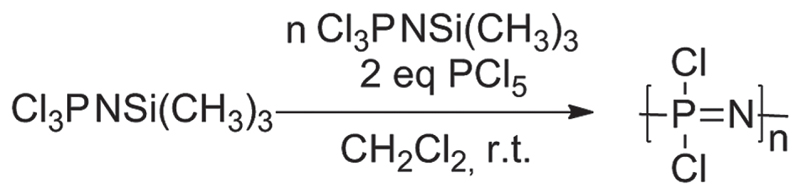Abstract
The preparation of novel macromolecular prodrugs via the conjugation of two platinum(IV) complexes to suitably functionalized poly(organo)phosphazenes is presented. The inorganic/organic polymers provide carriers with controlled dimensions due to the use of living cationic polymerization and allow the preparation of conjugates with excellent aqueous solubility but long-term hydrolytic degradability. The macromolecular Pt(IV) prodrugs are designed to undergo intracellular reduction and simultaneous release from the macromolecular carrier to present the active Pt(II) drug derivatives. In vitro investigations show a significantly enhanced intracellular uptake of Pt for the macromolecular prodrugs when compared to small molecule Pt complexes, which is also reflected in an increase in cytotoxicity. Interestingly, drug-resistant sublines also show a significantly smaller resistance against the conjugates compared to clinically established platinum drugs, indicating that an alternative uptake route of the Pt(IV) conjugates might also be able to overcome acquired resistance against Pt(II) drugs. In vivo studies of a selected conjugate show improved tumor shrinkage compared to the respective Pt(IV) complex.
Keywords: biodegradable, nanomedicine, platinum(IV) prodrugs, polymer therapeutics, polyphosphazenes
1. Introduction
Macromolecular prodrugs, the reversible chemical conjugation of drugs to polymers,[1] are a type of nanomedicine which have gained significant importance in recent years.[2] The major advantage of such polymer-drug conjugates over small drug-molecules is first and foremost the nanometer-sized dimensions (10–100 nm), which can be used to favorably alter biodistribution, for example, hindering renal clearance and extending plasma circulation times.[3] They have shown particular promise in combination with a wide-range of chemotherapeutics, whereby polymer therapeutics can be used to reduce side effects due to their decreased exposure to normal tissue and induce passive tumor targeting via the enhanced permeability and retention effect (EPR effect).[4]
Many proposed drug conjugates consist of nondegradable carbon-based polymers, for example, polyethylene glycols or N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers.[5] A major disadvantage of parenteral administration of such biopersistent polymers with hydrodynamic volumes above the renal clearance limit is, however, their long-term accumulation in the body, leading to symptoms similar to lysosomal storage disease.[6] This is of particular concern with the relatively large, repetitive doses required for the chemotherapeutic application of metal-based therapeutics. As a result, the focus has shifted to (bio)degradable polymers, where higher molecular weight polymers can be administered without long-term accumulation.[7] Alongside degradability, water solubility and multiple binding sites for drugs are essential for the design of macromolecular prodrugs. Furthermore, as size and architecture directly influence biodistribution,[8] precise control of the structure and molecular weight of the polymer also plays a critical role in selection of the macromolecular carrier. One group of polymers that potentially fulfill all these prerequisites are poly(organo)phosphazenes.[9]
Inorganic–organic hybrid poly(organo)phosphazenes consist of an alternating nitrogen and phosphorus atoms backbone, with organic substituents linked to the latter. These substituents play an important role in the subsequent hydrolytic stability of the polymer.[10,11] Thus by careful choice of substituents, the hydrolytic behavior and subsequently the degradation of the polymer can be fine-tuned.[12] This proven, tunable degradability to benign metabolites is an important feature of poly(organo) phosphazenes when considering their use in intravenous drug delivery.[13,14] Indeed, degradable poly(organo) phosphazenes have been investigated for a number of medical applications including photodynamic therapy,[15] tissue engineering[16,17] as well as their use as vaccine adjuvants.[18–21]
cis-Diamminedichloroplatinum(II), or cisplatin, is well known for its anticancer activity.[22] A multitude of derivatives have been developed and tested, first and foremost oxaliplatin and carboplatin, but there is still a need for new platinum drugs to overcome the toxicity and resistance of their predecessors.[23] Indeed, promising preclinical studies of a 25 kDa HPMA-DACH (diaminocyclohexane)platinum(II) conjugates (ProLindac) have shown superior efficacy compared to oxaliplatin, an effect attributed to distinctly enhanced delivery of platinum to the malignant tissue.[24] Conjugates with a variety of Pt(II) complexes have also been shown with other polymers,[25] including the combination with biocompatible and degradable poly(organo)phosphazenes.[26–28]
In recent years, platinum(IV) complexes have also been thoroughly investigated as potential anticancer drugs, since they show some advantages over platinum(II) complexes, for example, their kinetic inertness and the added axial ligands working as either a functional moiety or as a linker.[23] This results in an improved therapeutic index and lower side effects.[29] Systemic toxicity is reduced since platinum(IV) complexes act as prodrugs undergoing intracellular reduction via loss of their axial ligands.[30] However, there are indications that platinum(IV) compounds are not specifically activated only in tumor tissue but also in healthy tissues, especially in presence of hemeproteins.[31–33] Thus, it is of interest to further enhance the tumor targeting of platinum(IV) drugs. Since platinum(IV) complexes are expected to be activated inside the cell by loss of their axial ligands, covalent coupling to macromolecular agents, such as polymers, could be the key to a targeted tumor therapy with reduced adverse effects[34,35] and increased anticancer activity.[36] One approach includes delivering a platinum(IV) complex as a core crosslinker of pegylated methacrylate/polystyrene-based micelles which showed good tumor inhibiting properties.[37] The use of oligonucleotide gold nanoparticles with platinum(IV) complexes has also been reported, accomplishing improved results compared to cisplatin with various types of cancer.[38] Furthermore, it is well known that such macromolecular carriers enter cells via the endocytic route,[39] in which significantly lower pH values are encountered. The use of macromolecular, reducible platinum(IV) conjugates can thus exploit this pathway for a triggered intracellular release.[37,38,40–42]
In this contribution we investigate the coupling of platinum(IV) complexes to Jeffamine-substituted poly(organo)phosphazenes, which offer carriers with long-term hydrolytic degradability,[43] multiple loading sites,[44] as well as high aqueous solubility. Furthermore, contrary to the previously reported Pt(II) poly(organo) phosphazene conjugates, living cationic polymerization was used in an attempt to ensure controlled synthesis and defined macromolecules. Subsequently, the macromolecular prodrugs were tested with regard to their cell uptake, cytotoxicity, and in vivo activity.
2. Experimental Section
2.1. Materials and Methods
The synthesis of the monomer, precursor, and polymer was carried out under argon. Prior to use, all glassware was dried in an oven at 120 °C. The under vacuum-sublimed PCl5 was stored in a glove box under argon. Prior to use, Et3N was distilled and dried over molecular sieves. The mono-boc protection of 2,2′-(ethylenedioxy)-bis-(ethylamine)[45] and the synthesis of the monomer Cl3PNSi(CH3)3[46] were carried out according to previously reported procedures. Jeffamine M-1000 is an amino capped statistical poly(ethylene oxide-co-propylene oxides), PEO-PPO-NH2, donated by Huntsman Performance Products (Netherlands), with an approximate molecular weight of 1000 g mol−1 and an ethylene oxide/propylene oxide ratio of 19/3. K2[PtCl4] was obtained from Johnson Matthey (Switzerland). Dialysis membranes (Spectra/Por 1, 6000–8000 Da) were purchased from SpectrumLabs. Reactions involving platinum(II) agents were performed with glass-coated magnetic stirring bars under light protection; doubly distilled osmosis water was used for reactions in water. All other chemicals were purchased from Sigma Aldrich or Fisher Scientific and used as received.
