Abstract
Steroid 17-hydroxylase 17,20-lyase (cytochrome P450c17, P450 17A1, CYP17A1) catalyzes two major reactions: steroid 17-hydroxylation followed by the 17,20-lyase reactions. The most severe mutations in the cognate CYP17A1 gene abrogate all activities and cause combined 17-hydroxylase/17,20-lyase deficiency (17OHD), a biochemical phenotype that is replicated by treatment with the potent CYP17A1 inhibitor abiraterone acetate. The adrenals of patients with 17OHD synthesize 11-deoxycorticosterone (DOC) and corticosterone but no 19-carbon steroids, similar to the rodent adrenal, and DOC causes hypertension and hypokalemia. Loss of 17,20-lyase activity precludes sex steroid synthesis and leads to sexual infantilism. Rare missense CYP17A1 mutations minimally disrupt 17-hydroxylase activity but cause isolated 17,20-lyase deficiency (ILD), Mutations in the POR gene encoding the required cofactor protein cytochrome P450-oxidoreductase causes a spectrum of disease from ILD to 17OHD combined with 21-hydroxylase and aromatase deficiencies, sometimes including skeletal malformations. Mutations in the CYB5A gene encoding a second cofactor protein cytochrome b5 also selectively disrupt 17,20-lyase activity and cause the purest form of ILD. The clinical manifestations of these conditions are best understood in the context of the biochemistry of CYP17A1.
Keywords: hypertension; androgen; mineralocorticoid; 17-hydroxylase/17,20-lyase; 46XY DSD; infertility; primary amenorrhea
Graphical Abstract
1. Physiology, genetics, and biochemistry of CYP17A1
1.1. Physiology
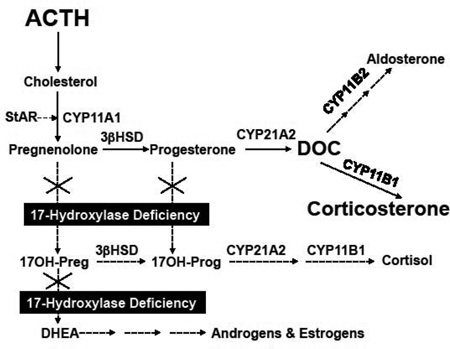
All vertebrates that exhibit sexual dimorphism and reproduction require the 17,20-lyase activity of CYP17A1 to synthesize 19-carbon androgens and subsequently 18-carbon estrogens (Figure 1A). CYP17A1 genes are expressed in the gonads of all these organisms for this purpose. Zebrafish [1] and trout [2] contain 2 CYP17A1 genes, which are both expressed under different regulation, and 1 enzyme has only 17-hydroxylase activity while the other also has 17,20-lyase activity. Based on its location in the steroidogenic pathways, CYP17A1 is the exclusive gateway to sex steroid production. As will be explored below, the substrates for the 17,20-lyase reaction are 17-hydroxysteroids—the products of the 17-hydroxylase reaction, which CYP17A1 also catalyzes. In fact, the 17-hydroxylase activity is only required in animal physiology to generate intermediates for subsequent conversion to androgens. For example, rodents express CYP17A1 only in the gonads but not in the adrenal glands. Rats and mice produce corticosterone as their dominant glucocorticoid rather than cortisol for this reason (Figure 1B). Thus, the 17-hydroxylase reaction would be completely dispensable if CYP17A1 could generate 17-ketosteroids directly from 17-deoxypregnanes such as pregnenolone.
Figure 1.
Major pathways of adrenal steroid biosynthesis. Panel A shows the pathways in the normal human adrenal, and panel B shows altered pathways in 17OHD. Dashed arrows show minor or reduced pathways, and size of text indicates relative abundance for cortisol, aldosterone, androgens and estrogens, corticosterone, and DOC (11-deoxycorticosterone).
Nevertheless, the balance of enzyme activities and substrate preferences in the adrenal varies amongst species, as do sensitivities of their nuclear hormone receptors for various steroids, plasma steroid binding capacities, and pathways of steroid catabolism. As a result, human beings need adrenal 17-hydroxylase activity to produce cortisol and to maintain glucocorticoid and mineralocorticoid homeostasis. Based on this analysis, complete deficiency of CYP17A1, like all forms of congenital adrenal hyperplasia, features both consequences of hormone deficiency—what is lacking after the block—and hormone excess—what accumulates upstream of the block. The hormone deficiency is really only the gonadal component, lack of androgens and estrogens, which causes sexual infantilism and pubertal failure. The absence of 17,20-lyase activity in the adrenal results in deficiency of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), which prevents adrenarche and the development of pubic and axillary hair—not a significant matter in health and bodily function.
The lack of adrenal 17-hydroxylase activity, however, forces steroidogenesis to corticosterone rather than cortisol via 11-deoxycorticosterone (DOC), which in human beings is normally a very minor adrenal product. DOC, however, is a mineralocorticoid, which is slightly less potent than aldosterone. In the face of complete 17-hydroxylase deficiency (17OHD), nascent pregnenolone is converted to progesterone and then to DOC and corticosterone. Circulating corticosterone rises from typical concentrations of <400 ng/dL (~10 nM) to nearly 40,000 ng/dL (~1 µM), which adequately substitutes for cortisol for supplying glucocorticoid activity, even if >90% is protein-bound (Table 1). In parallel, circulating DOC concentrations rise from <20 ng/dL (~0.6 nM) to >300 ng/dL (~10 nM), which saturates the mineralocorticoid receptor under most circumstances. Consequently, adrenal 17OHD does not really result in glucocorticoid deficiency despite the lack of cortisol synthesis, but the important physiologic disturbance is low-renin hypertension from DOC excess.
Table 1.
