Abstract
This was a first double-blind, placebo-controlled pilot study to evaluate the efficacy of the novel antidepressant medication mirtazapine for treating both the depressive symptoms and the level of alcohol consumption of subjects with comorbid major depressive disorder and an alcohol use disorder (MDD/AUD). The results of two previous studies of mirtazapine in MDD/AUD subjects had suggested efficacy for mirtazapine for decreasing their level of depressive symptoms, but level of alcohol consumption had not been assessed in those studies. All subjects in this 12-week pilot study were randomized to either mirtazapine or placebo, and also received motivational enhancement therapy. Between-group analyses involving the outcome measures of depressive symptoms, level of alcohol consumption, and level of alcohol craving indicated no significant differences between groups, possibly because of limited sample size. However, within-group t tests in the mirtazapine group showed a significant decrease in depressive symptoms by week 2, also noted at all subsequent assessments (weeks 3, 4, 6, 8, 10, and 12) during the 12-week study. In contrast, no significant decrease in depressive symptoms was noted in the placebo group until week 8. No evidence of efficacy was found for mirtazapine for decreasing level of alcohol consumption in MDD /AUD subjects.
Keywords: mirtazapine, comorbid, major depression, alcohol use disorder
1. Introduction
Previous studies involving antidepressant medications among persons with co-occurring major depressive disorder and an alcohol use disorder (MDD/AUD) have focused on selective serotonin reuptake inhibitors (SSRIs) or tricyclic medications, and the results of those trials have been disappointing (Nunes and Levin, 2004, Cornelius et al., 2009). Similarly, a meta-analysis by Lovieno et al. (2011) concluded that the SSRIs have not shown efficacy in comorbid populations. Those same authors (Lovieno et al., 2011) also noted the complete lack of studies involving a number of newer antidepressants, such as mirtazapine, for treating comorbid populations. Importantly, mirtazapine was found to be superior to other antidepressant medications for treating depression in a large meta-analysis of 12 new-generation antidepressants (Cipriani et al., 2009). Mirtazapine is classified as a second generation antidepressant medication with a tetracyclic structure. It is unique in its pharmacological profile among the currently available antidepressants, unrelated to tricyclic antidepressants or SSRIs.
Our own research group recently conducted a first open label study evaluating the acute efficacy of mirtazapine for decreasing the depressive symptoms and the level of drinking of persons with comorbid MDD/AUD. The results of that study demonstrated robust within-group efficacy for mirtazapine for decreasing both the level of depressive symptoms and the level of alcohol consumption of that comorbid population (Cornelius et al., 2012). However, no placebo control group was utilized in that study, so the efficacy of mirtazapine versus placebo could not be assessed.
In the current report, we present findings from a first double-blind placebo-controlled pilot study to assess the efficacy of mirtazapine for decreasing both the depressive symptoms and the excessive alcohol consumption of persons with comorbid MDD/AUD. We hypothesized that mirtazapine would demonstrate evidence of within-group efficacy and between-group efficacy for decreasing depressive symptoms and alcohol use.
2. Methods
Prior to entry into this treatment protocol, written informed consent was obtained from all subjects after all procedures had been fully explained. The study was approved by the University of Pittsburgh Institutional Review Board. Fourteen participants were recruited for this pilot study. Participants were required to be between 18 and 55 years of age at baseline, meet criteria for both current MDD and an AUD, and be eligible for outpatient treatment, to be included in the study. At the baseline assessment, participants were evaluated for the DSM-IV diagnoses of an AUD and MDD, using the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). Inclusion criteria included the co-occurring presence of both current MDD and an AUD. Exclusion criteria included: (1) age less than 18 or over 55; (2) presence of psychotic symptoms or a diagnosis involving psychosis, (3) receiving psychotropic medication in the prior month; (4) current DSM diagnosis of dependence or abuse on substances other than alcohol, cannabis, nicotine, or caffeine; (5) current significant medical or neurological condition; (6) suicidal ideation in the last three months, or lifetime suicidal attempt; (7) positive pregnancy test or breastfeeding; (8) inability or unwillingness to use contraceptive methods; (9) inability to read or understand study forms; (10) pending incarceration; or (11) current participation in another research study.