NMR spectra of the monomer, precursor, and substituted polymer were recorded using a Bruker 300 MHz spectrometer. The signal of CDCl3 was used as internal reference for the 1H NMR measurements. The {1H}31P NMR measurements were carried out at 121 MHz and 85% phosphoric acid was used as external standard. NMR spectra of the final compounds and platinum precursors were recorded using a Bruker FT-NMR Avance III 500 MHz instrument at 500.32 (1H) and 202.44 ({1H}31P) MHz. External quantification of the average platinum content was performed by inductively coupled plasma mass spectrometry (ICP-MS). The digestion of samples was performed with a microwave system Discover SP-D (CEM Microwave Technology, Germany) using 2 mL of 32.5% subboiled nitric acid (≥65%, p.a., Fluka, Buchs, Switzerland). Samples were diluted with Milli-Q water (18.2 MΩ cm, Milli-Q Advantage) resulting in nitric acid concentration lower than 3% and platinum concentrations lower than 15 ng g−1. The total platinum content was determined with an ICP-quadrupole MS instrument Agilent 7500ce (Agilent Technologies, Waldbronn, Germany) equipped with a MicroMist nebulizer at a sample uptake rate of ≈0.25 mL min−1. Platinum and rhenium standards for ICP-MS measurements were derived from CPI International (Amsterdam, The Netherlands). Rhenium served as internal standard for platinum and concentrations were determined via the isotopes 185Re, 194Pt, and 195Pt. The ICP-MS was operated with 15 L min−1 argon as plasma gas, 0.95 L min−1 argon as carrier gas and with an radio frequency (RF) power of 1500 W. The size exclusion chromatography (SEC) measurements were carried out with a Viscothek GPCmax instrument equipped with a PFG column from PSS, (Mainz, Germany) 300 × 8 mm2, 5 μm particle size, and dimethylformamide (DMF) containing 10 × 10−3 M LiBr as eluent at a flow rate of 0.75 mL min−1 at 60 °C. A conventional calibration of the refractive index detector versus linear polystyrene standards was used to estimate the molecular weights. The zeta potential measurements and the dynamic light scattering (DLS) measurements were carried out with a Malvern ZetaSizer Nano-ZS analyzer (Malvern Instruments, UK) in a disposable capillary cell and polystyrene cuvette, respectively, at 25 °C. To yield a 1 mg mL−1 concentration, the samples were dissolved in deionized H2O and filtered through a 0.2-mm nylon filter. For the DLS, the 4 mW HeNe laser was set at λ = 633 nm with the detector angle at 173° for backscattering measurements, and the volume particle size distribution was used to determine the hydrodynamic diameter.
2.2. Synthesis
2.2.1. Polymers P-(a-c)
The poly(dichlorophosphazene) [PNCl2]n precursor was synthesized in a glove box at room temperature. The initiator PCl5 (37.1 mg, 0.18 mmol) and the monomer Cl3PNSi(CH3)3 (1.00 g, 4.46 mmol) were dissolved separately, each in anhydrous CH2Cl2 (5 mL). The monomer was then added to the initiator in solution, stirred for 16 h and the solvent was removed at reduced pressure to produce [PNCl2]n in a quantitative yield. {1H}31P NMR (121 MHz, CDCl3, δ): −18.13 ppm.
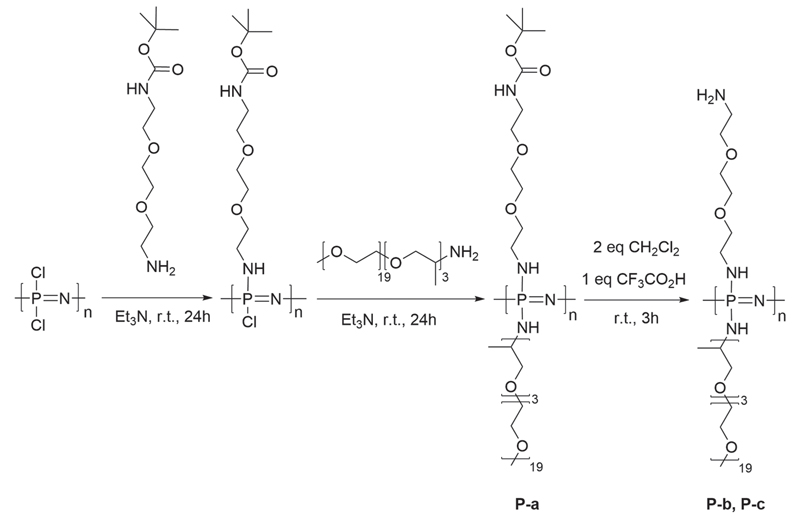
The [PNCl2]n precursor was then dissolved in anhydrous tetrahydrofuran (THF). Tertbutyl-2-(2-(2-aminoethoxy)ethoxy) ethylcarbamate (0.89 g, 3.56 mmol) in THF and Et3N (1 equivalent, 0.36 g) were then added and the solution stirred for 24 h. An excess of Jeffamine M-1000 (7.57 g, 7.57 mmol) was dissolved in THF and Et3N (1 equiv., 0.77 g), added to the solution containing the partially substituted precursor, and stirred in a glove box at room temperature for 24 h. After removal of the solvent at reduced pressure, the polymer was purified by dialysis (12 kDa cutoff) in H2O for 24 h followed by 72 h in EtOH. Removal of the solvent at reduced pressure gave a waxy solid. Yield: 3.13 g, 49%. 1H NMR (300 MHz, CDCl3, δ): 1.12 (br, 5.7H), 1.42 (s, 9H), 3.37(s, 3.3H), 3.63 (m, 73.8H) ppm; {1H}31P NMR (121 MHz, CDCl3, δ): 0.94 ppm (Figure SI-1, Supporting Information). Mn,theo = 6.8 × 104 g mol−1, SEC (linear polystyrene (PS) standards): Mn = 1.29 × 104 g mol−1, Mw/Mn = 1.42, DLS (H2O): dh = 5.6 nm.
Polymers P-b (Figure SI-2, Supporting Information) and P-c were prepared in an identical manner with different tertbutyl-2-(2-(2-aminoethoxy)ethoxy)ethylcarbamate:Jeffamine M-1000 ratios (Table 1).
Table 1.
Overview of synthesized and tested substances.
| Number | Description |
|---|---|
| Polymers | |
| P-a | Boc-protected poly(organo)phosphazene |
| P-b | Poly(organo)phosphazene with 24 mol% — NH2 (Linker:Jeffamine = 24:76) |
| P-c | Poly(organo)phosphazene with 44 mol% — NH2 (Linker:Jeffamine = 44:56) |
| Complexes | |
| 1 | Pt(IV) cisplatin derivative |
| 2 | Pt(IV) oxaliplatin derivative |
| Conjugates | |
| P-1 | Poly(organo)phosphazene loaded with Pt(IV) cisplatin derivative |
| P-2 | Poly(organo)phosphazene loaded with Pt(IV) oxaliplatin derivative |
For the deprotection of the boc-protected amino group, each polymer (P-a, P-b, and P-c) was dissolved in CH2Cl2 (100 mg polymer in 2 mL), trifluoroacetic acid was added (CH2Cl2:CF3CO2H = 2:1 ratio) and stirred for 3 h. The solvent was removed under reduced pressure. Polymers P-b and P-c were repurified by dialysis before testing.