Steroid changes in combined 17-hydroxylase/17,20-lyase deficiency
| Steroid | Normal Adult Range | 17OHD |
|---|---|---|
| Progesterone (ng/mL, follicular phase) | <0.2 | 2–40 |
| 17-Hydroxyprogesterone (ng/dL) | 50–200 | 10–100 |
| 11-Deoxycorticosterone (ng/dL) | <20 | 100–1000 |
| Corticosterone (ng/dL) | 100–800 | 4,000–40,000 |
| 11-Deoxycortisol (ng/dL) | 10–160 | <5 |
| Cortisol (µg/dL) | 2–25 | <2 |
| DHEAS (µg/dL, young adult) | 100–400 | <10 |
| Aldosterone (ng/dL) | 2–10 | <5 |
| Androstenedione (ng/dL) | 25–250 | <50 |
| Testosterone (ng/dL) | ||
| 46,XX | 10–50 | <20 |
| 46,XY young adult | 400–900 | <20 |
| Estradiol (pg/mL) | ||
| 46,XX follicular phase | 40–100 | <20 |
| 46,XY | 10–40 | <20 |
1.2. Genetics
The human CYP17A1 gene is located on chromosome 10q24.3 [3], spans 6.6 kb, and contains eight exons [4]. An identical 2.1 kb mRNA is transcribed from this gene in the both the adrenals and gonads [5]. From the 1.6 kb coding region, a 57 kDa polypeptide is translated. The protein resides in the smooth endoplasmic reticulum with the flavoprotein cofactor P450-oxidoreductase (POR). The enzyme system of CYP17A1 and POR catalyzes both the 17-hydroxylase and 17,20-lyase activities [6]. In cells with high 17,20-lyase activity, cytochrome b5 (b5) is also present with CYP17A1 and POR in the endoplasmic reticulum [7]. Collectively, POR and b5 are known as “redox partners,” since both are electron-transfer proteins. The importance of b5 in activating maximal 17,20-lyase activity will be discussed later.
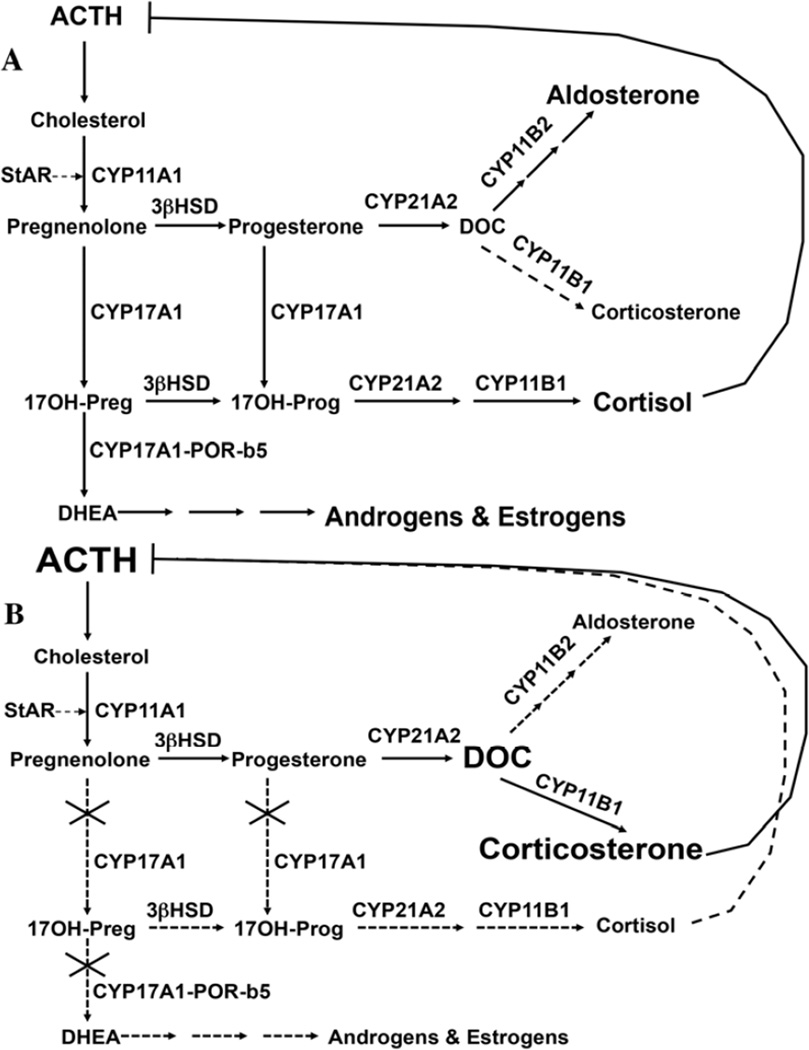
Over 100 mutations in the CYP17A1 gene have been associated with combined 17-hydroxylase/17,20-lyase deficiency (OMIM 202110), including point mutations, small insertions or deletions, splice site alterations, and rarely large deletions (Figure 2A). Although these mutations can be found throughout the gene, many occur near the C-terminus, emphasizing the importance of even the last 14 amino acids for enzyme activity. Splice site mutations can lead to “exon skipping” and truncated, inactive protein [8, 9]. Some frameshift mutations introduce premature stop codons, which also yield truncated proteins. The most commonly mutated residues include Y329 (to D, X, or frameshift TAC→AA with 418X), R362 (to C or H), and H373 (to L, N, or D) in exon 6; W406 (to R) in exon 7; and deletion of D487-S488-F489 or a CATC duplication within D487-S488 in exon 8. For some patients with a clinical and hormonal diagnosis of 17OHD, no CYP17A1 mutations have been identified [10]. Cases of incomplete 17-hydroxylase deficiency combined with partial 21-hydroxylase deficiency can result from mutations in POR, but the biochemical and phenotypic spectrum of POR deficiency can be quite variable [11, 12].
Figure 2.
Cartoon of CYP17A1 gene showing the location of common mutations and mutations causing isolated 17,20-lyase deficiency. Exons are shown as numbered rectangles connected by introns as solid horizontal line and are approximately drawn to scale. Mutations found in isolated 17,20-lyase deficiency are shown below the gene cartoon.
In Brazil, CYP17A1 deficiency appears to be the second most common cause of congenital adrenal hyperplasia, due to founder mutations R362C and W406R [13]. In a large series from China, the aforementioned Y329 frameshift and D487-F489 deletions accounted for >80% of the affected alleles in 26 affected individuals [14]. These positions appear to be mutational “hot spots,” as the same mutations have been identified in other ethnic groups in Asia [15] and elsewhere. A duplication of four nucleotides at amino acid 478, which induces a frameshift and premature stop codon, has been found in Dutch Frieslanders and in Canadian Mennonites [16], and a phenylalanine 53 deletion has been identified in Japan and elsewhere [17].
A special case of CYP17A1 dysfunction is isolated 17,20-lyase deficiency (ILD, Figure 2B). In these rare cases, missense mutations preferentially impair 17,20-lyase activity and leave 17-hydroxylase activity largely unaffected. Mutations R347H or C and R358Q map to the enzyme surface, and these mutations impair interactions of CYP17A1 with POR and more importantly with b5 [18–21], thus explaining a selective deficiency of 17,20-lyase activity. Mutation E305G is located in the active site and also preferentially impairs the 17,20-lyase activity [22], yet homozygous patients bearing this mutation show some biochemical but not clinical evidence of 17-hydroxylase impairment, with increased excretion of DOC and corticosterone metabolites [23]. POR mutation G539R causes ILD, which is clinically and biochemically a phenocopy of ILD caused by CYP17A1 mutations [24, 25].
