Abstract
Objective
To evaluate the association between Non-alcoholic fatty liver disease (NAFLD) with renal stone disease detected on computed tomography (CT).
Method
A total 1812 patients who underwent abdomen-pelvis CT in July 2015 were included in this study. The inclusion criteria for NAFLD were as follows: (i) lower average Hounsfield unit (HU) of hepatic right lobe, left medial and lateral segment when compared with that of spleen, (ii) patients who having urolithiasis in kidneys, ureters and urinary bladder, and (iii) patients underwent abdomen-pelvis CT including noncontrast image. The statistical significance of the association between NAFLD and renal stone disease was assessed using Chi Square Test. The Odds ratios and 95% CI were calculated to assess the propensity of renal stones disease for NAFLD by using Logistic Regression analysis.
Results
The frequency of renal stone disease in patients with NAFLD was higher approximately 19% than those who having renal stone disease without NAFLD. In addition, the presence of NAFLD was linked with renal stone disease showing that detection rate of renal stone disease in patients with NAFLD was markedly high (odds ratio: 5, 95% CI, 3–8.2) (p < 0.05) in multivariate analysis.
Conclusion
The presence of significant association between NAFLD with renal stone disease and NAFLD may be considered to be an independent variable as a risk factor for renal stone disease.
Keywords: Non-alcoholic fatty liver disease, Renal stone disease, Computed tomography
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) has been recognized as a liver manifestation of the metabolic syndrome [1]. The prevalence of NAFLD from United States, Europe and Asia is reported to be up to third of the human population [2], [3]. NAFLD is defined as the presence of at least 5% of the fat component in the liver without any other liver disease including alcohol related liver disease, chronic viral hepatitis, use of medications resulting in hepatic steatosis such as tamoxifen, herb medication or other chronic liver disease such as autoimmune hepatitis. The guideline for NAFLD (endorsed as American Association for the Study of Liver Disease, American College of Gastroenterology and American Gastroenterological) defines significant alcohol use as current or recent alcohol consumption more than 21 drinks per a week in men and 14 drinks per a week in women [4]. NASH is a more progressive type of NAFLD and defined histologically by presence of hepatic cell injury with parenchymal steatosis [4].
Recent studies concluded that NAFLD has no direct association with renal function and mild renal function abnormality may have similar risk factors or disease process [5]. Renal stone disease is a common renal disorder associated with crystal deposition in the renal medulla and urinary tract. It is influenced by both intrinsic and extrinsic factors [6]. Recent epidemiological studies have demonstrated that renal stone disease has an association with obesity, diabetes mellitus, hypertension, and metabolic syndrome [7]. These results reveal that metabolic syndrome can result in changes in process of urine concentration and dilution, causing an increased risk of both uric acid and calcium oxalate stone formations [7]. In the basis of these studies, renal stone disease may be related to the metabolic syndrome and can be a component of the metabolic syndrome.
Recently, we noticed a concomitant diagnosis of both fatty liver and renal stones disease in same patient on the basis of computed tomography (CT) finding in routine daily practice. Literature reviews by using Pubmed articles demonstrate only two recent studies were performed and revealed the association between fatty liver and renal stone disease. Therefore, the purpose of our study was to evaluate the prevalence of renal stone disease in the patients with NAFLD by using the CT examination.
2. Material and methods
This prospectively collected and retrospectively evaluated study was approved by the institutional review board, and informed consent from patients was waived.
2.1. Study population
From July 1, 2015 to July 31, 2015, a total of 1812 patients who visited our institute with performed abdomen-pelvis CT initially eligible. The inclusion criteria for NAFLD group were as follows: (i) lower average Hounsfield unit (HU) of hepatic right lobe, left medial and lateral segment when compared with that of spleen [8], [9], (ii) patients having radiopaque stones in the urinary tract including kidneys, ureters or urinary bladder, and (iii) patients underwent abdomen-pelvis CT including noncontrast image. Control group were defined as follows: (i) patients underwent abdomen-pelvis CT including noncontrast image, and (ii) patients whose HU of liver parenchyma showed higher than that of spleen. Exclusion criteria were follows: (i) those who underwent abdomen-pelvis CT without noncontrast image, (ii) those who had suboptimal image quality of CT examination due to beam hardening artifact or respiration artifact, and (iii) those who having other liver disease including viral hepatitis, liver cirrhosis, hepatocellular carcinoma, metastasis from other primary cancer, splenectomy status or abundant alcohol consumption.
