Abstract
Background: The 7th edition of the American Joint Committee on Cancer (AJCC) staging system trialed a subdivision of T1 tumors into T1a (<1 cm) and T1b (1.0–2 cm). The 2009 American Thyroid Association (ATA) guidelines recommended total thyroidectomy for tumors >1 cm, and lobectomy for those ≤1 cm. These AJCC staging parameters remain a focus of debate, and ATA guidelines are in transition. The aim of this study was to determine if the T1 staging subdivision is associated with different treatment strategies and patterns of patient survival.
Methods: All adult patients with AJCC pT1 differentiated thyroid cancer (DTC) from the National Cancer Data Base (NCDB; 1998–2012) and Surveillance, Epidemiology, and End Results (SEER) program (2004–2012) were divided into two groups based on tumor size: T1a versus T1b. Demographic, clinical, and pathologic features were evaluated. Multivariate regression analysis was used to determine factors associated with undergoing total thyroidectomy and radioactive iodine. Cox proportional hazards models were performed to determine factors associated with overall and disease-specific survival.
Results: Among 149,912 DTC patients, 98,111 (65.4%) were T1a and 51,801 (34.6%) T1b in the NCDB; in SEER, among 18,381 patients, 11,208 (61.0%) had T1a and 7173 (39.0%) T1b tumors. Patients with T1b cancers were younger (48 vs. 51 years T1a) and more likely to have private insurance (76.2% vs. 74.1%), no comorbidities (86.0% vs. 83.8%), and undergo treatment at academic medical centers (41.4% vs. 40.3%; all p < 0.01). They also were more likely to undergo total thyroidectomy (87.7% vs. 74.3%), and had more lymphovascular invasion (10.2% vs. 3.3%), positive surgical margins (7.9% vs. 3.8%), metastatic lymph nodes (35.8% vs. 23.8%), and distant metastases (0.4% vs. 0.3%; all p < 0.01). Factors associated with radioactive-iodine use included younger patient age, lower income, having insurance, positive surgical margins, and T1b stage (p < 0.01). After adjustment, overall (p = 0.23) and disease-specific survival (p = 0.93) were similar among patients with T1a versus T1b tumors.
Conclusion: These results illustrate that patients with pT1a versus pT1b tumors undergo different treatment strategies. Based on the newly published 2015 ATA guidelines, whereby either lobectomy or total thyroidectomy can be performed for low-risk tumors, it might be anticipated that treatment differences will diminish over time. Therefore, division of AJCC T1 staging into T1a versus T1b subgroups might become obsolete over time.
Introduction
Thyroid cancer is the most common endocrine malignancy, with an estimated incidence of 62,450 new cases in 2015, resulting in approximately 1950 deaths (1). Differentiated thyroid cancer (DTC), including papillary, follicular, and Hürthle cell cancer histologies, represents the overwhelming majority of cases. The overall five-year survival rate of patients with DTC is approximately 98%, with a 10-year overall survival of >90% when appropriate treatment is employed (2). The optimal extent of surgery and use of radioactive iodine (RAI) for patients with DTC has been controversial.
Due to the significant role that tumor size plays in patient management and outcomes, it is critical to scrutinize size criteria carefully as the basis for many treatment decisions. DTC in general has an excellent prognosis. It is not clear whether the subdivision of T1 DTC tumors (≤2 cm) into microcarcinomas (<1 cm; T1a) and tumors 1–2 cm (T1b) is necessary to inform treatment and prognosis discussions.
In 2007, Bilimoria et al. published a large population-level study that included 52,173 patients with papillary thyroid cancer (PTC) who underwent either total thyroidectomy or lobectomy. The study in part illustrated that overall survival at 10 years for PTC was not significantly different based on tumor size until it reached a threshold of 4 cm (3). The 7th edition of the Union for International Cancer (UICC)/American Joint Committee on Cancer (AJCC) tumor, node, metastases (TNM) staging system made a new subdivision of T1 tumors (≤2 cm intrathyroidal tumors) into T1a (≤1 cm) and T1b (1–2 cm) groups (4). The American Thyroid Association (ATA) published its 2009 guidelines with the recommendation of total thyroidectomy for tumors >1 cm, and thyroid lobectomy reserved only for the majority of those tumors <1 cm that did not present in the setting of prior cervical radiation or familial PTC (5). The aim of this study was to determine if the T1 staging subdivision is associated with different treatment strategies and patterns of patient survival.
