Abstract
Innate lymphoid cells (ILCs) are a new family of immune cells that play important roles in innate immunity in mucosal tissues, and in the maintenance of tissue and metabolic homeostasis. Recently, group 2 ILCs (ILC2s) were found to promote the development and effector functions of Th2-type CD4+ T cells by interacting directly with T cells or by activating DCs, suggesting a role for ILC2s in regulating adaptive immunity. However, our current knowledge on the role of ILCs in humoral immunity is limited. In this study, we found that ILC2s isolated from the lungs of naïve BALB/c mice enhanced the proliferation of B1- as well as B2-type B cells and promoted the production of IgM, IgG1, IgA, and IgE by these cells in vitro. Soluble factor(s) secreted by ILC2s were sufficient to enhance B cell Ig production. By using blocking antibodies and ILC2s isolated from IL-5-deficient mice, we found that ILC2-derived IL-5 is critically involved in the enhanced production of IgM. Furthermore, when adoptively transferred to Il7r−/− mice, which lack ILC2s and mature T cells, lung ILC2s promoted the production of IgM antibodies to a polysaccharide antigen, 4-hydroxy-3-nitrophenylacetyl Ficoll, within 7 days of airway exposure in vivo. These findings add to the growing body of literature regarding the regulatory functions of ILCs in adaptive immunity, and suggest that lung ILC2s promote the B cell production of early antibodies to a respiratory antigen even in the absence of T cells.
INTRODUCTION
Group 2 innate lymphoid cells (ILC2s) belong to a growing family of innate lymphoid cells (ILCs) that play important roles in innate immunity and the maintenance of tissue and metabolic homeostasis (1). Although ILC2s do not express antigen receptors, ILC2s react rapidly to infection and tissue injury by reacting to cytokines such as IL-33 secreted by tissue cells. Mirroring the function of their adaptive counterpart Th2 cells, activated ILC2s produce a large quantity of type 2 cytokines, such as IL-5 and IL-13, and initiate innate type 2 immune responses (2). The growing body of literature now suggests that ILC2s modulate adaptive immunity by regulating the development and effector functions of CD4+ T cells (3–7). Indeed, ILC2s enhance CD4+ T cell function through direct cellular contact via MHC class II and OX40L molecules and by secreting soluble factors, such as IL-4 (4–6, 8). Alternatively, ILC2s promote migration and chemokine production by dendritic cells (DCs), and thus promote CD4+ T cell functions (3, 7). In spite of our growing knowledge on the effects of ILC2s on T cell-mediated adaptive immunity, information has been limited regarding the role of ILC2s in humoral immunity.
Antibody responses can be generally divided into two arms: T cell-dependent antibody responses that are mediated primarily by B2 cells, and T cell-independent antibody responses that are mediated by marginal zone B cells and B1 cells (9). The critical roles of T cells in promoting humoral immune responses to protein antigen have been known for many years. In addition, both T cell-dependent and -independent antibody responses can be regulated by innate immune cells, such as invariant natural killer T (iNKT) cells, DCs, and granulocytes (9). More recently, Magri et al. identified splenic ILC3-like cells that enhance antibody production by marginal zone B cells (10). Moro et al. showed that adipose tissue-derived ILC2s support self-renewal of B1 cells and promote production of IgA (11), suggesting the ability of certain ILCs to regulate B cell function and Ig production.
The aim of this study was to better understand the effects of ILC2s on B cells, in particular the regulation of T-cell independent antibody responses. We performed a series of experiments in vitro using isolated ILC2s and B cells, and in vivo using an airway polysaccharide antigen exposure model in mice. Our results indicate that lung ILC2s promote the B cell production of early antibodies to a respiratory antigen even in the absence of T cells. Soluble factor(s) secreted by ILC2s, such as IL-5, likely play a key role.
MATERIALS AND METHODS
Mice and reagents
BALB/cJ, C57BL/6 and C57BL/6 Il7r−/− mice were from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 Il5−/− mice were kindly provided by Dr. Kiyoshi Takatsu (University of Toyama, Toyama, Japan). Female mice ages 6–12 weeks were used in all experiments. All animal experiments and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee, and were performed according to established guidelines.
Fluorescence-labeled antibodies to CD3 (145-2C11), CD25 (PC61; 7D4), CD44 (IM7), CD14 (rmC5-3), CD11b (M1/70), CD16/CD32 (2.4G2), CD45R/B220 (RA3-6B2), and CD23 (B3B4), purified anti-CD40 (HM40-3), and purified anti- ICOS (7E.17G9) were purchased from BD Biosciences. Fluorescence-labeled anti-ICOS (7E.17G9) was from Miltenyi Biotec. Anti-IL-5 (TRFK4), anti-IL-13 (eBio1316H), anti-IL-6 (BMS178), anti-IL-9 (16-7093), anti-GM-CSF (MMGM-CSFB2.6), and recombinant IL-33 were from eBioscience. Control antibodies were purified goat IgG, rat IgG (both from BD Biosciences), or mouse IgG (eBioscience). Recombinant mouse IL-7 and IL-25 and blocking polyclonal anti-OX40 ligand antibody were from R&D Systems. Recombinant mouse IL-4 was from PeproTech. LPS (L4516) was from Sigma Aldrich. Antibodies to mouse IgG1, IgM, IgA, and IgE were from BD Pharmingen. 4-Hydroxy-3-nitrophenylacetic (NP) hapten conjugated to aminoethylcarboxymethyl-Ficoll (NP-Ficoll) and NP (16)-BSA were from Biosearch Technologies.
