Abstract
Background
Amazonian populations are exposed to diverse parasites and pathogens, including protozoal, bacterial, fungal, and helminthic infections. Yet much of our understanding of the immune system is based on industrialised populations where these infections are relatively rare.
Aim
We examine distributions and age-related differences in 22 measures of immune function for Bolivian forager-horticulturalists and US and European populations.
Subjects and Methods
Subjects were 6,338 Tsimane aged 0–90 years. Blood samples collected between 2004–2014 were analysed for 5-part blood differentials, C-reactive protein, erythrocyte sedimentation rate (ESR), and total immunoglobulins E, G, A, and M. Flow cytometry was used to quantify naive and non-naïve CD4 and CD8 T cells, natural killer cells, and B cells.
Results
Compared to reference populations, Tsimane have elevated levels of most immunological parameters, particularly immunoglobulins, eosinophils, ESR, B cells, and natural killer cells. However, monocytes and basophils are reduced and naïve CD4 cells depleted in older age groups.
Conclusion
Tsimane ecology leads to lymphocyte repertoires and immunoglobulin profiles that differ from those observed in industrialised populations. These differences have consequences for disease susceptibility and co-vary with patterns of other life history traits, such as growth and reproduction.
Introduction
The Amazon River basin is home to an astonishing degree of biodiversity. It is the world’s largest rain forest, and includes over 2,000 species of birds and mammals. An estimated 10% of the world’s species live in Brazil alone (Lewinsohn, 2005), and the Amazon basin is home to an estimated 400 indigenous tribes (http://www.survivalinternational.org/). Unfortunately for Amazonian peoples, this diversity includes an exceptional array of pathogens and parasites (Dunn et al., 2010), including soil-transmitted helminths and filarial nematodes, protozoa, amoebas, bacteria, viruses, lice and scabies, and mycoses (Salzano and Callegari-Jacques, 1988; Martin et al., 2013). Despite the recognition that a large number of micro- and macro-parasite species coexist in the Amazon biome where people have lived for millennia, little data exists documenting how immune function develops and senesces in Amazonian and other tropical populations. Characterisation of immune function in Amazonians is important both for documenting human biological diversity and for understanding and addressing the health challenges facing Amazonian peoples. Understanding how immune function responds to ecological conditions also bears on questions of human life history evolution, as immune responses and disease impact profiles of growth, reproduction, senescence and mortality (McDade, 2003; Blackwell et al., 2010, 2015; Pisor et al., 2013).
The Tsimane are an Amazonian population indigenous to the Bolivian lowlands. Previous studies have documented high prevalences of helminthic, protozoal, fungal, and other infections in Tsimane of all ages (Martin et al., 2013). Tsimane also have much higher levels of several immune markers, including immunoglobulin E (IgE) (Blackwell et al., 2011), total leukocyte count, and erythrocyte sedimentation rates (ESR) (Gurven et al., 2009). Here, we expand upon these findings to provide a “thick description” of Tsimane immune function by characterising levels of 22 immunological parameters in reference to clinical values from the United States and Europe. Since the immune system is multifaceted, there are a vast number of biomarkers that might be measured to fully characterise it, but past studies of the Tsimane and other populations have relied primarily on one or two measures of immune function. Thus, while these results are informative, they fail to provide a comprehensive picture of immune function in a traditional population. Moreover, reliance on single measures may sometimes lead to incorrect conclusions if they are taken to represent the immune system as a whole, since trade-offs may be evident between multiple branches of immunity (McDade, 2005; Blackwell et al., 2010). Below, we briefly review the aspects of immune function considered in this paper and then describe the pathogenic environments of the Tsimane and other Amazonian peoples. We then present our results describing Tsimane immune function and discuss the implications of these results for life history tradeoffs and the health of Amazonian peoples.
A multifaceted and plastic immune system
Although often conceptualised as a single system, the immune system is actually a complex suite of diverse responses specialised for different tasks. As such, these responses have different costs and benefits for dealing with and preparing for particular threats, and trade-offs exist between branches of immune function. Organisms are expected to regulate immune function carefully because responding to disease is costly: immune responses use energy that could be used for other fitness relevant demands; many responses have the potential to cause collateral damage to the organism itself (Ashley et al., 2012); and illness can involve opportunity costs due to sickness behavior (e.g. reduced productivity) (Stieglitz et al., 2015b). Across species, mounting an immune response decreases growth and reproduction, as resources used for immunity are not available for these competing demands (Sheldon and Verhulst, 1996; Klein and Nelson, 1999; Uller et al., 2006; Blackwell et al., 2010; Abrams and Miller, 2011). Since different aspects of immune defense have their own costs and benefits, across species natural selection will have acted on patterns of responsiveness, determining the relative proportions and strengths of responses to employ. Within species, natural selection is likely to have shaped immunological reaction norms determining how phenotypes vary in response to environmental cues (McDade, 2003; Ashley et al., 2012).
Differences in parasite and pathogen exposure have been a major force shaping genetic variation across human populations (Fumagalli et al., 2011; Karlsson et al., 2014), and most immune function parameters have a heritable component, including IgE levels (Grant et al., 2008), baseline C-reactive protein (CRP) (Retterstol et al., 2003; Wu et al., 2012), and lymphocyte percentages (Hall et al., 2000). However, heritability estimates for immune function parameters are generally in the range of 30% – 60%, even when environmental variance is low and pathogen exposure limited (Brodin et al., 2015). This suggests that most of the variance in human immune function is attributable to environmental factors or reaction norms.
Table 1 describes the diverse immunological parameters measured in this study. They can be categorised in a variety of ways, but one fundamental distinction is between innate and adaptive immunity. With short generation times, pathogens evolve faster than the hosts they infect. To counteract this, the adaptive immune system evolved to generate variation in immunity within individual lifetimes, by randomly reorganising gene segments for immunoglobulins and T cell receptors and then selecting the most useful variants from this large pool (Flajnik and Kasahara, 2010). Levels of immunoglobulins, T cells, and B cells can be used to characterise investment into adaptive immunity, with T and B cells further separated into naïve cells, which represent the size of the random pool generated in preparation for pathogenic threats an organism has not yet encountered, and non-naïve cells, which represent protection against pathogens previously encountered in an organism’s lifetime.
Table 1.
Immunological parameters measured in this study
| Name | Description |
|---|---|
| Immunoglobulins | Also known as antibodies. Small molecules secreted by plasma cells which bind to specific antigens on pathogens or parasites. Immunoglobulins possess a hypervariable region produced through random recombination of gene segments. |
| IgG | The most common class of antibody. Important in immunological memory of previously encountered antigens. The only class of antibody that can cross the placenta and protect a foetus. |
| IgE | In most populations, the least common class of antibody, but produced in much higher levels in many Amazonian populations (Blackwell et al., 2011; Martin et al., 2013). Produced in response to macroparasitic infections and also in allergic responses. |
| IgM | Predominates in the primary immune response to a novel antigen. |
| IgA | Often studied in its secretory form in saliva and other mucosal secretions. Serum IgA may serve as a second line of defense when mucosal membranes are breached (Woof and Kerr, 2004). |
| Leukocytes | All white blood cells |
| Neutrophils | Abundant granulocytes that respond to acute infections by migrating to sites of infection where they phagocytise invading microbes. |
| Basophils | The least common granulocyte. Bind IgE and aid in defense against macroparasites and in allergic responses. Migrate to the invasion sites of ectoparasites such as ticks and mosquitos. Secrete IL-4 and stimulate IgE production. |
| Monocytes | Phagocytic cells that migrate to infections and injuries where they differentiate into macrophages. |
| Eosinophils | Defend against macroparasites and contribute to allergic responses. Activated by cytokines secreted by Type 2 helper T cells |
| Lymphocytes | Characterised by the presence of CD45 and by size and granularity in flow cytometry. |
| Helper T cells (CD4) | Recognises specific antigen to signal CD8 and macrophages to kill. Signals B cells to produce antibody. Both CD4 and CD8 T-cells have unique T-cell receptors on their surfaces produced by rearrangement of gene segments. |
| Cytotoxic T cells (CD8) | Induce apoptosis in cells infected with viruses or other intracellular pathogens. |
| Naïve T cells | Both CD4 and CD8 T cells begin life as naïve cells which have not been previously exposed to antigen. Naïve cell pools consist of many different lineages with randomly generated T-cell receptors that have not been selected upon for antigen reactivity. |
| Non-naïve T cells | When naïve T cells encounter an antigen to which they can bind, they undergo clonal expansion, becoming effector, memory, and regulatory T cells. These daughter lineages retain the antigen specificity of the original clone. |
| B cells | Recognises specific antigens. Includes plasma cells which produce immunoglobulins. |
| Natural Killer Cells | Kills virally infected cells by recognising non-specific molecules. May also play a role in defense against helminths. |
| Inflammatory Markers | |
| Erythrocyte Sedimentation Rate (ESR) | The rate at which red blood cells (erythrocytes) sediment to the bottom of a capillary tube. Used as an inflammatory marker since it is primarily related to the presence of fibrinogen, which facilitates blood clotting and is elevated as part of the acute phase response. |
| C-reactive Protein (CRP) | An acute phase reactant produced in response to inflammation. Binds to dead or dying cells and bacteria to promote phagocytosis. Elevated in acute infection and for one to two weeks after. Is frequently elevated in Amazonian populations, though baseline levels may not be high (McDade et al., 2005, 2012; Gurven et al., 2008; Blackwell et al., 2010). |
Innate defenses are generic, non-specific defenses that do not need to be acquired over the lifetime. Innate defenses include both non-cellular defenses such as fever, CRP, and fibrinogen, and cellular defenses such as neutrophils, monocytes, and natural killer cells. Innate and adaptive immunity work together, and functional immune responses depend on both types of response. Yet, several lines of evidence suggest that organisms might vary their relative investment in and reliance on innate versus adaptive immunity depending on their life histories, ontogenetic influences, and environmental circumstances (McDade et al., 2016). For example, compared to specific responses, non-specific inflammatory responses are more likely to cause collateral damage through oxidative stress, and are associated with long-term risks of several chronic diseases, including atherosclerosis, cardiovascular disease, obesity, and depression (Ridker et al., 1998; Pearson et al., 2003; Stieglitz et al., 2015b). An inflammatory response is often the body’s first line of defense against infections and wounds, and the strength of the response is expected to vary based on ecological and phenotypic conditions. If an organism has low energy stores or high extrinsic mortality risk, prolonged inactivity and illness might otherwise limit mating opportunities or result in fatal energy shortages. Thus innate defenses which can quickly clear a pathogen, such as inflammation, may be preferred. In longer living organisms with lower mortality risk and sufficient energy stores for prolonged immune activation, defenses that limit collateral damage may be preferred, even if they take longer to achieve pathogen clearance (Martin et al., 2008; Previtali et al., 2012). For these organisms, investing in slower more specific defenses may take precedence over investing in immediate, high cost innate responses. Similarly, organisms exposed to a high diversity of pathogens and parasites should invest more in generating diverse pools of naïve T and B cells, and should maintain larger pools of memory cells, particularly if diverse exposure is coupled with repeated exposure to the same pathogens over time, or if exposures occur earlier in life when developmental trajectories are determined.