2.2.2. Platinum(IV) Complexes 1 and 2
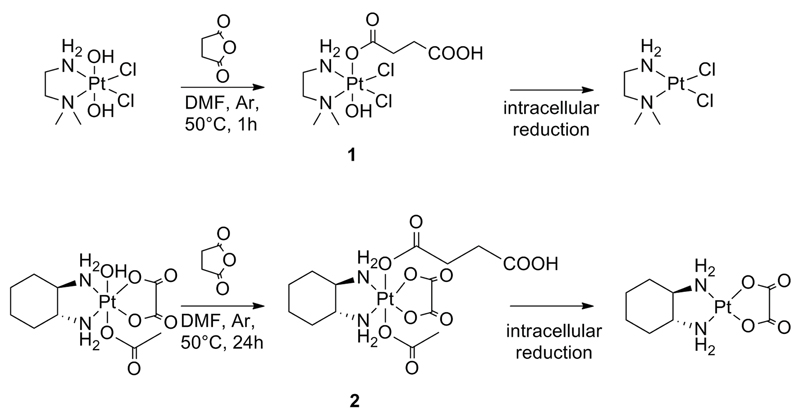
Platinum(IV) complexes (OC-6-54)-(3-carboxypropanoato)dichlorido(N,N-dimethylethane-1,2-diamine)hydroxidoplatinum(IV) (1)[47] and (OC-6-44)-acetato(3-carboxypropanoato)(1R,2R-diaminocyclohexane)oxalatoplatinum(IV) (2)[48] were synthesized according to previously reported procedures (Table 1).
(OC-6-54)-(3-carboxypropanoato)dichlorido(N,N-dimethylethane-1,2-diamine)hydroxidoplatinum(IV) (1)
A suspension of (OC-6-43)-dichlorido(N,N-dimethylethane-1,2-diamine) dihydroxidoplatinum(IV) (850 mg, 2.19 mmol) and succinic anhydride (440 mg, 4.4 mmol) was stirred in 12 mL of DMF abs. under an argon atmosphere at 50 °C for 1 h. A clear solution indicated completion of the reaction. The solvent was removed under reduced pressure; the crude product was dissolved in 10 mL of acetone and the pale yellow solid was precipitated with diethyl ether. The product was dried at reduced pressure. Yield: 772.2 mg, 73%. 1H NMR (DMSO-d6, δ): 12.08 (bm, 1H, OH), 9.41 (bs, 1H, NH2), 7.13 (bs, 1H, NH2), 2.83 (bm, 4H, H3/H4), 2.69 (s+d, 3H, H2a/H2b), 2.61 (s+d, 3H, H2a/H2b), 2.42 (m, 4H, H6/H7). (For numbered structure see Figure SI-3 in the Supporting Information.)
(OC-6-44)-acetato(3-carboxypropanoato)(1R,2R-diaminocyclohexane)oxalatoplatinum(IV) (2)
(OC-6-44)-acetato(1R,2R-diaminocyclohexane)hydroxido(oxalato)platinum(IV) (1 g, 2.11 mmol) and succinic anhydride (634 mg, 6.33 mmol) were suspended in 14 mL of DMF abs. and stirred for 24 h at 50 °C. After removal of DMF under reduced pressure, the residue was suspended in 15 mL of acetone. Methanol was added slowly until no more substance could be dissolved. The insoluble substance was filtered off; the filtrate was evaporated and the crude product was dissolved again in acetone. Precipitation of the product was performed with ethyl acetate and the white product was filtered, washed with diethyl ether, and dried at reduced pressure. Yield: 943.22 mg, 78%. 1H-NMR (DMSO-d6, δ): = 12.12 (bm, 1H, OH), 8.108.53 (bm, 4H, NH2), 2.58 (m, H1/H2), 2.41 (m, 2H, H12/H13), 2.12 (m, 3H, H3/H4/H5/H6), 1.97 (s, 2H, H10), 1.51 (m, 2H, H3/H4/H5/H6), 1.41 (m, 2H, H3/H4/H5/H6), 1.16 (m, 2H, H3/H4/H5/H6) ppm. (For numbered structure see Figure SI-3 in the Supporting Information.)
2.2.3. General Procedure for Synthesis of Conjugates P-1 and P-2
1,1′-Carbonyldiimidazole and the respective platinum complexes 1 and 2 were dissolved in DMF abs. and stirred for 10 min at 60 °C under an argon atmosphere. After cooling down to room temperature, the reaction mixture was flushed with argon in order to remove CO2. After deprotection of P-a, the polymer was dissolved in DMF under absolute conditions and added to the solution. After addition of Et3N, the reaction mixture was stirred for 24 h. The solvent was removed under reduced pressure; the product was dialyzed against methanol for 5 d and the obtained product dried at reduced pressure (Table 1).
Conjugate P-1
1,1′-carbonyldiimidazole (73 mg, 0.44 mmol), platinum complex 1 (193 mg, 0.40 mmol), deprotected P-a (400 mg, 0.29 mmol), Et3N (73 μL, 0.53 mmol). Yield: 117.7 mg, 29%. {1H}31P-NMR (DMSO-d6, δ): 0.88 ppm (Figure SI-4, Supporting Information). Mn,theo = 8.0 × 104 g mol−1, SEC (linear PS standards): Mn = 1.6 × 104 g mol−1, Mw/Mn = 1.85. DLS (H2O): dh = 6.5 nm.
Conjugate P-2
1,1′-carbonyldiimidazole (36 mg, 0.22 mmol), platinum complex 2 (114 mg, 0.20 mmol), deprotected P-a (200 mg, 0.15 mmol), Et3N (36 μL, 0.26 mmol). Yield: 37.3 mg, 18%. {1H}31P-NMR (DMSO-d6, δ): 0.44 ppm (Figure SI-4, Supporting Information). Mn,theo = 6.9 × 104 g mol−1, SEC (linear PS standards): Mn = 1.3 × 104 g mol−1, Mw/Mn = 1.36. DLS (H2O): dh = 4.2 nm.
2.2.4. General Procedures for Biological Studies
Cell culture
The ovarian carcinoma A2780 cell line and its cisplatin-resistant subline A2780/cis were purchased from Sigma and cultivated in RPMI-1640 cell media with 10% fetal calf serum (FCS). The colorectal carcinoma cell line HCT116 was donated by Dr. B. Vogelstein (John Hopkins University, Baltimore, MD), whereas oxaliplatin-resistant subline HCT116/oxR was established by stepwise drug selection of HCT116 as previously reported.[31] Both the parental and oxaliplatin-resistant cell lines were grown in McCoy cell media with 10% FCS. To maintain drug resistance, the sublines were cultivated under continuous selection pressure. Selection drugs were removed one week before the experiments were performed. Cell cultures were maintained at 37 °C in humidified atmosphere containing 5% CO2. Cultures were regularly checked for Mycoplasma contamination.
