Abstract
His Bundle pacing (HBP) restores electrical synchronization in left bundle branch block (LBBB), however, the underlying mechanisms are poorly understood. We examined the relationship between native QRS axis in LBBB, a potential indicator of the site of block, and QRS normalization in patients with LBBB. Data from patients (n=41) undergoing HBP at three sites were studied (68±13 years, 13 females). Study criteria included strictly defined complete LBBB, and successful implantation of a permanent HBP lead. Pre- and post-procedure electrocardiograms were reviewed independently by two blinded readers. QRS axis and duration were measured to the nearest 10° and 10ms, respectively. QRS narrowing or normalization was the primary endpoint. Of 29 patients meeting study criteria, 9 had frontal plane QRS axes between −60° and −80°, 10 from −40° to 0°, and 10 from +1° to +90°. QRS narrowing occurred in 24 patients (83%, 44±34ms, p<0.05). Percent QRS narrowing by axis were 26±19%, 29±25%, and 28±23%, respectively. No correlation between pre-pacing QRS axis and post-pacing narrowing was identified (r2 = 0.001, p = 0.9). In patients with or without QRS normalization following HBP, mean QRS duration were 155±21ms vs. 171±8ms, respectively, p=0.014). HBP induces significant QRS narrowing in most patients, and normalization in patients with shorter baseline QRS duration. In conclusion, the lack of correlation between native QRS axis and narrowing suggests that proximal His-Purkinje block causes most cases of LBBB, or that additional mechanisms underlie HBP efficacy. Further studies are needed to better understand how to predict those patients in whom HBP will normalize LBBB.
Keywords: His Bundle Pacing, QRS resynchronization, left bundle branch block
INTRODUCTION
Left bundle branch block (LBBB) causes asynchronous contraction of the LV, which may potentially lead to eventual global LV dysfunction.1–4 Cardiac resynchronization therapy (CRT) has emerged as a technique to overcome this electrical delay and has been associated with a reduction in all-cause mortality and non-fatal heart failure events.5–7,8 The idea of resynchronization and its clinical benefits has been extrapolated to support His bundle pacing (HBP) in patients with LBBB and cardiomyopathy. HBP has been shown to achieve significant QRS narrowing, with improved LV EF, New York Heart Association (NYHA) functional class, and cardiopulmonary reserve.9 The mechanisms underlying HBP-mediated improvement in conduction remain poorly understood and have been explained by the concept of longitudinal dissociation, that fibers within the His bundle are predestined for the left and right (Figure 1A) bundle branches. Given the well-described anatomy of the proximal His Purkinje system10–12, fibers exiting into the left posterior fascicle (LPF) prior to the site of block will result in earlier activation of the posterior-inferior LV, and hence a left or extreme left axis deviation (Figure 1B–C). Based on the concept of longitudinal dissociation and the anatomy of the HB, we hypothesized, that pre-procedure extreme left axis deviation indicates distal His-Purkinje block, and a lower likelihood that HBP would normalize the QRS duration.
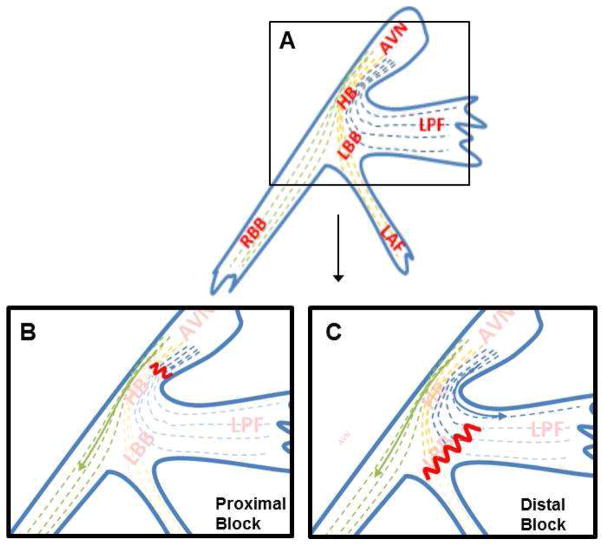
FIGURE 1. His-Purkinje Anatomy and Theory of Longitudinal Dissociation.