The Tsimane
The Tsimane are a rapidly expanding (3.6% annual growth rate (Gurven et al., 2014)) population of about 15,000 semi-sedentary forager-horticulturalists that live in over 90 villages along the Maniqui River and surrounding areas in lowland Beni, Bolivia. Tsimane subsist by hunting, fishing, and cultivation of plantains, rice, corn and manioc. Their diet consists of 74% plant products and 26% animal products, with <10% of calories coming from purchased foods (Martin et al., 2012). In general, Tsimane are short and lean, but not undernourished (Foster et al., 2005). Tsimane are predominately a natural fertility population, with a total fertility rate of 9.1 births per woman (Gurven et al., 2007; McAllister et al., 2012; Blackwell et al., 2015). Tsimane breastfeed their infants on-demand, with a mean weaning age of 19 months (Veile et al., 2014).
Tsimane are exposed to a wide array of pathogens and parasites, including high prevalences of hookworm (50%), giardia (37%), and roundworm (15%) that persist despite annual treatment by physicians working on the Tsimane Health and Life History Project (THLHP) (Blackwell et al., 2013; Martin et al., 2013). Common afflictions include upper and lower respiratory infection, gastrointestinal problems, skin infections, urinary tract infections, and traumatic injuries. During annual medical visits conducted by the THLHP (www.unm.edu/~tsimane), 75% of adults report being sick enough to remain in bed for the day at least once in the last three months (Gurven et al., 2012b). Tsimane are also exposed to a variety of sexually transmitted diseases (Stieglitz et al., 2012). Prior to 1990, approximately 14% of infants died in their first year (Gurven et al., 2007), and infant mortality remains relatively high in recent years at 4.6% (Kaplan et al., 2015). Using retrospective data, about half of Tsimane deaths between 1950–2000 were due to infectious disease (Gurven et al., 2007). A more recent analysis of deaths occurring between 2005–2010 similarly shows that 50% of deaths were due to infectious disease (unpublished data). Retrospective demographic interviews and recent THLHP medical exams utilising thoracic computed tomography scans also attest to untreated tuberculosis as a ubiquitous problem for older Tsimane.
Health in Amazonian populations
Although diversity in diet and residential mobility exists among indigenous Amazonian populations, with many living as sedentary or semi-sedentary slash-and-burn horticulturalists, others as peasant agriculturalists, or as mobile foragers, there are similarities common to most Amazonians inhabiting similar mixed neotropical, subtropical, and savanna biomes. Extremely high levels of IgE, indicative of parasite infections, have been documented in indigenous groups in Venezuela, Ecuador, and Bolivia (Blackwell et al., 2010; Martin et al., 2013). High prevalences of helminth infection, particularly Ascaris lumbricoides, have been documented in the Shuar of Ecuador, Yanomamo of Brazil, and many other Amazonian populations (Salzano and Callegari-Jacques, 1988; Hurtado et al., 2008; Cepon-Robins et al., 2014). Tuberculosis is prevalent and a source of morbidity and mortality in many indigenous South American populations (Hill and Hurtado, 1996; Hurtado et al., 2004). Skin diseases, including abscesses, leishmaniasis, and fungal infections are common (Salzano and Callegari-Jacques, 1988; Hern, 1991; Martin et al., 2013). Thus, although this study focuses on a single population, its results have broader implications for understanding health and well-being of numerous Amazonian populations.
The present study
Given their pathogenic environment we propose that Tsimane immune function is characterised by differences in the distributions and age profiles of many biomarkers when compared to US and European populations. Previous studies have documented high IgE, leukocyte counts, and ESR in Tsimane, but have not examined leukocyte subsets or other immunoglobulins, or described how all of these parameters differ by age cohorts. Here we measure and describe Tsimane child and adult blood differentials, CRP, ESR, and four classes of immunoglobulin (IgE, IgG, IgA, and IgM). We use flow cytometry to quantify naive and non-naïve CD4 and CD8 T cells, natural killer cells, and B cells. We then discuss how these measures may be related to Tsimane health and life histories, and potential implications for public health in both pre-industrial and industrialised populations.
Methods
Study population
Since 2002 the Tsimane have participated in the THLHP. All Tsimane residing in ~90 study villages are eligible to participate, and most choose to do so at least once. To date, 44,635 medical exams have been conducted along with 20,268 biospecimen collections from 8,569 individuals. Prior to 2010, data was collected in 33 villages by mobile medical teams, with all individuals present in the village encouraged to participate. However, since the Tsimane are a rapidly expanding population, there are many more young people than old. In an effort to increase sampling across older ages, beginning in 2010 an additional 44 communities were sampled and all individuals over age 40, as well as their dependent children, were offered transport to the town of San Borja (pop. ~25,000) for exams in our permanent clinic. Data for this study includes individuals from 77 communities, including ~90% of the population over age 40 and ~40% of adults and children under 40. The Tsimane ethnographic context and project details, including methods of demographic data collection, have been described in further detail elsewhere (Gurven et al., 2007; Blackwell et al., 2011).
Ethics approval
The study was reviewed and approved by the Gran Consejo Tsimane, the governing body overseeing Tsimane affairs and research projects, and by the IRBs of the University of California-Santa Barbara (UCSB) and the University of New Mexico. Informed consent was obtained at two levels. During a community meeting open to all residents, communities decided collectively whether the study would be conducted. To date, all communities that have been approached have approved the study. Individuals in the study also gave informed consent before each medical visit and interview. For minors, both parental consent and child assent was obtained.
Medical surveillance
Study participants were seen by the mobile THLHP biomedical team who visited Tsimane villages roughly once per year from 2004–2013. Beginning in 2010, some patients were also transported to our clinic in San Borja for exams. Patients seen by THLHP physicians were given routine physical exams (including medical history, symptom investigation and clinical diagnoses, blood pressure and temperature, anthropometrics). Following on-site analysis of blood and fecal samples for indicators of infection, physicians administered vitamins and medications as warranted.
Blood sample collection
For most participants blood was collected by venipuncture in a heparin-coated vacutainer. For infants <2 years old, blood was drawn with a capillary heel or finger prick, into a collection tube with heparin. Immediately following the blood draw, total leukocyte counts, lymphocyte/monocyte counts, hemoglobin, red blood cell count, and granulocyte count were determined with a QBC Autoread Plus dry hematology system (QBC Diagnostics), with a QBC calibration check performed daily to verify QBC performance. Relative fractions of neutrophils, eosinophils, lymphocytes, basophils, and monocytes were determined manually by microscopy with a hemocytometer by a certified Bolivian biochemist. ESR was calculated following the Westergren method (Westergren, 1957).
Total leukocytes as determined by hemocytometer counts were highly correlated with total leukocytes by QBC (r = 0.76, p <0.001), though QBC values were on average ~15% higher. Manual lymphocyte counts and neutrophil counts were also highly correlated with QBC counts (r = 0.84, p<0.001; r = 0.59, p<0.001). Over the course of 10 years of data collection, 7 certified biochemists performed 98% of the manual differentials. Each examined different patient samples, but obtained similar median differentials: neutrophils (42–54%), eosinophils (13–20%), basophils (0% for all biochemists), lymphocytes (28–34%), and monocytes (0–1%). In a direct comparison of three of these biochemists independently performing manual differentials on three patient samples, coefficients of variation (CVs) for neutrophils, eosinophils, basophils, lymphocytes, and monocytes were 2.18%, 1.85%, 0.26%, 1.99%, and 0.35%, respectively. Median erythrocyte sedimentation rates were also similar across biochemists (range: 24–31 mm/h).
Flow cytometry
25ul of fresh, whole, heparinised blood was incubated with multiple combinations of fluorescently labeled antibodies (eBioscience, Inc.) for CD4 (PE or FITC, Clone RPA-T4), CD8 (APC, RPA-T8), CD19 (APC, SJ25C1), CD56 (PE, CMSSB), and CD45RA (PerCP-Cy5.5, HI100). Following incubation for 30 minutes at 4°C, 500ul 1-step Fix/Lyse (eBioscience #00-5333-57) was added and blood was incubated an additional 30 min. Samples were then centrifuged, the supernatant was discarded, and cells were resuspended in 100ul PBS. Samples were read on an Accuri C6 Flow Cytometer (BD Accuri Cytometers). Given the large number of samples, FCS data files were exported from CFlow Plus (BD Accuri Cytometers) and imported into R 2.14.1. Cells were gated for lymphocytes using lymphGate from the Bioconductor package for R (http://www.bioconductor.org/) and florescence gates were determined using a combination of Bioconductor rangeGate and custom R scripting written to find low points in density functions. T cells were classified as CD4+CD8− helper or CD8+CD4− cytotoxic, and were further divided into naïve (CD45RA+) and non-naïve (CD45RA-) subsets. B cells were identified as CD19+ and natural killer cells as CD56+CD8−CD4−. Absolute counts were calculated by multiplying the relative percentages determined by flow cytometry by the total lymphocyte count obtained from the QBC Autoread Plus. CD4, CD8, Natural Killer, and B cell percents were measured in duplicate. Mean CVs across duplicates were 15.6%, 15.2%, 15.3% and 18.9%, respectively. Some individuals were also measured at multiple timepoints, indicating consistency in individual measures across time (see Results section).
Serum biomarkers
Serum immunoglobulins from samples collected in 2004–2006 were measured by TriCore Laboratories (Albuquerque, NM) using an Immulite 2000 (Siemens Corp; Deerfield, IL). An additional 652 IgE and IgG levels were quantified at the University of Oregon (UO) by ELISA (Bethyl Labs, Inc.: #E80-108, #E80-104 and #E101). IgE samples at UO were run in duplicate. Mean within and between plate CVs were 3.7% and 8.6%. Liquichek Immunology Controls (BioRad) were also run and fell within the manufacturers specifications. Distributions of IgE samples measured by ELISA did not differ from earlier distributions obtained by Tricore (Kolmogorov–Smirnov test: D = 0.05, p = 0.83). IgE values for individuals measured by both laboratories from samples taken at different times were also highly correlated (see Results).