Following completion of the baseline assessment, participants were treated using a double-blind, placebo-controlled study design. Participants were randomly assigned to receive mirtazapine or placebo administered in identical-looking opaque capsules. The study medication was taken once per day at bedtime. Subjects were given 15 mg of mirtazapine for the first two weeks of the trial and 30 mg for the remainder of the 12-week medication trial. Protocol assessments were conducted weekly in the first month and biweekly thereafter. Brief Motivational Enhancement Therapy (MET) was also provided at each assessment (Miller et al., 1992). MET has been shown to be an effective treatment of both the alcohol use and the depressive symptoms of persons with co-occurring MDD/AUD (Cornelius et al., 2009; Cornelius et al., 2010; Cornelius et al., 2011; Cornelius et al, 2012). Participant-rated depressive symptoms were assessed with the Beck Depression Inventory (BDI) (Beck et al., 1961). Drinking behavior was evaluated using the timeline follow-back method (TLFB) (Sobell, et al., 1988). Medication side effects were assessed using the Systematic Assessment for Treatment Emergent Events-Systematic Inquiry (SAFTEE/SI) (Levine et al., 1986). Recruitment for this pilot study was discontinued when time and money for the study were exhausted. The pilot study currently being reported was funded by a small grant (an R21 grant) rather than a larger R01 grant. Consequently, the length of the study (two years) was substantially shorter than the duration typically utilized in larger studies (five years), and the amount of money available per year was only a fraction of the money that would have been available in a study funded by a R01 mechanism.
2.1 Statistical Analysis
Descriptive statistics were calculated for all variables. Continuous baseline measures were compared by paired t tests and by analysis of variance. Categorical baseline measures were compared by chi-square analysis, corrected for continuity. Outcome measures were compared by repeated measures ANOVA, using demographic characteristics as covariates, and by t tests. Statistical analyses were completed utilizing an intent-to-treat study design. All tests of significance were 2-tailed. An alpha level <0.05 was used to indicate statistical significance. All analyses were conducted using the Statistical Package for the Social Sciences, version 22.0 (IBM Corp., Released 2013) and SAS 9.3 (SAS Institute, Cary, N.C.).
3. Results
A total of 14 subjects participated in the study. Subjects included 10 (71%) males and 4 (29%) females. Half of the sample participants were Caucasian and half were African American. The mean age of study subjects was 41.3 years (SD=8.8). At baseline, the mirtazapine and placebo groups were not significantly different in distribution of any demographic variables or variables used as outcome measures. At baseline, subjects demonstrated moderately severe depressive symptoms, with a mean BDI of 26.9 (SD=9.6), which is typical of our outpatient MDD/AUD patients. At baseline, subjects demonstrated prominent alcohol consumption, with a mean of 6.6 drinks per drinking day (SD=2.0 drinks). Drinking occurred, on average, 72% of days (SD=23%), and an average % heavy drinking days (five or more drinks per day) of 48%. An additional ten potential subjects signed informed consent for the study, but did not participate in the treatment study, because they were ruled out at baseline assessment. Reasons for exclusion from the study included the presence of psychotic symptoms (n=4), the absence of MDD at baseline (n=2), non-completion of the baseline assessment (n=2), the presence of cocaine abuse (n=1), and the discovery that the person was already participating in another research study (n=1).
Between-group analyses showed no significant differences in depressive symptoms or in level of alcohol consumption between the mirtazapine group and the placebo group at any time point. The repeated measures ANOVA group × time interaction analyses (the primary outcome assessment analyses involving level of depressive symptoms) failed to show a significant difference between the two treatment groups in levels of BDI depressive symptoms (F=0.71, p>0.68) (Table 2).
Table 2.