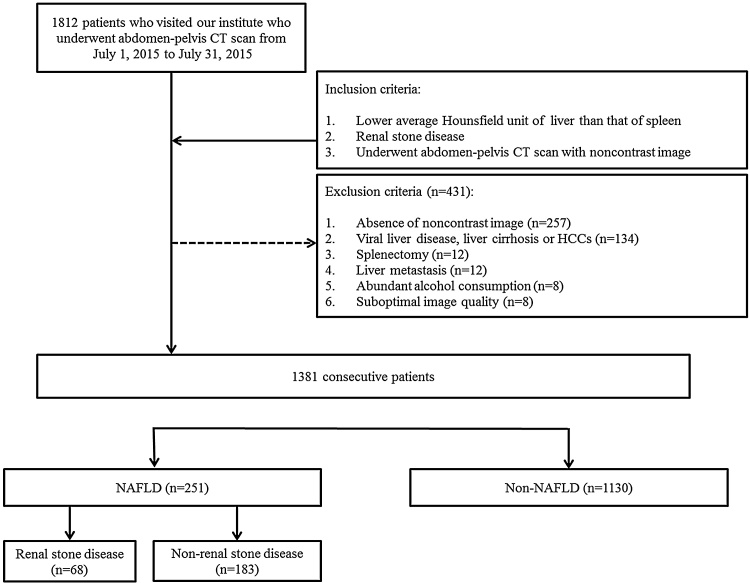
Of the 1812 cases, 431 were excluded due to absence of noncontrast image (n = 257), viral hepatitis, liver cirrhosis or hepatocellular carcinoma (n = 134), splenectomy status (n = 12), metastasis from other primary malignancy (n = 12), abundant alcohol consumption (n = 8) and suboptimal image quality (n = 8). Finally, 1381 cases (mean age: 55.8 years; range: 19–91 years; male:female = 684:697) were enrolled in this study. The case accrual process is summarized in Fig. 1.
Fig. 1.
Flowchart of the case accrual process. Computed tomography (CT); Hepatocellular carcinoma (HCC); Non-alcoholic fatty liver disease (NAFLD). NAFLD was defined as fatty liver showing lower Hounsfield Unit than that of spleen on CT.
2.2. CT protocols
A 128-detector row CT scanner (Definition AS+, Siemens Healthcare, Forchheim, Germany) was used to perform the abdomen-pelvis CT scan. All patients were in the supine position and were scanned from the lung base to the pubic symphysis. We performed a noncontrast scan. The scanning parameters were as follows: tube voltage, 120 kVp; collimation, 128 × 0.6 mm; rotation speed, 0.5 s; pitch, 0.8; reconstruction thickness, 3 or 5 mm; and no reconstruction interval. Automatic exposure control (caredose 4D, Siemens Healthcare) was activated to decrease the radiation dose, however, automatic tube potential modulation (careKV, Siemens Healthcare) was not switched on. Sagittal and coronal reformatted images were generated with a thickness of 3 mm.
2.3. Image analysis
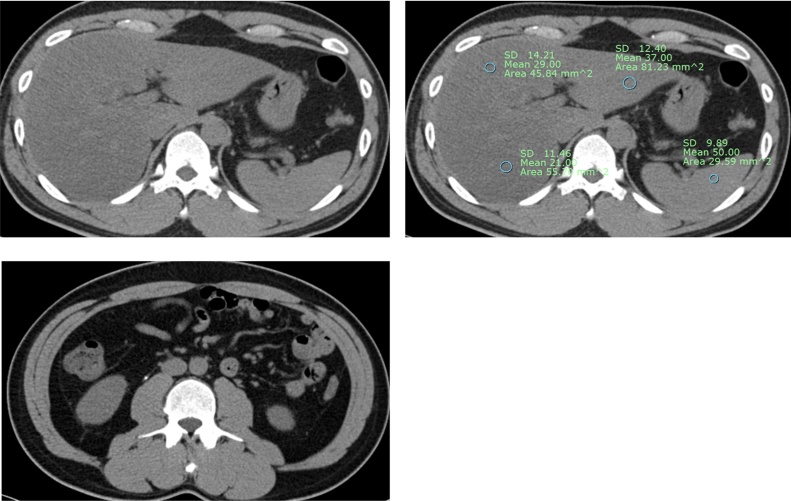
All imaging analysis was performed by a fellowship-complete academic radiologist with 3-year experience in reading abdomen-pelvis CT who was blinded to clinical and laboratory data. To obtain HU of liver parenchyma and spleen, the radiologist derived average HU value of liver parenchyma from three different hepatic segments including right hepatic lobe, left medial and lateral segment by measuring region of interest in each segment. Fatty liver was defined when average HU of liver parenchyma lower than that of spleen [8]. Then 4 weeks later, the radiologist reviewed electronic medical report of all enrolled patients to exclude those who having other liver disease including viral hepatitis, liver cirrhosis, hepatocellular carcinoma, metastasis from other primary cancer, splenectomy status or abundant alcohol consumption (Fig. 2).
