To the Editor:
The small GTPase Rab27a regulates a number of exocytic processes. In melanocytes, Rab27a interacts with melanophilin to mediate melanosome transport required for pigmentation (1). In Natural Killer (NK) cells and cytotoxic T lymphocytes (CTL), degranulation and thereby cell mediated-cytotoxicity require interaction of the active form of Rab27a with Munc13-4 (2; 3). Mutations in the human RAB27A gene cause the autosomal recessive Griscelli Syndrome type 2 (GS2) with diluted pigmentation and immunodeficiency that can progress to hemophagocytic lymphohistiocytosis (HLH) (4). Recently, six patients with GS2 and normal pigmentation have been reported, identifying Rab27a mutations that selectively disrupt Munc13-4 binding when re-expressed in HEK293 cells (5). Here, we present a new case of GS2 with no dilution of pigmentation and extend prior biochemical results through physiologic cell expression studies.
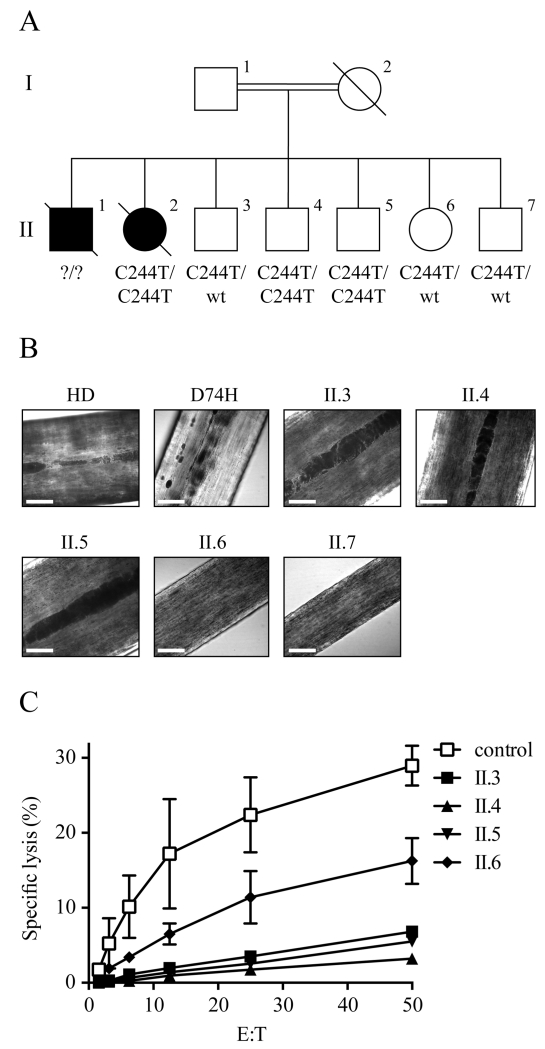
Death from HLH of two children of a first cousin consanguineous union led us to investigate the genetic background of the family (for clinical details see supplementary material). Targeted sequencing for the known HLH genes was unrevealing. Although pigmentation was normal, whole-exome sequencing identified a novel RAB27A mutation at c.244C>T (p.R82C) which was confirmed by Sanger sequencing and suggested the diagnosis of GS2. One of two deceased children for whom DNA was available (II.2) as well as two living siblings (II.4, II.5) were homozygous for c.244C>T. The other three living children (II.3, II.6 and II.7) were heterozygous for the mutation (Figure 1A). All five living siblings had unremarkable courses prior to, and at the time of this report.
Figure 1.
Rab27a R82C does not alter hair pigmentation but decreases NK cell mediated cytotoxicity. (A) Family pedigree. Parents are first-degree cousins. Patients II.1 and II.2 died. (B) DIC-images of hair from patients and a healthy donor (HD) (63×, scale bar is 25 μM). (C) NK cell cytotoxicity in PBMCs from patients and a healthy control measured in a standard 4h 51chromium release assay against K562 target cells. (n=2)
The atypical phenotype for GS2 prompted us to investigate the patients’ mutation further. Blood and hair samples were obtained with informed consent in compliance with the guidelines of the Institutional Review Boards of the Children’s Hospital of Philadelphia and Baylor College of Medicine. A GS2 patient with diluted pigmentation and progression to HLH (classical GS2) that had a homozygous RAB27A mutation (c.220G>C p.D74H) was included in this study (clinical details of this case to be reported elsewhere). We examined all patients’ hair using differential interference contrast (DIC) microscopy. The hair pigment of the typical GS2 patient was distributed irregularly in large clumps (Figure 1 B, D74H).
For all five homozygous and heterozygous R82C patients, hair pigmentation was more uniform with rare small clumps and not consistent with typical GS2 hair (Figure 1B). We studied the NK cell function of four out of the five living patients (II.3, II.4, II.5 and II.6) in detail in order to determine the effect of the Rab27a mutation on NK cell cytotoxicity. NK cell numbers in the patients were within low to normal ranges (Supplemental material). Standard 4h 51Cr release assays (performed as described previously (6) of peripheral blood mononuclear cells (PBMCs) against the human erythromyeloblastoid leukemia cell line K562 demonstrated that the NK cell mediated-cytotoxicity was reduced in all four siblings (Figure 1C). Both homozygous patients (II.4 and II.5) have normal NK cell numbers but reduced CD107a mobilization, which could explain the severely decreased cytotoxic NK cell function. The heterozygous siblings (II.3 and II.6) are less affected. The different cytotoxic responses observed for II.3 and II.6 could be caused by different percentages of NK cells detected for these patients (Supplemental material). In all four patients tested, the NK cell mediated cytotoxicity could be only partially rescued by IL-2 treatment (Figure E1). Impaired cytotoxicity without diluted pigmentation in the patients led to the hypothesis that the mutation in RAB27A at c.244C>T (p.R82C) differentially affects the downstream function of the protein in NK cells compared to pigment-producing cells. More specifically, this novel mutation would disrupt Rab27a binding to Munc13-4 but not affect interaction with melanophilin leading to impaired cytotoxic response with normal pigmentation as observed in the patients.