ILC2 isolation and culture
ILC2s were isolated from the lungs of naïve BALB/c or C57BL/6 mice as described previously (12). Briefly, lungs were minced and digested with a cocktail of collagenases (Roche Diagnostics) at 35.7 μg/ml and DNase I (StemCell Technologies) at 25 μg/ml at 37°C to obtain single cell suspensions. RBCs were lysed with ammonium chloride/potassium lysing buffer. Subsequently, lung cells were stained with PE-conjugated antibodies to CD3, CD14, CD11b, CD16/CD32, and B220, followed by magnetic depletion of PE+ cells with EasySep® PE selection kit as per the manufacturer’s instructions (StemCell Technologies). These lineage− (Lin−) cell-enriched lung cells were then stained with fluorescence-labeled antibodies to CD3, CD14, CD11b, CD16/CD32, B220, CD25, and CD44. ILC2s were isolated as the Lin−CD25+CD44hi cell population by FACS sorting (BD FACSAria®). ILC2s were resuspended in RPMI 1640 medium supplemented with 50 μM 2-ME, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FBS and then expanded by culturing in a 96-well tissue culture plate at 104 cells/well with a cocktail of IL-33 (10 ng/ml) and IL-7 (10 ng/ml). Fresh IL-33 and IL-7 were added to the culture every 3 or 4 days, and ILC2s were used for experiments after 1–2 weeks in culture. Before use, ILC2 were washed once with PBS to remove residual IL-33 and IL-7. Furthermore, supernatants of ILC2s that were cultured for 3 or 4 days were collected, pooled, and stored at –20 °C for culture with B cells (see below).
B cell isolation and culture
Splenic B cells were purified using a Negative Selection EasySep mouse B cell enrichment kit (StemCell Technologies) to more than 90% cell purity. For cell proliferation assays, B cells were labeled with CFSE using the CellTrace™ CFSE cell proliferation kit according to the manufacturer’s instructions (Invitrogen). Labeled B cells were cultured in a 96-well plate at 2×104 cells/well with or without ILC2s at 104 cells/well. The cells were stimulated with indicated concentrations of anti-CD40 antibody or LPS in the presence or absence of 10 ng/ml IL-4 for 4 days. Cells were then harvested, stained with PE-conjugated anti-CD19 or anti-B220, and counted by FACS. For B1 and B2 cell isolation, peritoneal lavage cells were collected by flushing the peritoneal cavity of naïve mice with 3–5 ml PBS. The cells were then stained with fluorescence-conjugated anti-CD23 and anti-B220 antibodies. B1 cells (B220lowCD23−) and B2 cells (B220+CD23+) were isolated by FACS sorting (Supplemental Figure 1) (11, 13). Purified B1 cells and B2 cells were cultured as described above. In some experiments, neutralizing anti-IL-5, anti-IL-6, anti-IL-9, anti-IL-13 and anti-GM-CSF antibodies were added at a concentration of 2 μg/ml. In separate experiments, the ILC2 culture supernatant was added to the B cell culture at 50 μl/well in a total volume of 200 μl/well. Cell-free supernatants were harvested from the B cell culture wells and analyzed for antibody concentrations. The remaining cells were harvested and stained with PE-conjugated anti-CD19 or anti-B220 antibody. After washing, cells were resuspended in 400 μl buffer and analyzed by FACS by collecting the cells at a constant time (1 minute).
Antibody ELISA
To determine the levels of Igs in B cell supernatants, ELISA plates were coated with antibodies to mouse IgG1, IgM, IgA, or IgE, blocked with 1% BSA, and incubated with culture supernatants or serum samples. After washing, the IgM and IgG1 plates were incubated with HRP-conjugated anti-mouse IgM or IgG1, followed by peroxidase substrate. The IgA and IgE plates were incubated with biotinylated secondary antibodies, followed by streptavidin and peroxidase substrate. In all assays, the absorbance was read in a microplate autoreader after stopping the reaction with hydrochloric acid. To determine the levels of antibodies specific for NP-Ficoll, ELISA plates were coated with NP (16)-BSA, blocked with BSA, and incubated with the diluted samples.
Adoptive cell transfer and airway antigen exposure in vivo
Lung ILC2s from C57BL/6 mice were expanded in vivo by i.p. injection with IL-25 and IL-33 (400 ng/mouse) daily for 4 days. Twenty-four hours after the last injection, lungs and mediastinal lymph nodes were collected. The lung single cell suspensions were prepared as described above (ILC2 isolation and culture) and then pooled with the lymph node cells that were dispersed by using a cell strainer and syringe. Lin− lung cells were enriched by staining with a cocktail of PE-conjugated lineage antibodies followed by immunomagnetic depletion of PE+ cells with EasySep® PE selection kit. These cells were then stained with fluorescence-labeled antibodies to CD3, CD14, CD11b, CD16/CD32, B220, and ICOS. Lin−ICOS+ ILC2s were sorted by FACS as described previously (12). By FACS analysis, we confirmed that these Lin−ICOS+ ILC2s are ST2+ (Supplemental Figure 2). Sorted ILC2s were resuspended in PBS and adoptively transferred by i.v. injection (1.5×105 cells/mouse) to recipient Il7r−/− mice (Day 0). On days 1, 3, and 6, mice were exposed intranasally (i.n.) to NP-Ficoll (50 μg) plus bromelain (10 μg). Twenty-four hours after the last exposure, sera were collected and the levels of total antibody and NP-Ficoll-specific antibody were determined by ELISA.
Statistical analysis
Statistical significance was assessed with Student’s t test; p< 0.05 was considered significant.