A) His-Purkinje anatomy with longitudinal dissociation. Fibers are longitudinally oriented and “predestined” from the His bundle to the bundle branches. B) Proximal left bundle branch block (LBBB), where the block is prior to the exit of any fibers of the left posterior fascicle (LPF). C) Distal LBBB, where some of the LPF fibers have exited before the level of the block. Sketch adapted from Rosenbaum. AVN=AV node, HB=His bundle, LBB=Left bundle branch, LPF=Left posterior fascicle, LAF=L anterior fascicle, RBB=Right bundle branch
METHODS
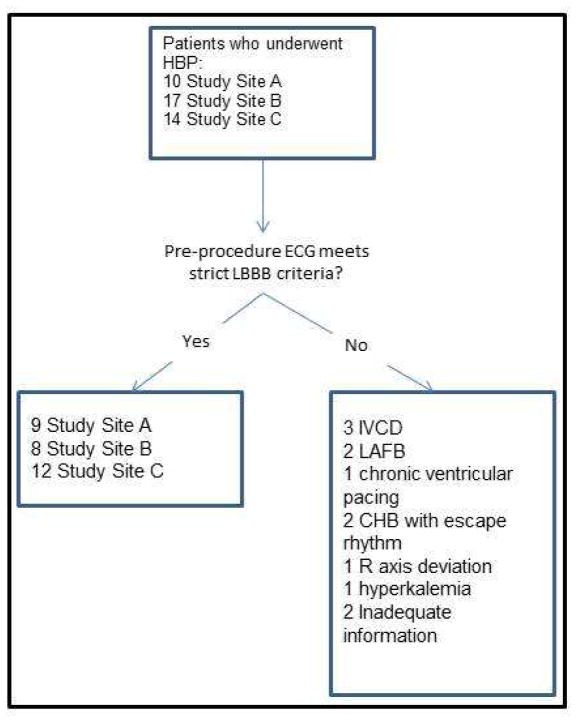
The study was approved by the institutional review board (IRB) at the 3 centers. Data from 41 patients who underwent permanent HBP at these sites were evaluated retrospectively, and included 17 patients from Geisinger Clinic (Site 1), 14 from University of Vermont (Site 2), and 10 from University of California- Los Angeles (Site 3). The study period included 7 years of implantation (2008 to 2015). Patients who met a strict criteria for complete LBBB (QRS duration greater than or equal to 140milliseconds (ms) in men or 130ms in women; a QS or RS in leads v1 or v3; and a mid QRS slowing or notching in ≥2 of the leads V1, V2, V5, V6, I, or aVL)13, had an indication for pacing or cardiac resynchronization, and who underwent a successful implantation of an HBP lead were included in this study (Figure 2). Criteria for LBBB were made from a standard 12-lead electrocardiogram (25mm/s paper speed) or electrocardiograms recorded using an electrophysiological mapping system (Cardiolab, GE Healthcare, OH, USA).
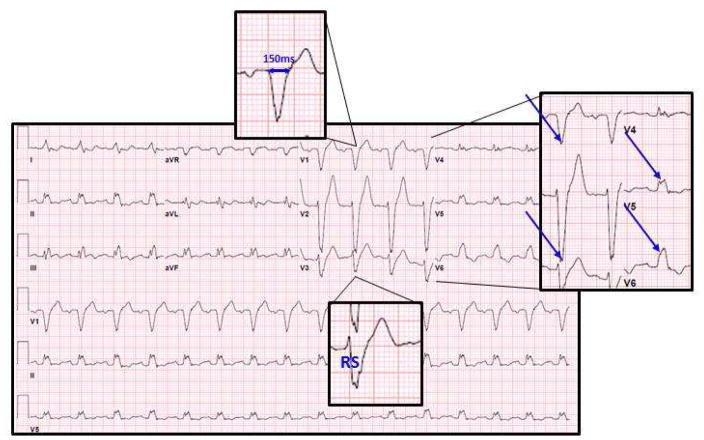
FIGURE 2. Strict Criteria for Complete Left Bundle Branch Block.