Methods
The Institutional Review Board granted exempt status for this retrospective analysis of the National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. The NCDB is administered jointly by the American College of Surgeons and the American Cancer Society. It was established in 1989, and at present contains data from >1500 Commission on Cancer–accredited institutions, and records from >30 million patients from all 50 states, Puerto Rico, and the District of Columbia. It is estimated that approximately 85% of all new thyroid cancer cases in the United States are captured in the database (6). This data set was used in describing patient demographic, tumor, and treatment characteristics and for calculating overall survival and adjusted Cox proportional hazards modeling.
The SEER database records demographic information, tumor site, histology, stage, grade, and treatments performed. The population residing within the areas served by the 18 SEER cancer registries is representative of approximately 28% of the general U.S. population (7). This database was uniquely used to determine disease-specific survival for the patient population of interest.
The NCDB participant user files from 1998 to 2012 and SEER registries from 2004 to 2012 were used to identify all thyroid cancer patients who underwent thyroid surgery. Patients with DTC, including papillary, follicular, and Hürthle cell histologies, were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography and histology codes. Aggressive histologic variants such as columnar/tall-cell and diffuse sclerosing subtypes were included in the study. Patients with multiple diagnoses of cancer were excluded to ensure that outcomes were not confounded by other diagnoses and/or treatments. Only patients with a tumor size of ≤2 cm were analyzed. These patients were divided into two groups based on their pathologic (p) tumor size: <1 cm (pT1a) and 1–2 cm (pT1b).
Patient variables, including age at diagnosis, race/ethnicity, sex, annual income, type and status of insurance, year of diagnosis, type of treatment facility, and comorbidities were extracted from the database. Comorbidity was represented by the modified Charlson/Deyo scoring system (1992) (8). Level of education and annual income were assigned by NCDB by matching the patient's ZIP code to previous U.S. Census data. The NCDB reports median annual income data in the patient's ZIP code in quartiles: the lower two quartiles were for median incomes of <$35,000, and the top two represented incomes of ≥$35,000.
Statistical analysis
NCDB analysis
Baseline characteristics and outcomes were compared between groups using the Kruskal–Wallis test for continuous variables and chi-square/Fisher's exact tests for categorical variables. The primary outcome was overall survival. Secondary endpoints included identification of factors associated with receipt of total thyroidectomy and RAI. To estimate the independent effect of pT1a versus pT1b status on overall survival, multivariable logistic and linear regression models were developed. They included the following variables, chosen a priori: pathologic T-stage, patient age, sex, race/ethnicity, insurance status, surgical margin status, presence of positive (metastatic) lymph nodes, histologic type of DTC, type of surgical procedure (thyroid lobectomy vs. total thyroidectomy), and use of RAI. Those who underwent thyroid lobectomy and subsequently had a completion thyroidectomy were coded as a total thyroidectomy in the database.
Overall survival was defined as the time from diagnosis to time of death or last follow-up, whichever occurred first. Patients with zero months of follow-up were excluded. Estimates and confidence intervals (CI) of overall survival proportions were computed using the Kaplan–Meier method, and survival distributions were compared across groups using the log-rank test. Cox proportional hazards modeling was used to evaluate independent predictors of overall survival. A two-sided p-value of <0.05 indicates statistical significance. All statistical analyses were performed using SPSS Statistics for Windows v22.0 (IBM Corp., Armonk, NY).
Multivariable logistic regression analyses were used to examine factors associated with receipt of total thyroidectomy and RAI treatment. Both models were adjusted for the effects of demographic, clinical, and pathologic factors, including patient age, sex, race/ethnicity, annual income, insurance status, and surgical margin status. Those patients with metastatic regional lymph nodes and distant metastases were excluded from both models after initial analysis in order to eliminate those patients who do not represent the standard presentation of patients with small, intrathyroidal, low-risk DTCs.