1.3. Biochemistry
Like all cytochrome P450 enzymes, CYP17A1 follows a catalytic cycle, including 2 one-electron transfers from reduced nicotinamide adenine dinucleotide phosphate (NADPH) via POR, binding of substrate and oxygen, formation of the reactive heme-iron complex with oxygen in concert with O-O bond scission and water release, and finally substrate oxidation [26]. The co-existence of two major activities in one enzyme was first demonstrated with the co-purification of 17-hydroxylase and 17,20-lyase activities from neonatal porcine testes [27]. Purified CYP17A1 disproportionately loses 17,20-lyase activity during purification as b5 is removed from the system, and addition of a stoichiometric amount of b5 restores the lost 17,20-lyase activity [28–30]. The effect of b5 is not subtle—a 10-fold increase in 17,20-lyase activity for 17-hydroxypregnenolone (17Preg) and 17-hydroxyprogesterone (17OHP) substrates [31] or a 3-fold stimulation with 5α–pregnane-3α,17α–diol-20-one, the best 17,20-lyase substrate for human CYP17A1 [32]. The importance of the b5 effect is illustrated in patients with mutations in the CYB5A gene, which causes the purest form of isolated 17,20-lyase deficiency [33, 34].
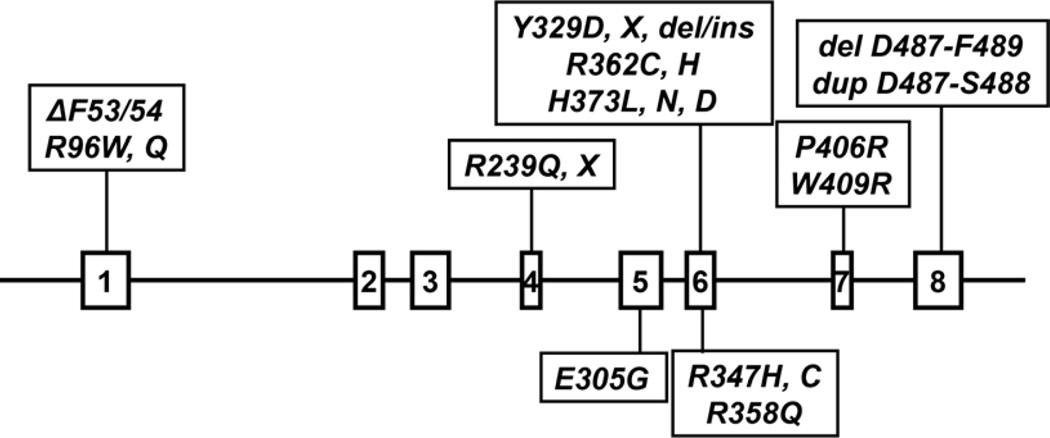
CYP17A1 from various species all appear to 17-hydroxylate both pregnenolone and progesterone with comparable efficiencies, and this property is true for the human enzyme [30, 31]. Human CYP17A1 also 16α–hydroxylates 20–25% of progesterone but not pregnenolone [35], and the presence of A rather than L at residue 105 allow for this high proportion of 16α–hydroxylation [36]. With progesterone substrate, human CYP17A1 also affords <1% DOC—the 21-hydroxylation product, and this fraction can be increased by deuterium incorporation at C-17 [37]. The x-ray crystal structures of modified wild-type human CYP17A1 with bound inhibitors [38] and mutation A105L with bound substrates [39] help to explain the diverse chemistry in this enzyme. The steroid rests against the I-helix perpendicular to the heme ring with the steroid D-ring closest to the heme iron and the A-ring pointing away to the center of the enzyme (Figure 3A). In this orientation, the hydrogen atoms on carbon atoms 16, 17, and 21 are all within the minimum distance to react with the iron-oxygen species, and the order of reactivity parallels the stability of carbon-centered radicals formed during catalysis [26, 40].
Figure 3.
Steroid-binding pocket in human CYP17A1. Images demonstrate proximity of hydrogen atoms at C-16, C-17, and C-21 of progesterone to heme ring (A) and hydrogen bonding (dots) of A-ring oxygen to side-chain of N202 in 17-hydroxypregnenolone (B) or 17-hydroxyprogesterone (C). Images were generated from the X-ray crystal structures of CYP17A1 mutation A105L with bound steroids (pdbid numbers 4NKX, 4NKY, 4NKZ) with program PyMol.
In contrast to the similar activities with 17-deoxypregnanes for the 17-hydroxylase reaction, CYP17A1 from various species often show strong preferences among 17-hydroxysteroid substrates for the 17,20-lyase reaction. Human CYP17A1 catalyzes the 17,20-lyase reaction approximately 50-times more efficiently with 17Preg substrate than with 17OHP, and b5 markedly stimulates both reactions [30, 31]. As a consequence, the human adrenal zona reticularis produces large amounts of DHEA, which is sulfated and circulates as DHEAS [41]. In the x-ray crystal structures of CYP17A1 mutation A105L with 17Preg (Figure 3B) or 17OHP (Figure 3C), the A-ring hydroxyl- or keto-group form a hydrogen bond with the carbonyl oxygen or amide hydrogen of N202, respectively [39]. Despite slightly different positioning of the steroid D-rings, these structures alone do not explain the difference in reactivity [26].
2. Clinical Presentation and Diagnosis
2.1. Clinical Presentation
Severe mutations of CYP17A1 cause complete 17OHD, which disrupts steroidogenesis in both the adrenals and the gonads. The production of both androgens and estrogens requires the 17,20-lyase activity of CYP17A1 and the availability of 17-hydroxysteroid substrates for this reaction, which are exclusive products of CYP17A1 as well. Thus, pubertal failure is one of the major features of 17OHD. In addition, individuals with both 46,XX and 46,XY karyotypes will have female external genitalia from absent testosterone (T) and dihydrotestosterone (DHT) synthesis in fetal life, but the 46,XY individuals will not have internal Müllerian structure, due to preservation of anti-Müllerian hormone from the testes. Occasionally, the 46,XY children are identified due to inguinal hernia or discrepancy between external (female) genitalia and chromosomal sex obtained from amniocentesis, performed for other reasons.
