Abstract
We report a case of epidemic typhus in a patient from the Batna region of Algeria, who presented with generalized febrile exanthema. The clinical diagnosis was confirmed by serological cross-adsorption followed by Western blotting. Our report emphasizes the threat of epidemic typhus in the highlands of Algeria.
CASE REPORT
In early July 2000, a 64-year-old woman came to the Department of Infectious Diseases of the Batna General Hospital in Batna, Algeria, for evaluation of fever and exanthema. She was a native Algerian who had always lived in the Batna area, a mountainous town in eastern Algeria at an elevation of 1,038 m. Symptoms had started 8 days prior to hospitalization with fever, asthenia, arthralgia, and headache. Four days after the onset of symptoms she had developed a generalized maculopapular skin rash. On admission she had a temperature of 40.2°C, a heart rate of 90 beats per min, a blood pressure of 120/80 mm Hg, and a maculopapulous rash involving the whole body except the face, palms, and soles, and she complained of severe asthenia, headache, and arthralgia. No inoculation eschar was found, nor were body lice found on her clothing. The clinical examination was otherwise normal. Laboratory findings showed a normal white blood cell count and a hemoglobin level of 100 g/liter. Liver enzyme levels were not assayed. The chest radiograph was normal. No antibiotic was administered, and the patient spontaneously recovered. Apyrexia was obtained on the sixth day of hospitalization. She was released from the hospital 10 days after admission and was lost to follow-up. Usual blood cultures remained negative. Serological tests for Salmonella enterica serovar Typhi, Borrelia recurrentis, Coxiella burnetii, Bartonella quintana, Francisella tularensis, and Anaplasma phagocytophilum were negative. For determination of antibodies to Rickettsia sp., we used as antigens for all serological tests R. prowazekii strain Breinl (ATCC VR-142), R. typhi strain Wilmington (ATCC VR-144), and R. conorii strain Malish (seven, ATCC VR-613). Immunofluorescence assay (IFA), cross-adsorption, and Western blotting were performed as previously described (7, 8). For Western blotting, purified R. prowazekii and R. typhi were suspended in sterile water and their concentrations were adjusted to 2 mg/ml with a UV spectrophotometer. The diagnosis of epidemic typhus was established by demonstrating higher titers of antibody to R. prowazekii (IFA titers: immunoglobulin G [IgG], 1:2,048; IgM, 1:512) than to other Rickettsia species (R. typhi IgG titer, 1:1,024 and IgM titer, 1:256; R. conorii IgG titer, 1:128 and IgM titer, 1:64) by IFA. This was confirmed by cross-adsorption followed by IFA and Western blotting. The latter technique demonstrated the specificity of antibodies to a high-molecular-weight antigen of R. prowazekii (Table 1; Fig. 1). No suitable specimen was available for culture. A nested PCR amplification from serum using primers specific for the citrate synthase (4) gene was attempted as previously described (1, 17) but was negative.
TABLE 1.
Results of serologic testing by IFA following cross-adsorption performed on our patient's sample
| Sample treatment | Titer of antibody to:
|
|||
|---|---|---|---|---|
|
R. prowazekii
|
R. typhi
|
|||
| IgG | IgM | IgG | IgM | |
| None (before adsorption) | 1:2,048 | 1:512 | 1:1,024 | 1:256 |
| Adsorption with R. prowazekii antigen | 0 | 0 | 0 | 0 |
| Adsorption with R. typhi antigen | 1:64 | 1:16 | 0 | 0 |
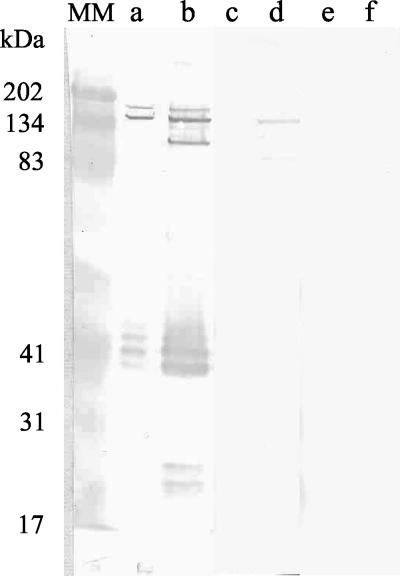
FIG. 1.
Western immunoblot assay of patient serum before and after cross-adsorption with R. prowazekii or R. typhi. Lanes: MM, molecular mass markers; a, c, and e, R. typhi antigen; b, d, and f, R. prowazekii antigen; a and b, untreated serum; c and d, serum adsorbed with R. typhi; e and f, serum adsorbed with R. prowazekii.