For IgG values run at UO, mean within and between plate CVs were 4.8% and 18.8%, respectively. Distributions between the two labs differed significantly (Kolmogorov–Smirnov test: D = 0.20, p < 0.001), but were similar enough that these differences do not change interpretation relative to other populations: e.g. percentiles for Tricore and UO, respectively, were 10th: 1,458 and 1,510, 25th: 1,719 and 1,680, 50th: 2,071 and 1,910, 75th 2,541 and 2,200, and 90th 2,964 and 2,492 mg/dL. As with IgE, IgG values for the same individuals measured at different times and analysed by different labs were correlated, though more weakly (see Results).
CRP was measured in two batches, a first batch (n=650) was measured at the UO, and a second (n=885) at UCSB’s Human Biodemography lab via enzyme immunoassay (Brindle et al., 2010). Results from both the UO and UCSB laboratories were validated against one another with matched samples, and then further validated against the University of Washington Medical Center (UWMC), which was responsible for analysing samples for the National Health and Nutrition Examination Survey (NHANES), which is used here as a comparison group. Twenty seven serum specimens ranging in CRP from 0.08− 27.33 mg/dL were sent to UWMC, where serum was analysed for CRP via nephelometry using the same protocols and equipment as NHANES III (Wong et al., 2001). Results from UWMC were highly correlated r2=0.973, p<0.001 and not statistically different according to Tukey’s HSD (Mean difference = 0.01, HDS= 0.0096). For UO samples, mean within plate CV was 3.8%. Between plates, CVs were 12.7% and 9.9% for the high and low controls. At UCSB, within and between plate CVs were 7.3% and 10.2% for the high and 5.3% and 9.2% for the low controls.
Sampling schedule and inclusion criteria
Given the longitudinal nature of medical surveillance, measures were added and removed during different sampling rounds, and sampling procedures were sometimes affected by logistical difficulties (e.g., weather related transportation issues, reagent availability). Final sample sizes therefore vary by measure, and some individuals were measured repeatedly in multiple years. Blood differentials were collected for all individuals at all medical exams. Immunoglobulins and CRP were measured on multiple subsets of random individuals, stratified to sample across a range of ages. Flow cytometry was measured on all individuals brought to the THLHP clinic in San Borja between late 2011 and 2014. Number of individuals and number of observations for each set of measures are as follows: IgE 811/902, IgG 1062/1286, IgA and IgM 604/604, CRP 1308/1828, blood differential 6338/11812, and flow cytometry 1434/1734. Number of observations by age group are given in Tables 2–4. The temporal overlap of immunological measures collected by the THLHP is shown in Figure 1. Since infections and other medical complaints are common, with <10% of individuals having no medical complaint at any medical visit, we have not excluded individuals with medical complaints from analysis.
Table 2.
Serum immunoglobulins and C-reactive protein by age group
| 0 to 5 | 6 to 11 | 12 to 17 | 18 to 49 | 50+ | ||
|---|---|---|---|---|---|---|
| IgE | Tsimane | 6963 (1677–20966) | 10077 (1836–28460) | 9091 (3440–21457) | 8949 (2246–23015) | 8731 (1856–25663) |
| IU/mL | Reference | 31 (3–399) | 63 (6–897) | 60 (5–895) | 52 (5–663) | 44 (4–638) |
| 18/18, p<0.001 | 91/91, p<0.001 | 51/51, p<0.001 | 467/467, p<0.001 | 269/269, p<0.001 | ||
|
| ||||||
| IgG | Tsimane | 1625 (1138–2506) | 1900 (1351–2643) | 1965 (1377–2741) | 1960 (1322–2878) | 2140 (1378–3540) |
| mg/dL | Reference | 686 (435–1026) | 961 (671–1329) | -- | 994 (691–1278) | 994 (691–1278) |
| 20/20, p<0.001 | 145/146, p<0.001 | n=100 | 679/685, p<0.001 | 328/329, p<0.001 | ||
|
| ||||||
| IgA | Tsimane | 140 (114–976) | 176 (106–827) | 199 (130–336) | 280 (157–512) | 351 (206–597) |
| mg/dL | Reference | 54 (22–110) | 102 (46–191) | -- | 171 (82–280) | 171 (82–280) |
| 18/18, p<0.001 | 91/94, p<0.001 | n=71 | 305/330, p<0.001 | 88/91, p<0.001 | ||
|
| ||||||
| IgM | Tsimane | 162 (111–858) | 164 (110–382) | 170 (114–326) | 176 (106–916) | 164 (104–930) |
| mg/dL | Reference | 93 (49–156) | 114 (58–199) | -- | 156 (67–305) | 156 (67–305) |
| 18/18, p<0.001 | 87/94, p<0.001 | n=71 | 202/331, p<0.001 | 55/90, p=0.045 | ||
|
| ||||||
| CRP | Tsimane | 0.84 (0.65–2.70) | 1.25 (0.18–21.10) | 1.21 (0.56–20.58) | 1.74 (0.31–13.61) | 2.30 (0.44–15.06) |
| mg/L | Reference | 0.30 (0.10–9.75) | 0.40 (0.10–8.18) | 0.40 (0.10–8.07) | 1.80 (0.20–16.50) | 2.50 (0.40–17.74) |
| 3/3, p=0.250 | 63/69, p<0.001 | 29/29, p<0.001 | 456/941, p=0.361 | 366/771, p=0.171 | ||
Reported values are the median and 5th and 95th percentiles, in parentheses. Test statistics show the proportion of Tsimane samples above the median reference value relative to the total sample size. P-value is the exact binomial test of the null that that the proportion above the reference median is 0.5. IgE and CRP references are from NHANES 2005–2006. IgG, IgA and IgM references are from Jolliff et al (1982) which gives values for ages under 12 and 18–62, but does not distinguish those above and below fifty or give values for ages 12–17. Reference ranges in Jolliff et al (1982) were reported as the 2.5th to 97.5th percentiles. Rather than report non-equivalent ranges, these ranges were used to estimate the 5th and 95th percentiles as described in Methods. Adjusted ranges are reported here and in Figures.
Table 4.
Lymphocyte subsets by age group
| 0 to 5 | 6 to 11 | 12 to 17 | 18 to 49 | 50+ | ||
|---|---|---|---|---|---|---|
| CD4 T cells | Tsimane | 1940 (820–4267) | 1178 (616–2637) | 1201 (579–1667) | 660 (343–1258) | 549 (289–1108) |
| Cells/μl | Reference | 2076 (951–3720) | 980 (579–1692) | 840 (465–1471) | 737 (496–1134) | 653 (327–1031) |
| 162/351, p=0.165 | 310/458, p<0.001 | 31/38, p<0.001 | 183/459, p<0.001 | 129/383, p<0.001 | ||
|
| ||||||
| Naïve CD4 | Tsimane | 1393 (432–3409) | 678 (271–1713) | 580 (266–1123) | 113 (31–424) | 66 (12–267) |
| Cells/μl | Reference | 1565 (639–2884) | 570 (272–1173) | 400 (197–927) | 380 (182–664) | 279 (66–614) |
| 146/349, p=0.003 | 275/456, p<0.001 | 31/38, p<0.001 | 29/457, p<0.001 | 18/381, p<0.001 | ||
|
| ||||||
| Non-Naive CD4 | Tsimane | 566 (275–1191) | 494 (246–1056) | 523 (240–742) | 515 (278–962) | 455 (231–924) |
| Cells/μl | Reference | 512 (315–836) | 410 (310–529) | 440 (269–559) | 357 (310–468) | 374 (254–405) |
| 219/349, p<0.001 | 317/456, p<0.001 | 28/38, p=0.005 | 374/457, p<0.001 | 266/381, p<0.001 | ||
|
| ||||||
| CD8 T cells | Tsimane | 1234 (492–2618) | 814 (319–1861) | 618 (221–1062) | 407 (197–904) | 390 (158–849) |
| Cells/μl | Reference | 1086 (533–2040) | 680 (311–1261) | 530 (289–1076) | 439 (215–760) | 302 (137–674) |
| 201/351, p=0.008 | 299/458, p<0.001 | 24/38, p=0.143 | 198/459, p=0.004 | 265/383, p<0.001 | ||
|
| ||||||
| Naïve CD8 | Tsimane | 1009 (381–2125) | 632 (244–1389) | 479 (174–794) | 230 (89–536) | 187 (65–500) |
| Cells/μl | Reference | 900 (423–1756) | 540 (265–1040) | 400 (208–835) | 194 (109–372) | 93 (43–211) |
| 203/349, p=0.003 | 287/456, p<0.001 | 23/38, p=0.256 | 285/457, p<0.001 | 339/381, p<0.001 | ||
|
| ||||||
| Non-Naïve CD8 | Tsimane | 175 (55–739) | 162 (49–496) | 149 (42–338) | 180 (68–406) | 181 (66–399) |
| Cells/μl | Reference | 186 (111–286) | 140 (47–221) | 130 (81–241) | 245 (105–387) | 209 (94–463) |
| 161/349, p=0.164 | 268/456, p<0.001 | 23/38, p=0.256 | 132/457, p<0.001 | 150/381, p<0.001 | ||
|
| ||||||
| Natural Killer | Tsimane | 324 (109–1258) | 452 (153–1158) | 413 (166–1027) | 568 (218–1319) | 610 (225–1268) |
| Cells/μl | Reference | 394 (137–1223) | 230 (79–591) | 190 (53–624) | 184 (100–294) | 184 (74–472) |
| 140/351, p<0.001 | 394/458, p<0.001 | 34/38, p<0.001 | 445/459, p<0.001 | 373/383, p<0.001 | ||
|
| ||||||
| B Cells | Tsimane | 1380 (570–2882) | 759 (372–1576) | 654 (338–1283) | 340 (168–698) | 302 (115–671) |
| Cells/μl | Reference | 1128 (453–2581) | 480 (229–1015) | 300 (83–684) | 189 (103–369) | 163 (75–447) |
| 221/351, p<0.001 | 383/458, p<0.001 | 38/38, p<0.001 | 420/459, p<0.001 | 335/383, p<0.001 | ||
|
| ||||||
| CD4/CD8 Ratio | Tsimane | 1.6 (0.8–3.5) | 1.5 (0.8–2.9) | 1.6 (0.9–3.4) | 1.7 (0.8–3.2) | 1.5 (0.7–3.1) |
| Reference | 1.7 (1.1–2.7) | 1.5 (0.9–2.4) | 1.5 (0.8–3.0) | 1.8 (0.9–3.1) | 2.3 (1.1–4.8) | |
| 158/351, p=0.069 | 236/458, p=0.544 | 22/36, p=0.243 | 180/459, p<0.001 | 53/383, p<0.001 | ||
Reported values are the median and 5th and 95th percentiles, in parentheses. Test statistics show the proportion of Tsimane samples above the median reference value relative to the total sample size. P-value is the exact binomial test of the null that that the proportion above the reference median is 0.5. Reference ranges for cell counts for age groups under 18 are from the US (Shearer et al., 2003). References for ages 18+ are from Swiss (Bisset et al., 2004). References for CD4/CD8 ratios are from English and German subjects (Bofill et al., 1992; Jentsch-Ullrich et al., 2005). Published reference ranges were sometimes reported as either the 10th and 90th or the 2.5th to 97.5th. Rather than report non-equivalent ranges, these ranges were used to estimate the 5th and 95th percentiles as described in Methods. Adjusted ranges are reported here and in Figures.