Cytotoxicity assays
Cells were plated (2−4 × 103 cells per well) in 100 μL per well in 96-well plates. After a recovery period of 24 h, drugs, complexes, and conjugates were added in another 100 μL growth medium and cells were exposed for 72 h. The proportion of viable cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay following the manufacturer’s recommendations (EZ4U, Biomedica, Vienna, Austria). Cytotoxicity was calculated using the Graph Pad Prism software (La Jolla, USA) (using a point-to-point function) and was expressed as IC50 values calculated from full dose-response curves (drug concentrations inducing a 50% reduction of cell number in comparison to untreated control cells cultured in parallel).
Platinum accumulation
A2780 and A2780/cis cells (1.5 × 105 cells per well) were exposed to 10 × 10−6 M cisplatin, oxaliplatin, platinum complexes 1 and 2, and polymer conjugates P-1 and P-2 as indicated, at 37 °C for 3 h. Cell samples were lysed in tetramethylammonium hydroxide and diluted in 0.4 N HNO3. Total platinum accumulation concentrations were determined by ICP-MS (Agilent Technologies, 7700 Series). The system used for these measurements was equipped with an octopole reaction cell using helium as inert collision gas.[49] Values represent means of at least three independent experiments. To avoid unspecific binding to cell culture plastic, results were corrected for Pt levels of two blank wells containing no cells.
Clonogenic assay
A2780 and HCT116 cells (200 cells per well) were seeded into 24-well plates. Following 24 h recovery, cells were treated with the test compounds. At day seven of exposure, cells were washed twice with phosphate-buffered saline (PBS), fixed with methanol, stained with crystal violet, and analyzed for colony formation.
Animals
All animal experiments were approved by the local ethics commission and carried out according to the Austrian and FELASA guidelines for animal care and protection. Eight- to ten-week-old Balb/c or CB-17 scid/scid (SCID) mice (weighing 25–30 g) were purchased from Harlan Laboratories, San Pietro al Natisone, Italy. The animals were kept in a pathogen-free environment and every procedure was done in a laminar airflow cabinet.
Treatment experiments in vivo
Murine CT-26 cells (5 × 105 cells per well) were injected subcutaneously into the right flank of male Balb/c mice. Animals were treated with oxaliplatin (9 mg kg−1), complex 2 (13 mg kg−1), and polymer conjugate P-2 (80 mg kg−1) on days 4, 7, 11, and 14. Oxaliplatin was dissolved in 5% glucose solution, whereas all other drugs were dissolved in 0.9% sodium chloride solution. Animals were controlled for distress development every day and tumor size was assessed regularly by caliper measurement. Tumor volume was calculated using the formula: length × width2/2.
3. Results and Discussion
Living cationic polymerization was applied to synthesize the poly(dichlorophosphazene) [PNCl2]n precursor (Figure 1) due to its ability to yield polymers with controlled chain lengths.[50,51] The chlorine atoms of the [PNCl2]n precursor were then completely substituted (Figure 2) in a successive two-step nucleophilic substitution. First, mono-boc protected 2,2′-(ethylenedioxy)-bis(ethylamine) was attached to the polymer backbone to provide subsequent reaction sites for the platinum complexes. The remaining chlorine atoms were then replaced by Jeffamine, a monoamine PPO-PEO random copolymer (Mn = 1000) to give the highly branched, water soluble poly(organo)phosphazenes. Jeffamine was used for its excellent aqueous solubility and superior cell-uptake kinetics.[52] 1H NMR spectroscopy allowed the calculation of the respective percentage of reactive sites. The boc-protecting group was then removed to yield free amino groups for loading the platinum(IV) complexes onto the poly(organo)phosphazene carrier (Figure 2).
Figure 1.
Synthesis of poly(dichlorophosphazene): Polymer chain length n = 50 was synthesized by living cationic polymerization of the trichlorophosphoranimine (Cl3PNSi(CH3)3) monomer with PCl5.
Figure 2.
Synthesis of poly(organo)phosphazenes: Macrosubstitution of the [PNCl2]n with boc-protected amine linker and Jeffamine. Boc to PEO/PPO in various ratios. P-a was deprotected with CH2Cl2:CF3CO2H = 2:1 yielding polymers P-b and P-c.
To covalently link the complex to the carrier, two recently developed platinum(IV) complexes were chosen with only one free carboxylic acid in the axial position (Figure 3). The choice of monocarboxylated complex is essential to avoid polymer crosslinking. The monocarboxylated platinum complex 1 could be synthesized from a Pt(IV) derivative with two axial hydroxyl groups due to steric hindrance of the two methyl groups[47] and is designed to undergo intracellular reduction to the active Pt(II) cisplatin derivative (Figure 3). Complex 2 was prepared from a Pt(IV) oxaliplatin derivative with one axial hydroxo ligand, the second axial ligand being an inert moiety. Further reaction of the axial hydroxyl group with succinic anhydride gave the required monocarboxylated derivative for subsequent polymer conjugation.
Figure 3.
Synthesis of platinum(IV) complexes 1 and 2 followed by their proposed intracellular reduction to the active Pt(II) species.
The monocarboxylated complexes 1 and 2 were then coupled to the free amino groups of the poly(organo) phosphazene to give conjugates P-1 and P-2. ICP-MS measurements of the obtained conjugates showed the total amount of platinum to be ranging from 9.6 μg mg−1 (4 wt% of the conjugate) to 55.7 μg mg−1 (19 wt%). This reflects an amine conversion from 9% to 40%. The poor coupling ability could be due to a number of factors, including steric hindrance, and a more efficient coupling strategy will be investigated for future conjugates.
The degradation of poly(organo)phosphazenes with similar structures, as well as the biocompatibility of the degradation products, have been investigated and reported in detail elsewhere.[12] The rate of degradation can be tailored to give a wide range of degradation rates. The polymers used in this study were previously shown to have relatively slow hydrolytic degradation rates (several months) compared to similar poly(organo)phosphazenes, and were chosen in order to simplify the in vitro and in vivo studies, effectively decoupling the drug-release from the degradation of the polymer. To confirm this, polymer P-c was incubated at 37 °C in a pH 7.4 phosphate-buffered solution over the course of 11 weeks and showed only minimal degradation during that time span (see Figure SI-5 in the Supporting Information).
