Introduction
Sympathetic ophthalmia (SO) is an autoimmune, bilateral, granulomatous panuveitis occurring after accidental or surgical trauma to the eye.1 Systemic corticosteroids are first-line therapy for SO with immunomodulatory therapy employed for corticosteroid-sparing immunosuppression and chronic, refractory cases. Biologic response modifiers (BRMs) are a class of therapeutics that target specific cytokines mediating inflammation, and TNF-α antagonist BRMs have shown promise for uveitis.2 Herein, we report the first use, to our knowledge, of adalimumab for refractory pediatric SO leading to resolution of inflammation.
Case Report
A 5-year-old Caucasian girl suffered accidental trauma to the right eye resulting in corneoscleral laceration repaired the day of trauma. Post-operative visual acuity (VA) was hand-motions. Two weeks later, increasing inflammation concerning for endophthalmitis prompted intravitreal antibiotic injection and subsequent pars plana vitrectomy with lensectomy. Vision became no light perception post-operatively.
Nine weeks after her initial injury, the patient began experiencing photophobia and redness of her uninjured, left eye. Vision was 20/20 OS. An examination under anesthesia of the left eye documented anterior and posterior segment inflammation with white peripheral chorioretinal deposits concerning for sympathetic ophthalmia. Topical prednisolone acetate 1% and oral prednisone 60mg daily was started. Enucleation of the right eye was performed 11 weeks after the initial injury with histopathologic examination consistent with sympathetic ophthalmia (Figure 1). The patient was referred to our service for further management.
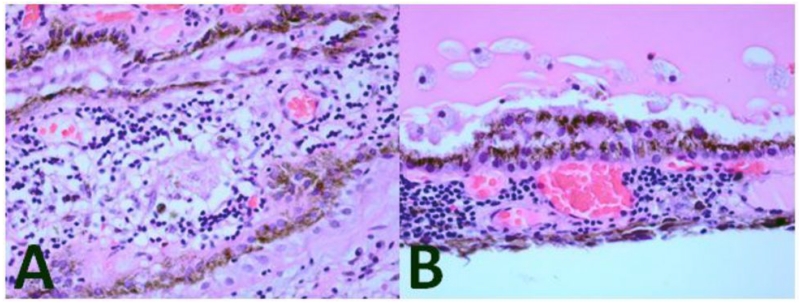
Figure 1. Enucleated Specimen.
The enucleated right eye contained (A) a diffuse chronic inflammatory infiltrate in the choroid including epitheliod histiocytes forming non-caseating granulomas and (B) Dalen-Fuchs nodules (hematoxylin and eosin, 100×).
On our initial examination, VA was 20/25 OS and intraocular pressure was 36 mmHg with rare anterior chamber cell and trace flare. Ophthalmoscopic examination showed 1+ vitreous cell without choroidal lesions. The patient developed weight gain and cushingoid habitus on oral prednisone. Topical timolol 0.5% was started for elevated IOP.
Oral prednisone was tapered to 10mg daily over a 12-week period in conjunction with initiating methotrexate 10mg subcutaneous injection (SQ) weekly. Despite dose escalation of methotrexate to 25mg SQ weekly over the following nine months, the patient continued to have low-grade anterior chamber inflammation, developed posterior synechiae (figure 2), and experienced flares up to 3+ anterior chamber cell when tapering oral prednisone below 10mg daily.
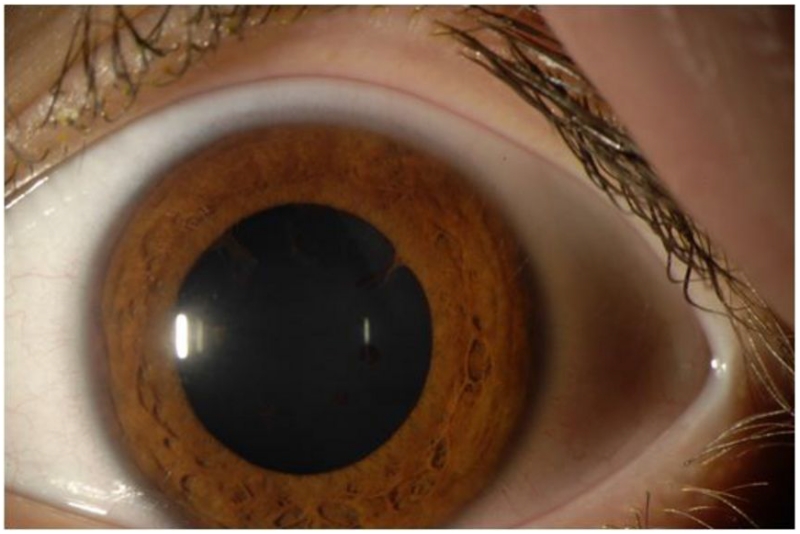
Figure 2. Anterior Segment Photograph.