Figure 1.
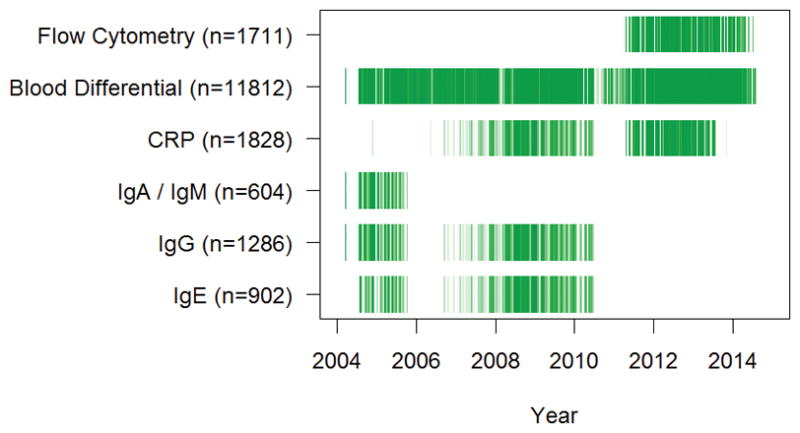
Tsimane sampling history. Darker shading indicates that more samples were analysed at a given time-point.
Analysis
Many blood markers have skewed distributions, making presentation of results in terms of mean and standard deviation problematic. For simplicity, we therefore present descriptive results in terms of the median value and the 5th and 95th percentile values. Since most individuals were measured only once, except for blood differentials where most were measured twice, we present the distribution of values for each measure and have not adjusted for repeat sampling of some individuals. Results are compared to values from published reference ranges (Hollinger and Robinson, 1953; Jolliff et al., 1982; Bofill et al., 1992; Wetteland et al., 1996; Shearer et al., 2003; Bisset et al., 2004; Jentsch-Ullrich et al., 2005) as well as values from NHANES 2005–2006 and 2009–2010 (http://www.cdc.gov/nchs/nhanes.htm). Some published reference values include non-equivalent ranges (e.g. 10th – 90th or 2.5th – 97.5th instead of 5th to 95th). For these reference ranges approximate 5th and 95th percentile values were estimated by assuming log-normality and using the following equation to adjust the provided values:
where x̃ is the median, value is the provided upper or low interval value, and Zscore is the number of standard deviations the provided percentile value is from the mean in a normal distribution (e.g. 1.96 for the 97.5th percentile). To test whether Tsimane values differ from reference medians we use an exact binomial test to determine whether the proportion of Tsimane values falling above or below the reference median is significantly different from 0.5.
For basophils and monocytes, a large number of individuals had zero counts. However, these zeros may have been due to sampling only 100 cells per individual for blood differentials. To estimate the most likely underlying distributions given the observed data and sampling, we fit zero-inflated Poisson models using map, in the rethinking package (McElreath, 2015), and then generated prediction medians and 95% intervals from the maximum a posteriori distribution. Intraclass correlation coefficients (ICCs) were estimated with ICCest in the ICC package, using only data for individuals with at least two observations. All analyses were conducted in R 3.2.1.
Results
Tsimane have elevated inflammatory biomarkers
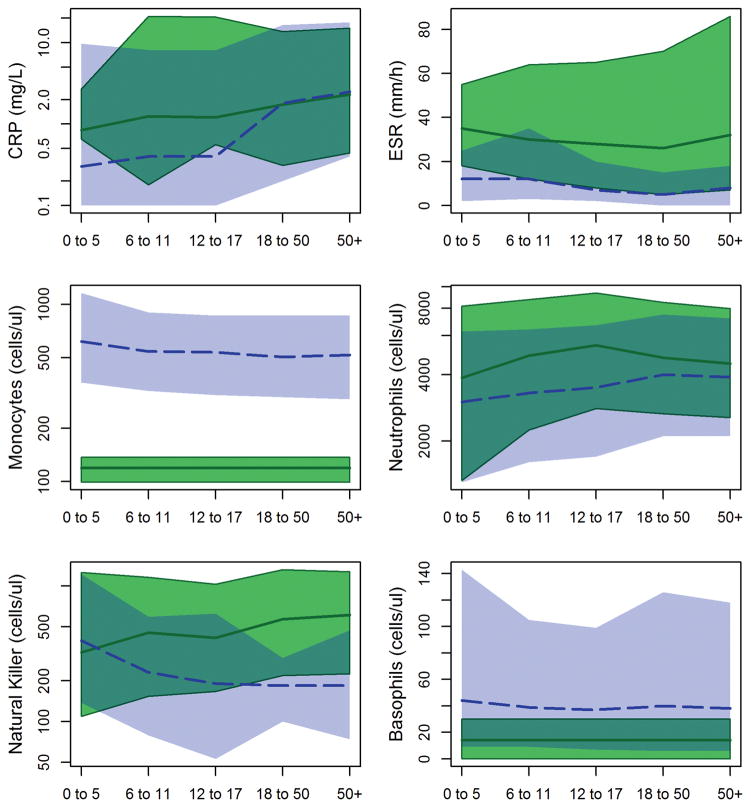
Tsimane children have elevated CRP compared to the US reference (Table 2; Figure 2). Median CRP in children <18 years old is 1.21 mg/L, compared to 0.40 mg/L in NHANES. Ninety four percent of Tsimane children have CRP greater than the NHANES median (exact binomial test of 50% proportion, p<0.001), and 9.9% of Tsimane children have CRP ≥ 10 mg/L, the cutoff commonly used as a clinical indicator of acute infection. Even excluding those with CRP ≥ 10 mg/L, median CRP in Tsimane <18 years old is 1.12 mg/L. In contrast, Tsimane ages 18–50 years have median CRP of 1.74 mg/L, and Tsimane age 50+ have median CRP of 2.30 mg/L, neither of which are significantly different from median values of 1.80 mg/L and 2.50 mg/L in NHANES.
Figure 2.
Innate immune responses in Tsimane (red) and reference populations (blue). Shading indicates the 5th and 9th percentiles, solid lines the median value. Note y-axes are logged except for ESR and basophils.
ESR is also significantly elevated compared to reference values. However unlike CRP, ESR is elevated across all age groups. Median ESR values are around 30 mm/h in all age groups (Table 3). In US populations, above 15–20 mm/h is generally considered elevated, though the cutoff varies slightly by age and sex (Wetteland et al., 1996). Ninety-five percent of Tsimane have ESR values exceeding these references medians.
Table 3.
Leukocyte subsets and erythrocyte sedimentation rates by age group
| 0 to 5 | 6 to 11 | 12 to 17 | 18 to 49 | 50+ | ||
|---|---|---|---|---|---|---|
| All leukocytes | Tsimane | 12000 (6800–21115) | 11900 (7255–19000) | 11450 (7038–17925) | 9600 (6000–15400) | 8625 (5300–13845) |
| Reference | 7900 (4900–12600) | 6900 (4400–10600) | 6600 (4200–10870) | 7000 (4400–11200) | 6700 (4300–10615) | |
| Cells/μl | Statistic | 1962/2198, p<0.001 | 2230/2323, p<0.001 | 1140/1176, p<0.001 | 3803/4426, p<0.001 | 1268/1572, p<0.001 |
|
| ||||||
| Lymphocytes | Tsimane | 5400 (2440–10693) | 4004 (2205–7146) | 3492 (1906–5622) | 2700 (1486–4660) | 2325 (1273–3996) |
| Cells/μl | Reference | 3700 (2000–6980) | 2600 (1600–4100) | 2200 (1400–3400) | 2100 (1300–3415) | 1900 (1000–3300) |
| Statistic | 924/1153, p<0.001 | 1257/1421, p<0.001 | 622/683, p<0.001 | 2694/3489, p<0.001 | 1126/1549, p<0.001 | |
|
| ||||||
| Neutrophils | Tsimane | 3872 (1323–8160) | 4865 (2240–8760) | 5434 (2801–9379) | 4774 (2657–8487) | 4480 (2551–7974) |
| Cells/μl | Reference | 3000 (1300–6280) | 3300 (1600–6400) | 3500 (1700–6700) | 4000 (2100–7500) | 3900 (2100–7200) |
| Statistic | 777/1153, p<0.001 | 1169/1421, p<0.001 | 606/683, p<0.001 | 2402/3489, p<0.001 | 979/1549, p<0.001 | |
|
| ||||||
| Basophils | Tsimane* | 14 (0–30) | 14 (0–30) | 14 (0–30) | 14 (0–30) | 14 (0–30) |
| Cells/μl | Reference | 44 (9–143) | 39 (9–105) | 37 (7–99) | 40 (6–126) | 38 (6–118) |
| Statistic | 1/1153, p<0.001 | 9/1418, p<0.001 | 12/683, p<0.001 | 48/3488, p<0.001 | 22/1549, p<0.001 | |
|
| ||||||
| Eosinophils | Tsimane | 1350 (125–5091) | 2277 (608–5984) | 1908 (356–5574) | 1528 (323–4108) | 1408 (403–3536) |
| Cells/μl | Reference | 190 (60–646) | 187 (56–718) | 157 (52–522) | 157 (50–457) | 170 (53–479) |
| Statistic | 1067/1153, p<0.001 | 1403/1421, p<0.001 | 665/683, p<0.001 | 3402/3489, p<0.001 | 1525/1549, p<0.001 | |
|
| ||||||
| Monocytes | Tsimane* | 119 (99–137) | 119 (99–137) | 119 (99–137) | 119 (99–137) | 119 (99–137) |
| Cells/μl | Reference | 618 (361–1161) | 541 (324–901) | 536 (308–866) | 504 (299–865) | 518 (291–871) |
| Statistic | 17/1150, p<0.001 | 25/1418, p<0.001 | 2/682, p<0.001 | 15/3486, p<0.001 | 16/1549, p<0.001 | |
|
| ||||||
| ESR | Tsimane | 35 (18–55) | 30 (12–64) | 28 (8–65) | 26 (5–70) | 32 (7–86) |
| mm/h | Reference | 12 (2–25) | 12 (3–35) | 7 (2–20) | 5 (0–15) | 8 (0–18) |
| Statistic | 1383/1402, p<0.001 | 1621/1710, p<0.001 | 828/869, p<0.001 | 3668/3892, p<0.001 | 1470/1569, p<0.001 | |
Reported values are the median and 5th and 95th percentiles, in parentheses. Test statistics show the proportion of Tsimane samples above the median reference value relative to the total sample size. P-value is the exact binomial test of the null that that the proportion above the reference median is 0.5, except for basophils test is the exact binomial test of the null that the proportion above zero is 0.37, the proportion in the reference population. Reference values are from NHANES 2009–2010. ESR reference values are approximate values estimated from (Hollinger and Robinson, 1953; Wetteland et al., 1996).