Beck Depression Inventory Total
| The GLM Procedure | |||||
| Repeated Measures Analysis of Variance | |||||
| Tests of Hypotheses for Between Subjects Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| TxArm | 1 | 10.151604 | 10.151604 | 0.02 | 0.8908 |
| gender | 1 | 16.719417 | 16.719417 | 0.03 | 0.8602 |
| race | 1 | 893.929381 | 893.929381 | 1.75 | 0.2158 |
| Error | 10 | 5119.654393 | 511.965439 |
| The GLM Procedure | |||||||
| Repeated Measures Analysis of Variance | |||||||
| Univariate Tests of Hypotheses for Within Subject Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F | Adj Pr > F | |
|---|---|---|---|---|---|---|---|
| G - G | H-F-L | ||||||
| time | 8 | 2572.412937 | 321.551617 | 10.58 | <.0001 | <.0001 | <.0001 |
| time*TxArm | 8 | 173.608732 | 21.701092 | 0.71 | 0.6784 | 0.5863 | 0.6584 |
| time*gender | 8 | 486.755999 | 60.844500 | 2.00 | 0.0566 | 0.1134 | 0.0680 |
| time*race | 8 | 109.386510 | 13.673314 | 0.45 | 0.8872 | 0.7704 | 0.8649 |
| Error(time) | 80 | 2431.363049 | 30.392038 | ||||
| Greenhouse-Geisser (G-G) Epsilon | 0.4961 |
| Huynh-Feldt-Lecoutre (H-F-L) Epsilon | 0.8632 |
Notes: GLM= Generalized Linear Model; DF= degrees of freedom, SS= sums of squares, TxArm= treatment arm, Pr= probability, Adj Pr= Adjusted probability
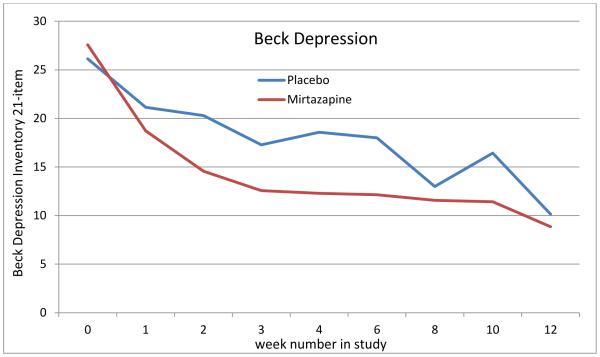
Within-group tests in the mirtazapine group showed a trend for a decrease in depressive symptoms (p<0.07) as early as week 1, with a 32.1% decrease in BDI depressive symptoms noted by week 1 in the mirtazapine group. A statistically significant within-group decrease in depressive symptoms among the mirtazapine group was noted in week 2 of the study (47.2% decrease, p=0.01), and a statistically significant within-group decrease in level of depressive symptoms compared to baseline levels of depression was also noted at all subsequent assessments (weeks 3, 4, 6, 8, 10, and 12) during the 12-week study. At week 4, five of the seven mirtazapine group subjects had demonstrated > 50% decrease in BDI depressive symptoms, while only one person in the placebo group showed a 50% decrease in BDI depressive symptoms at that time, which was a trend towards a statistically significant between-group difference between the mirtazapine group and the placebo group (mean change=52.9% in the mirtazapine group versus a 23.1% change in the placebo group, p=0.1). In contrast, no significant within-group decrease in depressive symptoms was noted in the placebo group until week 8, with no within-group decreases in depressive symptoms being noted in the placebo group at weeks 2, 3, 4, or 6. Both treatment groups showed significant decreases in depressive symptoms by the end of the clinical trial (week 12), but no between-group differences in depressive symptoms were noted at any time point.
During the 12-week course of the study, no significant between-group differences were noted on any measure of drinking (Table 4). Indeed, the level of drinking in the mirtazapine group was slightly higher than in the placebo group, though the difference between groups was not statistically significant. Also, craving for alcohol, as measured on the Obsessive Compulsive Drinking Scale (Anton, 2000), showed a significant decrease in each of the treatment groups during the course of the study, but no significant between group difference was noted at any time point (Table 3).
Table 4.
