Abstract
Acute ethanol intoxication is associated with Rapid Alterations in Neuroimmune Gene expression (RANGE), including increased Interleukin (IL)-6 and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), and suppressed IL-1β and Tumor necrosis factor (TNF) α, yet little is known about adaptations in cytokines across the first few ethanol exposures. Thus, the present studies examined central cytokines during intoxication (3 h post-ethanol) following 2, 4 or 6 intragastric ethanol challenges (4 g/kg) delivered either daily or every-other-day (EOD). Subsequent analyses of blood ethanol concentrations (BECs) and corticosterone were performed to determine whether the schedule of ethanol delivery would alter the pharmacokinetics of, or general sensitivity to, subacute ethanol exposure. As expected, ethanol led to robust increases in IL-6 and IκBα gene expression in hippocampus, amygdala and bed nucleus of the stria terminalis (BNST), whereas IL-1β and TNFα were suppressed, thereby replicating our prior work. Ethanol-dependent increases in IL-6 and IκBα remained significant in all structures—even after 6 days of ethanol. When these doses were administered EOD, modest IL-6 increases in BNST were observed, with TNFα and IL-1β suppressed exclusively in the hippocampus. Analysis of BECs revealed a small but significant reduction in ethanol after 4 EOD exposures — an effect which was not observed when ethanol was delivered after 6 daily intubations. These findings suggest that ethanol-induced RANGE effects are not simply a function of ethanol load per se, and underscore the critical role that ethanol dosing interval plays in determining the neuroimmune consequences of alcohol.
Keywords: ethanol, neuroinflammation, cytokines, repeated exposure, intermittent exposure, IL-6
1. Introduction
Alcohol consumption is a common pastime, with 51.0% of women and 62.2% of men in America reporting use of alcohol in 2013 (SAMHSA, 2015). While occasional limited consumption is a common social activity that poses relatively little health risk (German and Walzem, 2000, Kannel and Curtis Ellison, 1996), larger and more frequent doses of alcohol have widespread effects on multiple body systems. In 2013, 16.8% of women and 33.0% of men reported binge drinking, defined by NIAAA (2015) as approximately 4–5 drinks over the course of two hours (SAMHSA, 2015). Chronic consumption of such large doses of alcohol has been shown to lead to damage of the liver, brain, and other organs (Shukla et al., 2013, Ward et al., 2009).
Alcohol has a disruptive effect on numerous body systems, with altered function of the immune system contributing to pathologies seen in patients with chronic alcoholism. Indeed, there is a plethora of evidence for alcohol’s deleterious effects on the response to immune challenge in both human and animal models. For example, alcohol consumption in humans affects susceptibility to infectious disease, as well as the immune response to infections such as Hepatitis C (Wiley et al., 1998), bacterial pneumonia (Nelson and Kolls, 2002), and the progression of human immunodeficiency virus (HIV)-1 encephalitis (Potula et al., 2006), to name a few. In rodents, acute ethanol has been shown to generally suppress bacterial induction of cytokine production (Bhatty et al., 2011, Pruett et al., 2004), whereas chronic ethanol exposure seems to enhance the central and systemic cytokine response to an endotoxin challenge such as lipopolysaccharide (LPS) (Valles et al., 2003, Qin et al., 2008).
Even in the absence of a pathogen, however, ethanol has been shown to have direct consequences on immune-related factors. For instance, elevated serum levels of cytokines such as Tumor necrosis factor (TNF) α and interleukin (IL)-1β were associated with heavy chronic drinking, as was increased production of monocyte chemotactic protein-1 (MCP-1) in brain areas including the hippocampus, ventral tegmental area, and amygdala (He and Crews, 2008; for review, see Achur et al., 2010). In a rodent model, acute ethanol challenge elevated levels of TNFα, IL-1β, and IL-6 in the hypothalamus up to 48 hours after ethanol exposure (Emanuele et al., 2005), with in vitro ethanol administration to cultured astrocytes (50 to 200 mM) also increasing IL-6 expression (Sarc et al., 2011). Forced repeated exposure to ethanol for 10 days increased expression of brain TNFα and MCP-1 mRNA (Qin et al., 2008), whereas a chronic ethanol diet increased TNFα and IL-6 expression in the hypothalamus and pituitary of female rats (Emanuele et al., 2005).
More recently, data from our own laboratory has shown that acute ethanol administration (4 g/kg) elicits a robust and significant elevation in IL-6 and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) gene expression in the hippocampus, paraventricular nucleus of the hypothalamus (PVN) and amygdala during acute intoxication (i.e., 3 h after exposure), whereas IL-1β and TNFα tend to be suppressed during intoxication in most structures (Doremus-Fitzwater et al., 2014, 2015). For convenience, we now refer to these highly reproducible cytokine changes observed during acute intoxication (increased IL-6 and IκBα, decreased IL-1β and TNFα) as Rapid Alterations in Neuroimmune Gene Expression (RANGE) effects. Interestingly, a long-term history of moderate, voluntary, ethanol consumption (10 weeks of intermittent access to 20% ethanol) blunted the IL-6 response in the PVN but not the amygdala or hippocampus 3 hours after a 4 g/kg i.g. ethanol challenge (Doremus-Fitzwater, 2014). These intriguing findings suggest that ethanol-induced IL-6 responses may show substantial plasticity based on recent alcohol history, and that individual brain sites may be more (or less) capable of expressing such plasticity.
Thus, the overarching goal of the present series of studies was to examine adaptation in cytokine expression patterns across the first few ethanol exposures. In doing so, we focused our analyses on key limbic structures (hippocampus, amygdala, bed nucleus of the stria terminalis [BNST]), and a panel of pro-inflammatory cytokines downstream of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling (IL-1β, IL-6 and TNFα, as well as IκBα as a reporter of NF-κB activity) that are known to be ethanol responsive. In Experiment 1, we examined possible adaptation of ethanol-induced expression of central cytokines after 2, 4, or 6 ethanol exposures administered on a once-daily intubation schedule. Since intermittent exposure to ethanol, as well as other drugs of abuse, has been shown to induce unique responses such as behavioral sensitization (Marec et al., 2011, Legastelois et al., 2013, Abrahao et al., 2013), we then assessed the effects of intermittent (Every Other Day; EOD) ethanol exposure on central cytokine expression following 2, 4, or 6 ethanol exposures (Experiment 2). Given that the most marked ethanol-induced alterations in central cytokine expression were observed after 4 exposures to ethanol in both Experiments 1 and 2, Experiment 3 assessed potential differences in ethanol-related pharmacokinetics [blood ethanol concentrations (BECs) and corticosterone (CORT)] due to pattern of ethanol administration following 1 acute, 4 EOD, or 4 once-daily administrations of ethanol. Finally, in Experiment 4, we extended the analysis of BECs and CORT responses after 1 or 6 daily ethanol exposures as a final test of whether 6 daily ethanol administrations would produce metabolic tolerance.