The second major feature of 17OHD derives from the adrenal enzyme deficiency. Unlike all other forms of congenital adrenal hyperplasia, infants are not glucocorticoid deficient, even though their cortisol production is low. In the absence of adrenal 17-hydroxylase activity, corticosterone accumulates and substitutes for cortisol, similar to the physiology of the rodent adrenal. Consequently, adrenal crisis is very rare in 17OHD, and children escape diagnosis until adolescence for this reason. Instead, the precursor DOC accumulates, but manifestations of mineralocorticoid excess tend not to occur in infancy because the newborn kidney is rather insensitive to mineralocorticoids. Gradually and typically in adolescence, DOC excess causes hypertension and hypokalemia. Thus, the most common presentation of 17OHD is an adolescent girl without secondary sexual characteristics or menses and low-renin hypertension [42, 43]. The hypokalemia can be severe and cause muscle cramps or frank tetany, which can be the presenting symptom complex. In addition, like other forms of mineralocorticoid-mediated hypertension, the blood pressure is resistant to common antihypertensive agents yet responds well to mineralocorticoid-receptor antagonists (MRA) such as spironolactone.
In the differential diagnosis, 17OHD patients with 46,XX karyotypes resemble Turner syndrome with Müllerian structures and absent secondary sexual characteristics; however, the 17OHD patients lack the other Turner stigmata (lymphedema, wide carrying angle, cardiac defects) and are typically normal height or tall rather than short. The 17OHD patients with 46,XY karyotype somewhat resemble complete androgen insensitivity syndrome due to the blind vaginal pouch without Müllerian structures or body hair, but the 17OHD patients fail to feminize spontaneously and will respond to androgens if administered. Also on the differential diagnosis is P450-oxidoreductase deficiency (PORD), which itself has partial but variable deficiency of 17-hydroxylase and 17,20-lyase activities. Because of overlapping hormonal abnormalities in PORD and 17OHD, the two conditions can be difficult to distinguish in 46,XY individuals (Table 2). In contrast, PORD patients with 46,XX karyotypes often show inappropriate masculinization for complex biochemical reasons. Additional clues to PORD include elevated 17OHP and 21-deoxycortisol as in 21-hydroxylase deficiency, the presence of Antley-Bixler syndrome malformations in some but not all cases, and maternal virilization during pregnancy.
Table 2.
Differential diagnosis and distinguishing features
| Infant | |||||
|---|---|---|---|---|---|
| 46,XX/45,X | 17OHD | Turner | PORD | ||
| Turner Stigmata | Absent | Present | Absent | ||
| Skeletal Anomolies | Absent | Absent | ±Present | ||
| Genitalia | Prader 1 | Prader 1 | Prader 1–4 | ||
| Gonadotropins | High | High | High | ||
| 17OHP | Low/Nl | Nl | High | ||
| 46,XY | 17OHD | AIS/5αRD | PORD | 17βHSD3D | Gon Dys |
| Skeletal Anomolies | Absent | Absent | ±Present | Absent | Absent |
| Genitalia | Prader 1–3 | Prader 1–2 | Prader 2–4 | Prader 1–3 | Prader 1–2 |
| 17OHP | Low/Nl | Nl | High | Nl | Nl |
| Testosterone | Low | Nl Male | Low | Low | Low |
| Androstenedione | Low | Nl Male | Low | High | Low |
| Cortisol | Low | Nl | Low | Nl | Nl |
| DOC | High | Nl | Nl | Nl | Nl |
| Corticosterone | High | Nl | Nl or High | Nl | Nl |
| Adolescent & Adult | |||||
| 46,XX/45,X | 17OHD | Turner/GonDys | PORD | ||
| Blood Pressure | High | Nl or High | Nl | ||
| Stature | Nl or Tall | Short or Nl | Nl | ||
| Estradiol | Low | Low | Low | ||
| Cortisol | Low | Nl | Low | ||
| DOC | High | Nl | Nl | ||
| 46,XY | 17OHD | AIS | 17βHSD3D/5αRD | Gon Dys | |
| Blood Pressure | High | Nl | Nl | Nl | |
| Pubertal Virilization | Absent | Absent/Partial | Present | Absent | |
| Testosterone | Low | Nl Male | Low/Low Nl | Low | |
| Cortisol | Low | Nl | Nl | Nl | |
| DOC | High | Nl | Nl | Nl | |
Unlike most other forms of congenital adrenal hyperplasia, a true nonclassic form of 17OHD has not been well defined – meaning normal cortisol production and prenatal sexual development but discernable genetic and biochemical abnormalities with subtle clinical manifestations. One could speculate that such patients might have what would appear to be low-renin “essential” hypertension with low aldosterone, but because DOC is rarely measured in clinical practice, a genetic condition would not be suspected. The closest case so far described is a child with mild undervirilization, normal blood pressure, and biochemical evidence of partial 17OHD [44]. This child is a compound heterozygote for a frameshift mutation in R36, creating a stop codon at residue 107, and W121R, which shows 60% 17-hydroxylase and 16% 17,20-lyase activity of the wild-type enzyme. Indeed, the rs1004467 polymorphism in the CYP17A1 gene has been identified as a susceptibility allele for hypertension in several studies [45, 46], but this single-nucleotide polymorphism does not change the coding region of the enzyme. Females with mild 17OHD might have irregular menses and subfertility, while affected males might have low-normal testosterone with slightly elevated gonadotropins and possibly oligospermia.
Additional manifestations of 17OHD include ovarian cysts and cyst rupture in 46,XX patients [47, 48]. The mechanism of cyst formation is thought to be chronically elevated gonadotropins without an estrogen-triggered ovulatory surge. Occasionally, 46,XX patients with 17OHD have spontaneous but irregular menses [49, 50]. At this time, there are no published examples conclusively documenting a successful pregnancy in which either partner has 17OHD. Cases of 46,XX women with 17OHD have been described in whom embryos were obtained after adrenal-derived progesterone suppression with dexamethasone prior to superovulation with gonadotropins, oocyte retrieval, and in vitro fertilization, but these embryos have not afforded live births [51].
Boys with isolated 17,20-lyase deficiency are usually ascertained at birth because of impaired virilization [18, 22, 52], and girls with this condition have been identified as siblings of affected boys [52] or during evaluation of primary amenorrhea without hypertension [53]. The boys show varying degrees of poor genital development with hypospadias, bifid scrotum, and micropenis. Both girls and boys with this condition do not progress normally through puberty and are likewise infertile.
