Summary
Inter-individual behavioral variation is thought to increase fitness and aid adaptation to environmental change, but the underlying mechanisms are poorly understood. We find that variation between individuals in neuromodulatory input contributes to individuality in short-term habituation of the zebrafish (Danio Rerio) acoustic startle response (ASR). ASR habituation varies greatly between individuals, but differences are stable over days and are heritable. Acoustic stimuli that activate ASR-command Mauthner cells also activate dorsal raphe nucleus (DRN) serotonergic neurons, which project to the vicinity of the Mauthner cells and their inputs. DRN neuron activity decreases during habituation in proportion to habituation and a genetic manipulation that reduces serotonin content in DRN neurons increases habituation, whereas serotonergic agonism or DRN activation with ChR2 reduces habituation. Finally, level of rundown of DRN activity co-segregates with extent of behavioral habituation across generations. Thus, variation between individuals in neuromodulatory input contributes to individuality in a core adaptive behavior.
eToc
The mechanisms of vertebrate behavioral individuality are poorly understood. Pantoja et al. find that differences between individuals in sensory-evoked neuromodulatory input modulate behavior and co-segregate with behavioral differences in a sensory-evoked adaptive behavior. Thus, variation between individuals in neuromodulation contributes to behavioral individuality.
Introduction
One of the most interesting aspects of the study of animal behavior is the wide variation in animal-to-animal behavior. Since neuromodulatory input can act at multiple points in a neural circuit to control output and switch circuit dynamics and behavioral state (Gordus et al., 2015; Lee and Dan, 2012; Marder, 2012), we considered that neuromodulatory variation could contribute to behavioral variation between individuals. We examined this possibility in zebrafish (Danio rerio). Zebrafish display complex behaviors and patterns of neural activity in circuits that receive neuromodulatory input (Brustein et al., 2003; Thirumalai and Cline, 2008; Woods et al., 2014). Zebrafish populations are outbred, highly polymorphic and could therefore have behavioral variation more similar to wild animal populations and humans than do the inbred genetic models that are commonly used in behavioral studies (Brown et al., 2012; Howe et al., 2013).
We examined two types of locomotor behavior that are easily quantifiable in hundreds of animals: the habituation of the zebrafish acoustic startle response (ASR) (Burgess and Granato, 2007) and spontaneous swim activity (Lambert et al., 2012). Habituation is a critical adaptive behavior: a non-associative form of learning characterized by decreased probability of a behavioral output when the same stimulus is presented repeatedly. Habituation increases at shorter inter-stimulus interval (ISI) and decreases at higher intensities of the sensory stimulus (Rankin et al., 2009). During short-term habituation, which occurs in seconds to minutes, the motor output changes dramatically due to changes in sensory processing that may occur at multiple levels of a circuit and involve several neural mechanisms, while circuit anatomy remains unchanged (Ramaswami, 2014).
The ASR is evolutionarily conserved in vertebrates and characterized by a short-latency defensive behavior elicited by strong and sudden acoustic stimuli. In zebrafish, the escape behavior is expressed by a fast unilateral C-bend of the tail, which turns the animal so that it can swim away from the startling stimulus (Colwill and Creton, 2011). This behavioral output is regulated by the balance between sensory excitatory glutamatergic inputs and glycinergic feedforward inhibitory networks that converge on the Mauthner cells (M-cells), a pair of large reticulospinal hindbrain interneurons. A single action potential in one M-cell suffices to elicit the C-bend escape behavior in the contralateral direction (Korn and Faber, 2005). Intriguingly, differences in ASR habituation levels have been associated with different personality types and behavioral disorders in humans, including schizophrenia and anxiety disorders (Blanch et al., 2014; Braff, 1992).
The neuronal populations that comprise the ASR circuit are extensively modulated by neuromodulatory inputs. First, vestibulo-auditory (VIIIth nerve) afferents and the M-cell are subject to synaptic modulation by hypothalamic dopaminergic projections (Mu et al., 2012). Second, serotonergic varicosities are present in the vicinity of M-cell dendrites (McLean and Fetcho, 2004a) and axon cap, two locations in the M-cell where serotonin receptors are present (Curtin et al., 2013; Whitaker et al., 2011).
We tested the hypothesis that larval zebrafish display stable inter-individual differences in core adaptive behaviors that depend on differences in neuromodulatory activity. We find that inter-individual differences in ASR habituation, unlike differences in the spontaneous swim behavior, are stable over days and heritable. These differences are associated with differences in the rate at which M-cell firing drops off in response to repeated sound stimulation. DRN serotonergic neurons, which project into the vicinity of the M-cells, are also activated by acoustic stimuli and lower habituation is associated with individuals that sustain sound-evoked activity in larger number of serotonergic neurons for longer times during the repeated stimuli or are activated with channelrhodopsin-2 (ChR2) during sound stimulation. This suggests that serotonergic input to the M-cells and/or their inputs acts to blunt ASR habituation. Consistent with this interpretation, a serotonergic agonist decreases the frequency of individuals with high ASR habituation and a genetic manipulation that reduces the serotonin content of DRN neurons increases ASR habituation. Finally, individual differences in sound-evoked DRN neural activity co-segregate with heritable individual differences in ASR habituation. We propose that inter-individual differences in sensory-evoked behaviors are shaped by differences in neuromodulatory activation by sensory inputs.
Results
Large variation between individuals in ASR habituation behavior
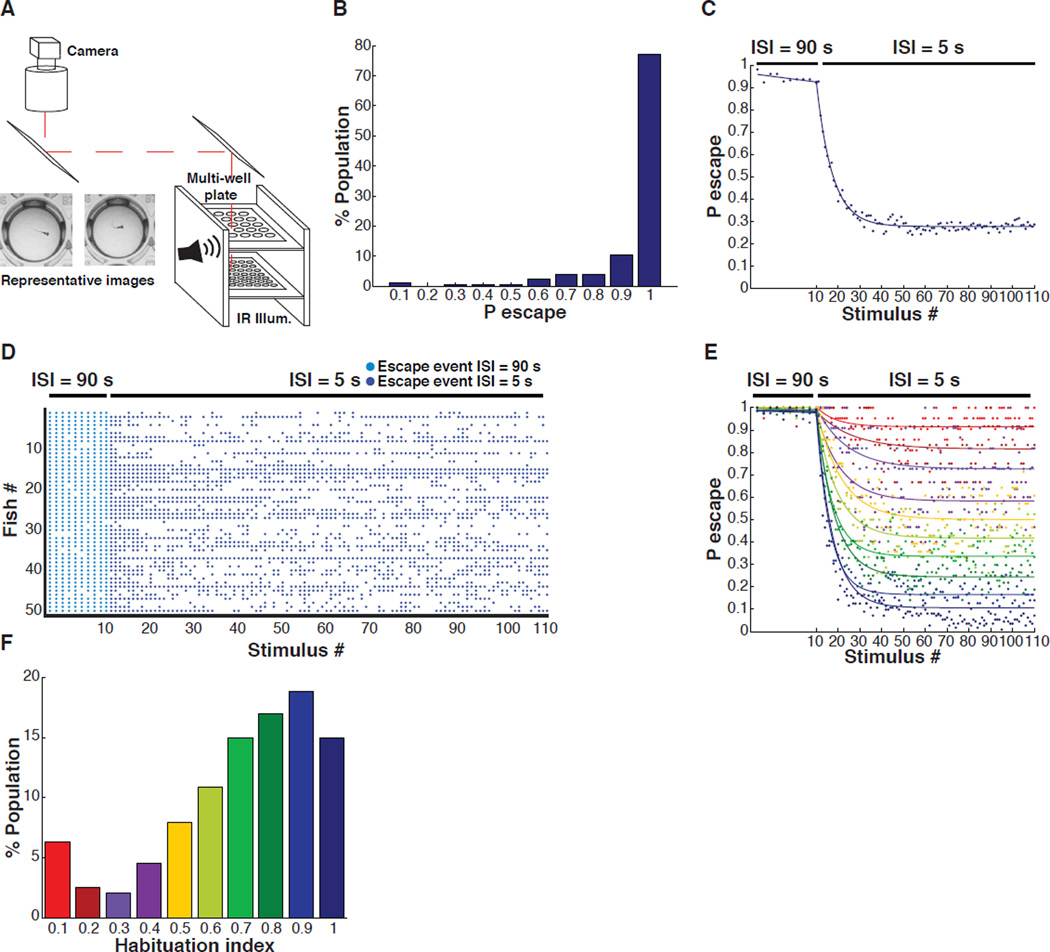
To characterize ASR short-term habituation in zebrafish, we used a high-throughput behavioral apparatus (Levitz et al., 2013) to monitor many individual larvae simultaneously. Forty-eight wild-type AB strain larval zebrafish placed individually in wells of a multi-well plate were imaged simultaneously at 30 frames per second (fps) under infrared illumination from an LED array and stimulated with brief, precisely timed acoustic-vibration stimuli (3 ms, 900 Hz, ~95 dB) from speakers that were coupled to the plate (Figure 1A). Behavioral movies were analyzed with custom-written software that automatically detects the C bend behavior (Levitz et al., 2013). First, we characterized the naive responsiveness of healthy larvae under the minimally habituating inter-stimulus interval (ISI) of 90 s (Figure S1A). The majority of individuals (56 – 90 %) obtained from independent crosses and tested on multiple experimental days responded to at least 9 out of the first 10 stimuli, (Pinitial > 0.9), while a small minority (2.5%) responded to fewer than 3 stimuli. Habituation was examined only in the stably responsive Pinitial > 0.9 group when the stimulation was switched to the much shorter ISI of 5 s (Figure 1B and 1C).
Figure 1. High-throughput analysis of larval zebrafish startle behavior.