Results
Lung ILC2s enhance B cell proliferation
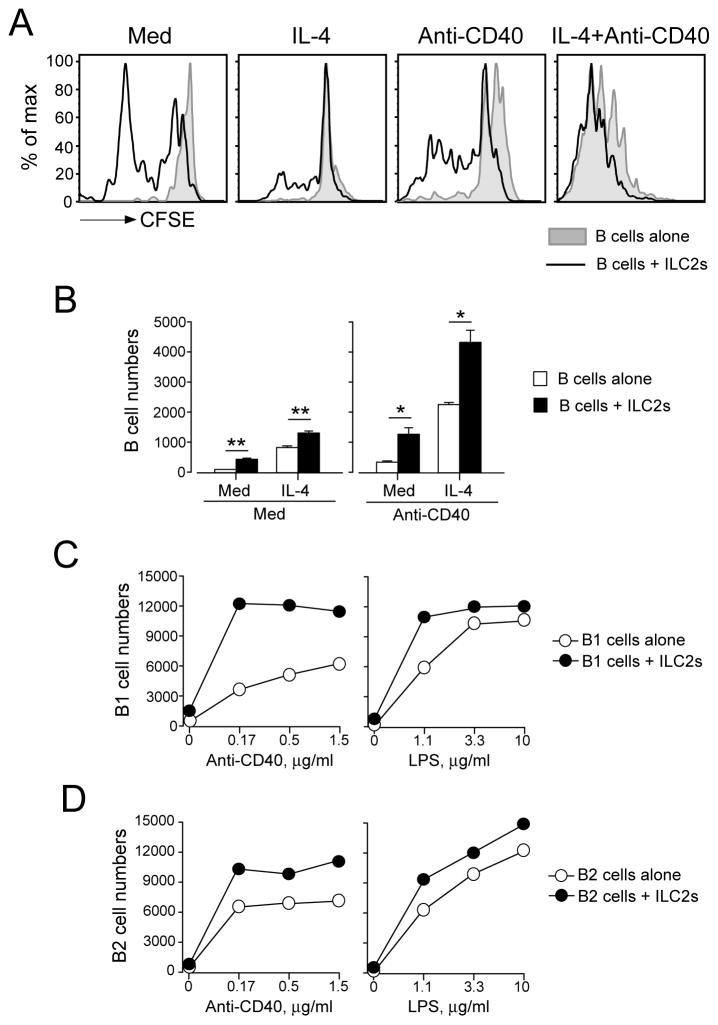
To investigate the effects of ILC2s on B cell proliferation, we first used an in vitro culture system. ILC2s were isolated from the lungs of naïve BALB/c mice, expanded and maintained with IL-33 plus IL-7, and then washed before use. We have previously shown that these in vitro-expanded ILC2s are lineage negative, CD44+CD25+ and ST2+ (5). B cells were isolated from the spleens of naïve BALB/c mice. To analyze whether ILC2s affect B cell proliferation, we took two different approaches. First, we cultured CFSE-labeled B cells alone or together with ILC2s and then analyzed the B cell division profiles by FACS (Figure 1A). Second, we cultured unlabeled B cells with or without ILC2s for 4 days and then counted the B cells in the cultures (Figure 1B). Since cytokine IL-4 and a co-stimulatory molecule CD40 play critical roles in B cell proliferation and class switch (14), we examined the effects of ILC2s on B cell proliferation in the presence or absence of IL-4 or anti-CD40 antibody. ILC2s significantly enhanced proliferation of B cells that were cultured without IL-4 or anti-CD40 as judged by CFSE staining and cell numbers (Figure 1A and B, p<0.01). IL-4 alone or anti-CD40 antibody alone induced modest proliferation of B cells, and the response was significantly enhanced in the presence of ILC2s (p<0.01 and p<0.05). In addition, a combination of IL-4 and anti-CD40 induced robust B cell proliferation, which was also further enhanced by ILC2s (p<0.01)
FIGURE 1.
Lung ILC2s enhance the proliferation of B lymphocytes. Lung ILC2s and splenic or peritoneal B cells were isolated from naïve BALB/c mice. Panel A: CFSE-labeled splenic B cells were cultured at 2×104 cells/well with or without ILC2s (104 cells/well) for 4 days. IL-4 (10 ng/ml) and/or anti-CD40 (1.5 μg/ml) were added to some cultures as indicated. The proliferation profiles of B cells were analyzed by FACS. Panel B: Splenic B cells were cultured with or without ILC2s for 4 days with medium (Med) alone or in the presence of IL-4 and/or anti-CD40. The numbers of CD19+ B cells were determined by FACS. Data (mean±SEM, n=3) are representative of two independent experiments. *, p<0.05; **, p<0.01 between the groups indicated by horizontal lines. Panel C and Panel D: Peritoneal B1 cells and B2 cells were cultured at 2×104 cells/well with or without ILC2s (104 cells/well) in the presence of serial dilutions of anti-CD40 antibody or LPS plus IL-4 (10 ng/ml) for 4 days. The number of B220+ cells was determined by FACS. Each data point represents the cell numbers from one well. Data are representative of two independent experiments.
To examine whether ILC2s have similar effects on B1 and B2 subpopulations of B cells, we isolated B1 cells (B220lowCD23−) and B2 cells (B220highCD23+) from the peritoneal cavity of naïve BALB/c mice and cultured them with or without ILC2s. Two common B cell agonists, anti-CD40 antibody and LPS, were used together with IL-4 to stimulate these B cells. Both B1 cells and B2 cells proliferated when stimulated with anti-CD40 or LPS in a concentration-dependent manner (Figure 1C and D). Consistent with a previous report (11), ILC2s enhanced the proliferation of B1 cells and shifted the dose-response curves of anti-CD40 or LPS to the left (Figure 1C); approximately one-third to one-tenth of the concentrations of anti-CD40 or LPS was required to achieve maximum proliferation in the presence of ILC2s as compared to the absence of ILC2s. Furthermore, ILC2s also enhanced the proliferation of B2 cells (Figure 1D), although the effects were not as pronounced as B1 cells. Additional replicate data are provided in Supplemental Figure 3. Collectively, these data indicate that ILC2s promote the proliferation of B cells of different subsets and from different organs.