2.2. Diagnosis
The two scenarios most commonly encountered in which the diagnosis of 17OHD is entertained include the infant with 46,XY karyotype and female or ambiguous genitalia and inguinal or abdominal testes, or the adolescent girl with primary amenorrhea and absent secondary sexual characteristics with hypertension and hypokalemia [42]. In the first case, the infant will have had the karyotype and initial laboratory findings of low T with high gonadotropins. At that point, androgen insensitivity and 5α–reductase deficiency are essentially excluded, so the differential diagnosis primarily includes various forms of gonadal dysgenesis, and 17β–hydroxysteroid dehydrogenase type 3 deficiency. Cosyntropin stimulation testing reveals the adrenal steroidogenic defect: low cortisol, DHEA, and 17OHP but high DOC and corticosterone [13, 54]. As in all cases of enzyme deficiency, the most informative tests are the high analytes above the block, particularly corticosterone. Progesterone is also above the block and is high in 17OHD [55] and even higher in PORD [56, 57], but low 17OHP and very high DOC distinguishes 17OHD from PORD [58]. Finally, the other hypertensive form of congential adrenal hyperplasia is 11-hydroxylase deficiency (11OHD), because DOC also accumulates, but 11-deoxycortisol is low in 17OHD and high in 11OHD. Androgens are also elevated in 11OHD but low in 17OHD [13, 59].
For the adolescent girls with primary amenorrhea, the initial evaluation will suggest a form of gonadal dysgenesis: high gonadotropins, low T, and low estradiol (E2), regardless of karyotype. The 46,XY cases will also lack a uterus and might have palpable testes in the inguinal regions. While the focus in these cases is on the gonads and reproductive development, the critical step in making the correct diagnosis is the consideration of a simultaneous defect in adrenal steroidogenesis, which is hinted in the majority of cases from the presence hypertension and/or hypokalemia. Steroid analysis, basal and after cosyntropin stimulation, will reveal the biosynthesis defect as described for infants.
The diagnosis of isolated 17,20-lyase deficiency is primarily considered for newborn boys with undervirilization. The presumptive diagnosis of gonadal dysgenesis will be consistent with initial laboratory tests, which show low AD and T and often high gonadotropins. Among the distinguishing laboratory features is the elevated 17OHP/AD ratio at baseline or with hCG stimulation, which is typically >50 in affected cases [18]. A second clue to the diagnosis in the neonate is a low DHEAS, which usually >100 µg/dL but falls sharply after birth [41]. The low DHEAS indicates both an adrenal and gonadal defect and focuses the differential diagnosis on enzymes common to both glands, including CYP17A1. For girls, isolated 17,20-lyase deficiency is a very rare cause of pubertal failure but without hypertension as in 17OHD [53].
2.3 Abiraterone acetate and pharmacologic induction of 17OHD
The prostate gland requires androgens for its formation and growth, and prostate cancer likewise demonstrates androgen dependence in most cases. For this reason, surgical or medical castration has been used for decades to treat this disease, at least in the initial stages. Currently, the standard of care in metastatic disease is testicular suppression with long-acting gonadotropin-releasing hormone agonists or antagonists, which often induces remission or stable disease for months to years [60]. When the disease progresses despite therapy, the condition is called castration-resistant prostate cancer (CRPC), and evidence from the past decade has shown that traces of residual androgens are primarily responsible for disease progression [61, 62]. Because CYP17A1 activities are the essential for all biochemical pathways of androgen synthesis, CRPC has been treated with additional means to suppress these androgens, such as dexamethasone to suppress adrenal-derived androgens and ketoconazole, an inhibitor of multiple cytochrome P450 enzymes including CYP17A1 [63]. To specifically target CYP17A1, selective inhibitors of this enzyme have been developed, including abiraterone [64], given orally as the acetate [65], and galeterone. Abiraterone is a very potent active site-directed inhibitor, with a binding affinity of 1–3 nM for purified CYP17A1 [66].
Administration of abiraterone to men with CRPC with continued medical castration suppresses testosterone from a baseline of 5–20 ng/dL to <0.1 ng/dL in the majority of cases [65]. In the phase I and II trials, abiraterone was given without glucocorticoids, and the potent CYP17A1 inhibition caused DOC and corticosterone to rise dramatically to concentrations found in 17OHD [65]. As a consequence, many men developed hypertension and hypokalemia, which was successfully treated with the selective mineralocorticoid-receptor antagonist eplerenone [67]. When dexamethasone was added to abiraterone, DOC and corticosterone fell back to baseline values. Abiraterone is indicated for use in CRPC in combination with glucocorticoids, typically prednisone, prednisolone, or dexamethasone to prevent the ACTH-driven mineralocorticoid excess seen in the phase I and II trials [63]. These data confirm that the hypertension and hypokalemia of 17OHD is derived from DOC and corticosterone accumulation. More importantly, this pharmacologic cause of 17OHD is now far more common than CYP17A1 mutations, and these patients are elderly males unlike typical cases of genetic 17OHD.
3. Treatment
3.1 Glucocorticoids
As discussed above, high corticosterone production compensates for cortisol deficiency, and as a result, patients with 17OHD do not experience clinical glucocorticoid insufficiency and rarely if ever experienced adrenal crises. Consequently, corticosteroid replacement is not essential, and pharmacologic glucocorticoids therapy can induce adrenal axis suppression with the risk of failure to mount an appropriate augmentation in corticosterone production during acute illness. Furthermore, chronic glucocorticoid therapy in Addison’s disease [68] and 21-hydroxylase deficiency [69, 70] is associated with deleterious impairments in bone health and cardiovascular risk factors. Nevertheless, glucocorticoid administration will lower DOC production and normalize blood pressure and potassium as observed in patients taking abiraterone. As a compromise, partial or incomplete replacement will substantially reduce but not normalize DOC yet mitigate long-term consequences of glucocorticoid therapy. Adjunctive treatments are likely to be necessary in this case, using mineralocorticoid antagonists as described in the next section, as glucocorticoid therapy alone might not achieve good blood pressure control [71].