SEER analysis
Disease-specific survival was analyzed for the patient population of interest. Similar to the analytic treatment of the NCDB, the CI of disease-specific survival was computed using the Kaplan–Meier method, and the log-rank test was used to compare survival distributions. Due to the limited number of patient deaths (events) attributable to pT1 thyroid cancers within the first 10 years after diagnosis, it was not possible to perform multivariable adjustment analysis of disease-specific survival, and only unadjusted analysis and modelling is presented. The NCDB and SEER databases were not combined at any point in the analysis. The SEER database was used specifically as a tool to address disease-specific survival among patients with pT1a versus pT1b tumors.
Results
A total of 149,912 patients met the study criteria and were included. Of these, 98,111 (65%) had pT1a DTC, and 51,801 (35%) had pT1b tumors. In SEER, 18,381 patients met the study criteria. Of these, 11,208 (61.0%) had pT1a tumors, and 7173 (31.0%) had pT1b tumors. Those patients in the NCDB with pT1a thyroid tumors were more likely to be female (81.7% vs. 79.8% pT1b), older (50 vs. 48 years), and white (88.1% vs. 88%; all p < 0.001). Patients in the NCDB with pT1b tumors were more likely to have lymphovascular invasion (10.2% vs. 3.3% for pT1a), undergo a total thyroidectomy (87.7% vs. 74.3%), have positive surgical margins (7.9% vs. 3.8%), metastatic regional lymph nodes (35.8% vs. 23.8%), distant metastases (0.4% vs. 0.3%), and receive RAI therapy (59.7% vs. 27.6%; all p < 0.001; Table 1).
Table 1.
Patient Demographic, Clinical, and Pathologic Characteristics by pT1 Status, NCDB 1998–2012
| Variable | pT1a, N = 98,111 (%) | pT1b, N = 51,801 (%) | p-Value |
|---|---|---|---|
| Demographic characteristics | |||
| Sex | <0.001 | ||
| Female | 80,124 (81.7) | 41,313 (79.8) | |
| Age | <0.001 | ||
| Mean ± SD | 50.6 ± 13.8 | 48.1 ± 14.5 | |
| Median | 50.0 | 48.0 | |
| Race/ethnicity | <0.001 | ||
| White | 84,825 (88.1) | 44,730 (88.0) | |
| Black | 6789 (7.0) | 3084 (6.1) | |
| Asian | 3756 (3.9) | 2381 (4.7) | |
| Other | 978 (1.0) | 637 (1.2) | |
| Annual income | <0.001 | ||
| <$35,000 | 23,020 (24.4) | 11,853 (23.9) | |
| ≥$35,000 | 71,182 (75.6) | 37,833 (76.1) | |
| Insurance status | <0.001 | ||
| None | 1984 (2.1) | 1182 (2.3) | |
| Government | 22,935 (23.8) | 10,922 (21.5) | |
| Private | 71,274 (74.1) | 38,655 (76.2) | |
| Comorbidity | <0.001 | ||