(A) Multi-well behavioral apparatus. (B) Frequency distribution of probability of escape values during the first 10 stimuli (ISI = 90 s) of habituation series. (C) Population average of the probability of sound-induced escapes during acoustic startle response (ASR) habituation (Stimuli # 1–10, Inter-Stimulus Interval (ISI) = 90 s; stimuli # 11–110, ISI = 5 s; n = 441). (D) Representative acoustic startle responses of 50 individual larvae ordered in the y-axis with each dot representing a sound-induced escape. (E) Average ASR probability curves for population in figure 1B binned by habituation index (HI). (F) Frequency distribution of HIs of same population as in figure 1C.
We quantified the habituation index (HI), which we defined as the steady-state probability of response at the end of a series of stimuli at the 5 s ISI (Pafter) divided by the initial probability (Pinitial) at the minimally habituating ISI of 90 s (HI = 1 – Pafter / Pinitial). HI values close to 1 indicate strong habituation, while values close to 0 indicate weak habituation. At 6 days post-fertilization (dpf), the average population behavior showed strong habituation at the 5 s ISI (HI ~ 0.7) (Figure 1C). However, the behavior varied considerably between individuals (Figure 1D), with approximately 10% of fish showing little or no habituation (HI < 0.2) (Figure 1E and 1F), and approximately 45% showing strong habituation (HI > 0.8) (Figure 1E and 1F).
Degree of ASR habituation behavior is stable over days and is heritable
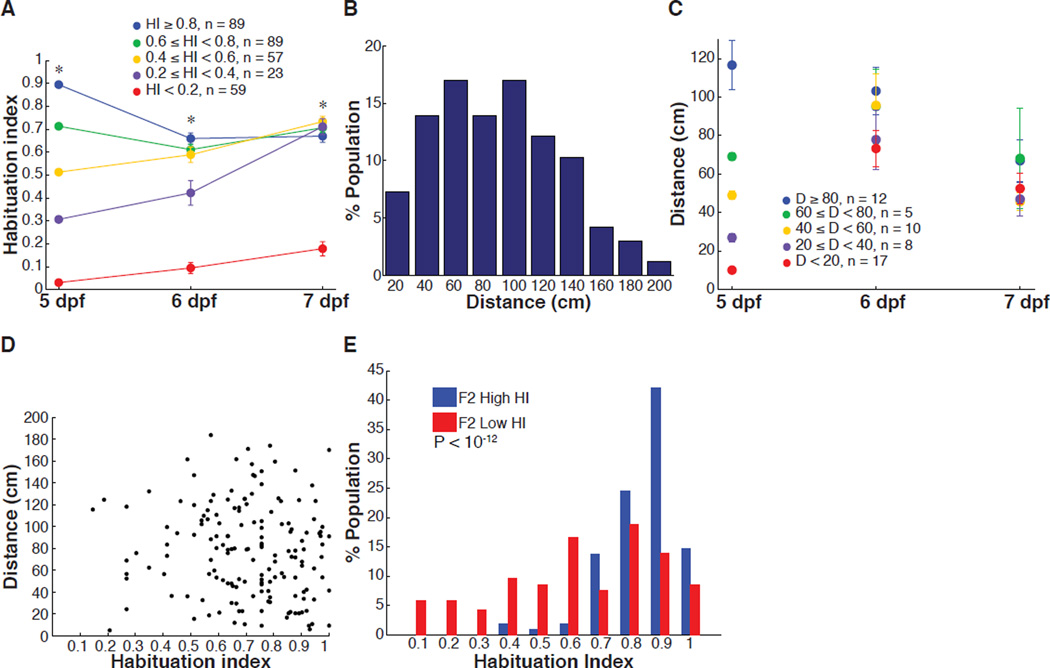
To determine if the level of behavioral habituation is a stable characteristic of each fish or varies over time, larval zebrafish were tested daily at 5, 6 and 7 dpf. Fish with intermediate and high HIs at 5 dpf converged in behavior and remained statistically indistinguishable two days later (Figure 2A). In contrast, fish with low HIs (< 0.2) at 5 dpf retained a significantly lower HI, when compared to the rest of the population, at both days 6 and day 7 (Figure 2A). This result shows that diversity between individuals at the high and low ends of ASR habituation is stable over days.
Figure 2. Longitudinal analysis of ASR habituation and spontaneous swim behavior.
(A) Repeated measurement of HI over 3 days. The HI value measured on 5 dpf was used to group larvae. HI value of HI < 0.2 group is significantly smaller than all groups at all time points, remaining groups are not significantly different in comparison to one another on 7 dpf (* = P < 10−9; two-tailed Mann-Whitney test, nHI<0.2 = 59, n0.2≤HI<0.4 = 23, n0.4≤HI<0.6 = 57, n0.6≤HI<0.8 = 89, nHI≥0.8 = 89, error bars, s.e.m.). (B) Frequency distribution values for individual larvae for total displacement in 10 min (n = 165). (C) Repeated measurement of distance swum over 3 days. Mean values for displacement of individual larvae. The distance value measured on day 5 was used to group larvae. The difference between groups on 7 dpf is not statistically significant (P > 0.1; two-tailed Mann-Whitney test, nD<20 = 17, n20≤D<40 = 8, n40≤D<60 = 10, n60≤D<80 = 5, nD≥80 = 12, error bars, s.e.m.). (D) Graph shows habituation index value versus total displacement values for individual larvae (6 dpf, n = 110). Pearson’s correlation coefficient, r = −0.0285, P = 0.71. (E) Highly responsive F2 Low HI, 6 dpf larvae have significantly lower HI than highly responsive F2 High HI larvae (z = 7.08, P < 10−12; two-tailed Mann-Whitney test; nF2 High HI = 102, nF2 Low HI = 186).
We wondered if persistent differences between individuals also characterize other larval zebrafish behaviors. We addressed this by examining spontaneous swimming behavior. As with ASR habituation, the level of spontaneous activity was widely distributed across the population (Figure 2B), yet differences in spontaneous activity were not stable from one day to the next (Figure 2C). We found no correlation (Pearson’s correlation coefficient, r = −0.0285, P = 0.71) between levels of spontaneous activity and ASR habituation in experiments in which the behaviors were measured sequentially (Figure 2D). Thus, individual constancy and diversity between individuals appear to be properties of ASR habituation and not of spontaneous swimming.
To assess if HI differences between individuals are heritable, we raised siblings from one parental cross that we split into two groups: one group selected for having a stably low HI (HI < 0.4), as measured over two days, and the other group for having a stably high HI (HI > 0.9). Both groups were also selected for high responsiveness, with Pinitial > 0.9 under non-habituating conditions (ISI = 90 s). Six sets of F1 progeny were obtained by F0 inbreeding of two low HI group couples (F1Low HI) and four high HI couples (F1High HI). These sets of progeny were then assayed at 6 dpf. The progeny of the low and high HI crosses were both shifted toward higher initial responsiveness, consistent with their common selection from the subset of parentals that were highly responsive (Pinitial > 0.9) (Figure S2A). We observed both increased frequency of individuals with Pinitial > 0.9 and decreased frequency of individuals with Pinitial < 0.1 in the low HI crosses, but only decreased frequency of individuals with Pinitial < 0.1 in the high HI crosses (Figure S2A), which suggests an association between basal ASR and ASR habituation. F1s of low HI F0 couples had substantially lower HI than did F1s of the high HI F0 couples (Figure S2B). Moreover, the F1s were also shifted lower and higher in HI, respectively, compared to the parental distribution, although this difference was only statistically significantly for the low HI crosses (Figure S2B). We found that the difference in HI was maintained, and indeed made more extreme, after a second round of selection in the F2 generation of F1 couples selected for high or low HI (low HI couple #2 and high HI couple #3 (Figure S2)) (MedianF2 Low HI = 0.63, MedianF2 High HI = 0.86, P < 10−12) (Figure 2E). Thus, the short-term habituation of the ASR is a heritable quantitative trait.
Mauthner cell activity during ASR habituation
To investigate if neural mechanisms participate in shaping the broad diversity in ASR habituation behavior we constructed a two-headed microscope for simultaneous and synchronized high-speed behavioral measurement (1000 fps) and neuronal calcium imaging (20–40 fps) in a single head-fixed larva (Figure 3A–3F). Acoustic stimuli were presented by two speakers resting on the imaging stage. The high speed of the behavioral camera and short duration of the acoustic stimulus (1000 Hz, 1–10 ms) allowed precise determination of zebrafish escape behavior latency and kinematics.