3.2 Mineralocorticoid receptor antagonists and antihypertensives
All forms of ACTH-dependent mineralocorticoid excess respond well to mineralocorticoid receptor antagonist therapy, because a rise in renin due to treatment does not enhance production of the pathogenic steroid. In 17OHD, spironolactone at 50–200 mg/d given in 1 or 2 divided doses is often ideal therapy. Because these patients are phenotypically female, the typical side effects seen in man, including gynecomastia and erectile dysfunction, are not an issue. Breast tenderness can occur with spironolactone, and 46,XX individuals might experience vaginal spotting due to progesterone antagonism. Eplerenone is a selective mineralocorticoid receptor antagonist, given at similar doses to spironolactone as an alternative. Potassium-sparing diuretics such as amiloride, 5–20 mg/d, are very effective in controlling the hypokalemia but are not as effective as mineralocorticoid receptor antagonists in controlling the blood pressure. Furthermore, mineralocorticoid receptor antagonists directly block mineralocorticoid actions on target tissue such as the heart and kidney, preventing end-organ damage. For example, a combination regimen might be hydrocortisone 10 mg every morning with spironolactone 50 mg once or twice daily. Such a regimen should normalize blood pressure and potassium without inducing adrenal axis suppression and the potential for adrenal crisis during illness.
Blood pressure and serum potassium should be the two major parameters for titrating therapy, particularly in the initial months. Plasma renin activity can be used as a surrogate readout of mineralocorticoid receptor antagonism, but only in the chronic phase, as renin might not rise for months to years following chronic suppression [71, 72]. Additional agents for blood pressure control include calcium channel blockers such as amlodipine 2.5–10 mg per day, which has the main side effect of peripheral edema.
3.3 Estrogen replacement, progestin withdrawal, androgen supplementation, and surgery
For the typical patient with 17OHD who is phenotypically female and associates with the female gender, estrogen replacement is initiated at an appropriate time during adolescence or upon diagnosis if an adult. The trend in recent years has been to use estradiol for estrogen replacement, either oral or transdermal. Similar to inducing secondary sexual characteristics for other disorders, the initial dose should be very low, with gradual increases to a full adult dose. For example, oral estradiol is initiated at 0.5 mg/d for several months and advanced to 1–2 mg/d over 1–3 years, or transdermal is started at 25 µg/d and gradually advanced to 75–100 µg/d. For patients with an intact uterus, progestin withdrawal may be induced with 5–10 days of micronized progesterone 100–200 mg, medroxyprogesterone acetate 5–10 mg, or norethindrone acetate 2.5–5 mg. Note that absence of withdrawal bleeding does not necessarily signify inadequate estrogen exposure but might derive from persistently high adrenal-derived progesterone as in other forms of CAH. For this reason, transient intensification of glucocorticoid therapy at the completion of the progestin course might be necessary every 1–3 months to prevent endometrial overgrowth.
For the rare 46, XY individual with partial 17OHD reared as a male, androgen replacement is typically necessary, as testicular testosterone synthesis will not rise sufficiently during puberty. At the age of expected puberty, intramuscular testosterone enanthate or cypionate is administered at 50 mg per month with dose escalations every 3–6 months to an average adult dose of about 200 mg every 2 weeks. Testosterone undecanoate is now available in several countries for administration every 10–12 weeks. Testosterone undecanoate has the advantage of very stable serum testosterone concentrations with infrequent administration, but the volume of drug is large, and the agent must be administered under medical supervision. For chronic therapy, transdermal hydroalcoholic 1–2% testosterone gels have become popular at doses of 25–100 g/d depending on preparation and absorption. I have used oral DHEA, 25 mg/d, to induce pubertal hair development in a woman with 17OHD over the period of 2 years to provide her a more normal appearance. Alternatively, a non-aromatizable androgen such as oxandrolone 1–10 mg/d allows for separate titration of estrogen dose.
Surgery of the external genitalia is rarely necessary, except for the 46,XY individuals with mild disease who might need repair of hypospadias. More commonly, 46,XX individuals with 17OHD might need surgery for ruptured or painful ovarian cysts. It is unclear whether can gonadotropin suppression with GnRH agonists should be advised to prevent this sequela.
Highlights.
CYP17A1 has 17-hydroxylase and 17,20-lyase activities
Severe 17-hydroxylase deficiency presents with hypertension and pubertal failure
High 11-deoxycorticosterone causes hypertension in 17-hydroxylase deficiency
High corticosterone production prevents adrenal crisis in 17-hydroxylase deficiency
Abiraterone acetate therapy causes pharmacologic 17-hydroxylase deficiency
Acknowledgments
I thank Dr. Hwei-Ming Peng for assistance with preparing Figure 3. This work was supported by grant R01GM086596 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pallan PS, Nagy LD, Lei L, Gonzalez E, Kramlinger VM, Azumaya CM, Wawrzak Z, Waterman MR, Guengerich FP, Egli M. Structural and kinetic basis of steroid 17α,20-lyase activity in teleost fish cytochrome P450 17A1 and its absence in cytochrome P450 17A2. J Biol Chem. 2015;290:3248–3268. doi: 10.1074/jbc.M114.627265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou LY, Wang DS, Kobayashi T, Yano A, Paul-Prasanth B, Suzuki A, Sakai F, Nagahama Y. A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology. 2007;148:4282–4291. doi: 10.1210/en.2007-0487. [DOI] [PubMed] [Google Scholar]
- 3.Matteson KJ, Picado-Leonard J, Chung B, Mohandas TK, Miller WL. Assignment of the gene for adrenal P450c17 (17α–hydroxylase/17,20 lyase) to human chromosome 10. J Clin Endocrinol Metab. 1986;63:789–791. doi: 10.1210/jcem-63-3-789. [DOI] [PubMed] [Google Scholar]