Tsimane basophil and monocyte values are the posterior distributions estimated from zero-inflated Poisson models of the observed distributions.
Tsimane have elevated lymphocytes, neutrophils and eosinophils, but lower monocytes and basophils
Tsimane total leukocyte (WBC) counts are consistently elevated relative to US references (Table 3). Median WBC values by age group range from 12.0×103 cells/μl in children <6 years old to 8.6×103 cells/μl in adults aged 50+. Comparable values in NHANES are 7.9 and 6.7×103 cells/μl, making Tsimane values approximately 1.5 times higher. Elevations are apparent in most, but not all WBCs. Lymphocytes and neutrophils are elevated by 1.2–1.6 times reference medians (Table 3; Figure 2). Eosinophils, which respond to parasitic infections, are the most dramatically elevated, with counts ~7 times higher than reference values in young children and adults, and ~10 times higher in juveniles and adolescents (Figure 3). Ninety-seven percent of Tsimane have eosinophil values higher than the NHANES median, and 89% have counts higher than the NHANES 95th percentile.
Figure 3.
Humoral and TH2 activated immune responses in Tsimane (red) and reference populations (blue). Shading indicates the 5th and 9th percentiles, solid lines the median value. Note y-axes are logged.
In contrast, monocytes and basophils are rarely detected in Tsimane samples (Figure 2). Seventy two percent of Tsimane have no monocytes observed in manual cell counts, and <1% have basophils observed (Table 3). This is unlikely to be due to human error, as low monocyte and basophil counts have been observed by seven different certified biochemists consistently over ten years. However, rare monocytes and basophils might have been overlooked since biochemists counted only 100 cells to determine differentials. To address this, we used a zero-inflated Poisson model to estimate the likely posterior distribution for cell counts given observations with counts of 100 cells. For monocytes, the median of the posterior is 119 cells/μl (95% prediction interval: 99 – 137 cells/μl). For basophils, the posterior distribution is estimated to be only 14 cells/μl (95% prediction interval: 0 – 30 cells/μl). Both of these values are significantly below reference ranges (Figure 2), with basophils ~20% of reference values and monocytes ~35%.
Tsimane have elevated natural killer cell counts
In American and European populations, natural killer cell concentrations decline with age. For Tsimane, precisely the opposite is observed as concentrations are higher in older age groups. Tsimane children <5 years old have natural killer cell counts that resemble reference populations, while juveniles and adolescents have roughly double the number of cells seen in age-matched Americans and Europeans, and adults roughly triple the number (Table 4; Figure 1).
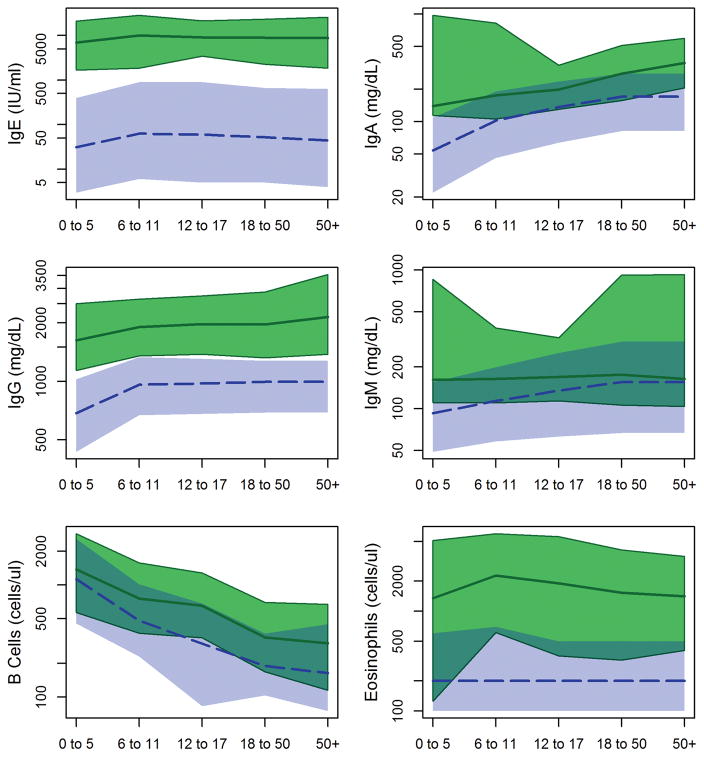
Tsimane have elevated immunoglobulin and B cell levels throughout life
Tsimane have elevated levels of all major classes of immunoglobulins across all ages (Table 2; Figure 3). Total level of serum IgM, the class of antibody produced immediately upon exposure to a novel antigen, is elevated in Tsimane children ≤ 5, with median concentration 1.7 times higher than US children and a positively skewed distribution. Relative elevation of IgM declines by age group, but is significantly higher at all ages (Figure 3). Median concentrations of serum IgE, a class of antibodies produced in response to macroparasitic infections such as helminths, are 150–200 times higher in Tsimane than in age-matched Americans, with 100% of Tsimane having IgE above reference medians. Distributions of total serum IgG and IgA levels are approximately double US reference distributions, with <1% of Tsimane having IgG lower than the reference median and 6% having lower IgA than the reference median.
Consistent with the pattern of elevated immunoglobulins, Tsimane adolescents and adults have roughly twice as many B cells as American and Swiss reference populations (Table 3; Figure 3). In both Tsimane and reference populations, B cell counts are lower in older age groups; however, this decline is attenuated among Tsimane. (Table 4).
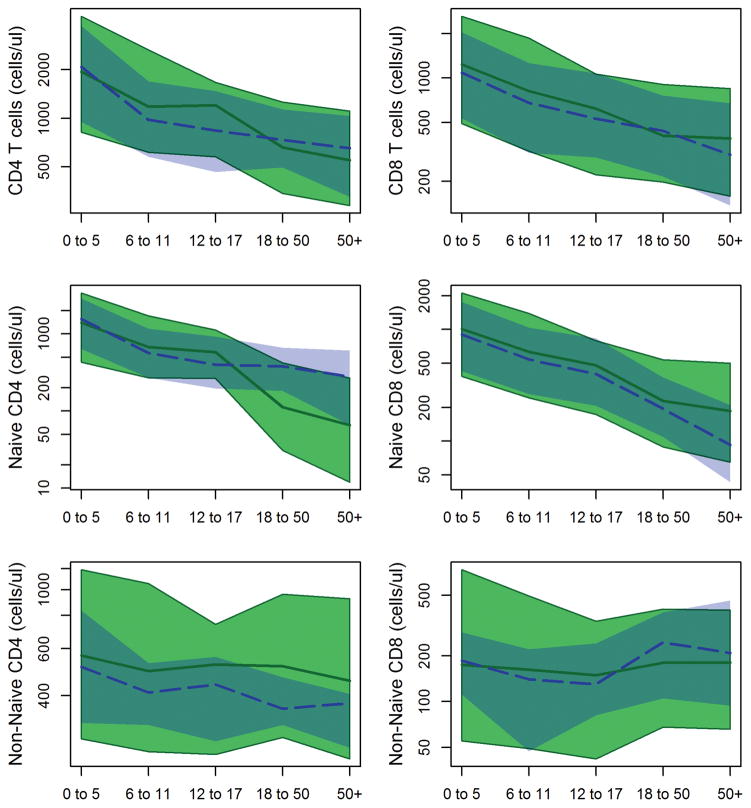
Tsimane naïve CD4 T-cell pool declines with age, while the naïve CD8 T-cell pool is maintained
Tsimane children ≤ 5 years old do not have significantly different numbers of CD4 helper T-cells than reference populations (Table 4; Figure 4). However, Tsimane have significantly more CD4 cells from ages 6–18 years, and significantly fewer cells in both adult age groups. When naïve and non-naïve (i.e. memory) pools are examined separately, we find that Tsimane have significantly elevated non-naïve CD4 cells at all ages. Naive CD4 cells show a pattern of increased production through childhood, followed by greater depletion in adulthood; Tsimane children ages 6–17 years have significantly more naïve CD4 cells than reference populations, while adult Tsimane have significantly fewer cells.
Figure 4.
Naïve and non-naïve T cell counts in Tsimane (red) and reference populations (blue). Shading indicates the 5th and 9th percentiles, solid lines the median value. Note y-axes are logged.
Across age groups, median Tsimane total CD8 T-cell counts are moderately higher than in reference populations, though with considerable overlap in distributions. Across all age groups, Tsimane naive CD8 counts are about 20% higher than a Swiss reference sample. Non-naïve CD8 cells are moderately higher in mid-childhood, and appear to maintain similar levels into adulthood. This stands in contrast to the reference sample where non-naïve CD8 cells are higher in adults, exceeding Tsimane values.
Ratios of CD4 to CD8 cells are commonly used as a diagnostic tool for immunosuppression, and in particular in monitoring HIV progression. Overall Tsimane CD4/CD8 ratios are similar to reference values, with medians ranging from 1.5 to 1.7 across age groups (Table 4). In both adult groups, Tsimane CD4/CD8 ratios are moderately lower than in reference populations, reflecting the slightly higher CD8 counts and slightly lower CD4 counts in these age groups.