Drinks per drinking day
| The GLM Procedure | |||||
| Repeated Measures Analysis of Variance | |||||
| Tests of Hypotheses for Between Subjects Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| TxArm | 1 | 15.9580321 | 15.9580321 | 0.87 | 0.3717 |
| gender | 1 | 14.7950900 | 14.7950900 | 0.81 | 0.3890 |
| race | 1 | 20.0786948 | 20.0786948 | 1.10 | 0.3189 |
| Error | 10 | 182.4545451 | 18.2454545 |
| The GLM Procedure | |||||||
| Repeated Measures Analysis of Variance | |||||||
| Univariate Tests of Hypotheses for Within Subject Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F | Adj Pr > F | |
|---|---|---|---|---|---|---|---|
| G - G | H-F-L | ||||||
| time | 8 | 100.4376594 | 12.5547074 | 4.07 | 0.0004 | 0.0074 | 0.0009 |
| time*TxArm | 8 | 6.5170015 | 0.8146252 | 0.26 | 0.9756 | 0.8988 | 0.9653 |
| time*gender | 8 | 29.3626256 | 3.6703282 | 1.19 | 0.3158 | 0.3302 | 0.3203 |
| time*race | 8 | 32.8582675 | 4.1072834 | 1.33 | 0.2403 | 0.2752 | 0.2491 |
| Error(time) | 80 | 246.8338022 | 3.0854225 | ||||
| Greenhouse-Geisser (G-G) Epsilon | 0.4983 |
| Huynh-Feldt-Lecoutre (H-F-L) Epsilon | 0.8699 |
Notes: GLM= Generalized Linear Model; DF= degrees of freedom, SS= sums of squares, TxArm= treatment arm, Pr= probability, Adj Pr= Adjusted probability
Table 3.
, Craving-Obsessive Compulsive Drinking Scale
| The GLM Procedure | |||||
| Repeated Measures Analysis of Variance | |||||
| Tests of Hypotheses for Between Subjects Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| TxArm | 1 | 217.349740 | 217.349740 | 0.81 | 0.3892 |
| gender | 1 | 175.269140 | 175.269140 | 0.65 | 0.4377 |
| race | 1 | 259.226283 | 259.226283 | 0.97 | 0.3488 |
| Error | 10 | 2682.670543 | 268.267054 |
| The GLM Procedure | |||||||
| Repeated Measures Analysis of Variance | |||||||
| Univariate Tests of Hypotheses for Within Subject Effects |
| Source | DF | Type III SS | Mean Square | F Value | Pr > F | Adj Pr > F | |
|---|---|---|---|---|---|---|---|
| G - G | H-F-L | ||||||
| time | 8 | 1290.282821 | 161.285353 | 7.57 | <.0001 | 0.0001 | <.0001 |
| time*TxArm | 8 | 81.768939 | 10.221117 | 0.48 | 0.8671 | 0.7451 | 0.8380 |
| time*gender | 8 | 171.293983 | 21.411748 | 1.01 | 0.4388 | 0.4150 | 0.4337 |
| time*race | 8 | 62.241602 | 7.780200 | 0.37 | 0.9358 | 0.8266 | 0.9127 |
| Error(time) | 80 | 1703.980620 | 21.299758 | ||||
Notes: GLM= Generalized Linear Model; DF= degrees of freedom, SS= sums of squares, TxArm= treatment arm, Pr= probability, Adj Pr= Adjusted probability, G-G= Greenhouse-Geisser, H-F-L= Huynh-Feldt-Lecoutre
Mirtazapine was well tolerated in the study. There were no serious adverse events during the study. Only one potential medication side effect, weight gain, occurred significantly more commonly in the treatment group than placebo group during the course of the study. The mirtazapine group gained an average of 2.36 kg (SD=1.69 kg; range= 0 to 4.08) compared to the placebo group’s average of 0.57 kg (SD=1.24 kg; range= −1.36 [weight loss] to 1.79) (independent t test=0.04). In contrast, there were no significant differences between the mirtazapine and placebo groups in reports of sedation at any week across the entire study.
4. Discussion
This report provides data from what we believe is the first double-blind, placebo-controlled study evaluating the safety and efficacy of the second generation antidepressant medication mirtazapine for the treatment of both the depressive symptoms and the level of alcohol use of persons with comorbid MDD/AUD. Mirtazapine was well tolerated in our study. The current study was largely negative for both depressive symptoms and alcohol consumption in the absence of a significant time × group interaction in the repeated measures ANOVA. Nonetheless, a within-group improvement in depressive symptoms was noted earlier in the mirtazapine group than in the placebo group, being noted as a trend at week 1 and as a statistically significant difference at week 2 and at all subsequent assessments (weeks 2, 3, 4, 6, 8, 10, and 12), while in the placebo group a significant within-group decrease in depressive symptoms was not noted until week 8. Thus, our current preliminary findings demonstrate early and sustained within-group efficacy for mirtazapine for decreasing the level of depressive symptoms in MDD/AUD. This difference in the timing of the response of depressive symptoms to mirtazapine versus to placebo could be clinically significant for some patients, because some patients may not be willing to wait two months or longer for a therapeutic response to their treatment, whereas a response to mirtazapine might be seen in as little as two weeks. Therefore, despite the negative between-group findings of the current study, the significant within-group findings concerning depressive symptoms suggest some level of promise for mirtazapine for decreasing the level of depressive symptoms of MDD/AUD patients. Our current findings concerning the rapid onset of action of mirtazapine for decreasing depressive symptoms is consistent with the results of a review by Watanabe et al., (2008), which found that a rapid onset of effect for treating depressive symptoms is typical of treatment with mirtazapine.