- 4.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene encoding P450c17 (steroid 17α–hydroxylase/17,20 lyase): Similarity to the gene for P450c21. DNA. 1987;6:439–448. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 5.Chung BC, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shively JE, Miller WL. Cytochrome P450c17 (steroid 17α–hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci U S A. 1987;84:407–411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuber MX, Simpson ER, Waterman MR. Expression of bovine 17α–hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS-1) cells. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 8.Costa-Santos M, Kater CE, Dias EP, Auchus RJ. Two intronic mutations cause 17- hydroxylase deficiency by disrupting splice acceptor sites: direct demonstration of aberrant splicing and absent enzyme activity by expression of the entire CYP17 gene in HEK-293 cells. J Clin Endocrinol Metab. 2004;89:43–48. doi: 10.1210/jc.2003-031020. [DOI] [PubMed] [Google Scholar]
- 9.Hwang DY, Hung CC, Riepe FG, Auchus RJ, Kulle AE, Holterhus PM, Chao MC, Kuo MC, Hwang SJ, Chen HC. CYP17A1 intron mutation causing cryptic splicing in 17α- hydroxylase deficiency. PLoS One. 2011;6:e25492. doi: 10.1371/journal.pone.0025492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolthers OD, Rumsby G, Techatraisak K, Honour JW, Hindmarsh PC. 17-Hydroxylase/17,20 Lyase Deficiency Diagnosed during Childhood. Horm Res. 2002;57:133–136. doi: 10.1159/000057964. [DOI] [PubMed] [Google Scholar]
- 11.Miller WL. P450-oxidoreductase deficiency: A new disorder of steroidogenesis with multiple clinical manifestations. Trends Endocrinol Metab. 2004;15:311–315. doi: 10.1016/j.tem.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Sun S, Liu Y, Zhang H, Jiao Y, Wang W, Li X. New, recurrent, and prevalent mutations: Clinical and molecular characterization of 26 Chinese patients with 17α- hydroxylase/17,20-lyase deficiency. J Steroid Biochem Mol Biol. 2015;150:11–16. doi: 10.1016/j.jsbmb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Fardella CE, Zhang LH, Mahachoklertwattana P, Lin D, Miller WL. Deletion of amino acids Asp487-Ser488-Phe489 in human cytochrome P450c17 causes severe 17α–hydroxylase deficiency. J Clin Endocrinol Metab. 1993;77:489–493. doi: 10.1210/jcem.77.2.8345056. [DOI] [PubMed] [Google Scholar]
- 16.Imai T, Yanase T, Waterman MR, Simpson ER, Pratt JJ. Canadian Mennonites and individuals residing in the Friesland region of the Netherlands share the same molecular basis of 17α–hydroxylase deficiency. Hum Genet. 1992;89:95–96. doi: 10.1007/BF00207050. [DOI] [PubMed] [Google Scholar]
- 17.Miura K, Yasuda K, Yanase T, Yamakita N, Sasano H, Nawata H, Inoue M, Fukaya T, Shizuta Y. Mutation of cytochrome P-45017α gene (CYP17) in a Japanese patient previously reported as having glucocorticoid-responsive hyperaldosteronism: with a review of Japanese patients with mutations of CYP17. J Clin Endocrinol Metab. 1996;81:3797–3801. doi: 10.1210/jcem.81.10.8855840. [DOI] [PubMed] [Google Scholar]
- 18.Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20 lyase deficiency. Nature Genet. 1997;17:201–205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- 19.Geller DH, Auchus RJ, Miller WL. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5 . Mol Endocrinol. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- 20.Naffin-Olivos JL, Auchus RJ. Human cytochrome b 5 requires residues E48 and E49 to stimulate the 17, 20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- 21.Peng HM, Liu J, Forsberg SE, Tran HT, Anderson SM, Auchus RJ. Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5. J Biol Chem. 2014;289:33838–33849. doi: 10.1074/jbc.M114.608919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 23.Tiosano D, Knopf C, Koren I, Levanon N, Hartmann MF, Hochberg Z, Wudy SA. Metabolic evidence for impaired 17α-hydroxylase activity in a kindred bearing the E305G mutation for isolate 17,20-lyase activity. Eur J Endocrinol. 2008;158:385–392. doi: 10.1530/EJE-07-0712. [DOI] [PubMed] [Google Scholar]
- 24.Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab. 2008;93:3584–3588. doi: 10.1210/jc.2008-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller WL. The syndrome of 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97:59–67. doi: 10.1210/jc.2011-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimoto FK, Auchus RJ. The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1) J Steroid Biochem Mol Biol. 2015;151:52–65. doi: 10.1016/j.jsbmb.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajin S, Shively JE, Yuan P, Hall PF. Microsomal cytochrome P450 from neonatal pig testis: Two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry. 1981;20:4037–4042. doi: 10.1021/bi00517a014. [DOI] [PubMed] [Google Scholar]
- 28.Onoda M, Hall PF. Cytochrome b 5 stimulates purified testicular microsomal cytochrome P450 (C21 side-chain cleavage) Biochem Biophys Res Commun. 1982;108:454–460. doi: 10.1016/0006-291x(82)90850-6. [DOI] [PubMed] [Google Scholar]
- 29.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b 5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Robichaud P, Akhtar ME, Akhtar M. Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b 5 . Biochem J. 1998;332:293–296. doi: 10.1042/bj3320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auchus RJ, Lee TC, Miller WL. Cytochrome b 5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 32.Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418:151–160. doi: 10.1016/j.abb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010;95:994–999. doi: 10.1210/jc.2008-1745. [DOI] [PubMed] [Google Scholar]
- 34.Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, Krone N, Arlt W. A missense mutation in the human cytochrome b 5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97:E465–E475. doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swart P, Swart AC, Waterman MR, Estabrook RW, Mason JI. Progesterone 16α-hydroxylase activity is catalyzed by human cytochrome P450 17α-hydroxylase. J Clin Endocrinol Metab. 1993;77:98–102. doi: 10.1210/jcem.77.1.8325965. [DOI] [PubMed] [Google Scholar]
- 36.Swart AC, Storbeck KH, Swart P. A single amino acid residue, Ala 105, confers 16α-hydroxylase activity to human cytochrome P450 17α-hydroxylase/17,20 lyase. J Steroid Biochem Mol Biol. 2010;119:112–120. doi: 10.1016/j.jsbmb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimoto FK, Zhou Y, Peng HM, Stidd D, Yoshimoto JA, Sharma KK, Matthew S, Auchus RJ. Minor activities and transition state properties of the human steroid hydroxylases cytochromes P450c17 and P450c21, from reactions observed with deuterium-labeled substrates. Biochemistry. 2012;51:7064–7077. doi: 10.1021/bi300895w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrunak EM, DeVore NM, Porubsky PR, Scott EE. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J Biol Chem. 2014;289:32952–32964. doi: 10.1074/jbc.M114.610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizrachi D, Wang Z, Sharma KK, Gupta MK, Xu K, Dwyer CR, Auchus RJ. Why human cytochrome P450c21 is a progesterone 21-hydroxylase. Biochemistry. 2011;50:3968–3974. doi: 10.1021/bi102078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 42.Biglieri EG, Kater CE. 17α-hydroxylation deficiency. Endocrinol Metab Clin North Am. 1991;20:257–268. [PubMed] [Google Scholar]