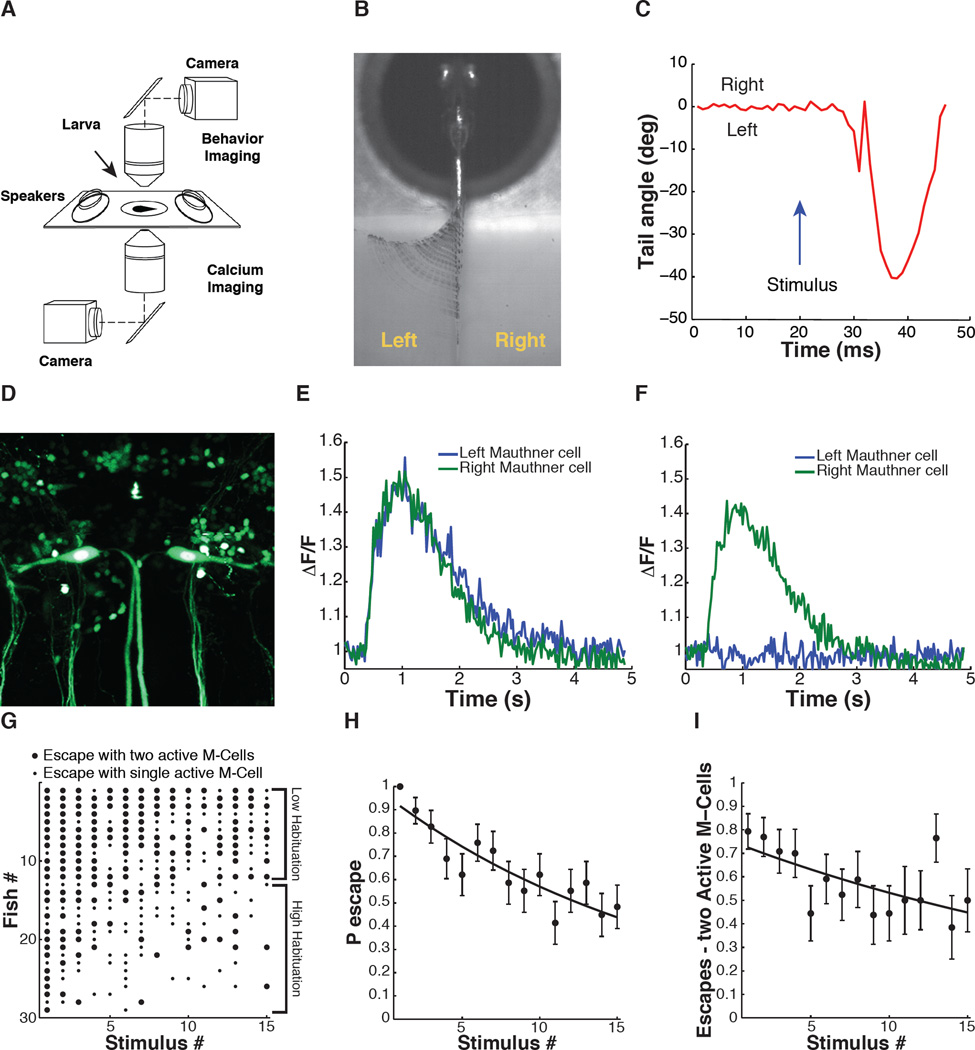
Figure 3. Simultaneous analysis of escape behavior and M-cell calcium signals during ASR habituation in 6 dpf zebrafish larvae.
(A) Simultaneous behavior (2.5× obj., 1000 fps) and GCaMP5 calcium imaging of M-cells (40× obj., 40 fps) with two independent light-paths. (B) Representative image of time-series projection (ventral view) of sound-induced escape behavior. (C) Tracking of tail movement during response to acoustic stimulus at t = 20 ms. (D) Representative image (dorsal view) of tg(s1181t:Gal4;UAS:kaede) transgenic zebrafish line. Scale bar 25 µm. (E and F) GCaMP5 calcium activity traces from M-cells in response to acoustic stimulus at 400 ms. (G) Acoustic startle responses of individual larvae ordered along y-axis by probability of escape over the stimulus series. Each dot represents a sound-induced escape event: large dot represents escape with two M-cells active; small dot represents escape with one M-cell active. (H) Decrease in ASR probability during stimulus series (F-statistic vs constant model: 41.5, P < 10−9, adjusted R-squared = 0.085). (I) ASR habituation accompanied by significant decrease in the fraction of escapes with two active M-cells (F-statistic vs constant model: 9.15, P < 0.01, adjusted R-squared = 0.028). Statistics: linear regression model analysis. Nlarvae = 29. Error bars, s.e.m.
M-cells are the command-like neurons for the ASR network and receive multiple inputs that control the properties of the ASR behavior. We hypothesized that the firing properties of M-cells might differ between individuals with different levels of ASR habituation. To test this hypothesis, we combined detection of ASR escape behavior with calcium imaging in M-cells using the tg(s1181t:Gal4;UAS:GCaMP5) transgenic line, to drive expression of the genetically encoded calcium indicator GCaMP5 in M-cells and only a few other hindbrain and spinal neurons (Akerboom et al., 2012; Scott et al., 2007) (Figure 3D). A larva was embedded in agar, which was cut away to free the tail to obtain a head-fixed preparation where tail motion could be observed (Figure 3B). Head-fixed zebrafish are generally less responsive to sensory stimuli when compared to freely swimming fish. To maximize the number of behavioral responses obtained from each larva, and to minimize photobleaching of GCaMP5 during the extended habituation assay, zebrafish were tested at an ISI of 90s, enabling use of short (5 s) bouts of fluorescence excitation, separated by longer periods (85 s) of time for diffusion-based recovery of the GCaMP5 signal. Acoustic-vibration stimuli were given at a fixed intensity that reliably elicited short-latency C-bend responses that are characteristic of the ASR (Figure 3B and 3C). In trials in which sound stimuli elicited escapes, we observed large M-cell calcium transients, sometimes in only one M-cell and sometimes concurrently in both (Figure 3E and 3F).
Head-fixed larval zebrafish exposed to a series of 15 sound stimuli at an ISI of 90 s showed high diversity in ASR habituation (Figure 3G and 3H), resembling the diverse behavior seen under the free swimming conditions (Figure 1B–1F). ASR latency duration was characteristic of M-cell dependent short latency ASR (in 332 out 334 of escapes, latency < 14 ms) and did not significantly change during the stimulus series (Figure S3A). Analysis of sound-induced M-cell calcium fluorescence signals showed a decrease in peak calcium amplitudes in individual M-cells (Figure S3B), no significant changes in calcium decay times (Figure S3C) and an overall decrease in the integral of sound-evoked M-cell calcium signals during habituation (Figure S3D). Despite the fact that firing of one M-cell is sufficient to produce the escape behavior and occurred in 100% of escapes, in 54% of escapes two M-cells fired (Figure 3E–3G). Notably, the frequency of escapes with two M-cells firing was higher during the early stimuli of the habituation series and decreased later in the series, trending with the behavioral habituation (Figure 3H and 3I). Fish with sustained behavioral responses (low habituation) sustained a high probability of response in each of the M-Cells and a higher frequency of dual M-cell responses when compared to high habituation individuals (Figure 3G, compare fish #1–12 with fish #13–29). The kinematics of escapes with two M-cells active did not differ significantly from those of escapes with one M-cell active (Figure S3E). This result agrees with the extensive cross-inhibition between the M-cells (Satou et al., 2009). Thus, the probability of sound-evoked calcium responses in each M-cell decreases in parallel to the habituation of the ASR behavior.
Dorsal Raphe Nucleus activity during ASR habituation
The individual differences in the loss of all-or-none M-cell responses during ASR habituation lead us to wonder whether the excitatory drive to the M-cells, or their excitability, is subject to regulation by neuromodulatory inputs, and, if so, whether differences in neuromodulation between individuals could contribute to ASR habituation diversity. Serotonergic dorsal raphe nucleus (DRN) neurons project axons near the M-cell dendrites (McLean and Fetcho, 2004a) (Figure 4A and Movie S1 and S2), raising the possibility that serotonin may either act directly on the M-cells or on their inputs to modulate M-cell activity and ASR habituation. We therefore set out to measure the activity of the DRN neurons during the ASR. To perform calcium imaging in serotonergic DRN neurons, we used the tg(tph2:Gal4ff)y228 line, which expresses Gal4 under the control of a fragment of the promoter of the tryptophan hydroxylase 2 gene that drives expression in serotonergic neurons in the DRN and a few spinal neurons tg(tph2:Gal4ff)y228 (Yokogawa et al., 2012). We crossed this driver line to a UAS:GCaMP5 line (Figure 4B). Transgenic head-fixed larvae were subjected to the ASR habituation protocol, as described above.
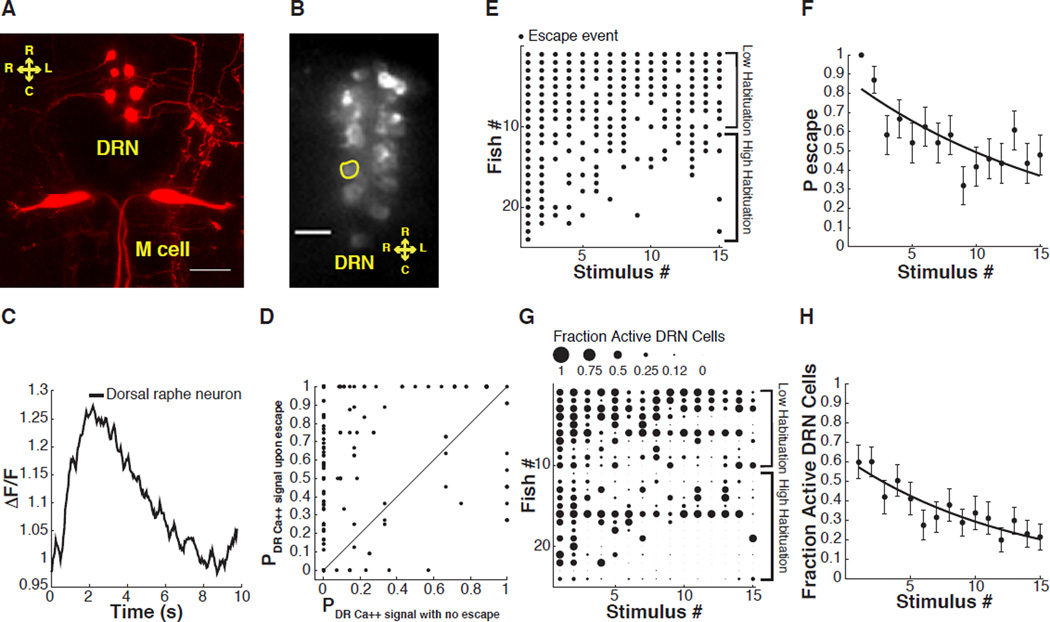
Figure 4. Simultaneous analysis of ASR behavior and dorsal raphe nucleus (DRN) neuron calcium signals in 6 dpf zebrafish larvae.