| 0 | 69,608 (83.8) | 43,510 (86.0) | |
| 1 | 11,281 (13.6) | 5997 (11.9) | |
| ≥2 | 2174 (2.6) | 1093 (2.1) | |
| Treatment-level characteristics | |||
| Facility type | <0.001 | ||
| Academic | 39,557 (40.3) | 21,438 (41.4) | |
| Comprehensive | 51,383 (52.4) | 26,770 (51.7) | |
| Community | 7125 (7.3) | 3580 (6.9) | |
| Facility location | |||
| Northeast | 24,770 (25.2) | 13,282 (25.6) | |
| Midwest | 23,885 (24.3) | 12,186 (23.5) | |
| South | 33,443 (34.2) | 17,268 (33.4) | |
| West | 16,013 (16.3) | 9065 (17.5) | |
| Clinical characteristics | |||
| Histology | <0.001 | ||
| Papillary | 96,633 (98.5) | 48,346 (93.3) | |
| Follicular | 1013 (1.0) | 2359 (4.6) | |
| Hürthle | 465 (0.5) | 1096 (2.1) | |
| Tumor size (mean ± SD) | 0.5 ± 0.3 | 1.5 ± 0.3 | <0.001 |
| Lymphovascular invasion* | 837 (3.3) | 1723 (10.2) | <0.001 |
| Extent of surgery | <0.001 | ||
| Total thyroidectomy | 72,923 (74.3) | 45,413 (87.7) | |
| Lobectomy | 21,073 (21.5) | 4806 (9.3) | |
| Positive margin status | 3528 (3.8) | 3955 (7.9) | <0.001 |
| Positive lymph nodes | 9581 (23.8) | 9998 (35.8) | <0.001 |
| Lymph nodes removed (mean ± SD) | 9.8 ± 21.5 | 10.0 ± 19.5 | 0.301 |
| Metastatic lymph nodes (mean ± SD) | 0.7 ± 2.2 | 1.3 ± 3.3 | <0.001 |
| Distant metastases | 289 (0.3) | 211 (0.4) | <0.001 |
| RAI therapy | 26,160 (27.6) | 30,216 (59.7) | <0.001 |
Variable not collected by the NCDB before 2010; values are presented as percentages of the known (after 2010). Percentages have been rounded and may not add up to 100%.
NCDB, National Cancer Data Base; SD, standard deviation; RAI, radioactive iodine.
Impact of pathologic T-stage on overall and disease-specific survival
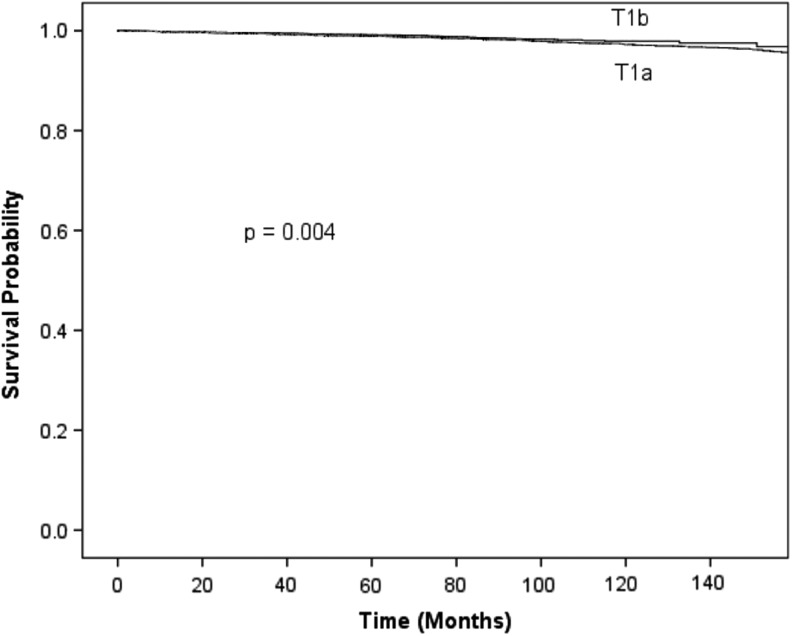
Unadjusted overall survival for patients with pT1a versus pT1b differentiated thyroid cancer is shown in Figures 1 and 2. In the NCDB, after multivariable adjustment, overall survival was similar between patients with pT1a and pT1b DTC (hazard ratio [HR] = 0.97 [CI 0.91–1.03]; p = 0.26; Table 2). Adjusted 10-year survival was similar for patients <45 years with T1a and T1b tumors in the NCDB (HR = 1.18 [CI 0.98–1.42]; p = 0.09; Table 3). In SEER, the unadjusted 10-year disease-specific survival was similar for patients with pT1a and pT1b tumors (99.2% vs 98.7%; p = 0.94). In the same database, unadjusted 10-year disease-specific survival was similar for patients <45 years with pT1a and pT1b tumors (99.8% vs 99.8%; p = 0.16).