Lung ILC2s enhance B cell antibody production
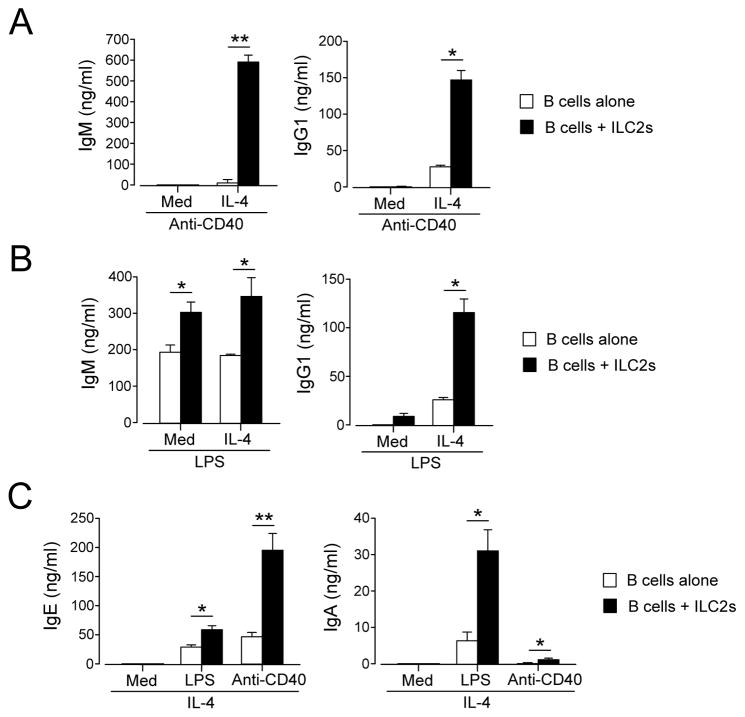
To investigate the effects of ILC2s on B cell Ig production, we cultured splenic B cells with or without ILC2s and quantitated Ig levels in the culture supernatants. B cells were stimulated with anti-CD40 or LPS in the presence or absence of IL-4. At 1.5 μg/ml, anti-CD40 did not induce detectable amounts of IgM or IgG1 in the absence of IL-4, and the addition of ILC2s did not affect Ig production (Figure 2A). When IL-4 was added in the culture, anti-CD40 induced low levels of IgM and IgG1 production; the addition of ILC2s dramatically enhanced production of these Ig subtypes (Figure 2A, p<0.01 and p<0.05). When B cells were stimulated with LPS at 5 μg/ml in the absence of IL-4 (Figure 2B), IgM, but not IgG1, was produced, and ILC2s significantly increased IgM levels. The addition of IL-4 showed no apparent effect on IgM production but enhanced IgG1 production. ILC2s robustly increased both IgM and IgG1 levels in the presence of IL-4. Collectively, these findings suggest that exogenous IL-4 is likely required to reveal the full spectrum of ILC2’s effects on B cell Ig production. Thus, we added IL-4 to all cultures in subsequent experiments.
FIGURE 2.
Lung ILC2s enhance Ig production by B lymphocytes. Lung ILC2s and splenic B cells were isolated from BALB/c mice. B cells were cultured at 2×104 cells/well with or without ILC2s (104 cells/well) for 4 days. IL-4 (10 ng/ml), anti-CD40 (1.5 μg/ml) or LPS (5 μg/ml) were added to some cultures as indicated. Ig levels in the supernatants were analyzed by ELISA. Data (mean±SEM, n=3) are representative of two independent experiments. *, p<0.05; **, p<0.01 between the groups indicated by horizontal lines.
To examine whether ILC2s affect production of other Ig subtypes by B cells, we analyzed the levels of IgE and IgA in the supernatants of B cells that were cultured with or without ILC2s in the presence of IL-4 (Figure 2C). Similarly to IgM and IgG1, IgE and IgA production by B cells stimulated with LPS or anti-CD40 was significantly enhanced in the presence of ILC2s.
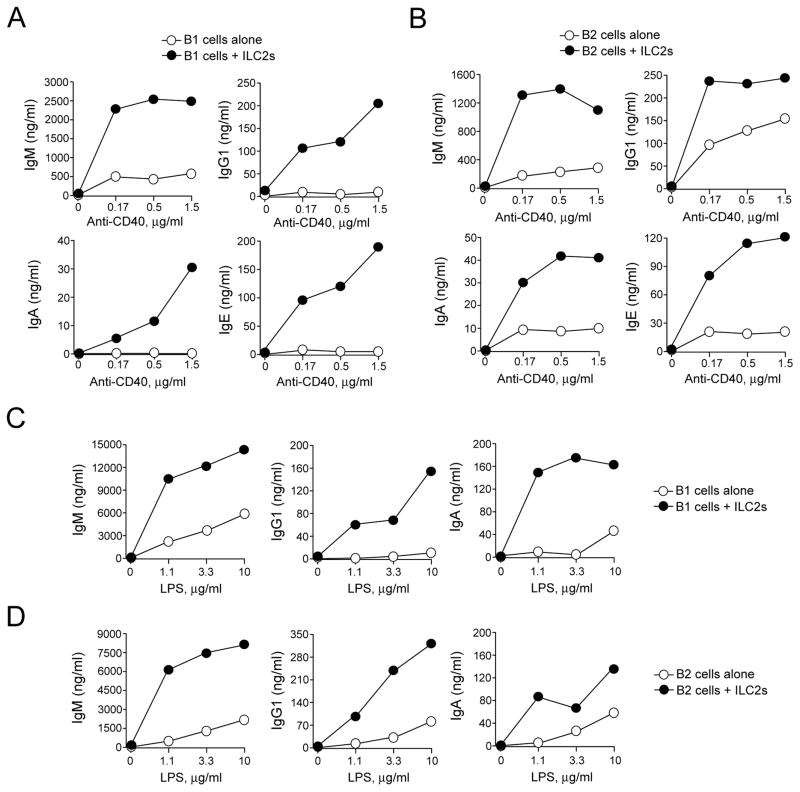
Since B1 and B2 cells are likely involved in distinct immune responses (9), we compared the ILC2’s effects on Ig production by B1 and B2 cells. When stimulated with anti-CD40 plus IL-4 in the absence of ILC2s, B1 cells produced IgM, but little IgG1, IgA, or IgE (Figure 3A). ILC2s dramatically enhanced the production of all Ig classes, including IgM, IgG1, IgA and IgE. Indeed, while IgG1, IgA, and IgE were nearly undetectable in the supernatants without ILC2s, the concentration-dependent response to anti-CD40 was apparent in the presence of ILC2s. Similarly, ILC2s enhanced the production of IgM, IgG1, IgA, and IgE by B2 cells stimulated with anti-CD40 (Figure 3B); the concentration of Ig produced by B2 cells was approximately twice to 5-times higher with ILC2s as compared to without ILC2s. In addition, both B1 and B2 cells stimulated with LPS plus IL-4 without ILC2s produced modest amounts of IgM, IgG1, and IgA (Figures 3C and 3D); more than a two-fold increase in the levels of these Ig was observed in the presence of ILC2s. Additional replicate data are provided in Supplemental Figure 4. Altogether, these findings suggest that ILC2s robustly promote production of various Ig subtypes by both B1 and B2 cells that are activated by immunological stimuli, such as LPS and anti-CD40, while ILC2s by themselves do not appear to induce Ig production.