(A) Image shows varicosities projecting from the DRN towards the Mauthner cell dendrites (maximum projection of a Z stack of confocal images, variegated individual shown for ease of demonstration). (B) Representative image of a confocal slice of DRN serotonergic neurons expressing GCaMP5. (C) Representative trace shows ROI normalized fluorescence in a single DRN neuron. (D) Probability of sound-evoked calcium responses for individual DRN neurons. Graph shows probability of sound-induced responses for individual cells during sound stimuli that did not induce escapes versus stimuli followed by escapes. (E) Acoustic startle responses of individual larvae ordered along y-axis by probability of escape over the stimulus series. Each dot represents a sound-induced escape. (F) Significant decrease in ASR probability (F-statistic vs constant model: 23, P < 10−5, adjusted R-squared = 0.06). (G) Fractional activation of DRN neurons in individual larvae ordered in the y-axis by probability of escape during stimulus series. The size of each dot is proportional to the number of cells activated by acoustic stimuli as a fraction of total number of analyzed individual neurons/fish. (H) Decrease in number of sound-activated DRN neurons as fraction of total number of analyzed individual neurons/fish during habituation (F-statistic vs constant model: 29.2, P < 10−6, adjusted R-squared = 0.073). Statistics: linear regression model analysis. 8–14 neurons/fish, nDRN neurons = 244, nlarvae = 24. Error bars, s.e.m.. Scale bar 25 µm.
Acoustic-vibration stimuli activated calcium signals in the DRN neurons (Figure 4B and 4C) with a similar temporal profile to responses that were shown earlier to be induced in these cells by water flow on the tail in another behavior paradigm (Yokogawa et al., 2012). Next, we asked if this line of fish retained the variability in ASR habituation that we described above. We found similar diversity in ASR behavior habituation in larvae expressing GCaMP5 in tph2 serotonergic neurons when compared to animals expressing GCaMP5 in the M-cells and that ASR latency did not change significantly during habituation (Figure 4E and 4F; Figure S4A). Sound-induced calcium signals in individual tph2 DRN neurons were longer lasting than those of the M-cells (compare Figure 3E and Figure S3C to Figure 4C and Figure S4C). As ASR habituation progressed, single DRN neuron calcium fluorescence peak amplitude and integral decreased, but calcium decay times did not significantly change (Figure S4B–S4D).
Responses to acoustic stimulation of the DRN neuron groups within each fish revealed great diversity between individuals. Most animals responded to early sound stimuli with DRN activity and the fraction of DRN cells that responded declined as the stimulus series progressed (Figure 4G and 4H; Figure S4E). Overall, individuals with low habituation showed sound-evoked activity in a larger number of DRN neurons than did individuals with high habituation (Figure 4E and 4G, compare fish #1–10 with #11–24). Indeed, DRN neuron calcium responses were evoked much more frequently when the sound stimulus also induced the ASR behavior (Figure 4D), suggesting a causal link. However, behavioral output was not required for sound-induced activity in the DRN neurons (Figure 4D).
Together, these results suggest that the sound-evoked activity of DRN neurons suppresses ASR habituation and that variability between individuals in this activity contributes to the diversity of habituation behavior.
Serotonin and dopamine regulate ASR habituation in opposite ways
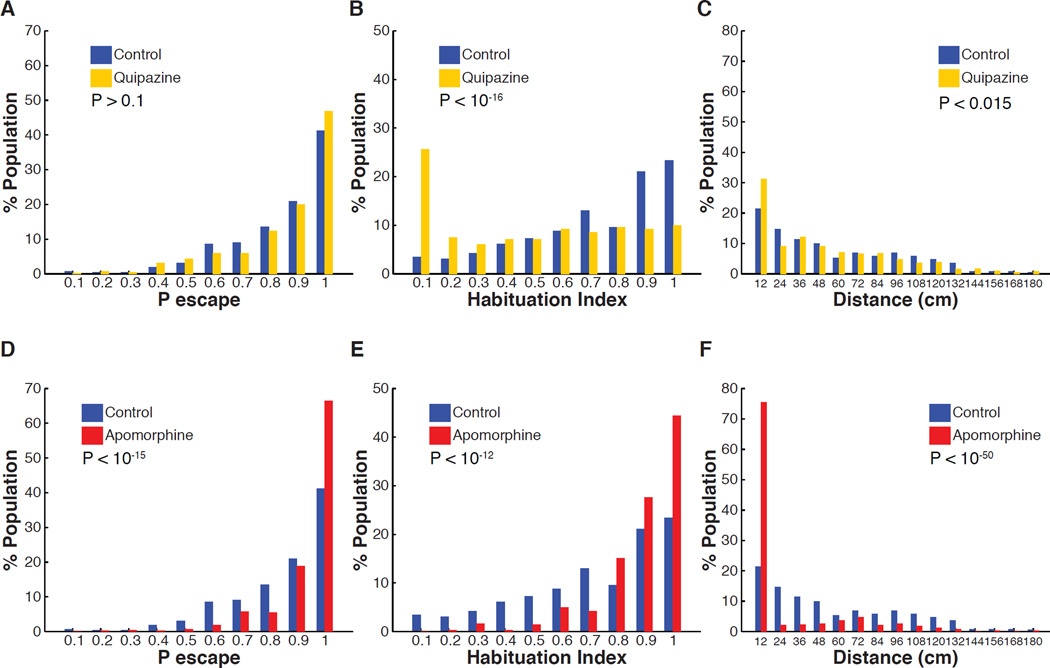
The previous results suggest that serotonin released by DRN neurons modulates ASR circuit function and escape behavior. We tested this pharmacologically first for serotonin and then for a second major neuromodulator, dopamine, which often works either in tandem with or in opposition to serotonin (Boureau and Dayan, 2010). Consistent with the observation that individuals with low habituation display high activity in serotonergic DRN neurons, we found that although treatment with the broad serotonergic agonist quipazine did not affect the initial probability of escape under non-habituating conditions (ISI = 90 s) (Figure 5A), it strongly reduced habituation of stably responsive larvae, Pinitial > 0.9, shifting the population distribution of HI values to lower levels (MedianHI Control = 0.76, MedianHI Quipazine = 0.56, P < 10−16) (Figure 5B). Strikingly, treatment with the broad dopaminergic agonist apomorphine increased the initial probability of escape (Figure 5D), but had the opposite effect of serotonin signaling, and strongly increased ASR habituation (MedianHI Control = 0.76, MedianHI Apomorphine = 0.91, P < 10−12) (Figure 5E). Thus, it appears that the distribution of individual ASR habituation phenotypes in a population is controlled in opposite ways by serotonin and dopamine. In contrast to the pronounced effect on ASR habituation at the population level, serotonergic agonism only slightly decreased spontaneous activity levels at the population level (Figure 5C) (MedianDistance Control = 44.3 cm, MedianDistance Quipazine = 38.4 cm, P < 0.015). Similarly to previous findings, dopaminergic agonism strongly decreased spontaneous activity (Figure 5F) (MedianDistance Control = 44.3 cm, MedianDistance Apomorphine = 0 cm, P < 10−6) (Thirumalai and Cline, 2008).
Figure 5. Effect of serotonin and dopamine agonists on ASR habituation and spontaneous swim behavior.
(A–C) Treatment with serotonin receptor agonist (quipazine 50 µM). (A) Quipazine treatment does not significantly affect ASR probability under non-habituating conditions (nControl = 420, nQuipazine = 420, z = 1.53, P > 0.1). (B) Quipazine treatment shifts the habituation index (HI) frequency distribution to lower values (less habituation) (nControl = 261, nQuipazine = 281, z = 8.4, P < 10−16). (C) Quipazine treatment shifts the frequency distribution of total displacement during 10 minutes to lower values when compared to control group (nControl = 359, nQuipazine = 394, z = 2.5 P < 0.015). (D–F) Treatment with dopamine receptor agonist (apomorphine 15 µM). (D) Apomorphine treatment increases initial ASR probability (nControl = 420, nApomorphine = 420, z = 8.2, P < 10−15). (E) Apomorphine treatment shifts HI frequency distribution to higher values (greater habituation) (nControl = 261, nApomorphine = 358, z = 7.38, P < 10−12). (F) Apomorphine treatment significantly shifts the frequency distribution of total displacement values during 10 minutes to lower values when compared to control group (nControl = 359, napomorphine = 383, z = 15.12, P < 10−50).