Epidemic typhus, caused by Rickettsia prowazekii, is a potentially fatal disease that has caused large outbreaks in the past and continues to threaten populations in various areas of the world (18, 19, 21). The most important outbreak since World War II occurred in 1997 in Burundi and involved more than 40,000 patients (18). Epidemic typhus is normally associated with wars and other crowded, unsanitary conditions such as those observed during human catastrophes when normal hygiene is disrupted. Its incidence is highest during colder months. In addition, a recrudescent and attenuated form of the disease (Brill-Zinsser disease) may occur up to 40 years post acute infection and serve as the source of future outbreaks (20). Although the disease is mainly prevalent in the highland and colder areas of Africa, Asia, and Central and South America (15, 16, 22), small outbreaks or sporadic cases of epidemic typhus have been described in industrialized countries (10, 11, 13, 21). R. prowazekii, which is currently on the B list of potential bioterrorism agents maintained by the U.S. Centers for Disease Control and Prevention, is transmitted to humans by Pediculus humanus humanus, the human body louse, which is infected while feeding on the blood of infected patients. Human infection results from contamination of scratches with the feces of infected body lice. Clinically, epidemic typhus presents as a febrile illness with marked headache, myalgia, and a generalized maculopapular rash. When untreated, it is often lethal. Herein, we describe a case of epidemic typhus that occurred in a mountainous region of Algeria in 2000.
We present a serologically confirmed case of epidemic typhus that emphasizes the potential public health problem posed by this disease in Algeria. The serological reference method for the diagnosis of typhus is IFA (7). However, cross-reactions among typhus group rickettsiae are common and may prevent the identification of the infecting species (5). We have previously demonstrated that a combination of IFA and Western blotting results has a sensitivity of 83% in differentiating between R. prowazekii and R. typhi infections (8) and that cross-adsorption is the reference test to identify the causative agent (8). In the present report, our patient had antibody titers to R. prowazekii twofold higher than those of antibody to R. typhi and the cross-adsorption procedure demonstrated a specific antibody response against R. prowazekii. Specific antibodies were directed against rOmpB, a surface protein antigen possessing species-specific epitopes (2-4). Therefore, we are confident that this patient suffered from epidemic typhus. However, we could not definitely determine whether the patient suffered from primary or recrudescent (Brill-Zinsser disease) typhus. Clinically, the patient presented with symptoms typically seen in both forms of epidemic typhus (12, 15) such as high-grade fever, headache, arthralgia, and a generalized maculopapular rash excluding the face, palms, and soles. Several arguments were in favor of Brill-Zinsser disease, including the mild evolution within 14 days from onset to recovery and the absence of body lice on the patient upon examination. However, we could not eliminate the diagnosis of primary typhus as detection of lice requires careful examination of the clothes (19). Because epidemic typhus was not initially considered among the differential diagnoses, the patient's clothes were not specifically searched for body lice and the patient was not specifically interviewed for a history of louse infestation. In addition, the patient did not report any medical history of either epidemic typhus or a febrile illness consistent with epidemic typhus. Regarding serology, Brill-Zinsser disease has been associated with low or absent titers of IgM antibody to R. prowazekii (12). However, in 1994, we have observed that elevated IgM antibody titers could also be present in recrudescent typhus (4). Therefore, we could not clearly discriminate which form of epidemic typhus the patient suffered from.
Typhus has been epidemic in northern Africa, where the role of lice as vectors was first described by Nicolle et al. in Tunis (14). In Algeria, a large typhus outbreak occurred during World War II (6), and subsequently the disease was endemic until 1970 (9). After that date, no case was reported in this country until 1999, when we reported the case of an Algerian patient who had been diagnosed as having epidemic typhus on returning from travel to Msila, a town in the highlands of eastern Algeria (13). In the present report, our patient also contracted typhus in a mountainous area of eastern Algeria close to Msila. Therefore, the highlands of eastern Algeria, like the highlands of central Africa or South America (16, 18), may be foci of epidemic typhus. As the patient never presented to the outpatient clinic and was lost to follow-up, we had no information on the sanitary conditions under which she lived. We also lack information regarding similar symptoms that may have been present in contacts of this patient. As this patient constituted a potential source of an outbreak, another epidemiological investigation was conducted in March 2004 but the patient remained untraceable.
Our report highlights the presence of epidemic typhus in the highlands of eastern Algeria. We suggest that epidemic typhus should be routinely considered in the differential diagnosis of febrile exanthemas in inhabitants of or travelers to this area, in particular in adults living under conditions favorable for contact with body lice.
Acknowledgments
We thank Patrick Rozmajzl for grammatical review of the manuscript.
REFERENCES
- 1.Carl, M., C. W. Tibbs, M. E. Dobson, S. Paparello, and G. A. Dasch. 1990. Diagnosis of acute typhus infection using the polymerase chain reaction. J. Infect. Dis. 161:791-793. [DOI] [PubMed] [Google Scholar]
- 2.Ching, W. M., G. A. Dasch, M. Carl, and M. E. Dobson. 1990. Structural analyses of the 120-kDa serotype protein antigens of typhus group rickettsiae. Comparison with other S-layer proteins. Ann. N. Y. Acad. Sci. 590:334-351. [DOI] [PubMed] [Google Scholar]
- 3.Dasch, G. A., and A. L. Bourgeois. 1981. Antigens of the typhus group of rickettsiae: importance of the species-specific surface antigens in eliciting immunity, p. 61-69. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, Inc., New York, N.Y.