Immunological values are consistent for individuals across multiple measurements
Differences between Tsimane and reference populations might be due to consistent differences between individuals or due to frequent acute infections in the sample. We investigated the stability of immunological measures by calculating the ICCs for each measure for individuals with at least two measurements. ICCs for monocyte and basophil counts were low due to very little variance in these measures (most individuals had zero cells detected and the difference between zero and one was essentially a random draw). Apart from these two measures, ICCs ranged from 0.20 to 0.81 (Table 5), indicating that between 20% and 81% of the variance in these measures was due to between individual differences. The lowest ICC was for neutrophil count (ICC = 0.20, CI 0.17–0.23), possibly indicative of fluctuations from acute infections. The ICC for CRP, also commonly used as a marker of acute infection, was 0.49 (CI 0.42–0.55), suggesting both significant between and within individual differences. Naïve CD4 (ICC = 0.73, CI 0.67–0.78) and CD8 (ICC = 0.72, CI 0.65–0.77) cells were highly stable across measurements, as were B cells (ICC = 0.68, CI 0.61–0.74). Immunoglobulin E levels were incredibly stable, even with measurements an average of four years apart (ICC = 0.81, CI 0.72–0.87).
Table 5.
Intraclass correlation coefficients
| Variable | ICC | N | k | Δ Years |
|---|---|---|---|---|
| IgE | 0.81 (0.72–0.87) | 91 | 2.0 | 3.95 |
| IgG | 0.38 (0.26–0.49) | 224 | 2.0 | 3.99 |
| Leukocytes | 0.37 (0.35–0.39) | 2846 | 2.9 | 2.24 |
| Lymphocytes | 0.49 (0.46–0.51) | 1917 | 3.0 | 2.05 |
| Neutrophils | 0.20 (0.17–0.23) | 1917 | 3.0 | 2.05 |
| Basophils | 0.13 (0.10–0.15) | 1916 | 3.0 | 2.05 |
| Eosinophils | 0.30 (0.27–0.33) | 1917 | 3.0 | 2.05 |
| Monocytes | 0.08 (0.05–0.10) | 1917 | 3.0 | 2.05 |
| CRP | 0.49 (0.42–0.55) | 473 | 2.1 | 2.48 |
| ESR | 0.35 (0.32–0.37) | 2095 | 3.0 | 2.16 |
| CD4 | 0.67 (0.60–0.73) | 255 | 2.1 | 1.24 |
| Naïve CD4 | 0.73 (0.67–0.78) | 251 | 2.1 | 1.24 |
| Non-Naïve CD4 | 0.30 (0.19–0.40) | 251 | 2.1 | 1.24 |
| CD8 | 0.69 (0.62–0.75) | 255 | 2.1 | 1.24 |
| Naïve CD8 | 0.72 (0.65–0.77) | 251 | 2.1 | 1.24 |
| Non-Naïve CD8 | 0.55 (0.46–0.62) | 251 | 2.1 | 1.24 |
| Natural Killer Cells | 0.30 (0.19–0.40) | 255 | 2.1 | 1.24 |
| B Cells | 0.68 (0.61–0.74) | 255 | 2.1 | 1.24 |
| log(CD4/CD8 Ratio) | 0.41 (0.31–0.51) | 255 | 2.1 | 1.24 |
ICC = estimate and 95% confidence intervals for the intraclass correlation coefficient, i.e. the proportion of variance attributable to between individual differences. N = number of unique individuals. k = mean number of observations per individual. Δ Years = mean time in years between observations. Analysis only includes individuals with at least two observations.
Discussion
Tsimane live in a highly pathogenic environment and are chronically infected with a wide range of pathogens and parasites. Our results show that this environment has dramatic consequences for immune function. Relative to industrialised populations Tsimane show elevations in most measures of immune function across all age groups, including levels of all four major classes of antibodies, levels of lymphocytes and many lymphocyte subsets, neutrophils, eosinophils, and ESR. Compared to reference populations, the most dramatic elevations are in immunological responses to extra-cellular parasitic infections, and in particular IgE and eosinophils (Figure 5). Elevations in natural killer cells may also be related to helminth infections, as some reports suggest that helminths, and in particular hookworm, may selectively activate natural killer cells and lead to expansions of natural killer cell lines (Korten et al., 2002; Hsieh et al., 2004; Anthony et al., 2007).
Figure 5.
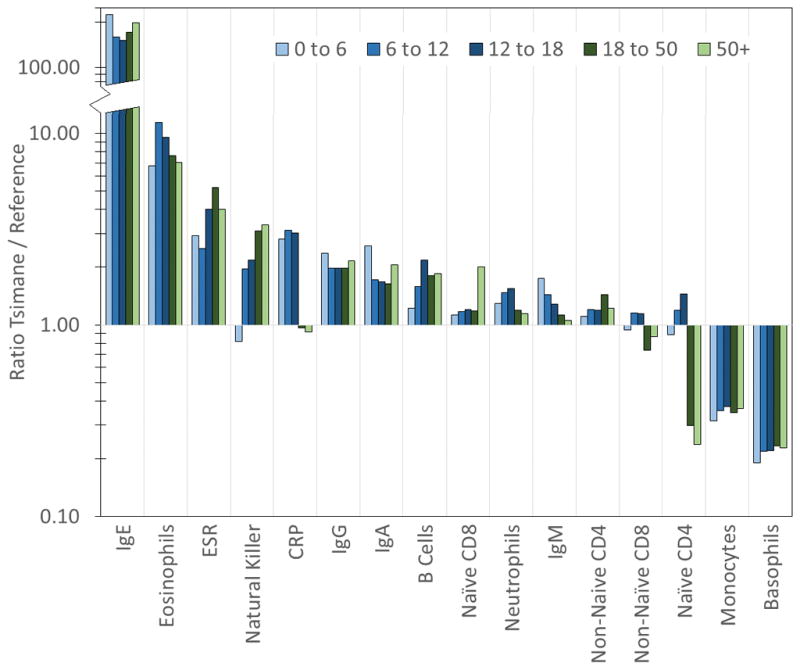
Ratio of median Tsimane immunological values to median values from reference populations, by age group. Note the y-axis is logged and discontinuous.
Other elevated immune parameters are harder to pin to specific infections because they are less specific to particular infections. Levels of other immunoglobulins and B cells likely represent general increases in humoral immunity, although they may also be secondary to shifts in T cell populations towards TH2 biased immune responses, again, possibly due to helminth infections (Maizels and Yazdanbakhsh, 2003). High ESR in the absence of elevated CRP has been reported as a component of a number of other conditions, including anaemia and pregnancy, but most predominant among these are conditions involving excess production of immunoglobulins, such as Schnitzler’s syndrome and Waldenstrom’s macroglobulinemia (Nashan et al., 1995; Ghobrial et al., 2003). Although these conditions involve cancerous expansion of B cell lines, Tsimane immunoglobulin levels are just as high, though presumably not of cancerous origin given that these are population wide elevations. ESR may also be elevated in some chronic conditions such as tuberculosis with also afflicts many Tsimane.
Monocytes and basophils are rarely observed in Tsimane blood differentials. Basophils are rare in venous blood samples in other populations as well; in the NHANES data basophils accounted for only 0.6% of leukocytes. In our study we estimate basophils account for only 0.1% of Tsimane leukocytes. We estimate monocytes represent 1.1% of leukocytes, far less than ~7% in reference populations. Basophils contain heparin, which slows clotting, and histamine to promote blood flow to tissues. They also have protein receptors that bind IgE. Monocytes differentiate into macrophages and dendritic cells to mount immune responses at localised sites of infection. Thus, both cell types exist in the blood primarily to mobilise and act at the sites of injury and invasion. We suggest that the low levels of basophils and monocytes in the blood may be a consequence of migration of these cells to the sites of helminth infections, injuries, and ectoparasitic invasions.
Age-Related Differences
Several markers show distinct differences across age groups. In reference populations, CRP, IgM, and neutrophil counts are higher in older ages, while in Tsimane median levels of these three markers are relatively consistent across age groups, meaning that Tsimane children have high relative levels (Figure 5). These three measures likely represent exposure to novel pathogenic stimuli. IgM is produced in response to novel antigen exposure. CRP is an acute phase reactant and part of the innate immune response, produced rapidly during early phases of infection. Neutrophils, similarly, are innate phagocytic cells that are on the “front line” of immune defenses. These results are therefore consistent with the observation that Tsimane are exposed to high levels of pathogens and parasites, beginning at young ages.
In reference populations and Tsimane, CD4, CD8, and B cells are highest at younger ages and lower in older ages. For T cells, where we have divided the sample into naïve and non-naïve cells, it is clear that this decline is driven by decreases in naïve cell pools. This is consistent with a life history perspective (McDade, 2003); children are born without immunological experience, and to acquire adaptive immunity they must generate a large pool of diverse naïve cells and then select from among this pool. As naïve cells are selected for antigen reactivity, libraries of memory T and B cells and immunoglobulins are generated. As children age, the demand for naïve cells declines, as fewer novel antigens are encountered. Naïve cells can be thought of as investments into future defense; given the costs of maintaining naïve cell pools, the payoffs to investment in naïve pools declines as they become both less necessary and the length of future lifespan declines.
Past results suggest that Tsimane infants have smaller thymic volumes than comparable infants from a Turkish sample, but larger thymic volume than Venezuelan Pumé infants of the same size (Veile et al., 2012). Despite variable thymic size across populations, it does not appear that the production of naïve T-cells is affected, as Tsimane children <5 years have comparable naïve T-cell counts when compared to US children. Unlike in mice, the majority of peripheral T cells in humans are actually produced through proliferation of naïve cells, rather than through thymic output (den Braber et al., 2012). The thymus then, is required for the production of T cells with novel receptors, but not for maintenance of the overall naïve pool.
Compared to reference populations, Tsimane show slightly higher naïve CD4 cells in mid-childhood and adolescence, followed by much lower naïve CD4 pools in adulthood. Previous reports of depleted CD4 cells have suggested that chronic helminth infection may be involved (Kalinkovich et al., 1998; Borkow et al., 2000; Tsegaye et al., 2003). This would certainly be consistent with the other immunological parameters we have measured. Whether this decrease is due to a change in thymic output or through reduced peripheral proliferation remains to be seen, and will require measurements of thymic size, or markers such as T cell receptor excision circles (Gruver and Sempowski, 2008). In contrast, naïve CD8 cells are retained at high levels.
There are two possible interpretations of this difference between CD4 and CD8 cells. One is that increased exposure to bacteria, helminths, fungi, and protozoa depletes naïve CD4 reserves. The other is that large pools of naïve CD4 cells may not be necessary, since novel helminths, protozoa, fungi, and bacteria may be rare, with many of these infections likely to be chronic or repeated throughout the Tsimane lifespan (e.g. giardia and helminths (Blackwell et al., 2013)). In contrast, naïve CD8 pools might be retained because novel viral infections are common, given the rapid pace of viral evolution and direct communicability between individuals. Given the high costs of maintaining immunity, we expect natural selection to have produced mechanisms for regulating these pools carefully in response to the pathogenic environment, perhaps by using early life immune activation of different cell lines as a cue for setting later allocation levels.