Reduction in DRN serotonin increases ASR habituation
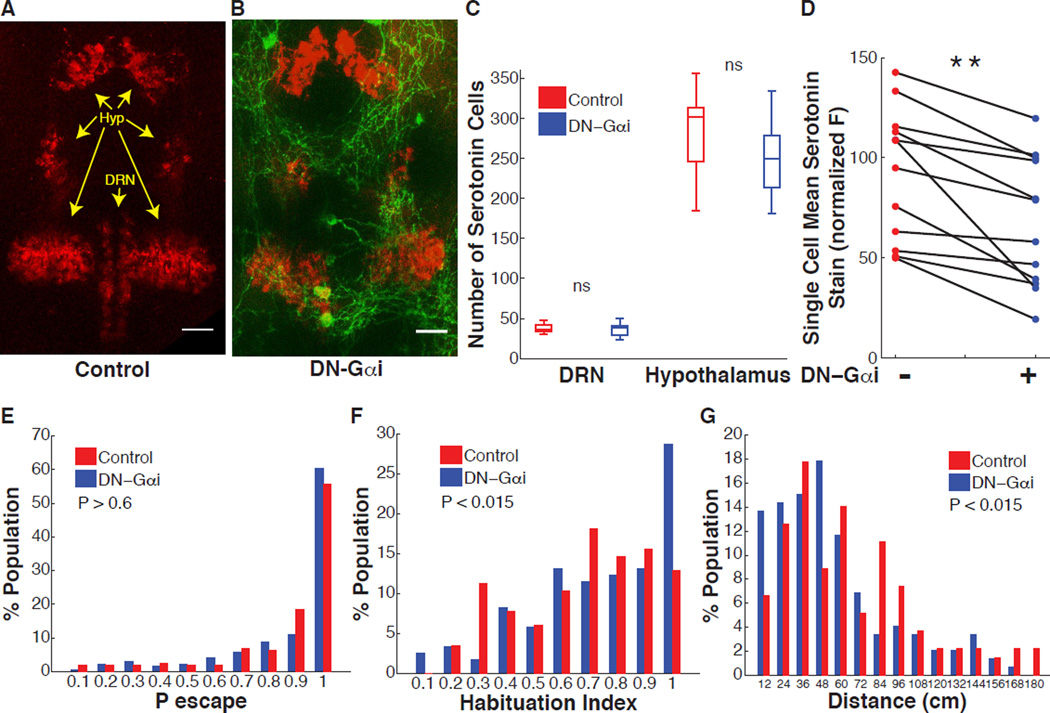
Our findings so far suggest a model in which serotonergic neuromodulation of the ASR circuit suppresses habituation of the ASR behavior and that differences between individuals in neuromodulation are associated with differences in ASR habituation. To test this model, we sought to disrupt the serotonergic DRN neurons and ask if this would result in greater habituation. Previous studies in rat cell cultures implicated the cAMP signaling pathway in regulating the development of serotonergic neurons (Rumajogee et al., 2005). We therefore set out to manipulate cAMP signaling in DRN serotonergic neurons. To accomplish this goal we built a transgenic line in which expression of a dominant negative peptide for the Gαi subunit of the heterotrimeric G protein complex (DNGαi) (Dell et al., 2013) is controlled by UAS sequences tg(tph2:Gal4ff;UAS:DNGαi,gap43-citrine). We crossed these with the transgenic line tg(tph2:Gal4ff)y228 to induce DNGαi expression in DRN neurons (Yokogawa et al., 2012). Immunofluorescence analysis of the tg(tph2:Gal4ff;UAS:DNGαi,gap43-citrine) line using an antibody directed against serotonin and intrinsic fluorescence of membrane targeted citrine fluorescent protein revealed that the DNGαi peptide is expressed specifically in serotonergic DRN neurons, but not in adjacent hypothalamic serotonergic neurons (Yokogawa et al., 2012) (Figure 6A and 6B). Expression of DNGαi was detected in ~20–50% of DRN neurons and occasionally in isolated cells in the spinal cord, consistent with earlier work (Yokogawa et al., 2012). In contrast to previously reported results in primary neuronal cultures (Rumajogee et al., 2005), DNGαi expression in the DRN did not affect the number of serotonergic neurons in the DRN (Figure 6C). Moreover, hypothalamic serotonergic neurons, which do not express the DNGαi peptide, were also not affected in number (Figure 6C). However, the fraction of DRN neurons that expressed the DNGαi peptide, which were marked green by the co-expressed citrine fluorescent protein, had lower amounts of serotonin than did neurons in the same DRN that were unmarked and did not express the DNGαi peptide (Movie S3). DNGαi expression significantly reduced serotonin content in DRN neurons in 12 out of 12 individual larvae examined by an average of ~30% (P < 0.001) (Figure 6D). Importantly, animals expressing DNGαi in DRN cells had similar serotonin levels in the hypothalamus when compared to control animals (Figure S5A).
Figure 6. Effect on ASR habituation of reduced serotonin content in DRN serotonergic neurons.
(A–B) Representative images of labeling with serotonin antibody (red) in control larva (A) and in tg(Tph2:Gal4ff;UAS:DNGαi,gap43-citrine) larva that co-expresses DN-Gαi and citrine fluorescent protein (green) in DRN serotonergic neurons (B). Scale bars 25 µm. (C) DRN DNGαi expressing larvae do not have significantly different numbers of serotonergic neurons in the DRN or hypothalamic serotonergic nuclei (nDN-Gαi = 11, nControl = 11, P > 0.9). (D) DRN neurons co-expressing DN-Gαi and citrine fluorescent protein (green) have significantly lower mean serotonin content than neurons in the same DRN that do not express (n = 12 larvae, ** = P < 0.001). (E) ASR probability under non-habituating conditions in DN-Gαi expressing larvae compared to control sibling larvae (nDN_Gαi = 171, nControl = 156, z = 0.43, P > 0.06.) (F) DN-Gαi larvae habituation index frequency distribution is shifted to higher values (more habituation) compared to control larvae (nDN_Gαi = 122, nControl = 116, z = 2.28, P = 0.011). (G) Frequency distribution of total displacement during 10 minutes in DN-Gαi larvae is shifted toward decreased displacement compared to control larvae (nDN-Gαi = 146, nControl = 106, z = 2.46 P =0.014). Statistics: unpaired, two-tailed Student’s t-test (C), paired, two-tailed Student’s t-test (D), two-tailed Mann-Whitney test (E–G).
Consistent with our findings above, we found that decreased serotonin content in DRN serotonergic neurons did not affect ASR probability under non-habituating conditions (Figure 6E), but significantly increased the frequency of individuals with high ASR habituation when compared to a population of control sibling larvae with no DNGαi expression (MedianHI Control = 0.76, MedianHI DN-Gαi = 0.83, P < 0.015) (Figure 6F). Tg(tph2:Gal4ff;UAS:DNGαi,gap43-citrine) transgenic larvae developed normally and were morphologically indistinguishable from control sibling larvae with no DNGαi expression (Figure S5B). Larvae with DNGαi expression were slightly decreased in median spontaneous swim activity levels compared to control sibling larvae (MedianD Control = 58.2 cm, MedianD DN-Gαi = 46.7 cm, P < 0.015) (Figure 6G). Similarly to our previous observation (Figure 2D), we did not observe significant correlation between spontaneous swim activity levels and HI in control larvae or in larvae expressing DNGαi under control of the tph2 promoter (Pearson’s correlation coefficient Distance vs HIControl, r = 0.0948, P = 0.23, Distance vs HIDN-Gαi, r = −0.0104, P = 0.89).
DRN activation with channelrhodopsin-2 suppresses ASR habituation
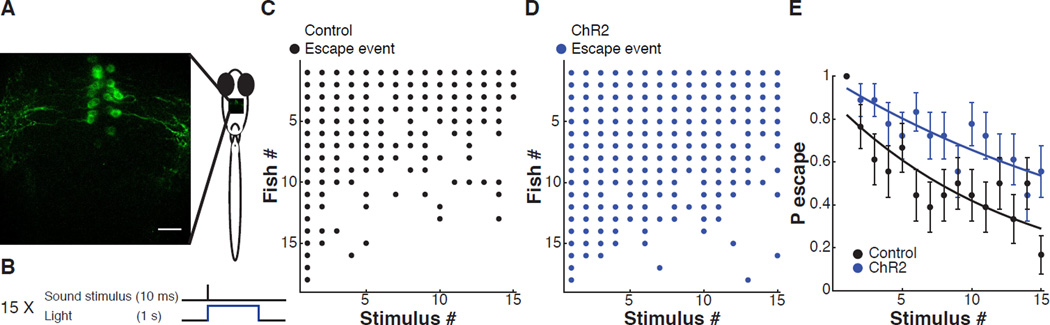
Having seen that reduction of the serotonin content of DRN neurons increased the fraction of individuals with high ASR habituation, we asked whether stimulating these cells to promote serotonin release would have the opposite effect and reduce habituation. To do this, we generated zebrafish larvae expressing YFP-tagged ChR2 (Lacoste et al., 2015) specifically in the DRN neurons. In tg(tph2:Gal4ff; 14xUAS-E1b:hChR2(H134R)-EYFP) larvae, ChR2 expression was restricted to the DRN and did not include other serotonergic populations (Figure 7A). We examined whether DRN activation with ChR2 modified ASR habituation in the head-fixed larval preparation, as above (Figure 3 and Figure 4). ChR2-expressing DRN neurons and neuronal processes were illuminated with laser light (488 nm, 1 s, 14 mW/mm2) administered through the microscope objective (40×/1.1), which yielded minimal light scattering to the eyes (Figure 7A). To mimic the pattern of DRN activation induced by acoustic stimuli, which activate DRN neurons at the same time as they activate the M-cell circuit, we paired the sound stimulus with optical activation of ChR2 in DRNs (Figure 7B).
Figure 7. Effect on ASR habituation of DRN activation with channelrhodopsin-2 (ChR2).
(A) Representative image showing ChR2-YFP expression in cell bodies and processes of DRN neurons in tg(tph2:Gal4ff; 14xUAS-E1b:hChR2(H134R)-EYFP) in 6 dpf larva. (B) Protocol showing single stimulus from habituation series, pairing illumination at 488 nm (to activate ChR2 in DRN neurons) with sound stimulation (to elicit the ASR). (C, D) Acoustic startle responses of all of the individual larvae ordered in the y-axis by probability of escape during the stimulus series, separated into two groups from the sibling larvae: not expressing ChR2-YFP (C, Control) and those expressing ChR2-YFP (D, ChR2). Each dot represents a sound-induced escape. (E) Plots of mean ± s.e.m. of data shown in (C) and (D) showing less habituation in siblings with ChR2 in DRN neurons (nChR2 = 18, nControl = 18, analysis of covariance, F-statistic: 28.24, P < 10−6).
We measured ASR habituation in individual larvae expressing ChR2 in DRN neurons and in sibling larvae that did not express ChR2 but which were exposed to the same light stimuli. We found that the ChR2-expressing fish showed lower habituation compared to their non-expressing sibling controls (analysis of covariance, F-statistic: 28.24, P < 10−6) (Figure 7C–7E). Thus, as shown in the previous section, ASR habituation is increased by reduction of DRN neuron serotonin content (and presumably release) and, as shown here, decreased by optogenetic stimulation of the DRN neurons (and presumably promotion of serotonin release).