Sample 12-lead ECG of patient who meets strict LBBB criteria. In this example male patient, the QRS duration is >140ms, there is an RS in lead v3, and there is mid-QRS slowing/notching in leads v1, v2, v5, and v6.
After femoral venotomy, a multipolar catheter was advanced to the region of the His bundle, and used to map a discrete His potential. Using standard lead and device implantation techniques, a defibrillator lead was placed into the right ventricle for patients undergoing CRT-D implantation. Next, using a SelectSite C304 or C315 catheter system (Medtronic, Minneapolis, MN, USA), a SelectSecure 3830 lead (Medtronic, Minneapolis, MN, USA) was secured to a site demonstrating a discrete His potential, guided by the multipolar catheter (Figure 3A). An atrial lead and generator were placed in the standard fashion. Device programming was at the discretion of the implanting physician.
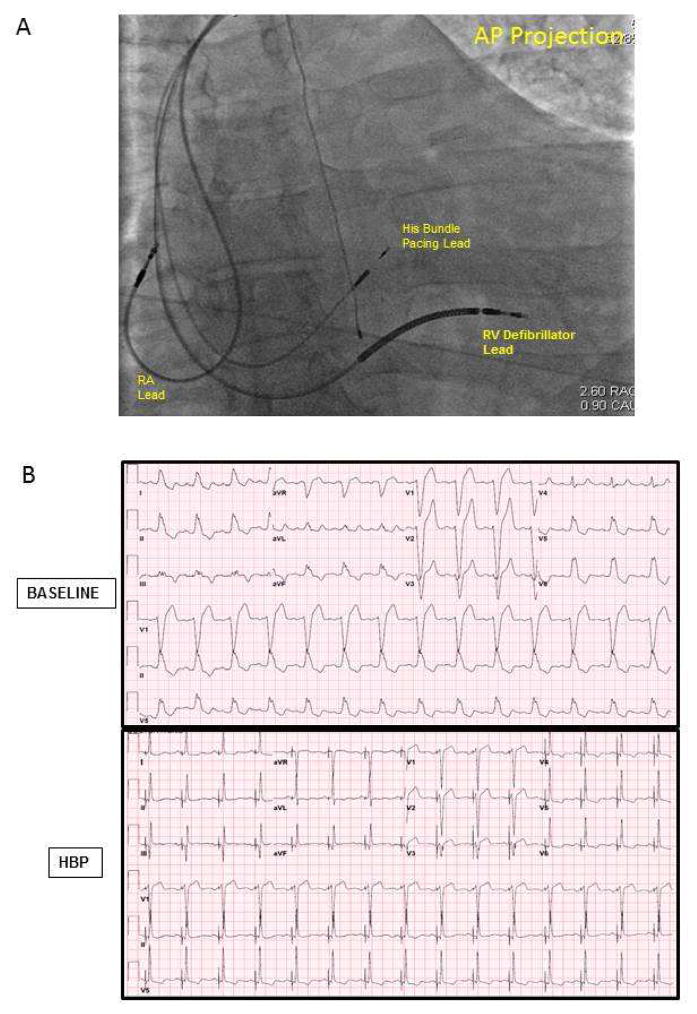
FIGURE 3. His Bundle Lead Placement and QRS Response to pacing.
A) Fluoroscopy image following His bundle lead placement. Also shown are the right ventricular (RV) defibrillator lead), and the right atrial (RA) lead. The fluoroscopic image is shown in the antero-posterior (AP) projection. B) Representative example of 12-electrocardiogram showing QRS narrowing pre- and C) post-His bundle Pacing.
Of the patients who met study criteria, pre-HBP procedure ECGs were evaluated by 2 blinded readers in an independent ECG Core Lab. Native QRS axis was measured to the nearest 10 degrees, and pre-procedure QRS duration was measured to the nearest 10ms. Post-procedure ECGs were de-identified. The same 2 blinded readers measured post-procedure QRS to the nearest 10ms. Any discordant measurements between readers were re-read in a group conference where a third blinded reader was present. On post-procedure ECGs and EP tracings, QRS was measured from the onset of the first deviation from baseline for selective HBP (after the “HV” interval) and from the onset of steepest deflection in non-selective HBP(Figure 4).
