547. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Alvaro I, Ortiz AM, Garcia-Vicuna R, Balsa A, Pascual-Salcedo D, Laffon A. Increased serum levels of interleukin-15 in rheumatoid arthritis with long- term disease. Clin Exp Rheumatol. 2003;21:639–642. [PubMed] [Google Scholar]
- 39.Kuczynski S, Winiarska H, Abramczyk M, Szczawinska K, Wierusz-Wysocka B, Dworacka M. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:231–236. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Sakai T, Kusugami K, Nishimura H, Ando T, Yamaguchi T, Ohsuga M, Ina K, Enomoto A, Kimura Y, Yoshikai Y. Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology. 1998;114:1237–1243. doi: 10.1016/s0016-5085(98)70430-5. [DOI] [PubMed] [Google Scholar]
- 41.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD, Sr, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanai T, Thomas EK, Yasutomi Y, Letvin NL. IL-15 stimulates the expansion of AIDS virus-specific CTL. J Immunol. 1996;157:3681–3687. [PubMed] [Google Scholar]
- 43.Conklyn M, Andresen C, Changelian P, Kudlacz E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76:1248–1255. doi: 10.1189/jlb.0504282. [DOI] [PubMed] [Google Scholar]
- 44.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, Lane HC. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, Creekmore SP, Waldmann TA, Roederer M. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 51.Walker JM, Maecker HT, Maino VC, Picker LJ. Multicolor flow cytometric analysis in SIV-infected rhesus macaque. Methods Cell Biol. 2004;75:535–557. doi: 10.1016/s0091-679x(04)75022-0. [DOI] [PubMed] [Google Scholar]
- 52.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr, Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grossman Z, Picker LJ. Pathogenic mechanisms in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2008;3:380–386. doi: 10.1097/COH.0b013e3282fbaae6. [DOI] [PubMed] [Google Scholar]
- 55.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuxman Bass JI, Diallo A, Nelson J, Soto JM, Myers CL, Walhout AJ. Using networks to measure similarity between genes: association index selection. Nat Methods. 2013;10:1169–1176. doi: 10.1038/nmeth.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PloS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, Ortaldo JR, Gupta S, Chen YQ, Giri JD, O’Shea JJ. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci USA. 1995;92:8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leone A, Rohankhedkar M, Okoye A, Legasse A, Axthelm MK, Villinger F, Piatak M, Jr, Lifson JD, Assouline B, Morre M, Picker LJ, Sodora DL. Increased CD4+ T cell levels during IL-7 administration of antiretroviral therapy-treated simian immunodeficiency virus-positive macaques are not dependent on strong proliferative responses. J Immunol. 2010;185:1650–1659. doi: 10.4049/jimmunol.0902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 65.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21:419–430. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 66.Lebrec H, Horner MJ, Gorski KS, Tsuji W, Xia D, Pan WJ, Means G, Pietz G, Li N, Retter M, Shaffer K, Patel N, Narayanan PK, Butz EA. Homeostasis of human NK cells is not IL-15 dependent. J Immunol. 2013;191:5551–5558. doi: 10.4049/jimmunol.1301000. [DOI] [PubMed] [Google Scholar]
- 67.Lowe M, Badell IR, Thompson P, Martin B, Leopardi F, Strobert E, Price AA, Abdulkerim HS, Wang R, Iwakoshi NN, Adams AB, Kirk AD, Larsen CP, Reimann KA. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira LE, Onlamoon N, Wang X, Wang R, Li J, Reimann KA, Villinger F, Pattanapanyasat K, Mori K, Ansari AA. Preliminary in vivo efficacy studies of a recombinant rhesus anti-alpha(4)beta(7) monoclonal antibody. Cell Immunol. 2009;259:165–176. doi: 10.1016/j.cellimm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riley JL, Westerheide SD, Price JA, Brown JA, Boss JM. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA) Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 70.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaccara S, Tebaldi T, Pederiva C, Ciribilli Y, Bisio A, Inga A. p53-directed translational control can shape and expand the universe of p53 target genes. Cell Death Differ. 2014;21:1522–1534. doi: 10.1038/cdd.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 73.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 76.Haustein S, Kwun J, Fechner J, Kayaoglu A, Faure JP, Roenneburg D, Torrealba J, Knechtle SJ. Interleukin-15 receptor blockade in non-human primate kidney transplantation. Transplant. 2010;89:937–944. doi: 10.1097/TP.0b013e3181d05a58. [DOI] [PubMed] [Google Scholar]
- 77.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 78.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi Y, Byrareddy SN, Albrecht C, Brameier M, Walter L, Mayne AE, Dunbar P, Russo R, Little DM, Villinger T, Khowawisetsut L, Pattanapanyasat K, Villinger F, Ansari AA. In vivo administration of a JAK3 inhibitor during acute SIV infection leads to significant increases in viral load during chronic infection. PLoS Pathog. 2014;10:e1003929. doi: 10.1371/journal.ppat.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 81.Stancovski I, Hurwitz E, Leitner O, Ullrich A, Yarden Y, Sela M. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci USA. 1991;88:8691–8695. doi: 10.1073/pnas.88.19.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finch DK, Midha A, Buchanan CL, Cochrane D, Craggs RI, Cruwys S, Grahames C, Kolbeck R, Lowe DC, Maltby J, Pattison DV, Vousden KA, Ward A, Sleeman MA, Mallinder PR. Identification of a potent anti-IL-15 antibody with opposing mechanisms of action in vitro and in vivo. Br J Pharmacol. 2011;162:480–490. doi: 10.1111/j.1476-5381.2010.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 84.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, Beach RK, Lifson JD, Felber BK, Pavlakis GN. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. J Biol Chem. 2013;288:18093–18103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wines BD, Powell MS, Parren PW, Barnes N, Hogarth PM. The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors Fc gamma RI and Fc gamma RIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol. 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 87.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thoma G, Druckes P, Zerwes HG. Selective inhibitors of the Janus kinase Jak3--Are they effective? Bioorg Med Chem Lett. 2014;24:4617–4621. doi: 10.1016/j.bmcl.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 91.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quigley M, Huang X, Yang Y. STAT1 signaling in CD8 T cells is required for their clonal expansion and memory formation following viral infection in vivo. J Immunol. 2008;180:2158–2164. doi: 10.4049/jimmunol.180.4.2158. [DOI] [PubMed] [Google Scholar]
- 93.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuca-Warnawin E, Burakowski T, Kurowska W, Prochorec-Sobieszek M, Radzikowska A, Chorazy-Massalska M, Maldyk P, Kontny E, Maslinski W. Elevated number of recently activated T cells in bone marrow of patients with rheumatoid arthritis: a role for interleukin 15? Ann Rheum Dis. 2011;70:227–233. doi: 10.1136/ard.2009.124966. [DOI] [PubMed] [Google Scholar]
- 95.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ, Schuurman J, van de Winkel JG, Parren PW, Gracie JA, Jongbloed S, Liew FY, McInnes IB. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- 96.Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J Investig Dermatol Symp Proc. 2013;16:S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.