2. Results
2.1 Experiment 1
2.1.1 Body & blood measures
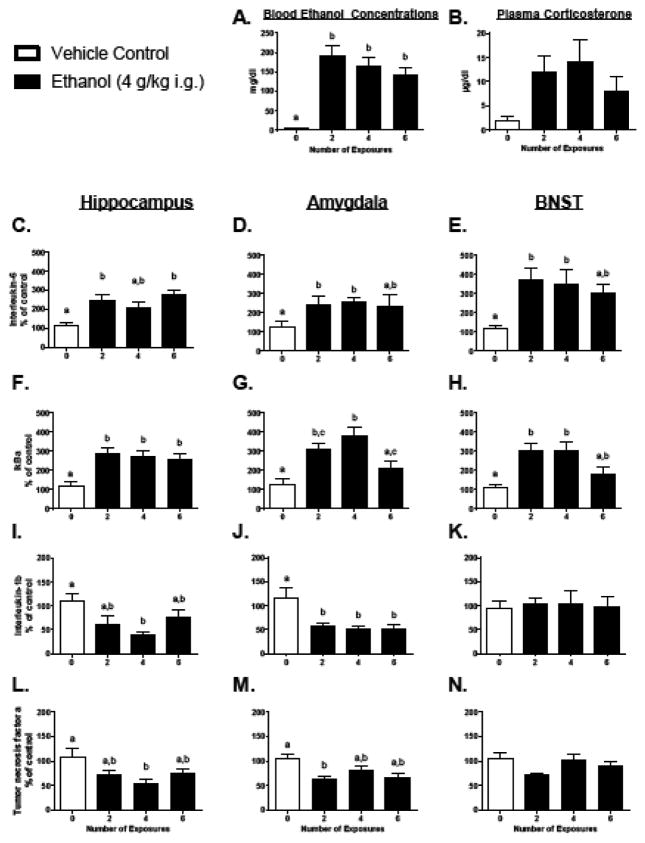
All data were analyzed using a one-way ANOVA that compared animals given 0, 2, 4, or 6 ethanol challenges. Three hours after the final intubation, plasma CORT concentrations (Figure 1B) exhibited by the ethanol-exposed groups were not significantly different from water-treated animals [F(3, 27) = 2.37, p = 0.093]. BECs (Figure 1A), however, were significantly elevated for all ethanol animals compared to vehicle-treated rats [F(3, 27) = 15.20, p < 0.001]. Although there was a trend for BECs to be reduced with an increasing number of ethanol exposures, neither CORT concentrations nor BECs were significantly different across the ethanol exposure groups. There was an effect of Group on percent change of body weight (Table 1) from the start of the experiment [F(3, 28) = 3.25, p < 0.05], with 4 exposures showing significant weight loss as compared to control (p < 0.05).
Figure 1. Central cytokine expression and blood measures following repeated ethanol exposure in Experiment 1.
Interleukin-6, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), Interleukin-1β, and Tumor Necrosis Factor α gene expression were assessed in the hippocampus, amygdala, and bed nucleus of the stria terminalis (BNST) in Experiment 1. All data are expressed relative to the ultimate control group (0 exposures). In the BNST and amygdala gene targets were normalized to Gylceraldehyde 3-phosphate dehydrogenase (Gapdh), whereas CyclophilinA was used as a reference gene for all hippocampal data. In this figure, as well as in all others, bars represent the mean of a particular group, with vertical lines indicating standard error of the mean (SEM). If a main effect of group was observed in the ANOVA for a particular gene of interest, then all bars were marked with at least one letter. Bars that share a common letter were considered statistically comparable, whereas bars that do not share a common letter were identified as significantly different using Tukey’s post hoc test (p < 0.05).
Table 1.
Experiment 1 & 3: changes in weight across exposure paradigms.
| Study | 0 Exposures | 2 Exposures | 4 Exposures | 6 Exposures | |
|---|---|---|---|---|---|
| Experiment 1: Massed Exposure | Start weight | 363.88±6.72 | 355.00±5.59 | 348.38±4.84 | 354.00±2.34 |
| Final weight | 370.38±7.99 | 357.50±7.79 | 331.50±5.90 | 338.38±3.04 | |
| % change | +1.79% a | +0.70% a,c | −4.84% b | −4.41% b,c | |
| Experiment 3: EOD Exposure | Start weight | 331.38±3.85 | 333.38±3.03 | 330.5±6.74 | 339.38±7.28 |
| Final weight | 366.25±4.39 | 361.75±4.07 | 359.63±7.52 | 363.13±8.32 | |
| % change | +10.52% | +8.51% | +8.81% | +7.00% |
Table displays starting weight (mean± SEM in grams), final weight, and directional % body weight change for each group across 0, 2, 4, or 6 exposures to ethanol (4 g/kg i.g) on either consecutive days for a total of 6 experimental days (Experiment 1), or intermittent every-other-day [EOD], for a total of 12 experimental days (Experiment 3). A lettering system was used to denote differences across groups in accordance with Tukey’s post-hoc test; groups that share a letter were statistically comparable (p < 0.05).
2.1.2 Central cytokine expression
In the hippocampus, IL-6 and IκBα (Figure 1C and F, respectively) were significantly elevated after 2, and 6 ethanol exposures [IL-6: F(3, 25) = 6.82, p < 0.01; IκBα: F(3, 25) = 9.53, p < 0.001]. Furthermore, IL-1β and TNFα (Figure 1I and L) were suppressed following 4 ethanol challenges, but were not different from vehicle controls after 2 or 6 exposures [F(3, 26) = 4.38, p < 0.05 and F(3, 26) = 3.97, p < 0.05, respectively]. There were no effects of ethanol administration on the expression of Hsp72 or Hmgb-1 (Table 2). C-fos expression (Table 2) was suppressed in all groups administered ethanol [F(3, 25) = 7.12, p < 0.01], and Mcp-1 (Table 2) was suppressed after 4 exposures, with a return to baseline levels by exposure 6 [F(3, 24) = 3.49, p < 0.05].
Table 2.
Experiment 1: Additional Hippocampal Gene Expression Data
| Gene target | 0 Exposures | 2 Exposures | 4 Exposures | 6 Exposures |
|---|---|---|---|---|
| c-Fos | 104.79±12.21 | 61.32±11.67* | 46.18±4.10* | 56.32±8.22* |
| Mcp-11 | 114.75±21.70 | 54.88±14.92 | 38.22±5.58* | 84.13±25.86 |
| Hsp722 | 101.61±7.20 | 132.09±10.97 | 120.69±10.13 | 115.83±8.76 |
| Hmgb-13 | 102.87±9.03 | 117.22±8.56 | 102.67±3.35 | 105.65±13.70 |
Gene expression levels (mean ± standard error of the mean) in the hippocampus following 0, 2, 4, or 6 exposures to ethanol are shown. Each group mean was first adjusted to the housekeeper, CyclophilinA, and then expressed as a percent change relative to the ultimate control group (0 Exposures). Values shown in bold and marked with an asterisk denote groups that differed significantly from the control.