- 4.Eremeeva, M. E., N. M. Balayeva, and D. Raoult. 1994. Serological response of patients suffering from primary and recrudescent typhus: comparison of complement fixation reaction, Weil-Felix test, microimmunofluorescence, and immunoblotting. Clin. Diagn. Lab. Immunol. 1:318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldwasser, R. A., and C. C. Shepard. 1959. Fluorescent antibody methods in the differentiation of murine and epidemic typhus fever: specific changes resulting from previous immunization. J. Immunol. 82:373-380. [PubMed] [Google Scholar]
- 6.Harries, C. V. 1953. Typhus fever; a historical review, with reference to the epidemics in Algeria and Naples during the Second World War. J. R. Nav. Med. Serv. 39:142-156. [PubMed] [Google Scholar]
- 7.La Scola, B., and D. Raoult. 1997. Laboratory diagnosis of rickettsioses: current approaches to the diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Scola, B., L. Rydkina, J. B. Ndihokubwayo, S. Vene, and D. Raoult. 2000. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin. Diagn. Lab. Immunol. 7:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeCorroller, Y., R. Neel, and R. Lecubarri. 1970. Exanthemic typhus in the Sahara. Arch. Inst. Pasteur Alger. 48:125-130. [PubMed] [Google Scholar]
- 10.Massung, R. F., L. E. Davis, K. Slater, D. B. McKechnie, and M. Puerzer. 2001. Epidemic typhus meningitis in the southwestern United States. Clin. Infect. Dis. 32:979-982. [DOI] [PubMed] [Google Scholar]
- 11.McDade, J. E., C. C. Shepard, M. A. Redus, V. F. Newhouse, and J. D. Smith. 1980. Evidence of Rickettsia prowazekii infections in the United States. Am. J. Trop. Med. Hyg. 29:277-284. [DOI] [PubMed] [Google Scholar]
- 12.Murray, E. S., G. Baehr, R. A. Mandelbaum, N. Rosenthal, J. C. Doane, L. B. Weiss, S. Cohen, and J. C. Snyder. 1950. Brill's disease. I. Clinical and laboratory diagnosis. JAMA 142:1059-1066. [Google Scholar]
- 13.Niang, M., P. Brouqui, and D. Raoult. 1999. Epidemic typhus imported from Algeria. Emerg. Infect. Dis. 5:716-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolle, C., C. Comte, and E. Conseil. 1909. Transmission expérimentale du typhus exanthématique par le pou de corps. C. R. Acad. Sci. 149:486. [Google Scholar]
- 15.Perine, P. L., B. P. Chandler, D. K. Krause, P. McCardle, S. Awoke, E. Habte-Gabr, C. L. Wisseman, Jr., and J. E. McDade. 1992. A clinico-epidemiological study of epidemic typhus in Africa. Clin. Infect. Dis. 14:1149-1158. [DOI] [PubMed] [Google Scholar]
- 16.Raoult, D., R. J. Birtles, M. Montoya, E. Perez, H. Tissot-Dupont, and H. Guerra. 1999. Survey of louse-associated diseases among rural Andean communities in Peru: prevalence of epidemic typhus, trench fever, and relapsing fever. Clin. Infect. Dis. 29:434-436. [DOI] [PubMed] [Google Scholar]
- 17.Raoult, D., P. E. Fournier, F. Fenollar, M. Jensenius, T. Prioe, J. J. de Pina, G. Caruso, N. Jones, H. Laferl, J. E. Rosenblatt, and T. J. Marrie. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344:1504-1510. [DOI] [PubMed] [Google Scholar]
- 18.Raoult, D., J. B. Ndihokubwayo, H. Tissot-Dupont, V. Roux, B. Faugere, R. Abegbinni, and R. J. Birtles. 1998. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 352:353-358. [DOI] [PubMed] [Google Scholar]
- 19.Raoult, D., and V. Roux. 1999. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 29:888-911. [DOI] [PubMed] [Google Scholar]
- 20.Raoult, D., V. Roux, J. B. Ndihokubwaho, G. Bise, D. Baudon, G. Martet, and R. J. Birtles. 1997. Jail fever (epidemic typhus) outbreak in Burundi. Emerg. Infect. Dis. 3:357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarasevich, I., E. Rydkina, and D. Raoult. 1998. Epidemic typhus in Russia. Lancet 352:1151. [DOI] [PubMed] [Google Scholar]
- 22.Walker, D. H., and D. B. Fishbein. 1991. Epidemiology of rickettsial diseases. Eur. J. Epidemiol. 7:237-245. [DOI] [PubMed] [Google Scholar]