3.1. Cellular Uptake of Platinum-Loaded Polymers
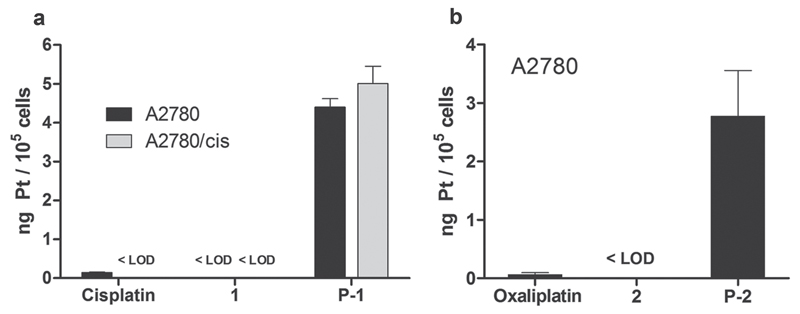
To investigate the cell-uptake behavior of the novel conjugates, A2780 cells were treated with cisplatin, complex 1, and the polymer conjugate P-1 for 3 h. ICP-MS analysis showed ≈30-fold enhanced platinum levels in cells after treatment with the conjugates when compared to cisplatin (Figure 4a). Notably, the respective platinum(IV) complex 1 was characterized by an even lower uptake than cisplatin (<limit of detection (LOD)). Similar uptake ratios were also obtained in experiments with oxaliplatin, platinum complex 2, and conjugate P-2 (Figure 4b). Furthermore, in order to investigate the impact of drug resistance, the cell-uptake behavior was also tested in cisplatin-resistant subline A2780/cis (Figure 4a). No difference in conjugate accumulation between A2780 and A2780/cis was observed, a noteworthy observation, since one of the mechanisms underlying the cisplatin resistance of A2780/cis is reduced cisplatin influx, also indicated by the cisplatin levels below the LOD in A2780/cis.[53] Consequently, these data indicate not only that conjugation to polymers is able to increase cellular platinum levels, but also to circumvent drug resistance based on possible differences in the uptake route, which could be expected to be endocytosis for polymers of this type.[39]
Figure 4.
Platinum accumulation in cell culture. Treatment of A2780 and A2780/cis cells with 10 × 10−6 M of the indicated drugs for 3 h. Values are expressed in ng of platinum per 105 cells ± SD (<LOD; below limit of detection).
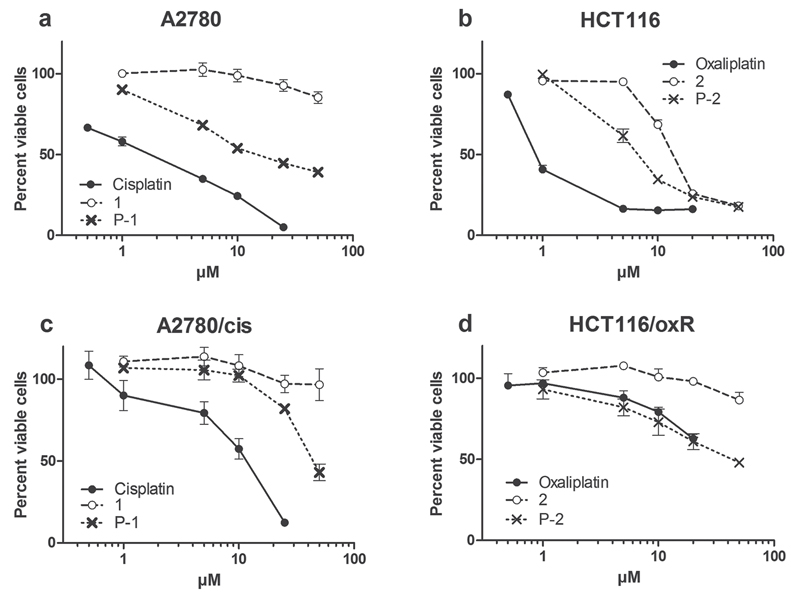
The cytotoxicity of the polymer conjugates P-1 and P-2 was also investigated in comparison to the respective free platinum(IV) complexes 1 and 2, as well as the established drugs cisplatin and oxaliplatin in A2780 and HCT116 cells, respectively (Figure 5). After 72 h exposure, both free platinum(IV) complexes 1 and 2 displayed distinctly lower cytotoxic activity in comparison to the respective platinum(II) drugs. This is in good agreement with results of previous studies,[47] as platinum(IV) drugs have to be considered as prodrugs, which need to be intracellularly reduced before exerting their anticancer activity, thus resulting in lower cytotoxicity. However, the anticancer activity of the platinum(IV) drugs was observed to increase significantly upon conjugation to the polymers. This is especially prevalent in case of polymer conjugate P-1 (Figure 5 as well as Tables 2 and 3), whereby the IC50 value in HCT116 cells was more than sixfold higher in case of platinum complex 1 than for polymer conjugate P-1. This effect can be explained by the enhanced cellular platinum accumulation observed for the platinum drug conjugate shown in Figure 4.
Figure 5.
Viability of cancer cells after treatment with novel platinum complexes and polymer conjugates. a) A2780 and c) A2780/cis ovarian cancer cells were treated with cisplatin, platinum complex 1, and polymer conjugate P-1 and b) HCT116 and d) HCT116/oxR colon carcinoma cells were treated with oxaliplatin, platinum complex 2, and polymer conjugate P-2. Viability changes as compared with the untreated control were measured by MTT assay after 72 h drug incubation. Values given are means ± SD of three experiments performed in triplicates. Untreated controls for all investigated drugs were normalized to 100%.
Table 2.
Cytotoxicity of platinum complex 1 and polymer conjugate P-1 versus cisplatin in cisplatin-sensitive versus -resistant cancer cells.
| Cisplatin |
Complex 1 |
Conjugate P-1 |
||||
|---|---|---|---|---|---|---|
| Cell line | IC50 [× 10−6 M] | RF | IC50[× 10−6 M] | RF | IC50 [× 10−6 M] | RF |
| A2780 | 2.4 ± 0.1 | – | > 50 | – | 16.3 ± 1.9 | – |
| A2780/cis | 12.5 ± 0.5 | 5.2 | > 50 | – | 45.6 ± 1.7 | 2.8 |
| HCT116 | 2.1 ± 0.1 | – | > 50 | – | 8.3 ± 0.9 | – |
RF: resistance factor (IC50 A2780/cis divided by IC50 of A2780).
Table 3.
Cytotoxicity of platinum complex 2 and polymer conjugate P-2 versus oxaliplatin in oxaliplatin-sensitive versus -resistant cancer cells.
| Oxaliplatin |
Complex 2 |
Conjugate P-2 |
||||
|---|---|---|---|---|---|---|
| Cell line | IC50[× 10−6 M] | RF | IC50 [× 10−6 M] | cell line | IC50[× 10−6 M] | RF |
| HCT116 | 0.9 ± 0.1 | – | 14.3 ± 0.1 | HCT116 | 0.9 ± 0.1 | – |
| HCT116/oxR | >20 | 28 | >50 | HCT116/oxR | >20 | 28 |
RF: resistance factor (IC50 A2780/cis divided by IC50 of A2780).
The cytotoxicity against the cisplatin-resistant subline A2780/cis and the oxaliplatin-resistant subline HCT116/oxR was also investigated. In accordance to previous publications, A2780/cis and HCT116/oxR displayed 5.2- and >20-fold resistance against cisplatin and oxaliplatin, respectively.[31,54] Notably, in both cell models the drug conjugates were influenced to a far lesser extent by the drug resistance phenotype. In the case of the oxaliplatin-resistance model, reduced cross-resistance was observed toward the free platinum(IV) drug. Consequently, it is not clear from these results, whether the reduced impact of drug resistance is based on a different route of uptake (endocytosis in case of polymers vs diffusion for the free platinum drugs) or other mechanisms.