To test this hypothesis in physiological relevant settings, we overexpressed tGFP-tagged Rab27a wild-type, R82C or D74H in the NK cell line NK92 and the melanoma cell line mel1106. The Rab27a variants were immunoprecipitated using anti-tGFP-coated magnetic beads and coimmunoprecipitation of melanophilin (mel1106) or Munc13-4 (NK92) was detected by immunoblotting. Rab27a R82C bound melanophilin, although to a lesser extent than the wild-type (Figure 2A). In contrast, no Munc13-4 was detectable in the Rab27a R82C precipitate (Figure 2B). Neither melanophilin nor Munc13-4 co-immunoprecipitated with Rab27a D74H (classical GS2 patient) (Figures 2A and 2B). Quantifying three independent experiments we detected that Rab27a R82C coprecipitated 2.7±1.4-fold more melanophilin than D74. The amount of Munc13-4 in the precipitate was comparable between the two Rab27a mutants (1.2±1-fold). The results supported the conclusion that the mutation at R82C inhibits interaction of Rab27a with Munc13-4, but only partially affects binding of Rab27a to melanophilin and that this selective effect causes the atypical GS2 with normal pigmentation.
Figure 2.
Rab27a mutation at R82C selectively disrupts binding to Munc13-4, but not melanophilin (MLPH). tGFP-tagged Rab27a wild-type (WT), D74H and R82C were expressed in mel1106 (A) and NK92 (B). Cells were lysed in CHAPS buffer and lysates incubated first with isotype control-coated beads (clone MPC-11) and subsequently with anti-tGFP-coated beads (clone 2H8). Whole cell lysates and precipitates were separated in SDS-PAGE (4-12%), proteins transferred onto nitrocellulose membrane and the membrane probed with anti-Rab27a (clone 1G7) and anti-MLPH (A) or anti-Munc13-4 (B) antibodies (n=3).
Our data are consistent with a recent report of patients with biallelic RAB27A mutations and normal pigmentation with absent or reduced degranulation and cytotoxicity (5). Those authors identified Rab27a mutations that reduced binding to Munc13-4 but did not affect binding to melanophilin in HEK293 cells. Here we report another homozygous Rab27a mutation (R82C) that selectively binds melanophilin in melanocytes, but not Munc13-4 in NK cells. Using NK and melanoma cell lines we have investigated the binding activity of the mutations to the endogenously expressed interaction partners in comparison to wild-type Rab27a. Thus, we confirm the previously described biology in a truly physiological context using cells that have full ability to mediate the functions of cytotoxicity or pigmentation. Our data indicate that the absent binding of Rab27a R82C to Munc13-4 in NK cells causes the cytotoxic deficiency observed in the patient PBMC and that the residual binding activity of R82C to melanophilin is sufficient to allow normal pigmentation in melanocytes.
Overall, our study underscores the importance of unbiased genetic sequencing paired with biological and functional analysis to determine causes of atypical presentation of immune deficiency in order to allow for the most appropriate and timely treatment of patients. Exceptions to canonical phenotypes of primary immunodeficiency are becoming increasingly common owing to increasing access to genomic diagnosis. The ability to pursue the biology of these extended phenotypes is essential to provide the proof to advance the basic immunology as well as the diagnoses needed to advance clinical care with confidence.
Supplementary Material
Acknowledgments
Declaration of all sources of funding: This work was supported by NIH-R01 AI067946 to JSO and funding from the Jeffrey Modell Foundation.
Abbreviations
- CTL
cytotoxic T lymphocyte
- FHL
familial hemophagocytic lymphohistiocytosis
- GFP
green fluorescent protein
- GS2
Griscelli syndrome type 2
- HLH
hemophagocytic lymphohistiocytosis
- IL
interleukin
- NK
Natural Killer
- PBMC
peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. The Journal of Biological Chemistry. 2002;277(28):25423–30. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 2.Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nature immunology. 2007;8(3):257–67. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 3.Neeft M, Wieffer M, de Jong AS, Nigroiu G, Metz CHG, van Loon A, Griffith J, et al. Munc13-4 Is an Effector of Rab27a and Controls Secretion of Lysosomes in Hematopoietic Cells. Molecular Biology of the Cell. 2005;16(2):731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nature genetics. 2000;25(2):173–6. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 5.Cetica V, Hackmann Y, Grieve S, Sieni E, Ciambotti B, Coniglio ML, et al. Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13-4 binding. The Journal of Allergy and Clinical Immunology. 2015;135(5):1310–8. e1. doi: 10.1016/j.jaci.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. The Journal of Clinical Investigation. 2012;122(10):3769–80. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.