FIG. 1.
Unadjusted overall survival for patients with pT1a versus pT1b differentiated thyroid cancer, NCDB 1998–2012. NCDB, National Cancer Data Base.
FIG. 2.
Unadjusted overall survival for patients <45 years old with pT1a versus pT1b differentiated thyroid cancer, NCDB 1998–2012.
Table 2.
Cox Proportional Hazards Model for Overall Survival in Patients with pT1 Differentiated Thyroid Cancer, NCDB 1998–2012
| Variable | Hazard ratio | CI | p-Value | |
|---|---|---|---|---|
| pT1a vs. pT1b | 0.96 | 0.91 | 1.02 | 0.23 |
| Demographic factors | ||||
| Patient age, per 10 years | 1.80 | 1.75 | 1.84 | <0.001 |
| Female sex | 0.58 | 0.55 | 0.61 | <0.001 |
| Race/ethnicity | ||||
| White* | ||||
| Black | 1.31 | 1.19 | 1.45 | <0.001 |
| Asian | 0.61 | 0.49 | 0.74 | <0.001 |
| Other | 0.49 | 0.31 | 0.77 | 0.002 |
| Annual household income | ||||
| <$35,000* | ||||
| ≥$35,000 | 0.72 | 0.68 | 0.76 | <0.001 |
| Insurance status | ||||
| Uninsured* | ||||
| Private | 0.66 | 0.58 | 0.76 | <0.001 |
| Government | 1.30 | 1.13 | 1.49 | <0.001 |
| Treatment characteristics | ||||
| Total thyroidectomy | 0.94 | 0.89 | 0.10 | 0.04 |
| Positive surgical margin | 1.22 | 1.09 | 1.38 | 0.001 |
| Metastatic lymph nodes | 1.54 | 1.42 | 1.67 | <0.001 |
| RAI | 0.73 | 0.68 | 0.77 | <0.001 |
Reference variable.
CI, confidence interval.
Table 3.
Cox Proportional Hazards Model for Overall Survival in Patients < 45 Years with pT1 Differentiated Thyroid Cancer, NCDB 1998–2012
| Variable | Hazard ratio | CI | p-Value | |
|---|---|---|---|---|
| pT1a vs. pT1b | 1.18 | 0.98 | 1.42 | 0.09 |
| Demographic factors | ||||
| Patient age, per 10 years | 1.70 | 1.47 | 1.96 | <0.001 |
| Female sex | 0.50 | 0.41 | 0.61 | <0.001 |
| Race/ethnicity | ||||
| White* | ||||
| Black | 1.38 | 1.05 | 1.82 | 0.02 |
| Asian | 0.52 | 0.30 | 0.90 | 0.02 |
| Other | 0.45 | 0.15 | 1.42 | 0.17 |
| Annual household income | ||||
| <$35,000* | ||||
| ≥$35,000 | 0.71 | 0.60 | 0.84 | <0.001 |
| Insurance status | ||||
| Uninsured* | ||||
| Private | 0.60 | 0.44 | 0.83 | 0.002 |
| Government | 2.18 | 1.55 | 3.06 | <0.001 |
| Treatment characteristics | ||||
| Total thyroidectomy | 0.98 | 0.80 | 1.20 | 0.85 |
| Positive surgical margin | 1.19 | 0.83 | 1.71 | 0.35 |
| Metastatic lymph nodes | 1.50 | 1.21 | 1.87 | <0.001 |
| Distant metastases | 1.16 | 0.16 | 8.31 | 0.88 |
| RAI | 0.70 | 0.58 | 0.84 | <0.001 |
Reference variable.
Impact of pathologic T-stage on surgical approach and RAI use
Using multivariable logistic regression analysis with adjustment for patient demographic, clinical, and pathologic characteristics, patients with pT1a tumors were less likely to undergo total thyroidectomy compared with patients with pT1b cancers (odds ratio [OR] = 0.430 [CI 0.417–0.444]; p < 0.001). Factors associated with increased use of total thyroidectomy among patients of all ages include female sex, black race, higher income, and insured status (Table 4).