The significant within-group decrease in depressive symptoms noted during the course of the current treatment trial is consistent with the within-group finding of an open label study by Yoon and colleagues (2006) conducted in South Korea and the between-group findings of a double-blind study by Altintoprak and colleagues (2008) conducted in Turkey. Both of those studies reported significant decreases in depressive symptoms in their studies of comorbid subjects involving mirtazapine. However, those two previous studies did not evaluate the level of alcohol use during the course of their studies, so we cannot compare the results of the current study to the results from those two previous studies on the important outcome variable of level of alcohol use. Our current findings also extend those previously reported findings by demonstrating no evidence of efficacy for mirtazapine for decreasing the level of alcohol consumption of that comorbid MDD/AUD population.
The results of this study should be interpreted in light of some limitations. The biggest limitation of this pilot study was its limited sample size. Large differences would have been required to demonstrate significant differences between groups with the current sample size. Also, since all of the subjects in this study were recruited as outpatients, it is unclear to what extent the results of this study apply to inpatients or to patients with additional co-occurring substance use disorders. In addition, we cannot rule out the possibility that some of the therapeutic effect that was noted in this clinical trial may have resulted from the brief motivation enhancement therapy used in study. Large double-blind placebo-controlled trials of mirtazapine appear to be warranted in persons with comorbid disorders.
Figure 1.
Depressive Symptoms as 21-item Beck Depression Inventory Means for Placebo and Mirtazapine Groups
Table 1.
Demographic and Clinical Characteristics of Randomized Subjects
| Total (n=14) | Placebo (n=7) | Mirtazapine (n=7) | χ 2 | sig | ||||
|---|---|---|---|---|---|---|---|---|
| Sex (% female) | 28.6% | 14.3% | 42.9% | 1.40 | 0.6 | |||
| Ethnic (% white) | 50.0% | 71.4% | 28.6% | 2.57 | 0.3 | |||
| Baseline | Mean | S.D. | Mean | S.D. | Mean | S.D. | t | sig |
| BDI Total | 26.9 | 9.6 | 26.1 | 11.7 | 27.6 | 7.7 | −0.27 | 0.8 |
| OCDS Total | 24.2 | 7.4 | 24.3 | 7.8 | 24.1 | 7.7 | 0.04 | 1.0 |
| Drinks per Week | 33.6 | 13.6 | 37.1 | 14.5 | 30.0 | 12.6 | 0.98 | 0.3 |
| Drinks per drinking day | 6.6 | 2.0 | 6.9 | 1.7 | 6.4 | 2.4 | 0.47 | 0.4 |
| Days of Alcohol Use per Week | 5.2 | 1.9 | 5.4 | 2.0 | 5.0 | 2.0 | 0.40 | 0.7 |
| Heavy Drinking Days / Week | 3.5 | 1.6 | 3.9 | 1.2 | 3.1 | 1.9 | 0.85 | 0.4 |
| Week 12 | ||||||||
| BDI Total | 9.5 | 6.6 | 10.1 | 8.7 | 8.9 | 4.4 | 0.35 | 0.7 |
| OCDS Total | 12.9 | 5.6 | 11.6 | 6.7 | 14.1 | 4.4 | −0.85 | 0.4 |
| Drinks per Week | 24.9 | 16.8 | 24.0 | 16.3 | 25.7 | 18.7 | −0.18 | 0.9 |
| Drinks per drinking day | 3.9 | 1.5 | 4.4 | 1.5 | 3.5 | 1.6 | 1.06 | 0.3 |
| Days of Alcohol Use per Week | 6.4 | 5.4 | 6.4 | 6.0 | 6.4 | 5.1 | 0.00 | 1.0 |
| Heavy Drinking Days / Week | 1.6 | 1.2 | 1.4 | 1.3 | 1.9 | 1.1 | −0.68 | 0.5 |
| Change from Baseline to Week 12 | ||||||||
| Δ BDI Total | −17.4 | 8.5 | −16.0 | 11.0 | −18.7 | 5.7 | 0.58 | 0.6 |
| Δ OCDS Total | −11.4 | 7.0 | −12.7 | 7.4 | −10.0 | 6.9 | −0.71 | 0.5 |
| Δ Drinks per Week | −8.7 | 20.4 | −13.1 | 19.6 | −4.3 | 21.8 | −0.80 | 0.4 |
| Δ Drinks per drinking day | −2.7 | 2.8 | −2.5 | 2.0 | −2.9 | 3.7 | 0.20 | 0.8 |
| Δ Days of Alcohol Use / Week | +1.2 | 4.5 | +1.0 | 5.1 | +1.4 | 4.2 | −0.17 | 0.9 |
| Δ Heavy Drinking Days / Week | −1.9 | 1.7 | −2.4 | 1.3 | −1.3 | 2.0 | −1.29 | 0.2 |
Note: BDI=Beck Depression Inventory, OCDS= Obsessive Compulsive Drinking Scale, S.D.=Standard Deviation, sig=statistical significance
Highlights.