Life history trade-offs and the costs of immune defense
Immune function is costly, and elevations in immunity are likely to have significant costs for Tsimane and other Amazonian populations. Fever, for example, increases metabolic rate by 13% for every degree increase in body temperature, while sepsis or systemic infection can increase metabolic costs by 50% (Lochmiller and Deerenberg, 2000). Protein synthesis increases during an immune response, from about 3.5g to 6g of protein per day per kilogram of body weight, an estimated expense of 285 kcal/day (McDade, 2003). Tsimane and other Amazonians are of small stature relative to international standards and are relatively lean but not underweight (Gurven et al., 2012a; Urlacher et al., 2015). Past studies of Tsimane and of Shuar, an Ecuadorian forager-horticultural population, have shown trade-offs between immune response and growth rates in children (McDade et al., 2008; Blackwell et al., 2010), and associations between IgE and the height of adolescents and adults (Blackwell et al., 2010). The results presented here suggest that high IgE is likely to be accompanied by elevations in many other immunological parameters, meaning that IgE levels may simply be indicative of much broader costs imposed by parasitic infections. This may help explain the strong inverse association between IgE and growth outcomes.
During growth, Amazonians also appear to prioritise weight gain over skeletal growth and height attainment (Blackwell et al., 2009; Stieglitz et al., 2015a), and they also seem to accumulate fat in central locations where it may be particularly important for fueling immune defenses (Urlacher et al., 2015). Tsimane also have high resting metabolic rates and total daily energy expenditures, as measured by doubly labeled water and respirometry (Gurven et al., 2016). All of these characteristics are likely to relate to the allocation of resources towards immune function, as additional energy is used and stored for maintenance rather than growth. Resources may also be directed away from some reproductive costs, at least in males, as Tsimane have low levels of testosterone (Trumble et al., 2012) and smaller prostate sizes (Trumble et al., 2015) compared to age matched men from industrialised populations.
Consequences for non-infectious disease
With effects on energy balance and mobilisation, it should be apparent that immune activation in Amazonians may have implications for fat deposition and the development of obesity and obesity-related diseases, particularly as these populations begin to integrate into markets and gain access to sanitation and hygiene that reduces pathogen exposure. Inflammatory processes have also been implicated in other metabolic and cardiovascular health outcomes. Inflammatory cytokines lead to inflammatory responses in adipose tissue, that promote local insulin resistance (Gustafson et al., 2007) and inflammation contributes to stress dependent hypertension (Marvar et al., 2012). Inflammation also mediates the pathogenesis of atherosclerotic plaques, with both innate and adaptive immunity responding to plaque antigens (McLaren et al., 2011; Wigren et al., 2012). Plaque antigens stimulate recruitment of monocytes to form macrophages that engulf lipids and transform them into lipid-laden foam cells. With plaque formation, the lesion does not resolve and a pro-inflammatory environment is created, which in turn recruits more monocytes, leading to formation of a lipid-rich necrotic core (Spagnoli et al., 2007; Moore and Tabas, 2011).
Notably, obesity, hypertension, atherosclerosis, and diabetes are largely absent in the Tsimane (Gurven et al., 2009, 2012a), despite apparent elevations in some inflammatory markers and a highly pathogenic environment. In the case of atherosclerosis, this may be due to recruitment of monocytes to non-arterial locations, meaning that monocytes that differentiate into macrophages do not respond to plaques. Tsimane blood lipid levels are also substantially lower than in other populations, which may be due in part to parasitic infections (Vasunilashorn et al., 2010). With effects on energy balance, pathogen stress may counteract obesity. Inflammation may also be better regulated, as helminths and other parasites induce regulatory and TH2 responses (McDade et al., 2012; Blackwell et al., 2015).
Limitations and future directions
We have characterised immune function in an Amazonian population exposed to high prevalences of pathogens and parasites. However, we have not directly tested for associations between particular infections and immunological effects, and we caution that conclusions about causality should be considered speculative until additional analyses, experiments, and observations can establish definitive links. Other environmental factors, such as diet, nutrition, and physical activity may play important roles in cross-population immunological differences. Moreover, some differences between Tsimane and industrialised populations may be due to genetic, rather than environmental differences. Analysis of a handful of Tsimane genes do show differences in the frequencies of some immune related genes, though how these link to phenotypes is somewhat unclear (Vasunilashorn et al., 2011). Future analysis of both genetic and environmental variance will be needed to elucidate how genes and environments interact to produce Tsimane immunological patterns. Finally, additional longitudinal data is needed to establish whether age patterns are due to developmental or cohort effects.
Conclusions
This comprehensive view of Tsimane immune function makes it clear that Tsimane differ from US and European populations on many immunological parameters. The most dramatic of these differences are likely related to chronic helminth infections, including elevated IgE and eosinophils. Other differences, such as elevated natural killer cells and depleted naïve CD4 cells may have a related etiology. These differences help us to understand patterns in Tsimane life history and health, and represent a first step in understanding cross-cultural diversity in immune function. While our focus here is on Tsimane immune function, chronic helminth infection, diverse bacterial and viral pathogens, and fungi are common features of many Amazonian populations, and so the overall portrait of immune function described here should be generalisble to other populations inhabiting similar infectious environments. By understanding how human immune function varies in response to ecological conditions, we can begin to elucidate the functional design of immunological reaction norms, and better understand how human immune function evolved. By understanding immune function as a plastic and dynamic system, we can begin to reason about how this system responds in both high pathogen and low pathogen environments, with consequences for the disease epidemiology of both infectious and non-infectious diseases.
References
- Abrams ET, Miller EM. The roles of the immune system in women’s reproduction: evolutionary constraints and life history trade-offs. Am J Phys Anthropol. 2011;146(Suppl):134–54. doi: 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley NT, Weil ZM, Nelson RJ. Inflammation: Mechanisms, Costs, and Natural Variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Gurven M, Sugiyama LS, Madimenos FC, Liebert MA, Martin MA, Kaplan HS, Snodgrass JJ. Evidence for a Peak Shift in a Humoral Response to Helminths: Age Profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Negl Trop Dis. 2011;5:e1218. doi: 10.1371/journal.pntd.0001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proc R Soc B. 2013;280:20131671. doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Pryor G, III, Pozo J, Tiwia W, Sugiyama LS, Pryor G. Growth and market integration in Amazonia: A comparison of growth indicators between Shuar, Shiwiar, and nonindigenous school children. Am J Hum Biol. 2009;21:161–171. doi: 10.1002/ajhb.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Snodgrass JJ, Madimenos FC, Sugiyama LS. Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am J Hum Biol. 2010;22:836–48. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. Helminth infection, fecundity, and age of first pregnancy in women. Science (80−) 2015;350:970–972. doi: 10.1126/science.aac7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill M, Janossy G, Lee Ca, MacDonald-Burns D, Phillips aN, Sabin C, Timms a, Johnson Ma, Kernoff PB. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol. 1992;88:243–252. doi: 10.1111/j.1365-2249.1992.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, Bentwich Z. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–1060. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor Ka. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362:112–20. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJL, Furman D, Shen-orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, Davis MM. Variation in the Human Immune System Is Largely Driven by Non-Heritable Influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepon-Robins TJ, Liebert MA, Gildner TE, Urlacher SS, Colehour AM, Snodgrass JJ, Madimenos FC, Sugiyama LS. Soil-transmitted helminth prevalence and infection intensity among geographically and economically distinct Shuar communities in the Ecuadorian Amazon. J Parasitol. 2014;100:598–607. doi: 10.1645/13-383.1. [DOI] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, Bregje de Boer A, Willems N, Schrijver EHR, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AFC, Ackermans MT, Miedema F, Borghans JAM, de Boer RJ, Tesselaar K. Maintenance of Peripheral Naive T Cells Is Sustained by Thymus Output in Mice but Not Humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Dunn RR, Davies TJ, Harris NC, Gavin MC. Global drivers of human pathogen richness and prevalence. Proc Biol Sci. 2010;277:2587–95. doi: 10.1098/rspb.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster Z, Byron E, Reyes-García V, Huanca T, Vadez V, Apaza L, Pérez E, Tanner S, Gutierrez Y, Sandstrom B, Yakhedts A, Osborn C, Godoy RA, Leonard WR. Physical growth and nutritional status of Tsimane’ Amerindian children of lowland Bolivia. Am J Phys Anthropol. 2005;126:343–351. doi: 10.1002/ajpa.20098. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admettla A, Pattini L, Nielsen R. Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution. PLoS Genet. 2011;7:e1002355. doi: 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial IM, Gertz MA, Fonseca R. Waldenström macroglobulinaemia. Lancet Oncol. 2003;4:679–685. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- Grant AV, Araujo MI, Ponte EV, Oliveira RR, Cruz AA, Barnes KC, Beaty TH. High heritability but uncertain mode of inheritance for total serum IgE level and Schistosoma mansoni infection intensity in a schistosomiasis-endemic Brazilian population. J Infect Dis. 2008;198:1227–1236. doi: 10.1086/591946. [DOI] [PubMed] [Google Scholar]
- Gruver aL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age? Longitudinal evidence among forager-horticulturalists. Hypertension. 2012a;60:25–33. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Supa AZ. Mortality experience of Tsimane Amerindians of Bolivia: regional variation and temporal trends. Am J Hum Biol. 2007;19:376–98. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Eid Rodriguez D, Vasunilashorn S, Kim JK, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci. 2008;63:196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, von Rueden C, Stieglitz J, Kaplan H, Rodriguez DE. The evolutionary fitness of personality traits in a small-scale subsistence society. Evol Hum Behav. 2014;35:17–25. doi: 10.1016/j.evolhumbehav.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: the role of transfers in shaping the evolved human life history. Exp Gerontol. 2012b;47:807–13. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Yetish G, Trumble B, Stieglitz J, Cummings D, Blackwell AD, Beheim B, Kaplan HS, Pontzer H. High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. 2016 doi: 10.1002/ajpa.23040. Submiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–83. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- Hall MA, Ahmadi KR, Norman P, Snieder H, MacGregor AJ, Vaughan RW, Spector TD, Lanchbury JS. Genetic influence on peripheral blood T lymphocyte levels. Genes Immun. 2000;1:423–7. doi: 10.1038/sj.gene.6363702. [DOI] [PubMed] [Google Scholar]