Fig. 2.
Noncontrast scan images in a 55-year-old man show diffuse fatty liver and right ureter stone on noncontrast image. He visited our hospitalwith acute right flank pain, and his laboratory test revealed microscopic hematuria.
2.4. Statistical analysis
To assess the association between fatty liver and renal stone disease, the Chi Square test was used. The Odds ratios and 95% CI were calculated to assess the propensity of renal stones disease for fatty liver patients by using Logistic Regression analysis. A P value less than 0.05 was considered to indicate asignificant difference. The statistical analysis was performed using Medcalc software for Window (Medcalc Software Version 11.3.8.0, Mariakerke, Belgium).
3. Results
Of the 1381 cases, 1038 were healthy group suggestive of no fatty liver or renal stone disease (75% of total number). Fatty liver were 251 patients (145 men, 106 women; mean age; 54.7 years; age range, 19–90 years) and renal stone disease were 160 patients (87 men, 73 women; mean age, 54.9 years; age range, 20–86 years). The frequency of renal stone disease in fatty liver patient group was 27% (n = 68) (p < 0.05). In contrast, only 8% was noted as renal stone disease in non-fatty liver patient group (n = 183) (p < 0.05) (Table 1). The frequency of renal stone disease in male fatty liver patient group was 31% (n = 45) and in female fatty liver patients group was 21.7% (n = 23) (p = 0.208). Diagnosis of fatty liver demonstrated an association with higher risk of developing renal stone disease (odds ratio: 5, 95% CI, 3–8.2) (p < 0.05) (Table 2). The mean and standard deviation of HU of liver was 51.9 ± 12 in patient with renal stone disease and fatty liver and 56.4 ± 11 in patient with renal stone disease without fatty liver, respectively (p < 0.05). The frequency of fatty liver in renal stone disease (n = 160) was 42.5% (n = 68), with the frequency of 54.4% and 45.6% in male and female, respectively (p = 0.208). In contrast, the frequency of fatty liver in patient without renal stone disease was 15%.
Table 1.
Total number and gender frequency of fatty liver and renal stone disease.
| Gender | Fatty liver | Renal stone disease |
Number | χ2 (p) | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Male | Negative | 497 (92.2) | 42 (7.8) | 539 (100) | 55.6 (p < 0.05) |
| Positive | 100 (69) | 45 (31) | 145 (100) | ||
| Female | Negative | 541 (91.5) | 50 (8.5) | 684 (100) | 16.8 (p < 0.05) |
| Positive | 83 (78.3) | 23 (21.7) | 591 (100) | ||
| Total | Negative | 1038 (91.9) | 92 (8.1) | 1130 (100) | 72 (p < 0.05) |
| Positive | 183 (72.9) | 68 (27.1) | 251 (100) | ||
Table 2.
Multivariate risk factor analysis for renal stone disease.
| Dependent variable | Independent variable | Odds ratio | P value |
|---|---|---|---|
| Renal stone disease | Gender | 1.127 | P = 0.491 |
| Age | 0.998 | P = 0.71 | |
| Hounsfield Unit | 1.01 | P = 0.294 | |
| Fatty liver | 4.996 | P < 0.05 |
4. Discussion
Our study showed that frequency of renal stone disease in patient with NAFLD was higher approximately 19% than those who having renal stone disease without NAFLD. In addition, the presence of NAFLD was linked with renal stone disease showing that detection frequency of renal stone disease in patients with NFALD was markedly higher (odds ratio: 5, 95% CI, 3–8.2) (p < 0.05) in multivariate analysis. On the basis of these findings, we suggested that there’s significant association between NAFLD with renal stone disease and NAFLD may be considered to be an independent variable as a risk factor for stone formation.