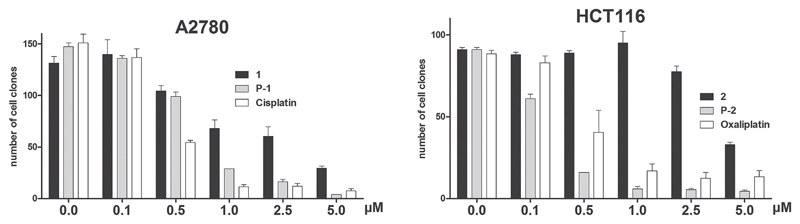
Since the ICP-MS data indicated an enhanced cellular platinum uptake by a factor of 30, but only a sixfold increased anticancer activity was observed after 72 h drug exposure, it was investigated whether prolonged drug exposure (extended time for drug reduction and activation) is able to enhance the anticancer activity of the test compounds. To this end, clonogenic assays were performed (Figure 6). After 7 d of drug incubation, the activity of all test drugs was increased in comparison to the 72 h experiment shown above. However, in case of the platinum(II) drugs only a factor 6 and 2 were observed for cisplatin and oxaliplatin, respectively. In contrast, the increase in activity was far more pronounced for the platinum(IV) drugs, and here especially for the drug-loaded polymers (increase in activity ≈30-fold). Consequently, the IC50 value of the oxaliplatin-loaded polymer conjugate P-2 was even lower than that of free oxaliplatin (IC50 of 0.25 × 10−6 M vs 0.47 × 10−6 M). This indicates that longer incubation time is required for activation of the platinum(IV) drugs and supports the hypothesis that 72 h drug exposure might not be sufficient to obtain the full cytotoxic potential of the investigated platinum(IV) complexes in cell culture.
Figure 6.
Viability of cancer cells after treatment with complexes and conjugates. A2780 ovarian cancer cells were treated with cisplatin, platinum complex 1 and polymer conjugate P-1, while HCT116 colon carcinoma cells were treated with oxaliplatin, platinum complex 2, and polymer conjugate P-2. Clonogenic survival was determined after exposure to the indicated drug concentrations for 7 d. Cell colonies were visualized by crystal violet staining.
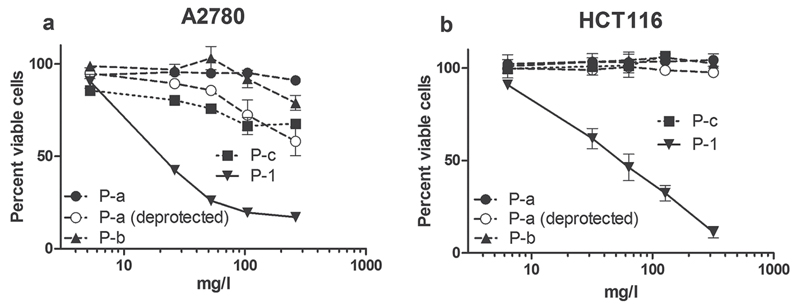
As discussed above, the conjugation of the Pt drugs to the amine groups of the polymer was incomplete (<50%) leading to a certain amount of free amino groups in the molecule, causing a small cationic charge at physiological pH (Zeta potential 10–20 mV). It has been reported that such cationic polymers can themselves cause some cytotoxicity.[55] Hence, in order to evaluate whether the enhanced anticancer activity of the drug conjugates is truly caused by the Pt prodrugs alone and not a toxic contribution of polymer components, cytotoxicity tests were also carried out with two nonloaded polymers (P-b and P-c) in comparison to polymer conjugate P-1 (Figure 7). Polymer P-b was chosen as one with rather low percentage of free amino groups (24 mol%, Zeta potential 24 mV), polymer P-c by a percentage of free amino groups (44 mol%, Zeta potential 35 mV), significantly higher than present on the loaded polymers. MTT assays performed after 72 h incubation showed that although polymer P-c and the deprotected version of P-a had some activity against A2780 in these tests, in both cell lines (A2780 and HCT116) the IC50 values were well above 50 × 10−6 M and, thus, more than 5- and 10-fold above the IC50 values of the platinum loaded conjugates. In addition, long-time incubation experiments with all of these unloaded polymers did not show any cytotoxic activity (Figure SI-6, Supporting Information). Thus, the anticancer activity can be clearly attributed to the platinum complex itself and not to the polymeric carrier.
Figure 7.
Viability of cancer cells after treatment with conjugate P-1 and unloaded polymers. A2780 ovarian cancer cells as well as HCT116 colon cancer cells were treated with polymer conjugate P-1, polymers P-a, P-a (deprotected), P-b, and P-c. Viability changes as compared with the untreated control were measured by MTT assay after 72 h drug incubation. Values given are means ± SD of three experiments performed in triplicates. Untreated controls for all investigated polymers were normalized to 100%.
3.2. Tolerability and Anticancer Activity In Vivo
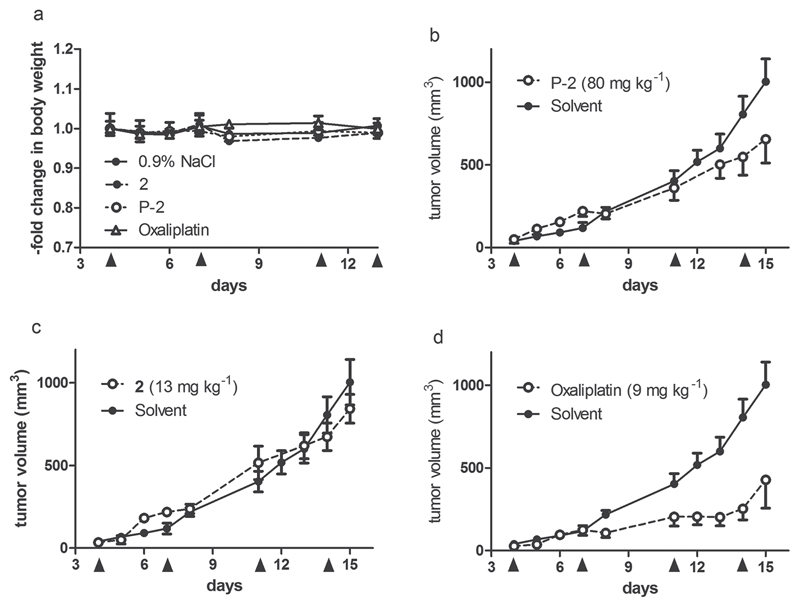
The tolerability and anticancer activity of the novel oxaliplatin conjugate P-2 was subsequently tested in vivo using the syngeneic murine tumor model CT-26. The use of this murine colon cancer cell model was necessary to allow testing under immune-competent conditions due to recently reported importance of the immune system for the anticancer activity of oxaliplatin.[31] The reference compound oxaliplatin was applied at its maximal tolerated dose of 9 mg kg−1. The conjugate, as well as the free platinum(IV) drug were applied at doses resulting in equimolar platinum concentrations. Drugs were administered twice a week for two weeks (Figure 8). The conjugate was very well tolerated without any signs of weight loss, which is especially noteworthy considering the high dose applied intravenously.
Figure 8.
Tolerability and anticancer activity in vivo. Murine CT-26 cells were injected subcutaneously into the right flank of male Balb/c mice. Subsequently, the animals were treated with the drugs intravenously at indicated concentrations on days 4, 7, 11, and 14. Data shown are derived from two independent experiments. While solvent controls are pooled (n = 6 mice), all drug-exposed groups contain 4 animals. a) Change in body weight during treatment. b–d) Impact of tumor growth by the indicated drugs.