Table 4.
Logistic Regression of Factors Associated with Total Thyroidectomy, NCDB 1998–2012
| Variable | Odds ratio | CI | p-Value | |
|---|---|---|---|---|
| pT1a vs. pT1b | 0.43 | 0.42 | 0.44 | <0.001 |
| Demographic factors | ||||
| Patient age, per 10 years | 0.96 | 0.95 | 0.97 | <0.001 |
| Female sex | 1.22 | 1.18 | 1.26 | <0.001 |
| Race/ethnicity | ||||
| White* | ||||
| Black | 1.07 | 1.02 | 1.13 | <0.001 |
| Asian | 1.01 | 0.94 | 1.08 | 0.86 |
| Other | 0.97 | 0.85 | 1.10 | 0.64 |
| Annual household income | ||||
| <$35,000* | ||||
| ≥$35,000 | 1.08 | 1.05 | 1.11 | <0.001 |
| Insurance status | ||||
| Uninsured* | ||||
| Private | 1.36 | 1.27 | 1.44 | <0.001 |
| Government | 1.25 | 1.17 | 1.34 | <0.001 |
Reference variable.
Adjusted analysis demonstrated that pT1a patients were significantly less likely to receive RAI compared with pT1b patients (OR = 0.277 [CI 0.270–0.285]; p < 0.001). Factors associated with increased RAI use included insured status and positive surgical margins (Table 5).
Table 5.
Logistic Regression of Factors Associated with RAI Use, NCDB 1998–2012
| Variable | Odds ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| pT1a vs. pT1b | 0.28 | 0.27 | 0.29 | <0.001 |
| Demographic factors | ||||
| Patient age, per 10 years | 0.94 | 0.93 | 0.95 | <0.001 |
| Female sex | 0.96 | 0.92 | 0.99 | 0.01 |
| Race/ethnicity | ||||
| White* | ||||
| Black | 0.75 | 0.71 | 0.79 | <0.001 |
| Asian | 0.93 | 0.87 | 0.99 | 0.03 |
| Other | 0.62 | 0.54 | 0.71 | <0.001 |
| Annual household income | ||||
| <$35,000* | ||||
| ≥$35,000 | 0.91 | 0.88 | 0.94 | <0.001 |
| Insurance status | ||||
| Uninsured* | ||||
| Private | 1.45 | 1.34 | 1.56 | <0.001 |
| Government | 1.24 | 1.15 | 1.34 | <0.001 |
| Treatment characteristics | ||||
| Positive surgical margin | 2.35 | 2.21 | 2.51 | <0.001 |
Reference variable.
Discussion
The current 7th edition of the AJCC guidelines subclassifies T1 intrathyroidal tumors (≤2 cm) into those <1 cm (T1a, or microcarcinomas), and those between 1 and 2 cm (T1b). This has been controversial. An important clinical question is whether patients with T1a tumors should be managed differently compared to patients with T1b tumors based on the expectation that patients with thyroid microcarcinomas have superior outcomes.
This study examined the association between this pathologic T1 subdivision, treatment strategies, and patient outcomes in a cohort of patients with DTC from the NCDB and SEER databases. After adjustment for numerous factors, including treatment differences observed, no difference was found between patients with pT1a and pT1b tumors in terms of overall or disease-specific survival. These findings call into question whether the subdivision of pT1 tumors is needed. The treatment differences observed may be the result of the 2009 ATA guidelines for the management of thyroid nodules and DTC, which recommended thyroid lobectomy for tumors ≤1 cm and total thyroidectomy for all tumors >1 cm (9).
Lack of survival differences found in this national-level study have been demonstrated in smaller institutional studies. In a study analyzing disease-specific survival at a single institution, Wang et al. could find no difference in disease-specific survival among 1522 patients with T1 DTC tumors categorized as T1a versus T1b (10). They demonstrated a five-year disease-specific survival of 100% for both groups, which is similar to the survival rates of 99.7% and 99.8% for T1a and T1b subgroups, respectively, observed in the present NCDB study (10). In a smaller institutional study of 269 patients, DeGroot et al. also reported no difference in survival between tumors measuring <1 cm and those with tumors 1–2 cm in size (11).