FIGURE 3.
Lung ILC2s enhance Ig production by B1 and B2 cells. Lung ILC2s and peritoneal B1 and B2 cells were isolated from BALB/c mice. B1 cells (Panels A and C) and B2 cells (Panels B and D) were cultured at 2×104 cells/well with or without ILC2s (104 cells/well) in the presence of serial dilutions of anti-CD40 or LPS plus IL-4 for 4 days. Ig levels in the supernatants were analyzed by ELISA. Each data point represents the Ig concentration from one well. Data are representative of three independent experiments.
Secretory product of ILC2s promotes B cell Ig production
We next investigated the mechanisms involved in the stimulatory effects of ILC2s on B cells. Previously, we found that direct cellular contact mediated by the OX40/OX40 ligand (OX40L) interaction plays a critical role in the ILC2 promotion of CD4+ T cell functions (5). However, the addition of blocking anti-OX40L antibody to ILC2-B cell culture did not affect IgM and IgG1 production (Supplemental Figure 5). Lung ILC2s also express high levels of ICOS (12), and B cells express the binding partner, ICOS ligand (ICOSL) (15, 16). Furthermore, the ICOS:ICOSL interaction in ILC2s promotes ILC2 survival and cytokine production (17). However, the anti-ICOS antibody showed no effect on the ability of ILC2s to enhance B cell IgM and IgG1 production (Supplemental Figure 5).
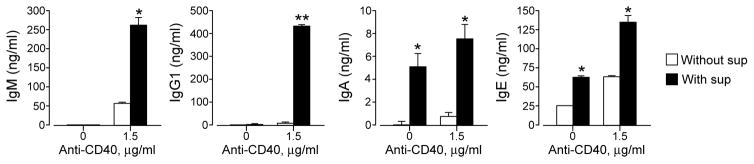
These findings led us to hypothesize that cytokines and other soluble factors that are secreted by ILC2s may play a key role. To test this hypothesis, we collected cell-free supernatants of ILC2s that had been cultured with IL-33 plus IL-7 for 3 or 4 days and added them to the culture of splenic B cells. No or minimal IgM, IgG1, or IgA was produced by splenic B cells in the absence of anti-CD40 or ILC2 supernatants (Figure 4). ILC2 supernatant alone without anti-CD40 significantly induced the production of IgA and IgE (p<0.05), but not IgM or IgG1. In the presence of anti-CD40 plus IL-4, ILC2 supernatants potently promoted the production of IgM and IgG1 (p<0.05 and p<0.01, respectively), suggesting a synergistic effect of the anti-CD40 and ILC2 supernatants.
FIGURE 4.
Supernatants of ILC2s promote B cell Ig production. Lung ILC2s and splenic B cells were isolated from BALB/c mice. B cells were cultured with or without pooled supernatants (sup) of ILC2s in the presence or absence of anti-CD40 antibody (1.5 μg/ml) plus IL-4 (10 ng/ml) for 4 days. Ig concentrations in the culture supernatants were analyzed by ELISA. Data (mean±SEM, n=3) are representative of three independent experiments. *, p<0.05; **, p<0.01 compared to cells cultured in the absence of ILC2 supernatants.
ILC2-derived IL-5 is involved in promoting IgM production
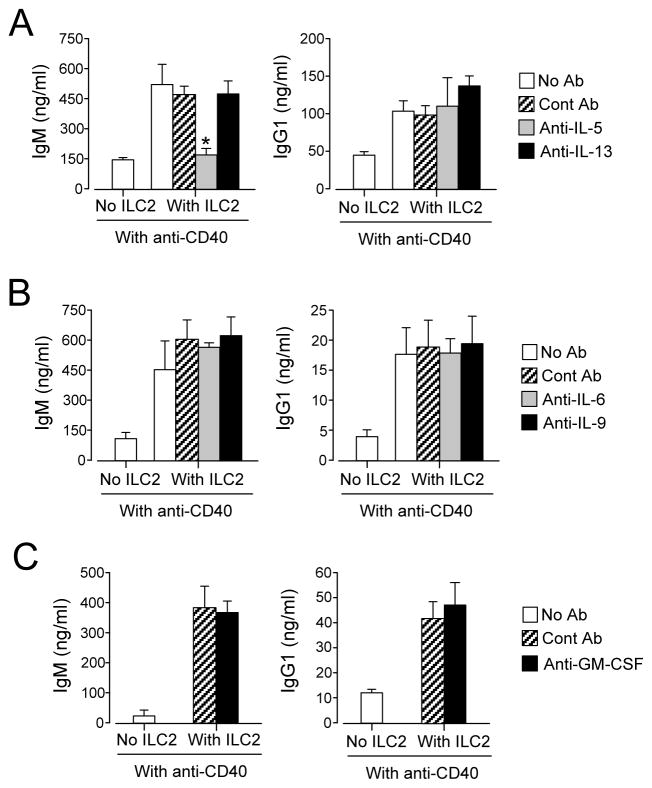
We performed subsequent mechanistic studies by focusing on the effects of ILC2s on anti-CD40-mediated IgM and IgG1 production, because these classes of antibodies responded most robustly to the addition of ILC2s. When stimulated with IL-33, lung ILC2s produce IL-5, IL-6, IL-9, IL-13 and GM-CSF (12). To identify which cytokines are involved, we added neutralizing antibodies to the B cell-ILC2 culture. Anti-IL-5 antibody significantly inhibited the ILC2-mediated upregulation of IgM production, but had no effect on IgG1 levels (Figure 5A, p<0.05). Anti-IL-6, anti-IL-9, anti-IL-13 and anti-GM-CSF antibodies did not have any effects on either IgM or IgG1 production (Figure 5B and 5C).