A chronic, low-grade inflammation including flare and posterior synechiae persisted in the left eye when oral prednisone was tapered.
Adalimumab 20 mg SQ every 2 weeks was initiated after a negative PPD reading, and within three months inflammation completely resolved with discontinuation of oral prednisone, prednisolone acetate, and timolol. After six months of stability on adalimumab, methotrexate was tapered and discontinued over six months.
After 18 months on adalimumab, VA was 20/25 OS with no evidence of recurrent inflammation, posterior synechiae, or fundus abnormalities.
Comment
Sympathetic ophthalmia is presumed to be an autoimmune, T-cell-mediated response to melanocyte self-antigens exposed during surgery or trauma. A cytokine-profiling study in an animal model resembling SO showed upregulation of TNF-α levels associated with photoreceptor damage.3 As TNF-α potentiates T-cell-mediated immunity, TNF-α antagonist therapy may provide a targeted approach for anti-inflammatory therapy.2
Gupta et al reported a case of pediatric SO refractive to multiple immunosuppressants treated with intravenous infliximab, a chimeric murine/human monoclonal antibody targeting TNF-α, with prolonged control of inflammation achieved on infliximab alone.4 Another case of an adult with SO refractory to multiple immunosuppressants achieved inflammation resolution with addition of adalimumab, a recombinant human monoclonal anti-TNF-α antibody dosed subcutaneously.5 In a series of 131 patients with refractory uveitis, addition of adalimumab reduced immunosuppressive load by 50% in 85% of patients.6
This is the first report, to our knowledge, of TNF-α blocker adalimumab’s use leading to resolution of inflammation in refractory pediatric SO. Addition of adalimumab led to long-term control with discontinuation of all other immunosuppressants for our patient. Although experience is limited to case reports, adalimumab could be considered for refractory SO and potentially other ocular autoimmune conditions where TNF-α is thought to play a role in its pathogenesis.
Acknowledgment
This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY) to the Emory Eye Center and an NEI Core Grant for Vision Research (P30 EY 006360), and the Knights Templar Educational Foundation of Georgia (SY, SAH). Dr. Angeles-Han was supported by the National Eye Institute of the National Institutes of Health under Award Number K23 EY021760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Yeh and Joon-Bom Kim had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsors
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest Disclosures
None reported.
References
- 1.Chang GC, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. 2011;26(4-5):316–20. doi: 10.3109/08820538.2011.588658. [DOI] [PubMed] [Google Scholar]
- 2.Yeh S, Faia L, Nussenblatt R. Advances in the diagnosis and immunotherapy for ocular inflammatory disease. Semin Immunopathol. 2008;30(2):145–64. doi: 10.1007/s00281-008-0109-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Rao NA. Mitochondrial oxidative stress initiates visual loss in sympathetic ophthalmia. Jpn J Ophthalmol. 2012;56(3):191–7. doi: 10.1007/s10384-012-0132-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SR, Phan IT, Suhler EB. Successful treatment of refractory sympathetic ophthalmia in a child with infliximab. Arch Ophthalmol. 2011;129(2):250–252. doi: 10.1001/archophthalmol.2010.358. [DOI] [PubMed] [Google Scholar]
- 5.Soheilian M, Jabbarpourbonyadi M, Soheilian R, Peyman GA. Bilateral uveitis after phakic intraocular lens implantation and management with adalimumab. J Catract Refract Surg. 2012;38(6):1094–6. doi: 10.1016/j.jcrs.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–81. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]