With regard to the anticancer activity, the platinum-loaded conjugate P-2 resulted in ≈35% reduced tumor burden on day 15. In contrast, no significant impact of the free platinum(IV) complex 2 on CT-26 tumor growth was observed. For the platinum(II) drug oxaliplatin, used as a positive control, a pronounced and highly significant antitumor effect was observed in accordance with previous reports.[31] Together with the complete lack of activity of the unloaded polymers P-a and P-a (deprotected) in independently performed experiments (data not shown), these results indicate that conjugation to polymers is able to distinctly increase the anticancer activity of the per se widely inactive platinum(IV) complex 2. Subsequent analysis is currently ongoing to evaluate whether these effects are based on enhanced tumor accumulation or prolonged serum plasma half life time. Moreover, it has to be considered that the fast growing CT-26 model might not provide enough time for complete drug release of the active platinum(II) species from the polymer. Consequently, it will be also of interest to perform experiments on other tumor models with slower tumor growth.
4. Conclusions
The preparation of degradable, water-soluble macromolecular Pt(IV) prodrugs is presented, designed to release the active Pt(II) species oxaliplatin and a cisplatin derivative upon intracellular reduction and simultaneous release from the polymer carrier. The use of living cationic polymerization allowed the preparation of conjugates with controlled Mn and relatively narrow molecular weight distributions, while macrosubstitution with free amino groups provided binding sites for the subsequent conjugation of monocarboxylated Pt(IV) complexes. In vitro studies showed a significantly enhanced (30-fold) cellular uptake of Pt upon treatment with the conjugates compared to the Pt(IV) complexes as well as compared to established platinum(II) drugs cisplatin and oxaliplatin used as reference. The higher uptake was also reflected in an increase in cytotoxicity of the polymer conjugates compared to the Pt(IV) complexes. The conjugates were, however, still considerably less cytotoxic than the established Pt(II) drugs, presumably due to slow reduction, although significantly higher cytotoxicity was observed for longer incubation times. Interestingly, drug-resistant sublines showed a significantly smaller resistance against polymer conjugates compared to clinically established platinum drugs, indicating that the enhanced uptake of the platinum(IV) conjugates might also be able to overcome the acquired resistance against platinum(II) drugs. Although in vivo studies in mice showed an improved tumor shrinkage compared to the free platinum(IV) complex upon conjugation to the poly(organo)phosphazene, the tumor suppression was less than would be desired. The low in vivo suppression could be due to a number of reasons and future work should concentrate on the improved reduction kinetics, as well as endosomal escape of the free Pt(II) drug, which appear from the results of this study to be too slow for such fast growing tumors. Furthermore, it should be investigated how the size of the conjugates can be optimized in order to take more advantage of the EPR effect.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Acknowledgements
The authors acknowledge financial support from the Austrian Science Fund (FWF) (Grant No. P24659-N28). P.H. acknowledges support from Grant P26603. This work was performed in the surrounding of the COST action CM1105. H. Henke would like to thank R. Kovacova (Prager Elektronik) for assistance with the zeta potential measurements. The authors are thankful to G. Zeitler and S. Van Schoonhoven for animal care as well as M. Galanski for his significant contributions to this work.
Contributor Information
Helena Henke, Institute of Polymer Chemistry, Johannes Kepler University Linz, Altenberger Straße 69, 4040 Linz, Austria.
Dr. Kushtrim Kryeziu, Institute of Cancer Research and Comprehensive Cancer Center, Department of Medicine I, Medical University Vienna, Borschkegasse 8a, 1090 Vienna, Austria Research Platform “Translational Cancer Therapy Research,” University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Dr. Jelena Banfić, Institute of Inorganic Chemistry University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria
Dr. Sarah Theiner, Research Platform “Translational Cancer Therapy Research,” University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria Institute of Inorganic Chemistry University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Prof. Wilfried Körner, Department of Environmental Geosciences, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria
Prof. Oliver Brüggemann, Institute of Polymer Chemistry, Johannes Kepler University Linz, Altenberger Straße 69, 4040 Linz, Austria
Prof. Walter Berger, Institute of Cancer Research and Comprehensive Cancer Center, Department of Medicine I, Medical University Vienna, Borschkegasse 8a, 1090 Vienna, Austria Research Platform “Translational Cancer Therapy Research,” University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Prof. Bernhard K. Keppler, Research Platform “Translational Cancer Therapy Research,” University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria Institute of Inorganic Chemistry University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Dr. Petra Heffeter, Institute of Cancer Research and Comprehensive Cancer Center, Department of Medicine I, Medical University Vienna, Borschkegasse 8a, 1090 Vienna, Austria Research Platform “Translational Cancer Therapy Research,” University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Prof. Ian Teasdale, Institute of Polymer Chemistry, Johannes Kepler University Linz, Altenberger Straße 69, 4040 Linz, Austria
References
- [1].Marinaro W, Stella V. In: Prodrugs. Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J, editors. Springer; New York: 2007. p. 989. [Google Scholar]
- [2].Duncan R. Nat Rev Drug Discov. 2003;2:347. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- [3].Haag R, Kratz F. Angew Chem Int Ed. 2006;45:1198. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- [4].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J Controlled Release. 2000;65:271. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [5].Duncan R. Curr Opin Biotechnol. 2011;22:492. doi: 10.1016/j.copbio.2011.05.507. [DOI] [PubMed] [Google Scholar]
- [6].Markovsky E, Baabur-Cohen H, Eldar-Boock A, Omer L, Tiram G, Ferber S, Ofek P, Polyak D, Scomparin A, Satchi-Fainaro R. J Controlled Release. 2012;161:446. doi: 10.1016/j.jconrel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- [7].Duncan R, Vicent MJ. Adv Drug Delivery Rev. 2010;62:272. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- [8].Fox ME, Szoka FC, Fréchet JMJ. Acc Chem Res. 2009;42:1141. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allcock HR. Chemistry and Applications of Polyphosphazenes. Wiley-Interscience; Hoboken, NJ: 2003. [Google Scholar]