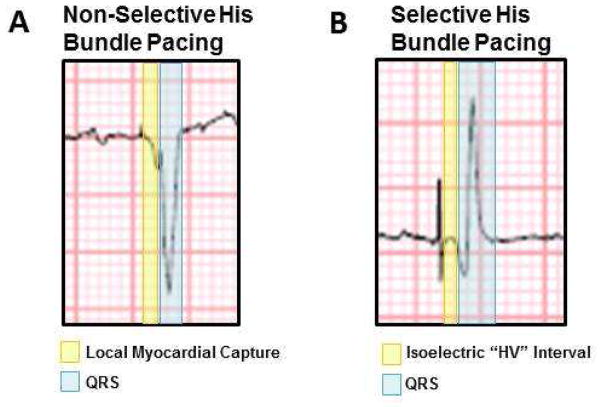
FIGURE 4. Quantification of QRS Duration in Nonselective and Selective His Bundle Capture.
Example of non-selective (A) and selective (B) His bundle pacing (HBP). In non-selective HBP cases, QRS was measured following the area of periventricular capture represented by a slurred area between the pacing spike and the steep deflection of the QRS. In selective pacing cases, QRS was measured at the start of the first deflection from the isoelectric period following the pacing spike.
The primary study outcome was the degree of QRS narrowing with HBP (absolute narrowing and percent narrowing) relative to the mean QRS axis in LBBB at baseline. The secondary outcomes were the relationship between QRS narrowing and QRS axis, and the relationship between QRS narrowing ischemic versus nonischemic cardiomyopathy.
Data are expressed as mean ± standard deviation. All indices reported were measured from consistent tracings without artifact or loss of capture. The study data were normally distributed, and paired comparisons were made using the Student T test. Data from ≥ 2 groups were compared using the one-way analysis of variance (ANOVA). A p value ≤ 0.05 was considered statistically significant. Correlation between QRS narrowing and mean native QRS axis or pre-procedure QRS duration was determined using linear regression. Correlation between QRS narrowing and type of cardiomyopathy was determined using one-way ANOVA, while normalization was evaluated using Fisher’s exact test. Analyses were performed using Systat version 13 (San Jose, CA, USA).
Results
Of the 41 patients studied, 29 patients fulfilled study criteria (including strict LBBB as defined above), and were included in the study. Sixteen patients were male and 13 were female (Figure 5). The frontal plane axis of 9 patients ranged from −60° to −80°, 10 from −40° to 0°, and 10 from +1° to +90°.
FIGURE 5. Study Population.
Schematic of inclusion data. Study Site A refers to UCLA, Study Site B to Geisinger, and Study Site C to University of Vermont.
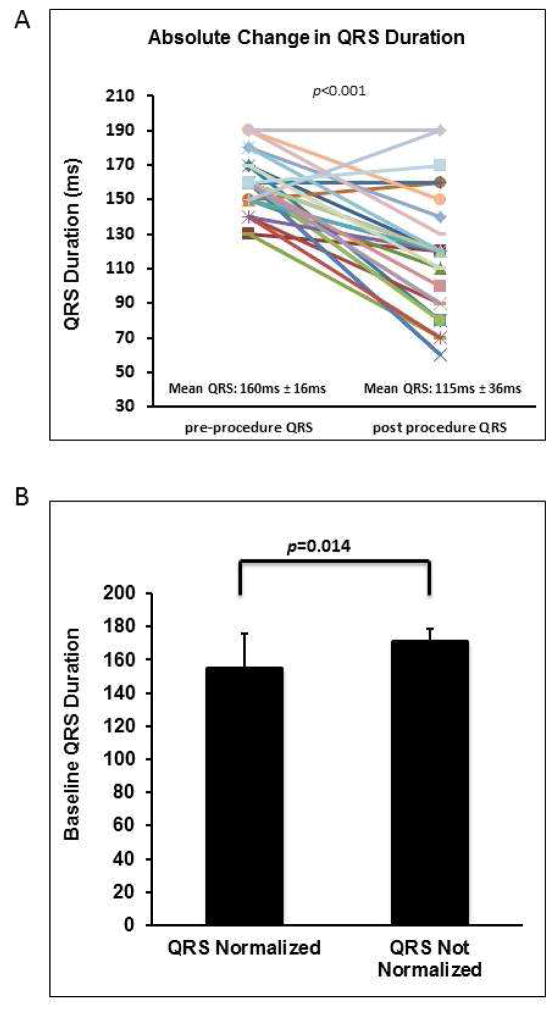
In 24 of the 29 study patients (83%), HBP resulted in some degree QRS narrowing, while 21 of 29 patients (72%) showed complete QRS normalization, as defined by a post-procedure QRS duration of ≤120ms. Overall, the mean QRS duration from pre- to post-procedure decreased from 160±16ms to 115±36ms respectively (p < 0.0001). Figure 6A depicts the change in QRS duration from pre- to post-HBP procedure. Mean pre-procedure QRS duration in patients who had QRS normalization was 155±21ms compared to those with no significant change or incomplete normalization with a mean pre-procedure QRS duration of 171±8ms (p = 0.014), as shown in Figure 6B. Mean QRS narrowing in patients with non-selective (n=8) vs. selective (n=21) HBP was 68±26ms vs. 36±33ms (p=0.024).