Monocyte chemotactic protein 1;
Heat shock protein 72;
High-mobility group box 1.
In the amygdala, IL-6 (Figure 1D) was significantly elevated after 2 and 4 exposures, whereas IL-1β (Figure 1J) was suppressed after 2 and 6 exposures [F(3, 24) = 5.58, p < 0.01 and F(3, 22) = 4.52, p < 0.05, respectively]. TNFα (Figure 1M) was suppressed after 2 ethanol challenges, with slight but insignificant suppression after 4 and 6 exposures [F(3, 26) = 4.29, p < 0.05]. IκBα expression (Figure 1G) was elevated slightly after 2 ethanol exposures, was significantly increased compared to vehicle controls following 4 exposures, but was no different from controls with 6 repeated ethanol intubations [F(3, 28) = 9.54, p < 0.001].
In the BNST, IL-1β (Figure 1K) and TNFα (Figure 1N) expression were unchanged in the ethanol-exposed rats, whereas IL-6 (Figure 1E) was significantly elevated across all ethanol-challenged groups, though by 6 exposures levels were similar to 0 groups as well as the ethanol-exposed groups [F(3, 28) = 4.08, p < 0.05]. In contrast, IκBα expression (Figure 1H) was significantly elevated after both 2 and 4 exposures, but not significantly different from vehicle controls or the ethanol groups following 6 ethanol exposures [F(3, 28) = 6.68, p < 0.01].
2.2 Experiment 2
2.2.1. Body & blood measures
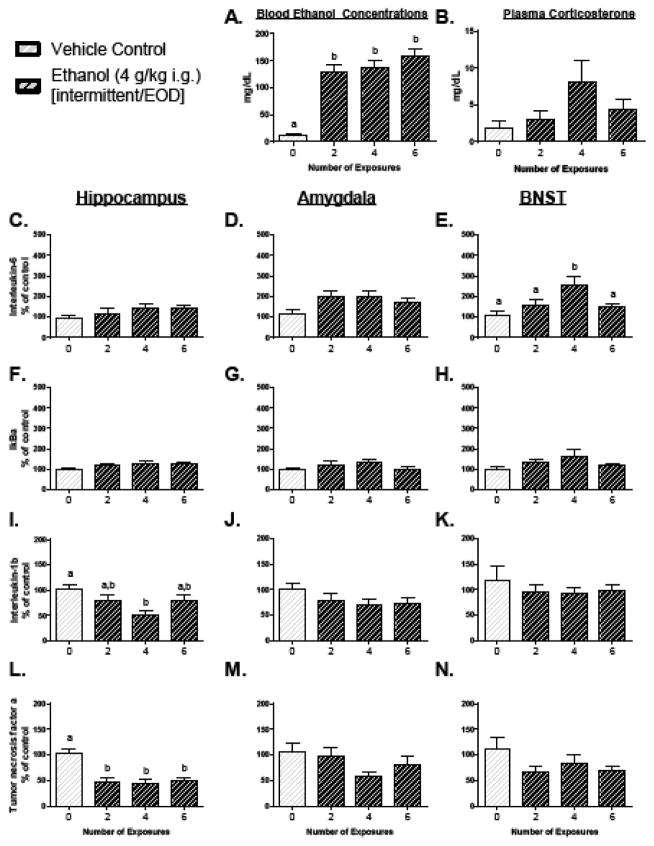
A one-way ANOVA (Group: 0, 2, 4, or 6 exposures) of plasma confirmed an elevation in BECs (Figure 2A) in all groups that were administered ethanol [F(3, 27) = 28.54, p < 0.0001], though no significant differences were observed between the ethanol-exposed groups. There was no effect of Group on CORT (Figure 2B). There was no significant change in body weight throughout this experiment (Table 1).
Figure 2. Central cytokine expression and blood measures following intermittent repeated ethanol exposure in Experiment 2.
Interleukin-6, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), Interleukin-1β, and Tumor Necrosis Factor α gene expression were assessed in the hippocampus, amygdala, and bed nucleus of the stria terminalis (BNST) in Experiment 2. All data are expressed relative to the ultimate control group (0 exposures), and are normalized to CyclophilinA. In this figure, as well as in all others, bars represent the mean of a particular group, with vertical lines indicating standard error of the mean (SEM). If a main effect of group was observed in the ANOVA for a particular gene of interest, then all bars were marked with at least one letter. Bars that share a common letter are statistically comparable, whereas bars that do not share a common letter were found to be significantly different according to Tukey’s post hoc test (p < 0.05).
2.2.2. Central cytokine expression
Ethanol induced no significant changes in cytokine expression in the amygdala (Figure 2D, G, J, M). Whereas intermittent ethanol exposure failed to significantly elevate expression of IL-6 and IκBα in the hippocampus (Figure 2C, and F), this EOD pattern of exposure did suppress TNFα (Figure 2L) in all groups receiving ethanol [F(3, 27) = 11.17, p < 0.0001; all p < 0.001 as compare to Vehicle]. Moreoever, IL-1β (Figure 2I) was suppressed in the 4 exposure group as compared to the Vehicle animals [F(3, 27) = 4.19, p < 0.05; p < 0.01 for post-hoc]. In the BNST, there were no effects of Group on IL-1β or TNFα expression (Figure 2K, N), but IL-6 (Figure 2E) was elevated as a result of ethanol [F(3, 27) = 4.06, p < 0.05] in the 4 exposures group as compared to Vehicle Controls (p < 0.01). No effects of ethanol were observed on IκBα (Figure 2H).
2.3 Experiment 3
BECs (Figure 3A) and serum CORT (Figure 3C) were assessed using a 3 (Group: 1st exposure [acute] vs 4th Daily vs 4th EOD) x 7 (Time Point) mixed ANOVA design. Analysis of BECs revealed a main effect of Time Point [F(6, 126) = 456.72, p < 0.0001] and Group [F(2, 21) = 3.81, p < 0.05]. Further examination using post-hoc testing determined that BECs remained significantly elevated through 6 h after intubation, but with levels not significantly higher than baseline observed at the next assessed time point of 12 h post-intubation. The 4th EOD group showed BECs that were significantly attenuated as compared to both the 1st exposure and 4th daily exposure groups (p < 0.05 for both).