Differences in DRN activity co-segregate with behavioral differences
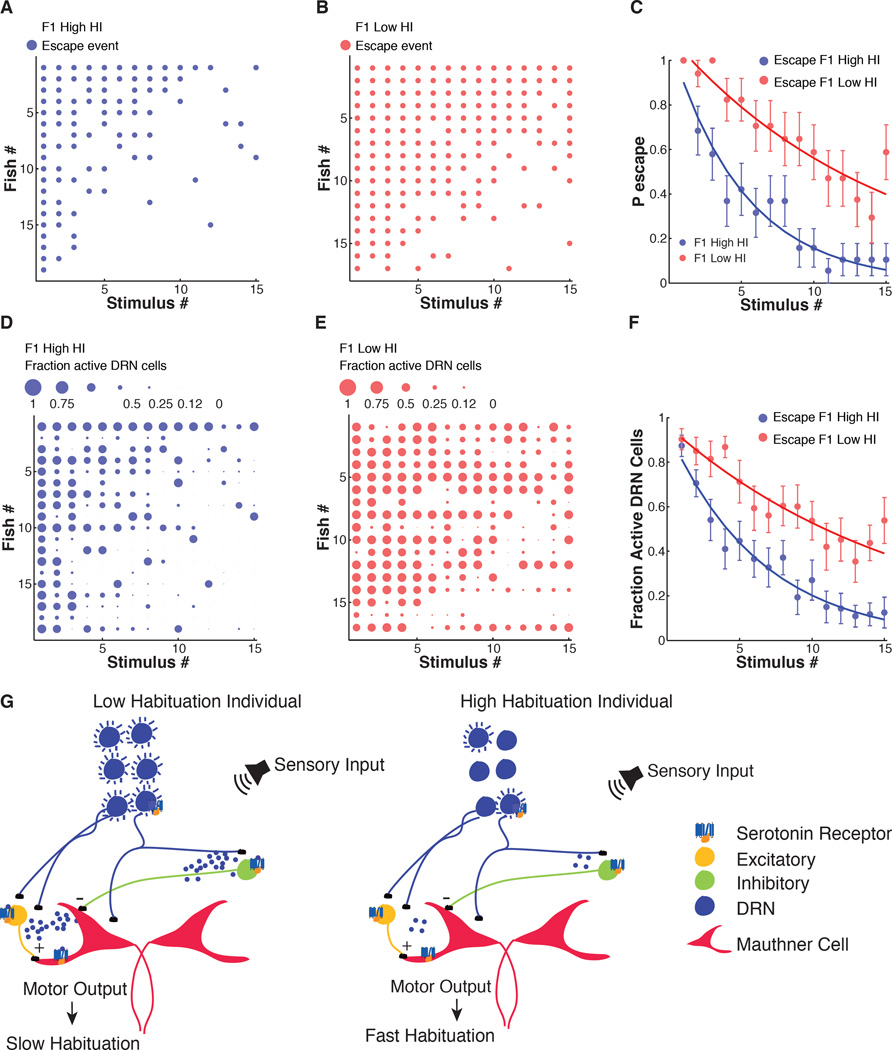
Having observed heritability in high versus low ASR behavioral habituation and having observed that the higher the behavioral habituation the faster the rundown of activity in serotonergic DRN neurons, we wondered if behavioral habituation and DRN neuron rundown would co-segregate between generations. To test this, we measured DRN neuron activity during ASR habituation, as above (Figure 4), in the F1 progeny of F0 animals that had been selected in our high-throughput apparatus (Figure S2, F0Low HI couple 2 or F0High HI couple 3).
We found that although the behavioral selection was done in freely swimming fish and subsequent testing in head-fixed fish, F1Low HI fish had significantly lower habituation rates than F1High HI fish (Figure 8A–8C). This result confirms that habituation is heritable, as shown above (Figure 2E and Figure S2) and suggests that the regulation of ASR habituation is similar in freely swimming and head-fixed preparations. Next, we compared DRN activity in the high and low habituation F1 groups. Consistent with the above evidence that DRN serotonergic neuronal activity suppresses ASR habituation, we found that sound-evoked activation of DRN serotonergic neurons was stronger and showed less rundown in F1Low HI larvae than in F1High HI larvae (Figure 8D–8F). This amounted to an ~180 % higher integrated area under the response curves during habituation (Figure 8F) in the low habituation group. Behavioral latencies were slightly increased in low HI larvae as a group when compared to high HI larvae at the end of the stimulus series (Figure S6A). Single DRN neuron mean calcium peak values decreased during ASR habituation in both high and low HI groups, but were significantly higher in the low HI group (Figure S6B). Single neuron mean calcium decay values did not change significantly during habituation and were not significantly different between groups (Figure S6C). The mean values of the integral of the calcium flux in DRN neurons also decreased during ASR habituation and were significantly higher in F1 low HI larvae (Figure S6D). In summary, differences in sound-evoked activation of DRN serotonergic neurons co-segregate with heritable individual differences in ASR habituation (Figure 8G).
Figure 8. ASR habituation behavior and DRN neuronal activity in F1 progeny of zebrafish selected for high or low habituation.
(A and B) Acoustic startle responses of individual larvae ordered in the y-axis by probability of escape during stimulus series. Each dot represents a sound-induced escape. (C) Slower habituation in F1Low HI zebrafish larvae compared to F1High HI group (F-statistic: 90.51, P < 10−19). (D and E) Fractional activation of DRN neurons in individual larvae ordered in the y-axis by probability of escape during stimulus series. The size of each dot is proportional to the number of cells activated by acoustic stimuli as a fraction of total number of analyzed individual neurons/fish. (F) Larger number of sound-activated DRN neurons as fraction of total number of analyzed individual neurons/fish in F1 Low HI zebrafish larvae compared to F1 High HI group (F-statistic: 86.45, P < 10−18). Statistics: analysis of covariance. 12–16 neurons/fish. NLow HI DRN neurons = 248, nHigh HI DRN neurons = 268, nLow HI larvae = 17, nHigh HI larvae = 19. Error bars, s.e.m. (G) Model illustrating how differential activation of DRN serotonergic neurons by acoustic stimuli contributes to differential habituation in the ASR circuit.
Discussion
ASR habituation is diverse, stable and heritable
Inter-individual differences in behavior are common in animal populations and underlie the related concepts of animal individuality and personality (Wolf and Weissing, 2012). Behavioral variability in individuals is due to genetic, developmental, pharmacological, environmental and social processes (Kappeler and Anthes, 2010; Laskowski and Bell, 2014). Inter-individual behavioral variation is widely observed across animal phyla and might be under selective pressure during animal evolution (Ayroles et al., 2015; Laurila et al., 2008). However, variation among individuals is often ignored when behavior is quantified as averages with associated dispersions (Geiler-Samerotte et al., 2013). In vertebrates, it has been challenging to study the extent of behavioral variation in outbred populations and the associated neural mechanisms that underlie behavioral variation at the individual level. In the present study we used zebrafish in an effort to identify properties of a neural circuit that determine behavioral individuality and generate behavior diversity at the population level.
Our high throughput behavioral analysis revealed a large diversity in the rate of ASR habituation between individual wild-type larval zebrafish. Interestingly, this large inter-individual behavioral diversity was present in laboratory conditions, where the role of environmental factors in determining behavioral differences is minimized. In fact, we found that differences in the rate of habituation between individuals are maintained over days and are heritable, indicating that ASR habituation has a genetic or epigenetic component. The genetic implications are consistent with recent findings from a forward genetic screen in larval zebrafish, which identified several mutants that affect ASR habituation without affecting startle performance (Wolman et al., 2015). Two of these genes were identified: one encoding the metabolic enzyme pyruvate carboxylase and the other the pregnancy-associated plasma protein A, a secreted metalloprotease. Although the mechanisms through which these genes affect ASR habituation are unclear, it is possible that polymorphisms in them contribute to the diversity of ASR habituation in wild-type populations.
In humans, differences in behavior are also heritable and variations in genes involved in dopamine and serotonin signaling are associated with differences in personality and heritable psychiatric conditions (Kishi et al., 2013; Ptacek et al., 2011). Furthermore, patients with a diagnosis of schizophrenia have ASR habituation deficits (Braff, 1992). Because the ASR habituation behavior is an example of an animal personality trait that is present at the zebrafish larval stage it represents an attractive vertebrate paradigm to investigate the neural bases of behavioral individuality.
In contrast to the properties of ASR habituation, which show stable differences between individuals, quantitation of the spontaneous swim locomotor behavior showed diversity among individuals but lack of stability over a period of days. Intriguingly, a recent study found that differences in spontaneous locomotor behavior were stable over days in adult zebrafish and more robust in female zebrafish than in males (Tran and Gerlai, 2013). Zebrafish sex-specific gonadal differentiation begins around one month post-fertilization (Takahashi, 1977). It is possible that the circuits controlling spontaneous locomotor behavior are subject to developmental control and that differences between individuals that are unstable early in life are crystalized as animals reach full sexual maturity. Comparison of the two behaviors in young and older adult zebrafish will be useful to address the mechanisms that lead to stability in behavioral parameters during development.
Inter-individual differences in ASR circuit properties
To determine if differences between individuals in properties of the ASR neural circuit account for variation in ASR habituation in a population, we monitored ASR-inducing M-cell calcium signals during a series of sound pulses that produced ASR habituation. During habituation, M-cell calcium fluxes decreased as the stimulus series progressed. Typically, the two M-cells were activated simultaneously early in the stimulus series and more than half of escapes in the entire series involved their simultaneous activity, as recently reported and found to be blocked by strychnine or MK-801, suggesting that change in glutamatergic and/or glycinergic input to M-cells or their inputs participates in ASR habituation (Marsden and Granato, 2015).
But what is the cause of the inter-individual difference in M-cell ability to follow repeated stimulation? Both dopamine and serotonin containing neurons have processes near the M-cells and these neuromodulators have been shown to influence the startle behavior (Lillesaar et al., 2009; McLean and Fetcho, 2004a, 2004b; Mu et al., 2012; Wolman et al., 2011). Previous studies in adult goldfish showed that serotonin inhibits M-cell activity pre- and post-synaptically (Mintz and Korn, 1991) and that in adult cichlid fish, serotonergic inputs modulate activity of M-cells and their inhibitory glycinergic inputs, while the 5-HTR2 antagonist ketanserin increases startle probability (Whitaker et al., 2011). In contrast to what was reported in cichlid fish, the 5-HT2R antagonists ritanserin and pirenperone enhance ASR habituation in larval zebrafish (Wolman et al., 2011). We found that treatment with quipazine does not affect ASR probability under non-habituating conditions, but strongly suppresses habituation under strongly habituating conditions. The results of Wolman et al. are consistent with our observation that treatment with quipazine decreases ASR habituation (Figure 5B), while the discrepancy between these results and those of Mintz et al. and Whitaker et al. might be due to experimental differences in ISI, and/or developmental or species differences. In our study we sought to determine if the activity of DRN serotonergic neurons is involved in the ASR individuality.