The treatment of DTC has continued to evolve based on a dynamic field of research. Adam et al. published a population-level analysis of 61,775 adult patients who underwent surgery from1998 to 2006, examining the impact that extent of surgery had on survival for patients with PTC. After multivariable adjustment for patient demographic, clinical, pathologic, and treatment characteristics, the authors found that overall survival was similar between those who underwent thyroid lobectomy only and those who underwent total thyroidectomy for intrathyroidal tumors between 1.0 and 4.0 cm (HR = 0.96 [CI 0.84–1.09]; p = 0.54). Similar results were seen after stratification based on tumor size: 1.0–2.0 cm (HR = 1.05 [CI 0.88–1.26]; p = 0.61) and 2.1–4.0 cm (HR = 0.89 [CI 0.73–1.07]; p = 0.21) (12). They concluded that tumor size alone should not be the only driver of treatment choice. Other tumor features, such as more aggressive tumor histology and extrathyroidal extension, may play a bigger role than size alone with regard to patient outcome (10,13–15). These findings were echoed by Ito et al. in their study of 2638 patients with clinical T1N0M0 papillary carcinomas. After adjustment for patient demographic, clinical, and pathologic characteristics such as multifocality and extrathyroidal extension, the authors found no difference in 10-year disease-free survival between T1a and T1b patients (97% for both groups) (13). In a population-level study of 29,512 patients, Wang et al. compared the overall survival of T1 patients with age-matched Americans without cancer, and determined that survival was comparable to that of the normative U.S. general population, regardless of treatment strategy undertaken (14).
Cancer recurrence has been another outcome of interest in the arena of DTC. The datasets used in the present study lack information regarding recurrence. However, other longitudinal institutional studies have shown no difference in recurrence rates between T1a and T1b tumors at five years (10,11), and up to 15 years following the time of diagnosis (16–18).
In light of many of these studies, the latest 2015 ATA guidelines (Recommendations 35B and 35C; Operative approach for a biopsy diagnostic for follicular cell-derived malignancy) revised surgical recommendations, stating that thyroid lobectomy alone may be sufficient initial treatment for low-risk, unifocal, intrathyroidal papillary and follicular carcinomas ≤4 cm, unless there are clear indications to remove the contralateral lobe (19). Patient preference remains a critical deciding factor. Guidelines can have clear effects on clinical practice patterns in the United States. Shortly after the release of the 2009 ATA guidelines, Roman et al. reported a 10% decrease in use of RAI for low-risk DTC that was associated with the change in recommendation (20). The current study may help to inform future practice guidelines and anticipated revisions of the AJCC staging system with regard to whether there is a need for T1a versus T1b subdivisions for DTC.
There are now data from an observational study by Miyauchi et al. suggesting that active surveillance may be appropriate for a subset of patients with T1a tumors instead of immediate surgery (21). No studies published to date have evaluated active surveillance for T1b tumors. Further analysis on proper patient selection and impact of long-term active follow-up versus surgery for T1 tumors is necessary, particularly set in the United States.
This study should be considered in light of limitations that are inherent to large national databases. There is a potential for coding errors, but data reporting in the NCDB and SEER is done using standardized abstraction methods and is highly scrutinized (22). The strength of this study lies in the large number of patients analyzed using two separate databases that encompass nationwide data, making the findings more generalizable. Another limitation of this study is that neither the NCDB nor SEER can determine the original intent of thyroidectomy, and whether the diagnosis of thyroid cancer was known preoperatively or established postoperatively based on surgical pathology results.
This study is one of the largest studies on low-risk DTC, and the first to evaluate survival and treatment strategies under the current AJCC staging system in a national analysis. Based on the 2015 ATA guidelines whereby either lobectomy or total thyroidectomy can be performed for low-risk tumors, it might be anticipated that treatment differences will diminish going forward. Therefore, division of the AJCC staging system for T1 tumors into T1a versus T1b subgroups may become obsolete over time.