Figure 3. Time course of serum corticosterone and ethanol concentrations in Experiments 3 and 4.
Experiment 3. (A) Blood ethanol concentrations [BECs] and (C) serum corticosterone were assessed across numerous time points post-ethanol challenge. Animals receiving ethanol (4g/kg) for the first time (solid circles) were compared to rats receiving their fourth exposure to the same dose of ethanol, but with one group exposed to ethanol once-daily for four consecutive days (open squares) and the other given ethanol once-daily in an every-other-day (EOD) pattern (hatched hexagons). For BECs: the pound symbol (#) represents a significant difference from baseline (when collapsed across Exposure Condition) according to Tukey’s post hoc, which was used to explore the main effect of Time Point (p < 0.05). The significant main effect of Group observed in the analysis of BECs is represented in the area under the curve inlay graph, wherein bars that share a common letter were considered statistically comparable, whereas bars that do not share a common letter were significantly different (p < 0.05 also using Tukey’s post hoc test). For corticosterone: an ampersand (&) represents time points at which the 1st exposure group was different from both the 4th EOD and 4th daily exposure groups, which did not differ from one another (determined by Tukey’s post-hoc test for the significant interaction of Group with Time Point; p < 0.05). Experiment 4. (B) Blood ethanol concentrations [BECs] and (D) serum corticosterone were assessed across a number of time points post-ethanol exposure, with rats given one acute exposure to 4 g/kg ethanol (solid circles) compared to animals that experienced their 6th consecutive once-daily ethanol challenge (open triangles). For BECs: the pound symbol (#) represents a significant difference from baseline (when collapsed across exposure condition) according to the Tukey’s post hoc test used to explore the main effect of Time Point (p < 0.05). For corticosterone: an asterisk (*) denotes a significant difference from baseline within the acute ethanol exposure group, whereas the double dagger (‡) represents a difference from baseline in the repeated exposure group.
Serum CORT analysis revealed a significant interaction between Group and Time Point [F(12, 126) = 9.73, p < 0.0001]. At 0.75 h and 1.5 h after ethanol intubation, the 1st exposure group revealed the higher concentration of CORT than both 4th daily and 4th EOD groups (p < 0.05 for all). There was no significant difference between the groups that had 4 ethanol exposures after these time points.
2.4 Experiment 4
CORT concentrations and BECs were assessed using a 2 (Group: 1st exposure [acute] vs 6th exposure [repeated]) x 7 (Time Point) mixed ANOVA design. In the analysis of BECs, a main effect of Time Point [F(6,60) = 64.22, p < 0.001] indicated that, relative to baseline, BECs were significantly elevated at 0.75, 1.5, 3, and 6 h after intubation (Figure 3B). By 12 h post-administration, however, BECs were no longer different than at baseline. No main effect of Group, nor a significant interaction of Group with Time Point, was observed in the analysis of BECs.
Serum CORT (Figure 3D) was significantly elevated by ethanol exposure [main effect of Time Point: F(6,60) = 44.05, p ≤ 0.001] at 0.75 and 1.5 h post-administration for both groups relative to their own baseline [Group x Time Point interaction: F(6, 60) = 4.91, p < 0.001]. At the onset of the dark cycle (12 h post-challenge), animals given an acute ethanol challenge showed a slight but significant increase in serum CORT, not seen in the 6th exposure group.
3. Discussion
This report presents a series of studies that examined the effects of schedule of ethanol delivery on central cytokine expression patterns across several brain regions including the hippocampus, amygdala, and BNST. These parametric studies are critical because there is at present a relative dearth of information regarding ethanol-induced alterations in central cytokines following sub-chronic exposure to ethanol, with virtually no studies examining the potential for adaptation in central cytokine responses across the first few ethanol exposures. These studies were predicated upon recent work from our laboratory that demonstrated robust, time-dependent increases in IL-6 expression in brain after acute ethanol exposure, which were modified in a site-selective manner by a prior history of long-term voluntary ethanol consumption (Doremus-Fitzwater et al., 2014). We now report several key new findings regarding adaptation in cytokine expression patterns that inform future studies relating to ethanol and cytokine expression in brain. (i) The present findings replicate and extend our prior work showing increased IL-6 and IκBα, along with suppressed IL-1β, and TNFα gene expression across multiple CNS sites after ethanol challenge —an effect we are referring to as Rapid Alteration in Neuroimmune Gene Expression (RANGE). (ii) The durability of the IL-6 response across as many as 4 daily ethanol exposures, and its relationship to Nf-κB signaling, suggest that this cytokine response does not adapt readily when a sub-chronic ethanol regimen is delivered daily (Exp. 1). (iii) Yet, when these doses are delivered every other day, the cytokine response is widely ablated across most areas, whereas the TNFα suppression in the hippocampus seems to persevere (Exp. 2). Together, these findings provide important insight into how unique ethanol exposure regimens —many of which are commonly employed in alcohol studies —might lead to differential outcomes on central cytokine expression.
Although the current results do not provide conclusive evidence of mechanisms involved in these ethanol effects on cytokines, they do provide a comprehensive analysis of the same inflammatory-related factors across a variety of brain regions. Thus, these experiments address the question of whether ethanol-induced alterations in cytokines are a brain-wide response to ethanol, or structurally dependent in nature. Together, the present results showed that, while cytokine expression in all three brain structures was affected by ethanol exposure, these effects varied between structures in persistence, magnitude, and directionality. In Experiment 1 for example, elevations in IL-6 were persistent across 6 ethanol exposures, with only modest adaptation beginning to emerge after 6 exposures in the amygdala and BNST. Interestingly, the greatest magnitude of IL-6 change was observed in the BNST among the structures examined here. In contrast, the BNST did not display IL-1β or TNFα effects, whereas the amygdala and hippocampus revealed significant suppression. Intriguingly, each brain structure demonstrated some unique measure of adaptation by the 6th exposure. For instance, the hippocampus showed recovery of the IL-1β and TNFα effect, whereas the BNST and amygdala demonstrated recovery of IκBα to baseline levels and partial attenuation of IL-6. These adaptations cannot simply be accounted for by differences in BECs, as all groups showed similar concentrations of ethanol in the blood.
While some of these effects were observed when ethanol was delivered intermittently in Experiment 2, the cytokine response was significantly diminished by the change in schedule of ethanol delivery. For instance, while the BNST IL-6 response was persistent with daily exposure, showing a mildly diminished response only by the 6th exposure, every other day delivery of ethanol resulted in only a slight IL-6 increase after 4 days of ethanol. The strong elevations seen after daily exposure in the hippocampus and amygdala were, however, entirely absent when ethanol was delivered every other day, again underscoring the site-specific nature of the cytokine response to ethanol. To further this observation, the expression of IL-1β and TNFα in the amygdala was affected by daily, not intermittent exposure, whereas similar patterns of expression for these cytokines in the hippocampus and BNST were seen regardless of schedule of ethanol. Thus, schedule of ethanol delivery, rather than ethanol load per se, appears to be a key determinant of neuroimmune responses evoked by ethanol challenge.