A first study to assess the efficacy of mirtazapine for treating MDD/AUD.
Mirtazapine was well tolerated in the study.
No significant differences between the treatment groups were noted for any outcome.
Within group results suggest efficacy for mirtazapine for decreasing MDD symptoms.
No evidence for mirtazapine decreasing alcohol consumption was found.
Acknowledgements
This research was supported in part by grants from the National Institute on Alcohol Abuse and Alcoholism (R21 AA022123, R21 AA022863, R01 AA013370, R01 AA015173, K24 AA15320); and from the National Institute on Drug Abuse (R01 DA019142, P50 DA05605, K02 DA017822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 25th Annual Meeting of the American Academy of Addiction Psychiatry (AAAP), Huntingdon Beach, California, December 3-6, 2015.
References
- Altintoprak AE, Zorlu N, Coskunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: A randomized, double-blind study. Human Psychopharmacol. 2008;23:313–319. doi: 10.1002/hup.935. [DOI] [PubMed] [Google Scholar]
- Anton RF. Obsessive-compulsive aspects of craving: Development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95(Suppl. 2):S211–S117. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, Brown SJ. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112:39–45. doi: 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy AB, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict. Behav. 2009;34:905–909. doi: 10.1016/j.addbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Douaihy AB, Bukstein OD, Daley DC, Wood DS, Kelly TM, Salloum IM. Evaluation of cognitive behavioral therapy/motivation enhancement therapy (CBT/MET) in a treatment trial of comorbid alcohol use disorder/ major depressive disorder adolescents. Addict. Behav. 2011;36:843–848. doi: 10.1016/j.addbeh.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Douaihy AB, Clark DB, Chung T, Wood DS, Daley D. Mirtazapine in comorbid major depression and alcohol dependence: An open-label trial. J. Dual Diagn. 2012;8:2000–2004. doi: 10.1080/15504263.2012.696527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp., Released . IBM SPSS Statistics for Windows, Version 22.0. IBM Corp.; Armonk, NY: 2013. [Google Scholar]
- Levine J, Schooler RN. SAFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacol. Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Lovieno N, Tedeschini E, Bentley KH, Evins AE, Papakostas GI. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: A meta-analysis of placebo-controlled randomized trials. J. Clin. Psychiatry. 2011;72:1144–1151. doi: 10.4088/JCP.10m06217. [DOI] [PubMed] [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Project MATCH Monograph Series. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Washington, DC: 1992. Motivational Enhancement Therapy Manual. [Google Scholar]
- Nunes EV, Levin FB. Treatment of depression in patients with alcohol or other drug dependence. A meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. Base SAS 9.3 Procedures Guide. SAS Institute, Inc.; Cary, N.C.: 2011. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan H, Amorim P, Janars J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(suppl. 20):22–33. [PubMed] [Google Scholar]
- Sobell LD, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, Furukawa TA. Mirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: Systematic review and meta-analysis. J. Clin. Psychiatry. 2008;69:1404–1415. doi: 10.4088/jcp.v69n0908. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: A multicentre, open label study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]