Acknowledgments
Grant support included the National Institutes of Health TL-1 clinical and translational science award (CTSA), 1UL1-TR001117-01 (NCATS).
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Author Disclosure Statement
J.A.S. is a Member of the Data Monitoring Committee of the Medullary Thyroid Cancer Registry, funded through UBC by GlaxoSmithKline, NovoNordisk, Astra Zeneca, and Eli Lilly. No competing financial interests exist for the remaining authors.
References
- 1.American Cancer Society. Cancer Facts & Figures, 2015. Available at: www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf (accessed November12, 2015)
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. 2014. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–271 [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, Sturgeon C. 2007. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 246:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer 2009 AJCC Cancer Staging Manual. Seventh edition. Springer, New York [Google Scholar]
- 5.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzeferri EL, Mclver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 6.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. 2009. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol 99:488–490 [DOI] [PubMed] [Google Scholar]
- 7.Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, Hu JC, Martin NE, Trinh QD, Alexander BM, Nguyen PL. 2014. Cancer-specific outcomes among young adults without health insurance J Clin Oncol 32:2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619 [DOI] [PubMed] [Google Scholar]
- 9.Puxeddu E, Filetti S. 2009. The 2009 American Thyroid Association guidelines for management of thyroid nodules and differentiated thyroid cancer: progress on the road from consensus- to evidence-based practice. Thyroid 19:1145–1147 [DOI] [PubMed] [Google Scholar]
- 10.Wang LY, Nixon IJ, Palmer FL, Thomas D, Tuttle RM, Shaha AR, Patel SG, Shah JP, Ganly I. 2014. Comparable outcomes for patients with PT1a and PT1b differentiated thyroid cancer: is there a need for change in the AJCC classification system? Surgery 156:1484–1490 [DOI] [PubMed] [Google Scholar]
- 11.Degroot LJ, Kaplan EL, McCormick M, Straus FH. 1990. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 71:414–424 [DOI] [PubMed] [Google Scholar]
- 12.Adam MA, John Pura, Gu L, Dinan MA, Tyler DS, Reed SD, Scheri R, Roman SA, Sosa JA. 2014. Extent of surgery for papillary thyroid cancer is not associated with survival. Ann Surg 260:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. 2010. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg 34:1285–1290 [DOI] [PubMed] [Google Scholar]
- 14.Wang TS, Goffredo P, Sosa JA, Roman JA. 2014. Papillary thyroid microcarinoma: an over-treated malignancy? World J Surg 38:2297–2303 [DOI] [PubMed] [Google Scholar]
- 15.Rossi R, Roti E, Trasforini G, Pansini G, Cavazzini L, Zatelli MC, Pearce EN, Braverman LE, Uberti EC. 2008. Differentiated thyroid cancers 11–20 mm in diameter have clinical and histopathologic characteristics suggesting higher aggressiveness than those < or = 10 mm. Thyroid 18:309–315 [DOI] [PubMed] [Google Scholar]
- 16.Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. 2004. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metabol 89:3713–3720 [DOI] [PubMed] [Google Scholar]
- 17.Cady B, Rossi R. 1988. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 18.Haigh PI, Urbach DR, Rotstein LE. 2005. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol 12:81–89 [DOI] [PubMed] [Google Scholar]
- 19.Haugen BR, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman BR, Feingold JH, Patel SG, Shaha AR, Shah JP, Tuttle RM, Epstein AJ. 2014. The 2009 American Thyroid Association guidelines modestly reduced radioactive iodine use for thyroid cancers less than 1 cm. Thyroid 24:1549–1550 [DOI] [PubMed] [Google Scholar]
- 21.Miyauchi A. 2016. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg 40:516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winchester DP, Stewart AK, Phillips JL, Ward EE. 2010. The National Cancer Data Base: past, present, and future. Ann Surg Oncol 17:4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]