While the experiments presented here did not explore potential mechanisms responsible for ethanol-induced RANGE, a plethora of other studies examining the effects of chronic ethanol exposure have demonstrated that ethanol has profound effects on neuroimmune signaling pathways (Crews et al., 2013a, Pascual et al., 2015, Li et al., 2010). Indeed, NF-κB has been identified as a driver of neuroimmune gene expression (Zou and Crews, 2010), an effect that may be downstream of HMGB1 signaling through TLR4 receptors (Crews et al., 2013a). Consistent with this, the present data show that IκBα expression was positively correlated with IL-6 (r = 0.86), whereas the relationship of IκBα to both TNFα and IL-1β was not significant (r = 0.02, 0.03 respectively). This intriguing finding suggests a significant departure from the simple expectation that NF-κB signaling (reported by increased IκBα gene expression) would show a strong positive correlation with all 3 of these cytokines, and is consistent with our previous work examining acute ethanol effects (Doremus-Fitzwater et al., 2015). Based on our present data, therefore, future studies examining more specific features of NF-κB signaling are of high priority for elucidating the seemingly paradoxical dissociation between IκBα and TNF/IL-1β.
In the present studies, we also probed gene expression levels for HMGB1, HSP72, and Mcp-1 (Table 1) because these signaling pathways have been shown to be important for long-term consequences of alcohol exposure and/or are upstream of cytokine gene regulation (Umhau et al., 2014; Whitman et al., 2013). Although on the surface these findings might be viewed as contradictory to prior published work, HMGB1 is not the only mechanism by which NF-κB signaling can be initiated. Indeed, one intriguing possibility is that ethanol-dependent activation of NF-κB signaling may be an immediate and durable consequence of alcohol exposure across the first few ethanol exposures, whereas alarmin signals such as HMBG1 and HSPs may require more protracted alcohol exposure across weeks, months, or even years to develop. Studies showing increased concentrations of HMGB1 have been measured in post-mortem analyses of the brains of alcoholics (Crews et al., 2013b), highlighting the potential importance of the HMGB1→TLR4→NF-κB→neuroimmune gene signaling pathway for ethanol-induced neuroimmune effects during life-long alcohol exposure or end-stage alcoholism. In this way, our work on the early phase (first few ethanol exposures) fills an important gap in our existing knowledge of neuroimmune consequences of alcohol exposure.
In comparing our findings to other studies, it is critical to acknowledge that most studies examining changes in neuroimmune genes and/or signaling pathways after ethanol exposure have used far more ethanol exposures or continuous exposure to doses that elicit loss of righting reflex (i.e., substantially longer and higher than those used here). In addition, most studies have examined neuroimmune effects that are expressed after ethanol clearance, and often during ethanol withdrawal, which clearly elicits a different pattern of cytokine changes (Doremus-Fitzwater et al., 2014). Timing, dose, and life history of alcohol are clearly important predictors of neuroimmune changes incurred by alcohol, and thus our emphasis on intoxication-related changes in neuroimmune gene expression are another unique feature of the present work. Future studies should extend these findings to changes in other cytokines that appear to be upregulated uniquely during alcohol withdrawal (i.e., Doremus Fitzwater et al., 2014) and will provide an important contrast to the results observed here during ethanol intoxication.
The propensity of ethanol to stimulate the HPA axis is well-documented (eg., Li et al., 2005, Thiagarajan et al., 1989). Not only is corticosterone a useful marker of sensitivity to ethanol (Glavas et al., 2007), but it also exerts a profound influence on neuroimmune gene expression in a variety of contexts (see Deak et al., 2015 for a recent review). In the current studies, ethanol-induced elevations in corticosterone were present after acute exposure, but have been shown to habituate with chronic (2 g/kg i.p. for 14 days) ethanol administration after 7 days of exposure (Spencer and McEwen, 1990). Consistent with these findings, assessment of serum corticosterone revealed modest signs of habituation, with the repeated ethanol exposure group exhibiting slightly lower peak corticosterone compared to animals given acute ethanol. These effects largely paralleled a subtle diminution of the BEC response observed during the 6th ethanol exposure. Although these modest shifts in BECs and corticosterone responses are likely not sufficient to account for the more marked adaptations in IL-6 expression observed here, future studies will be required to fully evaluate this issue mechanistically.
When considering the relationship between BECs and cytokine expression, one important conclusion that can be drawn is that the cytokine response to ethanol is mediated not only by ethanol load, but is also sensitive to the schedule of ethanol delivery. Whereas clear signs of metabolic tolerance were observed when ethanol was delivered on an EOD schedule, ethanol delivery on a daily schedule required a minimum of 6 exposures to achieve even a modest (and importantly, not statistically significant) metabolic tolerance (Experiment 4). Across these studies, however, the BECs at the time of cytokine assessments (3 h after exposure) exhibited some variability (~150–200 mg/dl), but the pattern of BECs across studies did not seem to meaningfully predict the changes in neuroimmune gene expression. This indicates that the periods of recovery afforded by the intermittent exposure paradigm allow not only for shifts in the metabolic tolerance to ethanol, but, to a larger extent, adaptations in ethanol-induced RANGE that are not altogether explained by ethanol load or pharamacokinetics. Whereas much work has been devoted to characterizing the behavioral and cognitive outcomes of intermittent models of ethanol exposure (Zhao et al., 2013, Pascual et al., 2014), this is the first demonstration that cytokine responses to ethanol are not simply a slave response to ethanol load, and that schedule of alcohol exposure may be a critical feature of alcohol-related cytokine changes.
Although the studies presented here have not tested the functional impact of ethanol-induced IL-6 expression, other studies have shown that IL-6 signaling in the hippocampus and cerebellum significantly influence synaptic plasticity and various features of cognitive function (see Gruol, 2014 for a recent review). For example, IL-6 signaling in the orbitofrontal cortex, and its activation of the JAK/STAT pathway, has been shown to have a direct impact on cognitive function and flexibility (Donegan et al., 2014). It has also been proposed that IL-6 signaling may be involved in adaptations following stress exposure via its modulatory effects on HPA axis output (Girotti et al., 2012). Together, these well-established functional effects of IL-6 in the CNS underscore the importance of better understanding patterns of adaptation in ethanol-induced cytokine expression in the CNS, and in particular, IL-6 expression patterns.