- Hern WM. Health and demography of native Amazonians: historical perspective and current status. Cad Saude Publica. 1991;7:451–480. doi: 10.1590/s0102-311x1991000400002. [DOI] [PubMed] [Google Scholar]
- Hill K. Aché life history: the ecology and demography of a foraging people. Aldine de Gruyter; New York: 1996. Hurtado AMBT-F of human behavior. [Google Scholar]
- Hollinger NF, Robinson SJ. A study of the erythrocyte sedimentation rate forwell children. J Pediatr. 1953;42:304–319. doi: 10.1016/s0022-3476(53)80186-1. [DOI] [PubMed] [Google Scholar]
- Hsieh GC-F, Loukas A, Wahl aM, Bhatia M, Wang Y, Williamson aL, Kehn KW, Maruyama H, Hotez PJ, Leitenberg D, Bethony J, Constant SL. A Secreted Protein from the Human Hookworm Necator americanus Binds Selectively to NK Cells and Induces IFN-Production. J Immunol. 2004;173:2699–2704. doi: 10.4049/jimmunol.173.4.2699. [DOI] [PubMed] [Google Scholar]
- Hurtado AM, Frey M, Hill K, Hurtado I, Baker J. The role of helminthes in human evolution: Implications for global health in the 21st century. In: Elton S, O’Higgins P, editors. Medicine and evolution: current applications, future prospects. Boca Raton, FL: Taylor and Francis Group; 2008. pp. 153–180. [Google Scholar]
- Hurtado AM, Hurtado I, Hill K. Public health and adaptive immunity among natives of South America. In: Salzano FM, Hurtado AM, editors. Lost paradises and the ethics of research and publication. Oxford University Press; New York: 2004. pp. 164–90. [Google Scholar]
- Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets’ reference ranges in an age- and gender-balanced population of 100 healthy adults--a monocentric German study. Clin Immunol. 2005;116:192–7. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Jolliff CR, Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, Fijan KJ, Fletcher LL, Shriner HC. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem. 1982;28:126–128. [PubMed] [Google Scholar]
- Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M, Bentwich Z. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–21. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Hooper PL, Stieglitz J, Gurven M. The Causal Relationship between Fertility and Infant Mortality: Prospective Analyses of a Population in Transition. In: Kreager Philip, Winne B, Ulijaszek S, Capelli C., editors. Population in the Human Sciences: Concepts, Models, Evidence. Oxford, UK: Oxford University Press; 2015. pp. 361–378. [Google Scholar]
- Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15:379–93. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Nelson RJ. Influence of social factors on immune function and reproduction. Rev Reprod. 1999;4:168–178. doi: 10.1530/ror.0.0040168. [DOI] [PubMed] [Google Scholar]
- Korten S, Volkmann L, Saeftel M, Fischer K, Taniguchi M, Fleischer B, Hoerauf A. Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: contribution to parasite control. J Immunol. 2002;168:5199–5206. doi: 10.4049/jimmunol.168.10.5199. [DOI] [PubMed] [Google Scholar]
- Lewinsohn TM. How Many Species Are There in Brazil? Conserv Biol. 2005;19:619–624. [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Fever and sickness behaviour vary among congeneric rodents. Funct Ecol. 2008;22:67–68. [Google Scholar]
- Martin M, Blackwell AD, Gurven M, Kaplan H. Make New Friends and Keep the Old? Parasite Coinfection and Comorbidity in Homo sapiens. In: Brinkworth J, Pechenkina K, editors. Primates, Pathogens, and Evolution. Springer; New York: 2013. pp. 363–387. [Google Scholar]
- Martin MA, Lassek WD, Gaulin SJC, Evans RW, Woo JG, Geraghty SR, Davidson BS, Morrow AL, Kaplan HS, Gurven MD. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern Child Nutr. 2012;8:404–18. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T Lymphocytes and Vascular Inflammation Contribute to Stress-Dependent Hypertension. Biol Psychiatry. 2012;71:774–82. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L, Gurven M, Kaplan H, Stieglitz J. Why do women have more children than they want? Understanding differences in women’s ideal and actual family size in a natural fertility population. Am J Hum Biol. 2012;24:786–99. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Georgiev AV, Kuzawa CW. Trade-offs between acquired and innate immune defenses in humans. Evol Med Public Heal. 2016;2016:1–16. doi: 10.1093/emph/eov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Leonard WR, Burhop J, Reyes-Garciá V, Vadez V, Huanca T, Godoy RA. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- McDade TW, Reyes-Garcia V, Tanner S, Huanca T, Leonard WR. Maintenance versus growth: Investigating the costs of immune activation among children in lowland Bolivia. Am J Phys Anthropol. 2008;136:478–484. doi: 10.1002/ajpa.20831. [DOI] [PubMed] [Google Scholar]
- McDade TW, Tallman PS, Madimenos FC, Liebert MA, Cepon TJ, Sugiyama LS, Snodgrass JJ. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. Am J Hum Biol. 2012;24:675–81. doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Yearb Phys Anthropol. 2003;46:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. Am J Hum Biol. 2005;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McElreath R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan. Boca Raton, FL: Chapman and Hall/CRC; 2015. [Google Scholar]
- McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the Pathogenesis of Atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashan D, Sunderkötter C, Bonsmann G, Luger T, Goerdt S. Chronic urticaria, arthralgia, raised erythrocyte sedimentation rate and IgG paraproteinaemia: a variant of Schnitzler’s syndrome? Br J Dermatol. 1995;133:132–134. doi: 10.1111/j.1365-2133.1995.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pisor AC, Gurven M, Blackwell AD, Kaplan H, Yetish G. Patterns of senescence in human cardiovascular fitness: VO2 max in subsistence and industrialized populations. Am J Hum Biol. 2013;25:756–69. doi: 10.1002/ajhb.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB. Relationship between pace of life and immune responses in wild rodents. Oikos. 2012;121:1483–1492. [Google Scholar]
- Retterstol L, Eikvar L, Berg K. A twin study of C-Reactive Protein compared to other risk factors for coronary heart disease. Atherosclerosis. 2003;169:279–282. doi: 10.1016/s0021-9150(03)00192-8. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma Concentration of C-Reactive Protein and Risk of Developing Peripheral Vascular Disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Salzano FM, Callegari-Jacques SM. South American Indians: A Case Study in Evolution. Oxford, UK: Clarendon Press; 1988. [Google Scholar]
- Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, et al. Lymphocyte subsets in healthy children from birth through 18 years of age:: The pediatric AIDS clinical trials group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of Inflammation in Atherosclerosis. J Nucl Med. 2007;48:1800–1815. doi: 10.2967/jnumed.107.038661. [DOI] [PubMed] [Google Scholar]
- Stieglitz J, Beheim BA, Trumble BC, Madimenos FC, Kaplan H, Gurven M. Low mineral density of a weight-bearing bone among adult women in a high fertility population. Am J Phys Anthropol. 2015a;156:637–648. doi: 10.1002/ajpa.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Blackwell AD, Quispe Gutierrez R, Cortez Linares E, Gurven M, Kaplan H. Modernization, Sexual Risk-Taking, and Gynecological Morbidity among Bolivian Forager-Horticulturalists. PLoS One. 2012;7:e50384. doi: 10.1371/journal.pone.0050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Trumble BC, Thompson ME, Blackwell AD, Kaplan H, Gurven M. Depression as sickness behavior? A test of the host defense hypothesis in a high pathogen population. Brain Behav Immun. 2015b doi: 10.1016/j.bbi.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Cummings D, von Rueden C, O’Connor KA, Smith EA, Gurven M, Kaplan H. Physical competition increases testosterone among Amazonian forager-horticulturalists: a test of the “challenge hypothesis”. Proc Biol Sci. 2012;279:2907–12. doi: 10.1098/rspb.2012.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Rodriguez DE, Linares EC, Kaplan HS, Gurven MD. Challenging the Inevitability of Prostate Enlargement: Low Levels of Benign Prostatic Hyperplasia Among Tsimane Forager-Horticulturalists. Journals Gerontol Ser A Biol Sci Med Sci. 2015:1–7. doi: 10.1093/gerona/glv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye A, Wolday D, Otto S, Petros B, Assefa T, Alebachew T, Hailu E, Adugna F, Measho W, Dorigo W, Fontanet AL, Baarle D, Van Miedema F. Immunophenotyping of blood lymphocytes at birth, during childhood, and during adulthood in HIV-1-uninfected Ethiopians. Clin Immunol. 2003;109:338–346. doi: 10.1016/j.clim.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Uller T, Isaksson C, Olsson M. Immune challenge reduces reproductive output and growth in a lizard. Funct Ecol. 2006;20:873–879. [Google Scholar]
- Urlacher SS, Blackwell AD, Liebert MA, Madimenos FC, Cepon-Robins TJ, Gildner TE, Snodgrass JJ, Sugiyama LS. Physical growth of the Shuar: Height, Weight, and BMI references for an indigenous amazonian population. Am J Hum Biol Early View. 2015 doi: 10.1002/ajhb.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, Finch CE. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22:731–40. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Finch C, Crimmins E, Vikman S, Stieglitz J, Gurven M, Kaplan H, Allayee H. Inflammatory Gene Variants in the Tsimane, an Indigenous Bolivian Population with a High Infectious Load. Biodemography Soc Biol. 2011;57:33–52. doi: 10.1080/19485565.2011.564475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veile A, Martin M, McAllister L, Gurven M. Modernization is associated with intensive breastfeeding patterns in the Bolivian Amazon. Soc Sci Med. 2014;100:148–158. doi: 10.1016/j.socscimed.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veile A, Winking J, Gurven M, Greaves RD, Kramer KL. Infant growth and the thymus: data from two South American native societies. Am J Hum Biol. 2012;24:768–75. doi: 10.1002/ajhb.22314. [DOI] [PubMed] [Google Scholar]
- Westergren A. Diagnostic tests: the erythrocyte sedimentation rate range and limitations of the technique. Triangle. 1957;3:20–25. [PubMed] [Google Scholar]
- Wetteland P, Røger M, Solberg HE, Iversen OH. Population-based erythrocyte sedimentation rates in 3910 subjectively healthy Norwegian adults. A statistical study based on men and women from the Oslo area. J Intern Med. 1996;240:125–131. doi: 10.1046/j.1365-2796.1996.30295851000.x. [DOI] [PubMed] [Google Scholar]
- Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta. 2012;413:1562–1568. doi: 10.1016/j.cca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–114. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- Woof JM, Kerr Ma. IgA function - Variations on a theme. Immunology. 2004;113:175–177. doi: 10.1111/j.1365-2567.2004.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, McDade TW, Kuzawa CW, Borja J, Li Y, Adair LS, Mohlke KL, Lange La. Genome-wide association with C-reactive protein levels in CLHNS: evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation. 2012;35:574–83. doi: 10.1007/s10753-011-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]