FIGURE 5.
IL-5 is involved in enhanced Ig production by B cells cultured with ILC2s. Lung ILC2s and splenic B cells were isolated from BALB/c mice. B cells (2×104 cells/well) were cultured with or without ILC2s (104 cells/well) in the presence of anti-CD40 antibody (1.5 μg/ml) plus IL-4 for 4 days. Neutralizing antibodies to IL-5 or IL-13 (Panel A), to IL-6 or IL-9 (Panel B) or to GM-CSF (Panel C), or control antibody (Cont Ab, 2 μg/ml) were added to the culture. *, p<0.05 compared to no antibody. In Panel A, data (mean±SEM, n=3) are a pool of three independent experiments. In Panel B and Panel C, data (mean± SEM, n=3) are representative of two to three independent experiments.
To verify the role of IL-5, we took a genetic approach. We isolated lung ILC2s from wild type (WT) mice or Il5−/− mice and cultured them with splenic B cells from WT mice. WT ILC2s significantly enhanced IgM production by B cells stimulated with either anti-CD40 or LPS (Figure 6A and 6B, p<0.05). ILC2s from Il5−/− mice exhibited a significantly decreased ability to promote IgM production as compared to WT ILC2 (p<0.05). On the other hand, Il5 deficiency did not affect IgG1 production. These findings suggest that ILC2-derived IL-5 plays a key role in promoting IgM production by B cells, whereas other molecules are likely involved in promoting IgG1 production.
FIGURE 6.
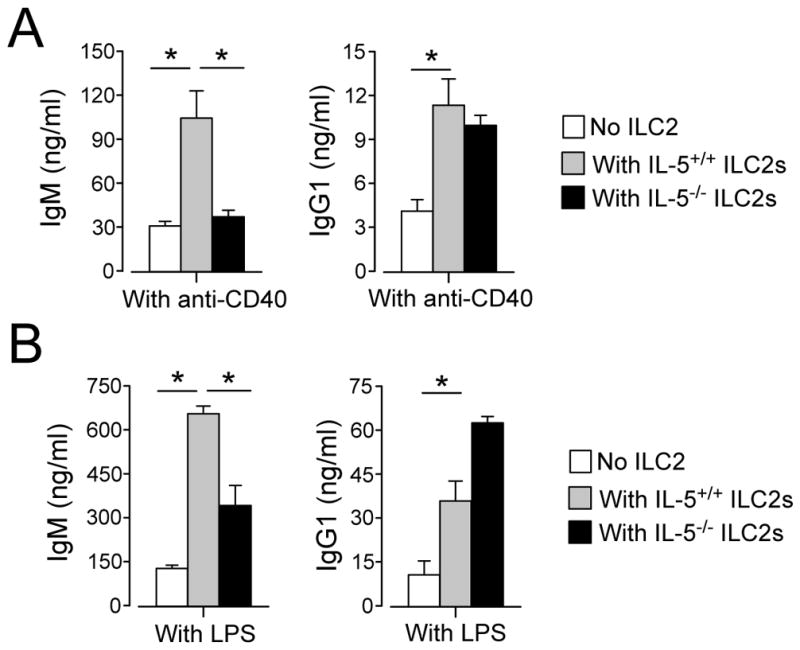
Lung ILC2s from IL-5-deficient mice fail to promote IgM production by B cells. Splenic B cells were isolated from C57BL/6 mice. ILC2s were isolated from C57BL/6 or C57BL/6 Il5−/− mice. B cells (2×104/well) were cultured with or without ILC2s (104/well) in the presence of anti-CD40 antibody (1.5 μg/ml, Panel A) or LPS (5 μg/ml, Panel B) plus IL-4 for 4 days. Ig concentrations in the culture supernatants were analyzed by ELISA. Data (mean±SEM, n=3) are representative of two independent experiments. *, p<0.05 between the groups indicated by horizontal lines.