- 43.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–119. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 44.Rubtsov P, Nizhnik A, Dedov I, Kalinchenko N, Petrov V, Orekhova A, Spirin P, Prassolov V, Tiulpakov A. Partial deficiency of 17α-hydroxylase/17,20-lyase caused by a novel missense mutation in the canonical cytochrome heme-interacting motif. Eur J Endocrinol. 2015;172:K19–K25. doi: 10.1530/EJE-14-0834. [DOI] [PubMed] [Google Scholar]
- 45.Dai CF, Xie X, Ma YT, Yang YN, Li XM, Fu ZY, Liu F, Chen BD, Gai MT. The relationship between the polymorphisms of the CYP17A1 gene, hypertension: A meta-analysis. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2015 doi: 10.1177/1470320315585683. [DOI] [PubMed] [Google Scholar]
- 46.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roger M, Merceron RE, Girard F, Canlorbe P, Dehennin L, Konopka P, Seneze J, Toublanc JE. Dexamethasone-suppressible hypercorticosteronism in two 46,XX subjects with ambiguous genitalia ovarian cysts Partial defect of 17α-hydroxylase or 17–20-desmolase. Horm Res. 1982;16:23–31. doi: 10.1159/000179481. [DOI] [PubMed] [Google Scholar]
- 48.ten Kate-Booij MJ, Cobbaert C, Koper JW, de Jong FH. Deficiency of 17,20-lyase causing giant ovarian cysts in a girl and a female phenotype in her 46,XY sister: case report. Hum Reprod. 2004;19:456–459. doi: 10.1093/humrep/deh065. [DOI] [PubMed] [Google Scholar]
- 49.Katayama Y, Kado S, Wada S, Nemoto Y, Kugai N, Furuya K, Nagata N. A case of 17a-hydroxylase deficiency with retained menstruation. Endocr J. 1994;41:213–218. doi: 10.1507/endocrj.41.213. [DOI] [PubMed] [Google Scholar]
- 50.Matsuzaki S, Yanase T, Murakami T, Uehara S, Nawata H, Yajima A. Induction of endometrial cycles and ovulation in a woman with combined 17α-hydroxylase/17,20-lyase deficiency due to compound heterozygous mutations on the p45017alpha gene. Fertil Steril. 2000;73:1183–1186. doi: 10.1016/s0015-0282(00)00500-8. [DOI] [PubMed] [Google Scholar]
- 51.Rabinovici J, Blankstein J, Goldman B, Rudak E, Dor Y, Pariente C, Geier A, Lunenfeld B, Mashiach S. In vitro fertilization and primary embryonic cleavage are possible in 17α–hydroxylase deficiency despite extremely low intrafollicular 17 beta-estradiol. J Clin Endocrinol Metab. 1989;68:693–697. doi: 10.1210/jcem-68-3-693. [DOI] [PubMed] [Google Scholar]
- 52.Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. Differential inhibition of 17α–hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab. 2002;87:5714–5721. doi: 10.1210/jc.2001-011880. [DOI] [PubMed] [Google Scholar]
- 53.Simsek E, Ozdemir I, Lin L, Achermann JC. Isolated 17,20-lyase (desmolase) deficiency in a 46,XX female presenting with delayed puberty. Fertil Steril. 2005;83:1548–1551. doi: 10.1016/j.fertnstert.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 54.Kater CE, Biglieri EG. Disorders of steroid 17α–hydroxylase deficiency. Endocrinol Metab Clin North Am. 1994;23:341–357. [PubMed] [Google Scholar]
- 55.Martin RM, Lin CJ, Costa EM, de Oliveira ML, Carrilho A, Villar H, Longui CA, Mendonca BB. P450c17 deficiency in Brazilian patients: biochemical diagnosis through progesterone levels confirmed by CYP17 genotyping. J Clin Endocrinol Metab. 2003;88:5739–5746. doi: 10.1210/jc.2003-030988. [DOI] [PubMed] [Google Scholar]
- 56.Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- 57.Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonca BB, Fujieda K, Miller WL. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 58.Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, Rose IT, O'Neil DM, Vijzelaar R, Smith MJ, MacDonald F, Cole TR, Adolphs N, Barton JS, Blair EM, Braddock SR, Collins F, Cragun DL, Dattani MT, Day R, Dougan S, Feist M, Gottschalk ME, Gregory JW, Haim M, Harrison R, Olney AH, Hauffa BP, Hindmarsh PC, Hopkin RJ, Jira PE, Kempers M, Kerstens MN, Khalifa MM, Kohler B, Maiter D, Nielsen S, O'Riordan SM, Roth CL, Shane KP, Silink M, Stikkelbroeck NM, Sweeney E, Szarras-Czapnik M, Waterson JR, Williamson L, Hartmann MF, Taylor NF, Wudy SA, Malunowicz EM, Shackleton CH, Arlt W. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012;97:E257–E267. doi: 10.1210/jc.2011-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kater CE, Biglieri EG. Distinctive plasma aldosterone, 18-hydroxycorticosterone, and 18-hydroxydeoxycorticosterone profile in the 21-, 17α-, and 11β-hydroxylase deficiency types of congenital adrenal hyperplasia. Am J Med. 1983;75:43–48. doi: 10.1016/0002-9343(83)91166-x. [DOI] [PubMed] [Google Scholar]
- 60.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 61.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mousses S, Wagner U, Chen Y, Kim JW, Bubendorf L, Bittner M, Pretlow T, Elkahloun AG, Trepel JB, Kallioniemi OP. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene. 2001;20:6718–6723. doi: 10.1038/sj.onc.1204889. [DOI] [PubMed] [Google Scholar]
- 63.Auchus ML, Auchus RJ. Human steroid biosynthesis for the oncologist. J Investig Med. 2012;60:495–503. doi: 10.231/JIM.0b013e3182408567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017α (17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 65.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 66.Garrido M, Peng HM, Yoshimoto FK, Upadhyay SK, Bratoeff E, Auchus RJ. A-Ring modified steroidal azoles retain similar potent and slowly reversible CYP17A1 inhibition as abiraterone. J Steroid Biochem Mol Biol. 2014;143:1–10. doi: 10.1016/j.jsbmb.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Attard G, Reid AHM, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 68.Jodar E, Valdepenas MP, Martinez G, Jara A, Hawkins F. Long-term follow-up of bone mineral density in Addison's disease. Clin Endocrinol (Oxf) 2003;58:617–620. doi: 10.1046/j.1365-2265.2003.01761.x. [DOI] [PubMed] [Google Scholar]
- 69.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantero F, Opocher G, Rocco S, Carpene G, Armanini D. Long-term treatment of mineralocorticoid excess syndromes. Steroids. 1995;60:81–86. doi: 10.1016/0039-128x(94)00018-8. [DOI] [PubMed] [Google Scholar]
- 72.BiglieriE G. 17α-Hydroxylase deficiency: 1963–1966. J Clin Endocrinol Metab. 1997;82:48–50. doi: 10.1210/jcem.82.1.3653. [DOI] [PubMed] [Google Scholar]