- [10].Allcock HR, Morozowich NL. Polym Chem. 2012;3:578. [Google Scholar]
- [11].Teasdale I, Brüggemann O. Polymers. 2013;5:161. doi: 10.3390/polym5010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilfert S, Iturmendi A, Schoefberger W, Kryeziu K, Heffeter P, Berger W, Brüggemann O, Teasdale I. J Polym Sci Part A: Polym Chem. 2014;52:287. doi: 10.1002/pola.27002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Teasdale I, Brüggemann O. Polyphosphazenes for Medical Applications. Smithers Rapra, Shrewsbury; UK: 2014. [Google Scholar]
- [14].Andrianov AK. Polyphosphazenes for Biomedical Applications. Wiley; Hoboken, NJ: 2009. [Google Scholar]
- [15].Feinweber D, Verwanger T, Bruggemann O, Teasdale I, Krammer B. Photochem Photobiol Sci. 2014;13:1607. doi: 10.1039/c4pp00251b. [DOI] [PubMed] [Google Scholar]
- [16].Rothemund S, Aigner TB, Iturmendi A, Rigau M, Husár B, Hildner F, Oberbauer E, Prambauer M, Olawale G, Forstner R, Liska R, et al. Macromol Biosci. 2015;15:351. doi: 10.1002/mabi.201400390. [DOI] [PubMed] [Google Scholar]
- [17].Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. Adv Funct Mater. 2010;20:2743. doi: 10.1002/adfm.201090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G. Proc Natl Acad Sci USA. 2009;106:18936. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mutwiri G, Benjamin P, Soita H, Townsend H, Yost R, Roberts B, Andrianov AK, Babiuk LA. Vaccine. 2007;25:1204. doi: 10.1016/j.vaccine.2006.10.011. [DOI] [PubMed] [Google Scholar]
- [20].Andrianov AK, Decollibus DP, Marin A, Webb A, Griffin Y, Webby RJ. J Pharm Sci. 2011;100:1436. doi: 10.1002/jps.22367. [DOI] [PubMed] [Google Scholar]
- [21].Andrianov AK, Marin A, DeCollibus DP. Pharm Res. 2011;28:58. doi: 10.1007/s11095-010-0133-7. [DOI] [PubMed] [Google Scholar]
- [22].Kelland L. Nat Rev Cancer. 2007;7:573. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- [23].Johnstone TC, Wilson JJ, Lippard SJ. Inorg Chem. 2013;52:12234. doi: 10.1021/ic400538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nowotnik DP, Cvitkovic E. Adv Drug Delivery Rev. 2009;61:1214. doi: 10.1016/j.addr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [25].Xiao H, Zhou D, Liu S, Qi R, Zheng Y, Huang Y, Jing X. Macromol Biosci. 2012;12:367. doi: 10.1002/mabi.201100320. [DOI] [PubMed] [Google Scholar]
- [26].Jadhav VB, Jun YJ, Song JH, Park MK, Oh JH, Chae SW, Kim I-S, Choi S-J, Lee HJ, Sohn YS. J Controlled Release. 2010;147:144. doi: 10.1016/j.jconrel.2010.07.101. [DOI] [PubMed] [Google Scholar]
- [27].Jun YJ, Kim JI, Jun MJ, Sohn YS. J Inorg Biochem. 2005;99:1593. doi: 10.1016/j.jinorgbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- [28].Song S-C, Lee SB, Lee BH, Ha H-W, Lee K-T, Sohn YS. J Controlled Release. 2003;90:303. doi: 10.1016/s0168-3659(03)00199-8. [DOI] [PubMed] [Google Scholar]
- [29].Zheng Y-R, Suntharalingam K, Johnstone TC, Yoo H, Lin W, Brooks JG, Lippard SJ. J Am Chem Soc. 2014;136:8790. doi: 10.1021/ja5038269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galanski M, Jakupec MA, Keppler BK. Curr Med Chem. 2005;12:2075. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- [31].Jungwirth U, Xanthos DN, Gojo J, Bytzek AK, Körner W, Heffeter P, Abramkin SA, Jakupec MA, Hartinger CG, Windberger U, Galanski M, et al. Mol Pharmacol. 2012;81:719. doi: 10.1124/mol.111.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carr J, Tingle M, McKeage M. Cancer Chemother Pharmacol. 2002;50:9. doi: 10.1007/s00280-002-0462-2. [DOI] [PubMed] [Google Scholar]
- [33].Carr J, Tingle M, McKeage M. Cancer Chemother Pharmacol. 2006;57:483. doi: 10.1007/s00280-005-0069-5. [DOI] [PubMed] [Google Scholar]
- [34].Callari M, Aldrich-Wright JR, deSouza PL, Stenzel MH. Prog Polym Sci. 2014;39:1614. [Google Scholar]
- [35].Scarano W, Duong HTT, Lu H, DeSouza PL, Stenzel MH. Biomacromolecules. 2013;14:962. doi: 10.1021/bm400121q. [DOI] [PubMed] [Google Scholar]
- [36].Hou J, Shang J, Jiao C, Jiang P, Xiao H, Luo L, Liu T. Macromol Biosci. 2013;13:954. doi: 10.1002/mabi.201300057. [DOI] [PubMed] [Google Scholar]
- [37].Duong HTT, Huynh VT, deSouza P, Stenzel MH. Biomacromolecules. 2010;11:2290. doi: 10.1021/bm100396s. [DOI] [PubMed] [Google Scholar]
- [38].Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. J Am Chem Soc. 2009;131:14652. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Duncan R, Richardson SCW. Mol Pharm. 2012;9:2380. doi: 10.1021/mp300293n. [DOI] [PubMed] [Google Scholar]
- [40].Yang J, Liu W, Sui M, Tang J, Shen Y. Biomaterials. 2011;32:9136. doi: 10.1016/j.biomaterials.2011.08.022. [DOI] [PubMed] [Google Scholar]
- [41].Pichler V, Mayr J, Heffeter P, Domotor O, Enyedy EA, Hermann G, Groza D, Kollensperger G, Galanksi M, Berger W, Keppler BK, et al. Chem Commun. 2013;49:2249. doi: 10.1039/c3cc39258a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Neumann W, Crews BC, Marnett LJ, Hey-Hawkins E. ChemMedChem. 2014;9:1150. doi: 10.1002/cmdc.201402074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wilfert S, Iturmendi A, Schoefberger W, Kryeziu K, Heffeter P, Berger W, Brüggemann O, Teasdale I. J Polym Sci Part A: Polym Chem. 2014;52:287. doi: 10.1002/pola.27002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Teasdale I, Wilfert S, Nischang I, Brueggemann O. Polym Chem. 2011;2:828. [Google Scholar]
- [45].Marom H, Miller K, Bechor-Bar Y, Tsarfaty G, Satchi-Fainaro R, Gozin M. J Med Chem. 2010;53:6316. doi: 10.1021/jm100289b. [DOI] [PubMed] [Google Scholar]
- [46].Wang B, Rivard E, Manners I. Inorg Chem. 2002;41:1690. doi: 10.1021/ic011125n. [DOI] [PubMed] [Google Scholar]
- [47].Pichler V, Valiahdi SM, Jakupec MA, Arion VB, Galanski M, Keppler BK. Dalton Trans. 2011;40:8187. doi: 10.1039/c1dt10301f. [DOI] [PubMed] [Google Scholar]
- [48].Abramkin S. Synthesis and Characterization of Novel Oxaliplatin Analogs. University of Vienna; 2012. PhD Thesis. [Google Scholar]
- [49].Heffeter P, Böck K, Atil B, Hoda MAR, Körner W, Bartel C, Jungwirth U, Keppler BK, Micksche M, Berger W, Koellensperger G. J Biol Inorg Chem. 2010;15:737. doi: 10.1007/s00775-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Henke H, Wilfert S, Iturmendi A, Brüggemann O, Teasdale I. J Polym Sci Part A: Polym Chem. 2013;51:4467. doi: 10.1002/pola.26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Honeyman CH, Manners I, Morrissey CT, Allcock HR. J Am Chem Soc. 1995;117:7035. [Google Scholar]
- [52].Peddada LY, Harris NK, Devore DI, Roth CM. J Controlled Release. 2009;140:134. doi: 10.1016/j.jconrel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Heffeter P, Jungwirth U, Jakupec M, Hartinger C, Galanski M, Elbling L, Micksche M, Keppler B, Berger W. Drug Resist Update. 2008;11:1. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [54].Pichler V, Heffeter P, Valiahdi SM, Kowol CR, Egger A, Berger W, Jakupec MA, Galanski M, Keppler BK. J Med Chem. 2012;55:11052. doi: 10.1021/jm301645g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lv H, Zhang S, Wang B, Cui S, Yan J. J Controlled Release. 2006;114:100. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.