Differences in neuromodulatory input between individuals
Differences in neuromodulation have been previously shown to be associated with differences in behavioral state in mouse (Tereshchenko et al., 2008; Zalocusky et al., 2016), Caenorhabditis elegans (Mersha et al., 2013), honeybee (Müller and Hildebrandt, 2002) and Aplysia californica (Glanzman et al., 1989). Moreover, studies in the sea slug Pleurobranchaea californica, in Drosophila and in C. elegans suggest that serotonergic modulation of behavior variability seems to be conserved during evolution (Flavell et al., 2013; Kain et al., 2012; Lillvis and Katz, 2013). We found that the sound stimulus that triggers the ASR also triggers activity in serotonergic DRN neurons whose nerve terminals project widely in the larval zebrafish brain and traverse the hindbrain in close proximity to M-cell dendrites and, hence, to their inputs (Lillesaar et al., 2009; Yokogawa et al., 2012). Animals in which a larger number of DRN neurons responded to the sound stimulus and dropped out more slowly during repeated sound stimulation had lower habituation. Consistent with this, ASR habituation was decreased by either application of a serotonin agonist or optogenetic stimulation of DRN neurons. In further support, a genetic manipulation that reduced the serotonin content of the DRN serotonergic neurons had the opposite effect and increased habituation. Thus, variability between individuals in the ability of DRN neurons to respond to and follow repeated bouts of startling sensory input appears to contribute to diversity of habituation rates.
The identity of the neuronal populations that are the targets of DRN inputs and mediate the behavioral impact of differential DRN activation by sensory stimuli remains unknown. We speculate that elements of the ASR circuit in the hindbrain mediate these effects (Figure 8G). First, we observed differential drop-off of M-cell activity between individuals during ASR habituation. Second, serotonin modulates M-cells and the inhibitory glycinergic neurons that control ASR circuit gain. Third, the DRN send projections that traverse the hindbrain. These results are consistent with a model in which individual differences in serotonergic signaling to the hindbrain M-cell circuit mediate the behavioral impact of differential DRN activation by sensory stimuli (Figure 8G). It is also possible that individual differences in DRN-dependent serotonergic signaling affecting additional nodes of the ASR circuit located in the mid-brain or forebrain, for example, participate in the control of ASR habituation individuality.
The cause of variability in DRN responses between individuals remains to be determined as does the question of whether it is cell autonomous or due to differences in synaptic input to the DRN neurons. Multiple stimuli activate DRN neurons in larval zebrafish (Cheng et al., 2016; Filosa et al., 2016; Yokogawa et al., 2012), however the neuroanatomy of the circuits that provide the synaptic inputs that mediate activation of the DRN is unknown. In adult zebrafish it has been proposed that the dorsal interpeduncular nucleus might send projections to the DRN (Agetsuma et al., 2010). In mice, the DRN receives inputs from multiple brain nuclei and multiple sensory modalities affect the properties of DRN neurons (Pollak Dorocic et al., 2014; Ranade and Mainen, 2009; Weissbourd et al., 2014). Moreover, transient activation of serotonergic neurons in the DRN acutely modulates behavioral states in mice and zebrafish (Cheng et al., 2016; Miyazaki et al., 2014; Yokogawa et al., 2012). Thus, it is possible that variation in DRN activation by sensory inputs might also contribute to inter-individual behavioral diversity in mammals. In Aplysia, sensory feedback participates in the control of behavioral variability by increasing intra-individual variability in motor neurons that participate in swallowing behavior control (Cullins et al., 2015). In fact, sensory inputs appear to participate in the control of behavioral variability at the population level and may increase or decrease behavioral variability at the individual level. Our work suggests that differential activation of neuromodulatory networks by sensory inputs may be a key mechanism through which sensory systems control behavioral variability (Figure 8G).
After a single round of behavioral selection for animals with low versus high habituation, we obtained F1 animals with significant differences in ASR habituation behavior and the strength and sustainability of serotonergic DRN neuron activity in response to sensory activation. This observation indicates that differences in the sensory activation of the DRN are heritable and may provide a neural substrate upon which selection could act to shape behavioral diversity at the population level. Observation of behavior and neural activity in single individuals also reveals that some individuals with high levels of DRN activity had high habituation (Figure 4 and Figure 7). Conversely, low DRN activity was not fully correlated with high habituation rates (Figure 4 and Figure 7). Previous studies have shown that dopaminergic inputs modulate several nodes of the ASR network (Mu et al., 2012; Pereda et al., 1992; Toro et al., 2015). Significantly, a dopamine agonist had the opposite effect of the serotonin agonist, and decreased habituation, despite increasing pre-habituation ASR probability, suggesting that individuality in this adaptive behavior may be determined by the operational balance between dopaminergic and serotonergic drive to the escape circuit.
In conclusion, our study shows that sensory-evoked responses in a neuromodulatory network can be dynamic, vary greatly between individuals and participate in the shaping of individuality in a core adaptive behavior in zebrafish, which may, for its parallels to the startle circuits of other vertebrates and importance of neuromodulation, be relevant to psychiatric disorders.
Experimental Procedures
Zebrafish transgenesis and high-throughput behavioral assay
The transgenic lines used in this study were generated with the Tol2 transposase transgenesis system. Detailed description of transgenesis procedures can be found in Supplemental Experimental Procedures. The high-throughput behavioral assay was performed in multi-wells, as described earlier (Levitz et al., 2013) and explained in detail in Supplemental Experimental Procedures.
Immunohistochemistry in larval zebrafish
We quantified the number of serotonergic cells and 5-HT content using immunohistochemistry against 5-HT (MAB352, 1:200, Millipore, Bedford, MA) in embryos obtained from crosses of tg(tph2:Gal4ff) fish to tg(cmlc2:Kal4/cry:Red;UAS:DNGαi) fish. Detailed protocols for immunohistochemistry and image analysis are presented in the Supplemental Experimental Procedures.
Head-mounted behavioral assay with calcium imaging and ChR2 activation
For behavioral experiments with simultaneous calcium imaging and sound stimulus presentation larvae were mounted in a glass well petri dish dorsal side down with free tail in 2% agar E3 solution. ChR2 activation was accomplished with 488 nm laser light (~ 14 mW/mm2) administered through a 40×/1.1 Zeiss objective, at a focal plane encompassing DRN neurons cell bodies and processes, and lasted for 1 s. ChR2 activation was initiated simultaneously with sound stimulus. Control fish without ChR2 expression were subjected to the same protocol. Detailed description of the experimental set up is included in the Supplemental Experimental Procedures. Animal experiments were approved by the University of California Animal Care and Use Committee.
Behavioral analysis
Behavioral analysis was performed using custom MATLAB scripts previously described and modified for the present study (Bianco et al., 2011; Levitz et al., 2013). Scripts are summarized in the Supplemental Experimental Procedures.
The Habituation Index (HI) is defined as the difference in escape probability to the last 40 high-level stimuli under habituating conditions (5 s ISI) with the first 10 high-level stimuli under non-habituating conditions (90 s ISI), divided by the probability of escape to the last 40 stimuli (HI = (Plast40 - Pfirst10)/Plast40). Only fish that responded 90% or more to the initial 10 high-level stimuli were used in calculating the HI.
Calcium imaging analysis
Analysis of calcium signals was performed with custom-written MATLAB scripts and is described in the Supplemental Experimental Procedures. All scripts used in this study are freely available upon request.
Statistics and data analysis
Data and statistical analysis were performed using MATLAB. Unless noted, all data points reported are mean ± s.e.m.. No statistical methods were used to estimate required sample sizes. Sample sizes used in this study are similar to those reported in similar studies previously published (Brustein et al., 2003; Whitaker et al., 2011; Wolman et al., 2011; Yokogawa et al., 2012). Investigators conducting the experiments were not blind to the groups being tested or analyzed. The 1- or 2-sided Mann-Whitney U test was used in comparing the nonparametric distributions of HIs amongst groups. The 2-sided Student’s t-test was used to compare distributions of serotonergic cell numbers and serotonin content in the DRN neurons. Linear regression model was used to determine variation of behavioral or calcium signals during stimulus series. The one-way analysis of covariance with linear fits for each group was used to compare groups with different habituation levels in experiments with head-mounted fish.
Supplementary Material
Highlights.
Acoustic startle response (ASR) habituation varies widely among individual zebrafish
Acoustic stimuli activate dorsal raphe nucleus (DRN), which projects to the ASR circuit
DRN activation decreases and DRN serotonin depletion enhances ASR habituation
Differences in DRN sensory activation and ASR habituation co-segregate
Acknowledgments
We thank, Didier Stainier for the cmlc2:Kal4;cry:red plasmid, Jonathan Raper for the Tol2-UAS:DNGαi1/2;UAS:citrine plasmid and Loren Looger for GCaMP5; Herwig Baier for the tg(s1181t:Gal4) zebrafish line and Harold Burgess for the tg(tph2:Gal4ff) zebrafish line; Einat Peled; Isaac Bianco and Florian Engert for MATLAB scripts for analysis of calcium signals and zebrafish tail tracking, respectively; Mel Boren, Kait Kilman and Jessie McNichols for fish care; Victor Hung, Kristian Dadakay, Holly Aaron, Jen-Yi Lee and the UC Berkeley Molecular Imaging Center for microscopes and technical support, and Gautam Agarwal, Helen Bateup, Henry Bourne, Brian Grone, Philipp Gut, Liam Holt, Joshua Levitz, Hans Luecke, Craig Miller, Nirao Shah and members of the Isacoff laboratory for helpful discussion. Support was provided by the National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (PN2EY018241) and the Human Frontier Science Program (RGP0013/2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, C.P. and E.Y.I.; Methodology and Formal Analysis, C.P., A.H. and E.C.; Investigation, C.P., A.H., E.C. and V.K.; Resources C.P. and A.C.; Supervision and Writing, C.P. and E.Y.I. All authors discussed the results and commented on the manuscript.