ILC2s enhance antigen-specific IgM responses in vivo
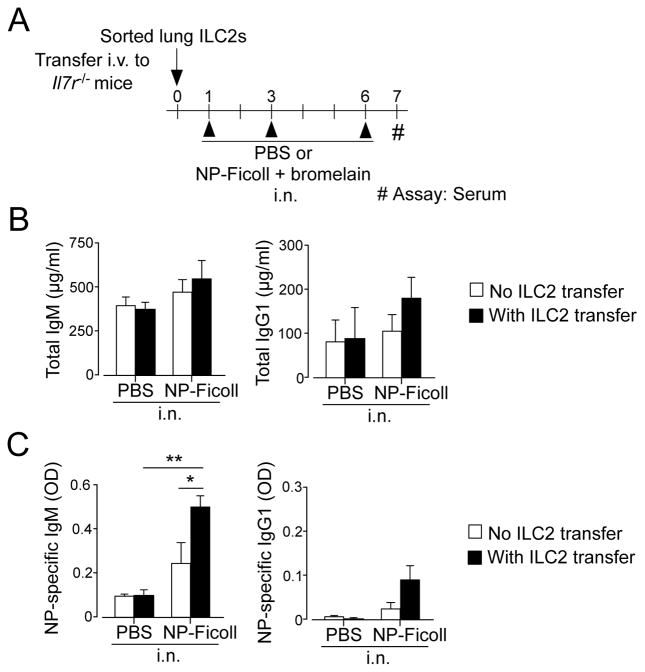
We took an adoptive transfer approach to examine whether lung ILC2s promote the production of specific antibodies to respiratory antigens in vivo. IL-7R-deficient mice are defective in both B and T lymphopoiesis and lack ILC2s (12, 18); however, they have sufficient B1 cells to generate IgM antibody responses (18). Therefore, we sought to investigate the IgM responses against a polysaccharide model antigen NP-Ficoll in IL-7R-deficient mice. Administration of innocuous protein via the airway is generally tolerogenic, and co-administration of adjuvants, such as proteases, is necessary to sensitize naïve animals (19, 20). Indeed, TNP-Ficoll, an antigen similar to NP-Ficoll, previously induced antigen-specific antibody responses only when it was administered to the airway together with the bacteria Brucella abortus but not by itself (21). Thus, we took a similar approach to induce NP-Ficoll-specific Ig responses by exposing mice intranasally (i.n.) to NP-Ficoll together with a cysteine protease, bromelain, as an adjuvant (22). We isolated lung ILC2s from non-sensitized WT C57BL/6 mice that had been injected i.p. with IL-33 and IL-25 and immediately transferred them i.v. to naïve Il7r −/− mice. These mice were then exposed i.n. to NP-Ficoll and bromelain. To examine early antibody responses, serum antibody concentrations were analyzed 6 days after initial antigen exposure (Figure 7A).
FIGURE 7.
ILC2s promote antigen-specific IgM antibody production in IL-7R-deficient mice. (Panel A) Experimental protocol: Lung ILC2s were isolated from C57BL/6 mice and transferred into C57BL/6 Il7r−/− mice. Mice were then exposed i.n. to NP-Ficoll plus bromelain on days 1, 3, and 6, and sera were analyzed on day 7. The levels of total IgM or IgG1 (Panel B) or NP-Ficoll-specific IgM or IgG1 in sera were analyzed by ELISA. Data (mean±SEM, n=2 in PBS and n=6 in NP-Ficoll) are representative of two independent experiments. *, p<0.05; **, p<0.01 between the groups indicated by horizontal lines.
Sera of Il7r−/− mice that were exposed to PBS contained approximately 400 μg/ml and 80 μg/ml of total IgM or IgG1, respectively, without ILC2 transfer (Figure 7B). Intranasal exposure to NP-Ficoll or adoptive transfer of ILC2s showed no significant effects on the levels of total IgM and IgG1, suggesting that ILC2s are unlikely to be involved in steady-state production of these Igs. Il7r−/− mice displayed low levels of NP-Ficoll-specific IgM antibody responses when exposed to NP-Ficoll without ILC2 transfer (Figure 7C). The adoptive transfer of ILC2s significantly increased the serum levels of NP-Ficoll-specific IgM antibody (p<0.05). On the other hand, no significant effects of exposure to NP-Ficoll or ILC2 transfer were observed in the levels of NP-Ficoll-specific IgG1 antibody. Collectively, these findings suggest that ILC2s promote the generation of IgM antibodies to a respiratory antigen even in the absence of T cells in vivo.
DISCUSSION
Innate immunity and adaptive immunity are intimate partners. Besides performing their specialized functions, innate immune cells and adaptive immune cells also interact with each other during the immune response. Previously, we and others reported that new innate immune cell ILC2s interact with CD4+ T cells and subsequently enhance Th2-type immune responses (4–6). ILC2s also act on other innate immune cells, such as DCs, and indirectly promote Th2-type immune responses (3, 7). This manuscript adds to this knowledge of the effects of ILC2s on adaptive immunity by demonstrating that ILC2s activate B cells and enhance the early antibody response to a respiratory antigen in vivo. Thus, the regulatory functions of ILC2s are not limited to the cellular arm of adaptive immunity but likely extend to humoral immunity as well.
Mature peripheral B cells consist of three different subsets, including innate-like B1 cells, follicular B2 cells, and marginal zone B cells (23). A previous study showed that adipose tissue-derived ILC2s support the self-renewal of B1 cells and enhance IgA production (11). The observations in this manuscript show that lung ILC2s enhance both B1 and B2 cell proliferation and promote the production of IgM, IgG1, IgA, and IgE. Indeed, by using a model polysaccharide antigen NP-Ficoll, adoptively transferred ILC2s to Il7r−/− mice promoted antigen-specific IgM antibody responses to a respiratory antigen. Because Il7r−/− mice do not have ILC2s, mature T cells, or B2 cells (12, 18), the stimulating effects of ILC2s on IgM antibody production (Figure 7) is likely independent of T cells and is mediated by the interaction between ILC2s (or their products) and B1 cells. This speculation is consistent with the in vitro observation that ILC2s potently enhance B1 cell proliferation and promote IgM production (Figure 1 and 3).
Since B1 and B2 cells are considered to mediate T cell-independent and T cell-dependent antibody responses, respectively, our in vitro results would suggest that ILC2s may regulate both T cell-dependent and T cell-independent antibody responses. We have not examined specifically whether ILC2s promote antibody responses to T cell-dependent antigens in vivo; however, this scenario can be reasonably expected, as judged by the fact that ILC2s enhance Th2-type CD4+ T cell functions (3–7). Indeed, in a papain-induced airway inflammation model, ILC2-deficient mice showed reduced levels of total serum IgE (3), suggesting the ability of ILC2s to promote T cell-dependent antibody responses.