There are, however, a few limitations to the work presented here. For instance, although it would have been advantageous to examine the PVN from these same studies, the PVN tissue was unfortunately lost due to equipment malfunction in Experiment 1. Without the possibility of comparing PVNs across experiment, the sensitivity of the PVN to schedule of ethanol delivery therefore cannot be ascertained at this time. Second, it should be noted that these studies were conducted within the light phase of the circadian cycle, whereas many voluntary consumption models often perform ethanol exposures during the dark phase. We recognize that there are large and distinct alterations in neuroimmune function across the circadian cycle and we have not yet explored time-of-day differences in our studies. Importantly, the majority of our prior studies examining alcohol-neuroimmune interactions have been conducted at the same time of day (early phase of lights on), and so one goal here was to be consistent with our prior work. In addition, most pre-clinical studies involving stress manipulations/measures are also conducted in the light phase so that discrete corticosterone responses to stress can be readily separated from circadian fluctuations in corticosterone. Finally, at the conceptual level, it is most common for humans who consume alcohol to initiate alcohol consumption towards the end of the normal wakeful period, which is probably more akin to what we have done here (i.e., alcohol intubations shortly after light onset, which perhaps corresponds to “staying up late to drink” for the rat). This approach is in stark contrast to many voluntary consumption models such as the “Drinking in the Dark” procedure, in which alcohol consumption peaks during the early hours of wakefulness and probably models alcohol intake in humans around brunch time. Finally, the present studies did not include a separate group of non-manipulated rats to control for minor stress associated with repeated intubations. From a practical standpoint, it is not possible to include such controls in every experiment because the studies can get very large and costly, and the number of statistical comparisons increases proportionally. Our decision to omit ultimate controls from these studies was informed by our prior work indicating that any mild corticosterone response evoked by the vehicle intubation had largely resolved at the time point of interest (3 h post-intubation) as indicated by plasma corticosterone levels in the normative range of basal samples (baseline levels of ~ 2 μg/dL; Doremus-Fitzwater et al., 2014). Indeed, baseline levels of corticosterone in the present studies also fell within the normative range of non-manipulated rats. While this does not negate the need for non-stressed controls, we believe that our current (and previous) results provide some assurance that our intubation procedures are minimally distressing.
Overall, these experiments fill an important gap in our knowledge regarding how ethanol-induced RANGE adapts across the first few ethanol exposures. Furthermore, these findings highlight the importance of not just ethanol load, but also the schedule, on which those ethanol exposures occur. Neuroimmune consequences of alcohol abuse have, in recent times, come to be seen as a treasure trove of missing links with relevance to the development of alcohol addiction (Montesinos et al., 2015; for review, see Cui et al., 2014) and other health outcomes related to alcohol abuse (for review, see Molina et al., 2015). In this way, neuroimmune signaling pathways may offer novel pharmacotherapeutic pathways that, with successful target validation studies, might be engaged to ameliorate the adverse of alcohol use and abuse.
4. Methods and Materials
4.1 Subjects
Adult male Sprague Dawley rats (300–375 g) were purchased from Harlan (Frederick, MD) and given 2 weeks to acclimate to the colony (22±1°C with 12:12 light–dark cycle, lights on 06:30am) prior to experimentation. Animals were pair-housed in standard Plexiglas cages with ad libitum access to food and water, except during behavioral testing. In all experiments, rats were handled (3–5 min) for 2 days prior to experimentation. In all studies, cage mates were assigned to the same experimental condition. At all times animals were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Animal Resources (1996), and with protocols approved by the IACUC committee at Binghamton University.
4.2 Ethanol administration
Ethanol (95%) was diluted fresh daily with tap water to make a 20% (v/v) solution for intragastric (i.g.) administration, with tap water used as the vehicle.
4.3 Blood and Tissue collection
In Experiments 1 and 2, animals were rapidly decapitated (unanesthetized) at the appropriate time point and trunk blood collected into EDTA-coated Vacutainers (BD Vacutainers, VWR cat. no. VT6450, Radnor, PA). Plasma was separated through refrigerated centrifugation and stored at −20°C. Brain tissue was harvested and stored as described previously (Hueston and Deak, 2014). Structures of interest (hippocampus, amygdala, BNST) were the foci of our investigations, as prior work has shown these brain areas to be responsive to stress (Hueston et al., 2011) and ethanol exposure (Doremus-Fitzwater et al., 2014). Brain structures were identified using a brain atlas (Watson & Paxinos, 2005) and punched as previously described (Blandino et al., 2013; Doremus-Fitzwater et al., 2015). In Experiments 3 and 4, repeated blood samples were collected using the tail clip method for later assessment of blood ethanol content and corticosterone concentrations in serum.
4.4 Blood ethanol concentration (BEC)
BECs were determined in 5-μl aliquots using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA) as previously described (Doremus-Fitzwater et al., 2015). The machine was calibrated every 15 samples using an industry standard (100 mg%).
4.5 Corticosterone (CORT) concentration
Plasma or serum concentrations of CORT were determined using a commercially available ELISA kit (Cat No: ADI-901–097; Enzo Life Sciences, Farmingdale, NY) as previously described (Hueston and Deak, 2014) with an assay sensitivity of 27.0 pg/ml and an inter-assay variability of 6.2%.
4.6 Real Time RT-PCR
Tissue was placed into a 2.0 ml Eppendorf tube containing 500 μL TrizolR RNA reagent (Invitrogen, Grand Island, NY) and a 5 mm stainless steel bead, and then homogenized using a Qiagen Tissue Lyser II (Qiagen, Valencia, CA). Following homogenization, RNA was extracted using Qiagen’s RNeasy mini kit (cat. no. #74106), according to manufacturer’s instructions. Synthesis of cDNA was performed as described previously (Doremus-Fitzwater, 2015) using QuantiTect® Reverse Transcription Kit (Cat. No. 205313, Qiagen, Valencia, CA). Probed cDNA amplification was performed and captured in real-time using the Bio-Rad (Hercules, CA, cat. no. 185-5485) CFX384 Real-Time PCR Detection System (Doremus-Fitzwater et al., 2015; Hueston and Deak, 2014). Primer sequences and accession numbers are listed in Table 3. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and CyclophilinA were used as reference genes, with expression of housekeeper initially analyzed as a separate target to examine its stability across experimental conditions. All data were adjusted relative to housekeeper using the 2−ΔΔCT transformation (Livak and Schmittgen, 2001).
Table 3.