Our results correspond well with those of previous studies [10], [11]. Einollahi et al. [10] investigated 11245 ultrasonography report in a cross sectional study. They found that the detection rate of renal stone disease was 17% in patients with NAFLD and 8% in patients without NAFLD, respectively. In addition, for NAFLD patients, detection or diagnosis of fatty liver was associated with a higher risk of developing renal stone disease (p < 0.0001), (odds ratio: 2.4, 95% CI 2.1–2.7). Even though the detection rate of renal stone disease was significantly higher in patients with NAFLD than that of patients without NAFLD in their study, however, it was lower than that of our study. A discrepancy between our study and theirs could be the difference of diagnostic performance between ultrasonography and CT. Published sensitivity and specificities of CT routinely performed with slice collimations of less than 3 mm approach 98%–100% [12]. Also, a meta-analysis reported a sensitivity of 97% and a specificity of 95% for the detection of obstructive ureterolithiasis with CT [13]. However, Ray et al. [14] performed a prospective evaluation of renal ultrasonography in the Emergency Department followed by CT examination for the detection of urolithiasis and they revealed that US performed poorly with an accuracy of 67–77% and the limitation of US in clinical practice. Thus, we believe that it might be difficult to properly evaluate urolithiasis by using US resulting in relatively lower detection rate of urolithiasis in patients. Paz et al. [11] retrospectively analyzed a total of 100 patients who were indicated for renal colic and performed noncontrast CT images and they reported 37.5% NAFLD in the positive urinary stone disease group as compared to 10% in the non-urinary stone disease group with statistical significance. Our study revealed that detection rate of NAFLD was 42.6% in positive renal stone disease and 15% in non-renal stone disease. Also, they revealed that detection rate of urolithiasis was 93.8% in NAFLD and 73.5% in non-NAFLD, respectively. Although the detection rate of renal stone disease was significantly higher in patients with NAFLD than that of patients without NAFLD in their study, however, detection rate of urolithiasis was extremely higher than that of our study. A discrepancy between our study and theirs could be the difference of inclusion criteria. All enrolled patients in their study were clinically suspected urolithiasis and it would lead to a selection bias.
It has well known that CT examination has a high sensitivity and specificity for detecting fatty liver and renal stone disease [12], [13]. In addition, CT examination also can quantify liver fat accumulation [8], [15]. In our study, we reveal that the attenuation of liver parenchyma in patients with renal stone disease significantly lower than that in patients without renal stone disease (p < 0.05). However, this disparity is too subtle to differentiate two groups for clinical physicians in visual assessment. Furthermore, HU was no significant association with renal stone disease in multivariate analysis. Thus, we suggest that the severity of fatty liver has no association with renal stone disease.
The association between NAFLD and renal stone disease has received little attention. In the review of recent literature, oxidative and systemic metabolisms are compatible with stone formation [16]. The kidneys is an highly vulnerable organ damaged by reactive oxygen species, likely due to the abundance of long chain poly unsaturated fatty acids in the composition of renal lipids and systemic oxidative species can result in peroxidation of lipid that may have an effect in calcium oxalate stone formation [16]. Further data suggest that fatty liver may result in changes in urinary constituents, leading to an increased incidence of stone formation [17].
The strong point of our study is that we validated high relevance between fatty liver with renal stone disease on the basis of CT findings that have a higher sensitivity and specificity to detect fatty liver and renal stone disease than that of other imaging modality such as ultrasonography which can miss the renal stone disease in daily routine practice. Furthermore, it is noteworthy that the detection rate of renal stone disease more increased in patients with NAFLD (odds ratio: 5, 95% CI, 3–8.2) (p < 0.05) than that of other study.
There were several limitations to our study. First, the possibility of selection bias should be considered because our results were based on a retrospective study design. However, we attempted to enroll all consecutive patients who met the inclusion criteria during the eligible period. Second, we did not performed interobserver and intraobserver reliability to evaluate NAFLD and renal stone disease.