ILC2s facilitate adaptive immune cell functions directly through several mechanisms, including cellular interactions through TCR/MHC class II and OX40/OX40L, and the paracrine secretion of soluble factors, such as IL-4 (1, 5, 8). The potent B cell stimulatory effects of ILC2 culture supernatant suggest that soluble factors secreted by ILC2s are sufficient to mediate the ILC2 effects on B cells. Activated lung ILC2s produce a variety of cytokines, including IL-4, IL-5, IL-6, IL-9, IL-13 and GM-CSF (1, 12). All of these cytokines have been shown to be involved in regulating B cell functions (14, 24–28). In particular, IL-4 plays an important role in regulation of B cell growth and Ig class switch and production (14). Our finding that ILC2s promote anti-CD40-induced IgM or IgG1 production only in the presence of exogenous IL-4 (Figure 2A) suggests that ILC2-derived IL-4 is unlikely to be sufficient to upregulate Ig production in this experimental setting. IL-5 is another important cytokine that regulates B cell growth and antibody production (29). Indeed, mice deficient in IL-5Rα showed decreased numbers of B1 cells in the peritoneal cavity and decreased serum levels of IgM, but not IgG1 (29, 30). IL-5 transgenic mice showed increased serum levels of polyreactive autoantibodies of IgM class (31). Consistent with these previous observations, the results of our antibody blocking experiments suggest that IL-5 produced by lung ILC2s promotes B cell IgM production (Figure 5). This conclusion was further supported by experiments involving the culture of B cells with IL-5-deficient ILC2s (Figure 6).
Currently, we do not know what soluble factors are involved in the enhanced production of IgG1 and other antibody classes. Our antibody blocking experiments (Figure 5) suggest that ILC2-derived IL-5, IL-13, IL-6, IL-9 and GM-CSF are unlikely to be responsible for this, although we cannot rule out the possibility that these cytokines may work additively or redundantly to promote IgG1 production. It has been shown that B cell-activation factor (BAFF), a proliferation-inducing ligand (APRIL) and IL-21 are involved in neutrophil-induced Ig production (32). However, IL-33-activated lung ILC2s are an unlikely source for these cytokines, since microarray gene analysis revealed no or extremely low level expression of BAFF, APRIL and IL-21 in these cells (Bartemes K, unpublished observations). Thus, further studies are necessary to identify the molecule(s) that are produced by ILC2s and responsible for enhanced production of IgG1 and other Ig classes.
T cell-independent antibody responses play a crucial role in the rapid clearance of pathogens, because these responses can develop as early as 1–3 days after exposure to blood-borne microorganisms (33). In the case of lung infection, a subset of B1 cells in the pleural space migrates to the lung, where they produce protective IgM against pneumonia in the early phase of infection (28). Furthermore, IL-33, a key cytokine involved in the activation of ILC2s, promoted the production of IgM antibodies to oxidized low-density lipoprotein in an IL-5-depedent manner and reduced the development of atherosclerosis in mice (34). As innate immune cells, ILC2s react promptly to infection, injury, stress, and metabolic changes (1, 2, 35). Therefore, the findings of our study suggest a model in which activated lung ILC2s interact with B1 cells, possibly those in the pleural cavity or lung parenchyma (36), to promote rapid antigen-specific IgM antibody production. Accordingly, the interaction between ILC2s and B1 cells may strengthen the first line of defense against certain pathogens. Achieving a better understanding of the molecular and cellular mechanisms involved in the interaction between ILC2s and B cells will be of central importance in furthering our knowledge of innate and adaptive immunity to pathogens and tissue homeostasis against insults.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health, R01 HL117823, and by the Mayo Foundation.
Abbreviations
- BAL
bronchoalveolar lavage
- DCs
dendritic cells
- ILCs
innate lymphoid cells
- ILC2s
group 2 ILCs
- iNKT
invariant natural killer T
- i.n
intranasal
- Lin−
lineage-negative
- Lin+
lineage-positive
- NP
4-hydroxy-3-nitrophenylacetic
- WT
wild-type
- ICOSL
ICOS ligand
- OX40L
OX40 ligand
- BAFF
B cell-activation factor
- APRIL
a proliferation-inducing ligand
Footnotes
Conflict of interest statement: All authors declare no conflict of interest.
Author contributions: L.D. and H.K. designed the studies and experiments, interpreted the data, and wrote the manuscript. L.D., K.B., and K.I. performed the experiments.
References
- 1.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 3.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 5.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon PG, McKenzie AN. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, McKenzie AN. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2015;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Ruckerl D, Seddon B, MacDonald AS, McKenzie A, Wilson MS. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunology. 2016 doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Chang PP. Innate B cell helpers reveal novel types of antibody responses. Nat Immunol. 2013;14:119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- 10.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Arostegui JI, Juan M, Yague J, Merad M, Fagarasan S, Cerutti A. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15:354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 12.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J Immunol. 2001;166:1531–1539. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 15.Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, Miyashiro JS, Jacobs KA, Collins M. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 17.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol. 2010;185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 19.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 20.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T, Saito H, Nakae S, Sudo K, Suto H, Ichikawa S, Ogawa H, Okumura K, Takai T. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013;190:4489–4499. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 21.Goud SN, Kaplan AM, Subbarao B. Primary antibody responses to thymus-independent antigens in the lungs and hilar lymph nodes of mice. Infection and Immunity. 1990;58:2035–2041. doi: 10.1128/iai.58.7.2035-2041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, Kephart GM, Kurabayashi M, Kita H. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192:4032–4042. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takatsu K. Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 1998;9:25–35. doi: 10.1016/s1359-6101(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 25.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 26.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai YH, Mosmann TR. Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol. 1999;162:78–87. [PubMed] [Google Scholar]
- 28.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med. 2014;211:1243–1256. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2011;87:463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J, Takaki S, Takatsu K. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 31.Tominaga A, Takaki S, Koyama N, Katoh S, Matsumoto R, Migita M, Hitoshi Y, Hosoya Y, Yamauchi S, Kanai Y, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 36.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.