Primer sequences and accession numbers
| Primer | Accession Numbers | Oligo | Sequence |
|---|---|---|---|
| β-Actin | NM_031144.3 | Forward | 5’-GTCGTACCACTGGCATTGTG-3’ |
| Reverse | 5’-GCCATCTCTTGCTCGAAGTC-3’ | ||
| C-Fos | NM_022197.2 | Forward | 5’-CCAAGCGGAGACAGATCAAC-3’ |
| Reverse | 5’-AAGTCCAGGGAGGTCACAGA-3’ | ||
| CyclophilinA | NM_017101.1 | Forward | 5’-GCGTCTGCTTCGAGCTGTTT-3’ |
| Reverse | 5’-CGTAGATGGACTTGCCACCA-3’ | ||
| Gapdh1 | NM_017008 | Forward | 5’-ATGACTCTACCCACGGCAAG-3’ |
| Reverse | 5’-AGCATCACCCCATTTGATGT-3’ | ||
| Hmgb-12 | NM_012963.2 | Forward | 5’-GGCGGCTGTTTTGTTGACAT-3’ |
| Reverse | 5’-ACCCAAAATGGGCAAAAGCA-3’ | ||
| Hsp723 | NM_031971.2 | Forward | 5’-GGCCTTGAGGACTTTGGGTT-3’ |
| Reverse | 5’-CTGGGAATGCAAAGCACACG-3’ | ||
| IκBα4 | NM_001105720.2 | Forward | 5’-CTGTTGAAGTGTGGGGCTGA-3’ |
| Reverse | 5’-AGGGCAACTCATCTTCCGTG-3’ | ||
| Il-1β5 | NM_031512 | Forward | 5’-AGGACCCAAGCACCTTCTTT-3’ |
| Reverse | 5’-AGACAGCACGAGGCATTTTT-3’ | ||
| Il-66 | NM_012589 | Forward | 5’-TAGTCCTTCCTACCCCAACTTCC-3’ |
| Reverse | 5’-TTGGTCCTTAGCCACTCCTTC-3’ | ||
| Mcp-17 | NM_031530.1 | Forward | 5’-TCTCTGTCACGCTTCTGGG-3’ |
| Reverse | 5’-TGCTGCTGGTGATTCTCTTG -3’ | ||
| Tnf-α8 | NM_012675 | Forward | 5’-GGGGCCACCACGCTCTTCTG-3’ |
| Reverse | 5’-CGACGTGGGCTACGGGTTG-3’ |
Primers, accession numbers, and sequences used in real-time RT-PCR for all gene expression studies.
Glyceraldehyde 3-phosphate dehydrogenase;
High-mobility group box 1;
Heat shock protein 72;
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha;
Interleukin-1 beta;
Interleukin-6;
Monocyte chemotactic protein 1;
Tumor necrosis factor alpha.
4.7 Statistical analyses
Data were analyzed with Statistica software using either between-subjects one-way ANOVA (Experiments 1 & 2) or repeated measures ANOVA (Experiments 3 & 4). Post-hoc testing was done using Tukey’s test for all observed main effects and interactions. An α-level of 0.05 was used as the criterion for determining statistical significance.
4.8 Specific methods Experiment 1: Effects of repeated ethanol exposure on CNS cytokine expression
Rats (n = 8/group; N = 32) were given 0, 2, 4, or 6 once-daily exposures to 4 g/kg EtOH (i.g.) on consecutive days. All rats received a total of 6 intubations, with vehicle given on non-ethanol days. Body weights were measured daily to establish volume of intubations and as a general assessment of growth/health. Intubations were administered at 0800-1000 daily. Rats not receiving ethanol (the 0 group) were administered 6 vehicle intubations and served as the ultimate controls. Trunk blood and brain tissue were collected 3 h after the final intubation. This time point was selected based on prior data showing highly reproducible effects of ethanol intoxication (evidenced by peak BECs) on the expression of cytokines of interest (Doremus-Fitzwater, 2014).
4.9 Specific methods Experiment 2: The impact of repeated ethanol exposure on brain cytokine expression following intermittent ethanol administration
Intermittent access to drugs of abuse results in altered behavioral responses in comparison to continued administration (Marec et al., 2011), and intermittent exposure administration procedures are commonly used to study alcohol effects (Pandey et al., 2015; Risher et al., 2015). Having assessed adaptations in cytokine responses that occur with repeated once-daily exposure to ethanol, we next examined whether the schedule of ethanol exposure would impact cytokine expression patterns in the CNS. To do this, rats (n = 8; N = 32) were given 2, 4, or 6 intermittent every other day (EOD) exposures to 4 g/kg i.g. ethanol, with an additional group of control animals (n = 8) receiving 6 EOD vehicle intubations. As in the previous study, intubations were performed between 0800-1000, with body weights taken the evening before intubations. Three hours after the final intubation, all rats were killed and trunk blood and brain tissue (hippocampus, amygdala, BNST) were collected as described above.
4.10 Specific methods Experiment 3: Effects of acute, repeated, or intermittent ethanol exposure on serum CORT and ethanol pharmacokinetics
Experiment 3 was designed to determine whether schedule-dependent changes in central cytokines might reflect differences in the pharmacokinetics (rate or peak) of BECs achieved by the daily versus intermittent ethanol exposures. To do this, rats (n = 8; N = 24) were given either an acute i.g. exposure to 4 g/kg ethanol, 4 once-daily, or 4 EOD exposures to the same dose. Four ethanol intubations were chosen for the number of exposures, as central cytokine alterations were most robust following this number of ethanol deliveries in both Experiments 1 and 2. Notably, in all groups rats received an equal number of intubations, with the acute exposure group given tap water on days 1 - 3 and then intubated with a single ethanol exposure on day 4. Following the final gavage on day 4, tail blood samples (≤ 100 μl) were taken at 0.75, 1.5, 3, 6, 12, and 24 h after intubation, as well as 30 min before ethanol administration (baseline sample).
4.11 Specific methods Experiment 4: Effects of acute versus repeated ethanol exposure on serum CORT and ethanol pharmacokinetics
The outcomes of the experiments described above suggested that rats given ethanol intubation on 4 consecutive days (relative to intermittent ethanol exposure) evinced no signs of metabolic tolerance. Experiment 4 was thus designed to extend these findings to 6 ethanol exposures in just the daily exposure group. Rats were given either an acute i.g. exposure to 4 g/kg ethanol (n = 5), or 6 once-daily i.g. intubations of 4 g/kg ethanol (n = 7) that were delivered on consecutive days. In both groups, rats received an equal number of intubations, with the acute exposure group intubated with tap water on days 1 - 5 and then given a single ethanol exposure on day 6. Following the final intubation on day 6, tail blood samples (≤ 100 μl) were taken at 0.75, 1.5, 3, 6, 12, and 24 h after intubation, as well as 30 min before ethanol administration (baseline sample).
Highlights.