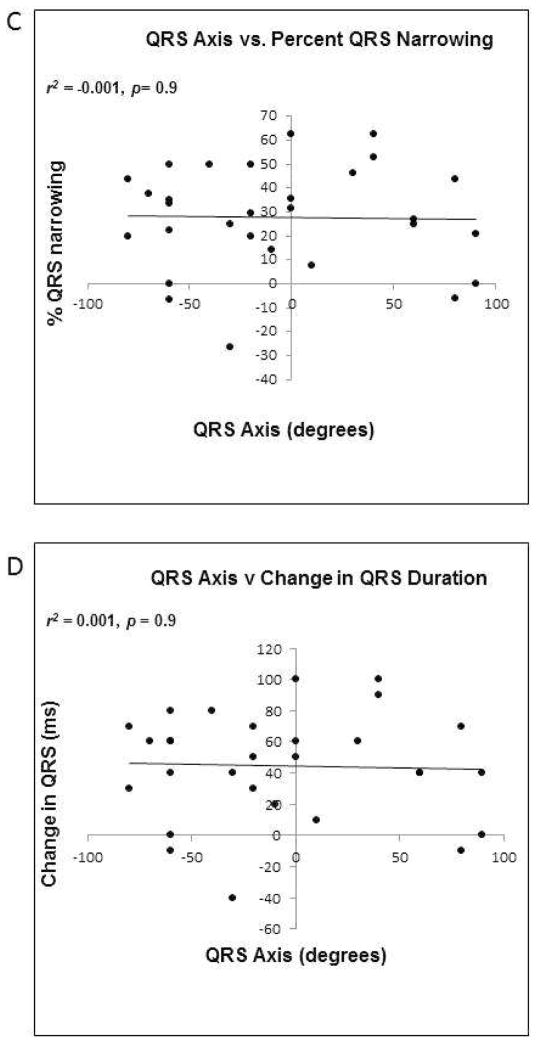
FIGURE 6. Relationship between QRS axis, duration and QRS narrowing with His bundle pacing.