In conclusion, the prevalence of renal stone disease in patients with NAFLD was significantly higher than that of patients without NAFLD and the presence of NAFLD may be considered to be an independent variable as a risk factor for renal stone disease.
Conflict of interests
Author declares that there are no conflict of interests.
Contributor Information
In Chul Nam, Email: sky_hall@naver.com.
Jung Hee Yoon, Email: radyjh@hanmail.net.
Seung Ha Park, Email: SH_Park@paik.ac.kr.
JiHwa Ryu, Email: rjhrad@empas.com.
Seung Ho Kim, Email: radiresi@gmail.com.
Yedaun Lee, Email: chosai81@gmail.com.
References
- 1.Hamaguchi M., Takeda N., Kojima T. Identification of individuals with non-alcoholic fatty liver disease by the diagnostic criteria for the metabolic syndrome. World J. Gastroenterol. 2012;18:1508–1516. doi: 10.3748/wjg.v18.i13.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006;40:S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 3.Targher G., Chonchol M., Pichiri I. Risk of cardiovascular disease and chronic kidney disease in diabetic patientswith non-alcoholic fatty liver disease: just a coincidence. J. Endocrinol. Invest. 2011;34:544–551. doi: 10.3275/7614. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of Non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Gastroenterology. 2012;124(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Li G., Shi W., Hug H. Nonalcoholic fatty liver disease associated with impairment of kidney functionin nondiabetes population. Biochem. Med. (Zagreb) 2012;22:92–99. doi: 10.11613/bm.2012.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan A.P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr. Nephrol. 2010;25:831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohjimoto Y., Iba A., Sasaki Y. Metabolic syndrome and nephrolithiasis. Acta Urol. Jpn. 2011;57:43–47. [PubMed] [Google Scholar]
- 8.Ricci C., Longo R., Gioulis E. Noninvasive in vivo quantitative assessment of fat content in human liver. J. Hepatol. 1997;27:108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 9.Hamer O.W., Aquirre D.A., Casola G. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 10.Einollahi B., Naghii M.R., Sepandi M. Association of nonalcoholic fatty liver disease (NAFLD) with urolithiasis. Endocr. Regul. 2013;47(1):27–32. doi: 10.4149/endo_2013_01_27. [DOI] [PubMed] [Google Scholar]
- 11.D. Paz, L. Guralnik, I.L. Haifa, et al. Association of renal stone (urolithiasis) with nonalcoholic fatty liver (NAFL), 10.1594/ecr2015/c-2056
- 12.Heidenreich A., Desgrandschamps F., Terrier F. Modern approach of diagnosis and management of acute flank pain: review of all imaging modalities. Eur. Urol. 2002;41:351–362. doi: 10.1016/s0302-2838(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 13.Niemann T., Kollmann T., Bongartz G. Diagnostic performance of low-dose CT for the detection of urolithiasis: a meta-analysis. Am. J. Roentgenol. 2008;191(2):396–401. doi: 10.2214/AJR.07.3414. [DOI] [PubMed] [Google Scholar]
- 14.Ray A.A., Ghiculete D., Pace K.T. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76(2):295–300. doi: 10.1016/j.urology.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Mendonça P.R., Lamb P., Kriston A. Contrast-independent liver-fat quantification from spectral CT exams. Med. Image Comput. Assist. Interv. 2013;16:324–331. doi: 10.1007/978-3-642-40811-3_41. [DOI] [PubMed] [Google Scholar]
- 16.Schwille P.O., Schmiedl A., Wipplinger J. Idiopathic recurrent calcium urolithiasis (IRCU): variation of fasting urinaryprotein is a window to pathophysiology or simple consequence of renal stones in situ? A tripartite study inmale patients providing insight into oxidative metabolism as possible driving force towards alteration of urinecomposition, calcium salt crystallization and stone formation. Eur. J. Med. Res. 2009;14:378–392. doi: 10.1186/2047-783X-14-9-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasca A., Ammendola C. Nephrolithiasis: metabolic defects and therapeutic implications. Urologia. 2014;81(1):1–11. doi: 10.5301/uro.5000058. [DOI] [PubMed] [Google Scholar]