References
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, Sassa T, Amo R, Shiraki T, Kawakami K, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles JF, Buchanan SM, O’Leary C, Skutt-Kakaria K, Grenier JK, Clark AG, Hartl DL, de Bivort BL. Behavioral idiosyncrasy reveals genetic control of phenotypic variability. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6706–6711. doi: 10.1073/pnas.1503830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Kampff AR, Engert F. Prey Capture Behavior Evoked by Simple Visual Stimuli in Larval Zebrafish. Front. Syst. Neurosci. 2011:5. doi: 10.3389/fnsys.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanch A, Balada F, Aluja A. Habituation in acoustic startle reflex: Individual differences in personality. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2014 doi: 10.1016/j.ijpsycho.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Boureau Y-L, Dayan P. Opponency Revisited: Competition and Cooperation Between Dopamine and Serotonin. Neuropsychopharmacology. 2010;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Gating and Habituation of the Startle Reflex in Schizophrenic Patients. Arch. Gen. Psychiatry. 1992;49:206. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Brown KH, Dobrinski KP, Lee AS, Gokcumen O, Mills RE, Shi X, Chong WWS, Chen JYH, Yoo P, David S, et al. Extensive genetic diversity and substructuring among zebrafish strains revealed through copy number variant analysis. Proc. Natl. Acad. Sci. 2012;109:529–534. doi: 10.1073/pnas.1112163109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Chong M, Holmqvist B, Drapeau P. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J. Neurobiol. 2003;57:303–322. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R-K, Krishnan S, Jesuthasan S. Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci. Rep. 2016;6:20788. doi: 10.1038/srep20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Gill JP, McManus JM, Lu H, Shaw KM, Chiel HJ. Sensory Feedback Reduces Individuality by Increasing Variability within Subjects. Curr. Biol. CB. 2015;25:2672–2676. doi: 10.1016/j.cub.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin PCP, Medan V, Neumeister H, Bronson DR, Preuss T. The 5-HT5A Receptor Regulates Excitability in the Auditory Startle Circuit: Functional Implications for Sensorimotor Gating. J. Neurosci. 2013;33:10011–10020. doi: 10.1523/JNEUROSCI.4733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell AL, Fried-Cassorla E, Xu H, Raper JA. cAMP-induced expression of neuropilin1 promotes retinal axon crossing in the zebrafish optic chiasm. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:11076–11088. doi: 10.1523/JNEUROSCI.0197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa A, Barker AJ, Dal Maschio M, Baier H. Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Neuron. 2016 doi: 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiler-Samerotte KA, Bauer CR, Li S, Ziv N, Gresham D, Siegal ML. The details in the distributions: why and how to study phenotypic variability. Curr. Opin. Biotechnol. 2013;24:752–759. doi: 10.1016/j.copbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J. Neurosci. Off. J. Soc. Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordus A, Pokala N, Levy S, Flavell SW, Bargmann CI. Feedback from Network States Generates Variability in a Probabilistic Olfactory Circuit. Cell. 2015;161:215–227. doi: 10.1016/j.cell.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain JS, Stokes C, de Bivort BL. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19834–19839. doi: 10.1073/pnas.1211988109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler PM, Anthes N. Animal behaviour evolution and mechanisms. Berlin; London; New York: Springer; 2010. [Google Scholar]
- Kishi T, Yoshimura R, Fukuo Y, Okochi T, Matsunaga S, Umene-Nakano W, Nakamura J, Serretti A, Correll CU, Kane JM, et al. The serotonin 1A receptor gene confer susceptibility to mood disorders: results from an extended meta-analysis of patients with major depression and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:105–118. doi: 10.1007/s00406-012-0337-4. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Lacoste AMB, Schoppik D, Robson DN, Haesemeyer M, Portugues R, Li JM, Randlett O, Wee CL, Engert F, Schier AF. A convergent and essential interneuron pathway for Mauthner-cell-mediated escapes. Curr. Biol. CB. 2015;25:1526–1534. doi: 10.1016/j.cub.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AM, Bonkowsky JL, Masino MA. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:13488–13500. doi: 10.1523/JNEUROSCI.1638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski KL, Bell AM. Strong personalities, not social niches, drive individual differences in social behaviours in sticklebacks. Anim. Behav. 2014;90:287–295. doi: 10.1016/j.anbehav.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila A, Lindgren B, Laugen AT. Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology. 2008;89:1399–1413. doi: 10.1890/07-1521.1. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Dan Y. Neuromodulation of Brain States. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Pantoja C, Isacoff EY, Gaub B, Reiner A, Trauner D. Optical Control of Metabotropic Glutamate Receptors. Nat. Neurosci. 2013 doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C, Stigloher C, Tannhäuser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J. Comp. Neurol. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- Lillvis JL, Katz PS. Parallel evolution of serotonergic neuromodulation underlies independent evolution of rhythmic motor behavior. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:2709–2717. doi: 10.1523/JNEUROSCI.4196-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Granato M. In Vivo Ca2+ Imaging Reveals that Decreased Dendritic Excitability Drives Startle Habituation. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J. Comp. Neurol. 2004a;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 2004b;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Mersha M, Formisano R, McDonald R, Pandey P, Tavernarakis N, Harbinder S. GPA-14, a Gα(i) subunit mediates dopaminergic behavioral plasticity in C. elegans. Behav. Brain Funct. BBF. 2013;9:16. doi: 10.1186/1744-9081-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I, Korn H. Serotonergic facilitation of quantal release at central inhibitory synapses. J. Neurosci. Off. J. Soc. Neurosci. 1991;11:3359–3370. doi: 10.1523/JNEUROSCI.11-11-03359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr. Biol. CB. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Mu Y, Li X-Q, Zhang B, Du J-L. Visual Input Modulates Audiomotor Function via Hypothalamic Dopaminergic Neurons through a Cooperative Mechanism. Neuron. 2012;75:688–699. doi: 10.1016/j.neuron.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Müller U, Hildebrandt H. Nitric oxide/cGMP-mediated protein kinase A activation in the antennal lobes plays an important role in appetitive reflex habituation in the honeybee. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:8739–8747. doi: 10.1523/JNEUROSCI.22-19-08739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc. Natl. Acad. Sci. U. S. A. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Dorocic I, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Ptacek R, Kuzelova H, Stefano GB. Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders. Med. Sci. Monit. 2011;17:RA215–RA220. doi: 10.12659/MSM.881925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M. Network Plasticity in Adaptive Filtering and Behavioral Habituation. Neuron. 2014;82:1216–1229. doi: 10.1016/j.neuron.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Ranade SP, Mainen ZF. Transient Firing of Dorsal Raphe Neurons Encodes Diverse and Specific Sensory, Motor, and Reward Events. J. Neurophysiol. 2009;102:3026–3037. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumajogee P, Vergé D, Darmon M, Brisorgueil M-J, Hamon M, Miquel M-C. Rapid up-regulation of the neuronal serotoninergic phenotype by brain-derived neurotrophic factor and cyclic adenosine monophosphate: relations with raphe astrocytes. J. Neurosci. Res. 2005;81:481–487. doi: 10.1002/jnr.20572. [DOI] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:6780–6793. doi: 10.1523/JNEUROSCI.0801-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull. Fac. Fish. HOKKAIDO Univ. 1977;28:57–65. [Google Scholar]
- Tereshchenko Y, Brandewiede J, Schachner M, Irintchev A, Morellini F. Novelty-induced behavioral traits correlate with numbers of brainstem noradrenergic neurons and septal cholinergic neurons in C57BL/6J mice. Behav. Brain Res. 2008;191:280–284. doi: 10.1016/j.bbr.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J. Neurophysiol. 2008;100:1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Trapani JG, Pacentine I, Maeda R, Sheets L, Mo W, Nicolson T. Dopamine Modulates the Activity of Sensory Hair Cells. J. Neurosci. 2015;35:16494–16503. doi: 10.1523/JNEUROSCI.1691-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Gerlai R. Individual differences in activity levels in zebrafish (Danio rerio) Behav. Brain Res. 2013;257:224–229. doi: 10.1016/j.bbr.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KW, Neumeister H, Huffman LS, Kidd CE, Preuss T, Hofmann HA. Serotonergic modulation of startle-escape plasticity in an African cichlid fish: a single-cell molecular and physiological analysis of a vital neural circuit. J. Neurophysiol. 2011;106:127–137. doi: 10.1152/jn.01126.2010. [DOI] [PubMed] [Google Scholar]
- Wolf M, Weissing FJ. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 2012;27:452–461. doi: 10.1016/j.tree.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Liss L, Granato M. Chemical modulation of memory formation in larval zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15468–15473. doi: 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Marsden KC, Bell H, Skinner J, Hayer KE, Hogenesch JB, Granato M. A genome-wide screen identifies PAPP-AA-mediated IGFR signaling as a novel regulator of habituation learning. Neuron. 2015;85:1200–1211. doi: 10.1016/j.neuron.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods IG, Schoppik D, Shi VJ, Zimmerman S, Coleman HA, Greenwood J, Soucy ER, Schier AF. Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:3142–3160. doi: 10.1523/JNEUROSCI.3529-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T, Hannan MC, Burgess HA. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:15205–15215. doi: 10.1523/JNEUROSCI.1019-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–646. doi: 10.1038/nature17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.