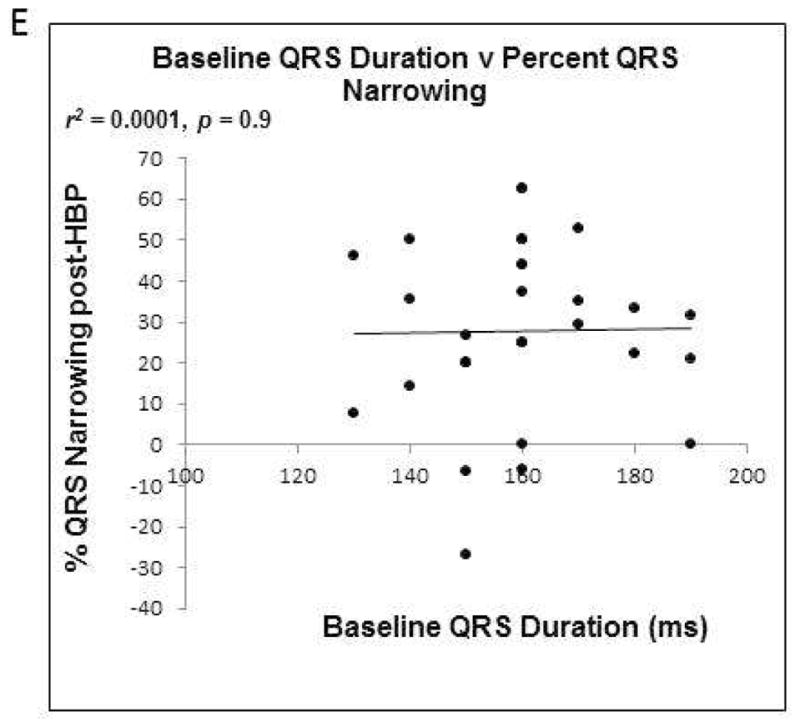
A) pre- and post-His bundle pacing (HBP) QRS duration for all included study patients. B) Relationship between QRS normalization and baseline QRS duration.
C) Correlation between QRS axis and percent QRS narrowing. D) Correlation between QRS axis and change in QRS duration. E) Correlation between baseline pre-HBP QRS duration and percent QRS narrowing.
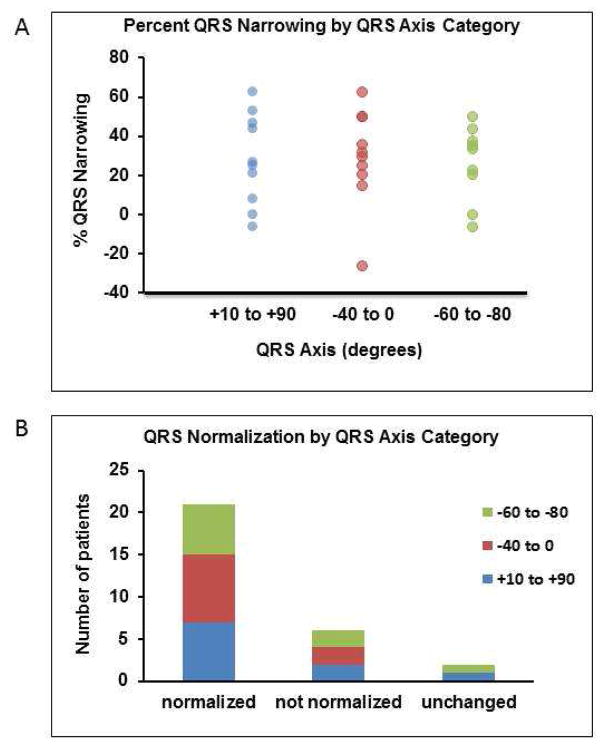
Across the study population, there was no correlation between pre-procedure QRS axis and post-procedure percent QRS narrowing (r2 = 0.001, p = 0.904, Figure 6C), absolute change in QRS duration (r2 = 0.001, p = 0.869, Figure 6D), or post-HBP QRS duration (r2= 0.0001, p = 0.968, Figure 6E). In patients with a QRS axis ranging between −60° and −80°, the QRS duration narrowed by 26±19%, while in those with a QRS axis between −40° and 0°, the QRS duration narrowed by 29±25%. In patients with a QRS axis of +1° to +90°, QRS duration narrowed by 28±23% with HBP. The means were not significantly different (p=0.958). QRS percent narrowing and normalization by QRS axis category are depicted in Figures 7A and 7B respectively.
FIGURE 7. Relationship between QRS axis and QRS normalization.
A) Percent QRS narrowing by QRS axis categorized by range. B) Normalization of QRS following HBP by QRS axis categorized by range.
Likewise, there was no correlation between frontal plane axis and percent QRS narrowing when evaluated by individual study site (Site 1: r2 = 0.19, p = 0.278; Site 2: r2 = 0.020, p = 0.663; and Site 3: r2 = 0.04, p=0.627).
In patients with ischemic cardiomyopathy mean absolute narrowing was 60±25ms compared to 29±31ms in those with non-ischemic cardiomyopathy (p=0.164), and post-procedure percent QRS narrowing was 38±16% compared to 19±20% in the ischemic and nonischemic groups respectively (p=0.2).