Alcohol significantly increased IL-6 and IkBa and reduced IL-1b and TNFa in the CNS
Increased IL-6 and IκBα persisted after 6 daily but not 6 intermittent EtOH exposures
Mild metabolic tolerance was observed after intermittent but not daily EtOH exposure
Neuroimmune consequences of EtOH were contingent upon schedule of EtOH delivery
These data shed important light on intoxication-related changes in neuroimmune genes
Acknowledgments
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institute of Health under Award Number P50AA017823 to T. Deak and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, Mccool BA, Souza-Formigoni MLO, Weiner JL. Locomotor sensitization to ethanol impairs nmda receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. The Journal of Neuroscience. 2013;33:4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achur R, Freeman W, Vrana K. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. Journal of Neuroimmune Pharmacology. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatty M, Jan BL, Tan W, Pruett SB, Nanduri B. Role of acute ethanol exposure and tlr4 in early events of sepsis in a mouse model. Alcohol. 2011;45:795–803. doi: 10.1016/j.alcohol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Hueston CM, Barnum CJ, Bishop C, Deak T. The impact of ventral noradrenergic bundle lesions on increased IL-1 in the PVN and hormonal responses to stress in male sprague dawley rats. Endocrinology. 2013;154:2489–2500. doi: 10.1210/en.2013-1075. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013a;73:602–12. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. Hmgb1/tlr receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry. 2013b;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol. 2014;118:1–12. doi: 10.1016/B978-0-12-801284-0.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015;18(4):367–80. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan JJ, Girotti M, Weinberg MS, Morilak DA. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal crotex. J Neurosci. 2014;34(3):953–62. doi: 10.1523/JNEUROSCI.3968-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater T, Buck HM, Bordner KA, Richey L, Deak T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res. 2014;38:2186–2198. doi: 10.1111/acer.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater T, Gano A, Paniccia JE, Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav. 2015;148:131–44. doi: 10.1016/j.physbeh.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele NV, Lapaglia N, Kovacs EJ, Emanuele MA. The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: Reproductive implications. Cytokine. 2005;30:109–115. doi: 10.1016/j.cyto.2004.11.004. [DOI] [PubMed] [Google Scholar]
- German JB, Walzem RL. The health benefits of wine. Annual Review of Nutrition. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- Girotti M, Donegan JJ, Morilak DA. Influence of hypothalamic IL-6/gp130 receptor signaling on the HPA axis response to chronic stress. Psychoneuroendocrinology. 2013;38(7):1158–69. doi: 10.1016/j.psyneuen.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic –hypothalamic–pituitary–adrenal regulation: Role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Gruol DL. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015;96(Pt A):43–54. doi: 10.1016/j.neuropharm.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased mcp-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the sprague dawley rat. Physiology & Behavior. 2011;104:187–198. doi: 10.1016/j.physbeh.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T. The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav. 2014;124:77–91. doi: 10.1016/j.physbeh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Kannel W, Curtis Ellison R. Alcohol and coronary heart disease: The evidence for a protective effect. Clinica Chimica Acta. 1996;246:59–76. doi: 10.1016/0009-8981(96)06227-4. [DOI] [PubMed] [Google Scholar]
- Legastelois R, Botia B, Naassila M. Blockade of ethanol-induced behavioral sensitization by sodium butyrate: Descriptive analysis of gene regulations in the striatum. Alcohol Clin Exp Res. 2013;37:1143–1153. doi: 10.1111/acer.12088. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of fosb/δfosb by voluntary alcohol intake: Effects of naltrexone. Alcohol Clin Exp Res. 2010;34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (crf) gene expression. Molecular and Cellular Neuroscience. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2 δδct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marec T, Marie-Claire C, Noble F, Marie N. Chronic and intermittent morphine treatment differently regulates opioid and dopamine systems: A role in locomotor sensitization. Psychopharmacology (Berl) 2011;216:297–303. doi: 10.1007/s00213-011-2223-6. [DOI] [PubMed] [Google Scholar]
- Molina PE, Katz PS, Souza-Smith F, Ford SM, Teng SX, Dodd TY, Maxi JK, Mayeux JP. Alcohol’s burden on immunity following burn, hemorrhagic shock, or traumatic brain injury. Alcohol Res. 2015;37(2):263–78. doi: 10.35946/arcr.v37.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodríguez-Arias M, Miñarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls J. Alcohol, host defense and society. Nature Reviews of Immunology. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- NIAAA. [Accessed 4/7/2016];Alcohol Facts and Statistics. 2015 Available at: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics.
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–19. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Pla A, Miñarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: A review with reference to human adolescent drinking. Alcohol and Alcoholism. 2014;49:187–192. doi: 10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Aragón CMG, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of tlr4 and tlr2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Potula R, Haorah J, Knipe B, Leibhart J, Chrastil J, Heilman D, Dou H, Reddy R, Ghorpade A, Persidsky Y. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. Am J Pathol. 2006;168:1335–1344. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: A global perspective and a new mechanism beginning with inhibition of signaling through tlr3. The Journal of Immunology. 2004;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong J, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5 doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher ML, Sexton HG, Risher WC, Wilson WA, Fleming RL, Madison RD, Moore SD, Eroglu C, Swartzwelder HS. Adolescent intermittent alcohol exposure: dysregulation of thrombospondins and dysnapse formation are associated with decreased neuronal density in the adult hippocampus. Alcohol Clin Exp Res. 2015;39(12):2403–13. doi: 10.1111/acer.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarc L, Wraber B, Lipnik-Stangelj M. Ethanol and acetaldehyde disturb tnf-alpha and il-6 production in cultured astrocytes. Human & Experimental Toxicology. 2011;30:1256–1265. doi: 10.1177/0960327110388533. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge ethanol and liver: New molecular developments. Alcohol Clin Exp Res. 2013;37:550–557. doi: 10.1111/acer.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–9. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Table 2.46B–Alcohol Use, Binge Alcohol Use, and Heavy Alcohol Use in the Past Month among Persons Aged 18 or Older, by Demographic Characteristics: Percentages, 2012 and 2013. [Accessed 4/7/2016];2013 Available at http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabsPDFWHTML2013/Web/HTML/NSDUH-DetTabsSect2peTabs43to84-2013.htm#tab2.46b.
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis: Exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–432. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt M, Solomon MG, Yuan P, Nugent A, Zarate CA, Drevets WC, Hall SD, George DT, Heilig M. Cerebrospinal fluid monocyte chemoattractant protein-1 in alcoholics: support for a neuroinflammatory model of chronic alcoholism. Alcoho Clin Exp Res. 2014;38(5):1301–6. doi: 10.1111/acer.12367. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ, Renau-Piqueras J, Guerri C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Re. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, De Witte P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol and Alcoholism. 2009;44:128–135. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37(12):2086–97. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis c infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural Brain Research. 2013;236:270–282. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews FT. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: Key role of nf-κb and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34(5):777–89. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]