Discussion
The major findings of the present study are 1) HBP results in QRS narrowing or normalization in a majority of patients with complete LBBB, independent of the site of block as estimated by the theory of longitudinal dissociation; and 2) patients with mean QRS duration <155±21ms were more likely to normalize LBBB than those with greater QRS duration. We observed that 83% of the study subjects demonstrated QRS narrowing, while 71% had complete QRS normalization following HBP. Native QRS axis did not predict QRS narrowing or normalization.
Traditionally, the efficacy of HBP has rested on the theory of longitudinal dissociation, the concept that fibers within the HB are predestined for the LB or RB, and as such, conduction block within the HB can be bypassed by pacing distally14–16. While it is possible that the high success rate of HBP observed in this study and several other recent studies17,18 suggests that the site of block in patients with strict LBBBs is within the proximal HB, it also raises the possibility that other mechanisms may explain how HBP works.
To date, the mechanism by which HBP works rests on early descriptions of the structural organization and electrophysiological properties of the His-Purkinje system from the 1970s. James and Sherf posited that within the HB, there are longitudinally-oriented cells that are separated by collagen sheaths that continue directly into the bundle branches11. Narula showed that pacing the distal HB could restore native QRS, thereby suggesting the block was within the HB and could be bypassed. Distal lesions likely involve complete blockage of the LAF and LPF, distal to where the HBP lead would be placed15. Barba-Pichardo et al. further demonstrated the potential and feasibility of HBP with the implantation of permanent HBP leads18,19. The inability to narrow QRS in some patients was attributed to infra-hisian block, possibly within the His or peripheral in the Purkinje system or bundles, the latter of which could not be bypassed and therefore not corrected19. In the present study, we found no relationship between baseline QRS duration and the degree of QRS narrowing in LBBB (Figure 6D), however, the baseline QRS duration of patients who showed normalization compared to those who did not, a QRS duration < 155±21ms was associated with QRS narrowing.
We examined whether frontal plane axis was predictive of a more proximal lesion within the His-Purkinje system. The LPF is composed of fibers dispersed over a broad area, exiting the His-Purkinje system as high as the level of the His bundle and extending down the left bundle12,20. Accordingly, it would be expected that the more distal the level a block is, more fibers of the LPF have exited, resulting in an ECG with LBBB and leftward axis deviation pattern (Figure 1B). As an extension, if the bulk of LPF fibers exit prior to the level of block, LAFB would result, and not LBBB. Such patients were not included in this study.
In this study, there was no correlation between extreme left axis deviation and refractoriness to HBP. Instead, the data showed a reduction in QRS duration by an average of 44ms, independent of frontal plane axis in 24 of 29 patients (83%). It is possible that the cases that did not narrow were attributable to block distal to the HB and that pacing anywhere along the HB would not be distal to that site of block, but it is also possible that the efficacy of HBP may not only depend on bypassing a structural block but in overcoming a functional block as well. An alternative or complementary, mechanism may be related to the pacing output required to capture tissue (including normal myocardium and/or His-Purkinje tissue) just distal to the site of block. In a crossover comparison between HBP and biventricular pacing, 21 of 29 (72%) of patients experienced QRS narrowing at the time of implant with HBP. Lustgarten et al. noted that the HBP capture threshold was higher, though insignificantly, compared to the LV lead threshold17. However, more recently, Vijayaraman et al. demonstrated even better success rates with voltages as low as 1.3 ± 0.7V21. They specifically demonstrated significantly higher rates of success in infranodal blocks than previously noted. This may be operator dependent, but may also reflect improved technology allowing better lead placement.
A limitation of this study was its sample size. This also limits the ability to control for potential confounders, such as study site, variations in technique and equipment, and direct versus indirect capture. Likewise, of the 29 patients evaluated, it could not be determined in 7 of them whether they had ischemic cardiomyopathy, nonischemic cardiomyopathy, neither, or both.
Table 1.
Demographics of patients included in study
| Number | % | |
|---|---|---|
| Total pre-procedure patients included | 29 | 100% |
| Age (Years) | 68±13 | |
| Women | 13 | 41% |
| LVEF (%) | ||
| • <25 | 9 | |
| • 25–35 | 9 | |
| • 36–45 | 3 | |
| • >45 | 6 | |
| Cardiomyopathy | ||
| • Nonischemic | 10 | 34% |
| • Ischemic | 8 | 28% |
| • Preserved ejection fraction | 4 | 14% |
| • Unknown | 7 | 24% |
| Frontal Plane Axis: | ||
| • Extreme left axis deviation (−0° to −80°) | 9 | 31% |
| • Horizontal to left axis deviation (−40° to 0°) | 10 | 34% |
| • Normal axis (+10° to +90°) | 10 | 34% |
Acknowledgments
The authors acknowledge the National Heart Lung and Blood Institutes of the National Institutes of Health for HL084261 to KS and HL125730 to OAA.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kass DA. Ventricular resynchronization: pathophysiology and identification of responders. Rev Cardiovasc Med. 2003;4(Suppl 2):S3–S13. [PubMed] [Google Scholar]
- 2.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 3.Barth AS, Aiba T, Halperin V, DiSilvestre D, Chakir K, Colantuoni C, Tunin RS, Dimaano VL, Yu W, Abraham TP, Kass DA, Tomaselli GF. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ Cardiovasc Genet. 2009;2:371–378. doi: 10.1161/CIRCGENETICS.108.832345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, Van Eyk JE. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3:78–87. doi: 10.1161/CIRCGENETICS.109.871236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. New Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg I, Kutyifa V, Klein HU, Cannom DS, Brown MW, Dan A, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Kautzner J, Klempfner R, Kuniss M, Merkely B, Pfeffer MA, Quesada A, Viskin S, McNitt S, Polonsky B, Ghanem A, Solomon SD, Wilber D, Zareba W, Moss AJ. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 10.Lev M. Fine structure of the His bundle. Circulation. 1971;44:304–305. doi: 10.1161/01.cir.44.2.304-a. [DOI] [PubMed] [Google Scholar]
- 11.James TN, Sherf L. Fine structure of the His bundle. Circulation. 1971;44:9–28. doi: 10.1161/01.cir.44.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Tawara S, Suma K, Shimada M. The conduction system of the mammalian heart : an anatomico-histological study of the atrioventricular bundle and the Purkinje fibers. Vol. 23. London: Imperial College Press; 2000. p. 229. [Google Scholar]
- 13.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Barba-Pichardo R, Manovel Sanchez A, Fernandez-Gomez JM, Morina-Vazquez P, Venegas-Gamero J, Herrera-Carranza M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace. 2013;15:83–88. doi: 10.1093/europace/eus228. [DOI] [PubMed] [Google Scholar]
- 15.Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56:996–1006. doi: 10.1161/01.cir.56.6.996. [DOI] [PubMed] [Google Scholar]
- 16.Scherlag BJ, Kosowsky BD, Damato AN. A technique for ventricular pacing from the His bundle of the intact heart. J Appl Physiol. 1967;22:584–587. doi: 10.1152/jappl.1967.22.3.584. [DOI] [PubMed] [Google Scholar]
- 17.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart rhythm. 2015;12:1548–1557. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Sharma PS, Dandamudi G, Naperkowski A, Oren JW, Storm RH, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart rhythm. 2015;12:305–312. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Barba-Pichardo R, Morina-Vazquez P, Fernandez-Gomez JM, Venegas-Gamero J, Herrera-Carranza M. Permanent His-bundle pacing: seeking physiological ventricular pacing. Europace. 2010;12:527–533. doi: 10.1093/europace/euq038. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum MB, Elizari MV, Lazzari JO. The hemiblocks : new concepts of intraventricular conduction based on human anatomical, physiological, and clinical studies. Oldsmar, Fla: Tampa Tracings; 1970. [Google Scholar]
- 21.Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic Insights Into Site of Atrioventricular Block: Lessons From Permanent His Bundle Pacing. JACC: Clin Electrophysiol. 2015;1:571–581. doi: 10.1016/j.jacep.2015.09.012. [DOI] [PubMed] [Google Scholar]