Abstract
The ovary of neonatal nonhuman primates contains the highest number of immature oocytes, but its cryopreservation has not yet been sufficiently investigated in all life stages. In the current study, we investigated cryodamage after vitrification/warming of neonatal ovaries from a nonhuman primate, the common marmoset (Callithrix jacchus). A Cryotop was used for cryopreservation of whole ovaries. The morphology of the vitrified/warmed ovaries was found to be equivalent to that of fresh ovaries. No significant difference in the number of oocytes retaining normal morphology per unit area in histological sections was found between the two groups. In an analysis of dispersed cells from the ovaries, however, the cell viability of the vitrified/warmed group tended to be decreased. The results of a comet assay showed no significant differences in DNA damage. These results show that cryopreservation of neonatal marmoset ovaries using vitrification may be useful as a storage system for whole ovaries.
Keywords: comet assay, common marmoset, neonate, ovarian tissue cryopreservation, vitrification
Introduction
Since the mammalian ovary contains a large number of immature oocytes (follicles), ovarian cryopreservation is expected to provide an effective means for germ cell storage and fertility preservation. There are two main methods used for cryopreservation in reproductive biology and medicine. One is slow freezing performed using a programmable freezer that undergoes a controlled temperature change based on a computer program, and the other is vitrification, a rapid cooling method in which the tissue is directly plunged into liquid nitrogen after equilibration in high concentrations of cryoprotectants. Vitrification is known to be a simple and convenient protocol for preserving embryos and oocytes. This technology has been put to practical use for both oocytes and preimplantation embryos of various mammalian species [5, 36].
In recent years, ovarian cryopreservation has attracted attention as a promising reproductive technology for fertility preservation in mammals, because the ovary includes a large number of immature oocytes (follicles). This approach has been attempted not only in laboratory animals, such as rodents, but also in domestic and wild animals [25] and humans [29]. The methods for both slow freezing [3, 14, 17, 26, 34, 37, 38, 40] and vitrification [1, 9, 10, 12, 32,33,34,35, 40] have been investigated in nonhuman primates. The vitrification method is at least as effective as slow freezing in viability after thawing [40]. Furthermore, it is easy and more convenient than slow freezing because devices such as a programmable freezer are not required. These investigations have mainly targeted the follicles of adults or have, in rare cases, utilized prepubertal ovarian tissues [37, 38], but they have not utilized neonatal stage tissues.
The neonatal ovaries include a large number of primordial oocytes (follicles), more than are contained in adult ovaries. For instance, human ovaries at birth store approximately 130,000 to 500,000 primordial oocytes, and the number of ovarian follicles decreases gradually with the increase in age after puberty [7, 8]. Not all primordial oocytes stored in the ovaries achieve growth, maturation, and ovulation during reproductive life. A certain percentage of oocytes are present in dormant primordial follicles, and others undergo follicle atresia via an apoptotic process [19]. In fact, more than 99% of all follicles fail to reach the preovulatory stage, with most of these follicles undergoing atresia. Ovarian cryopreservation at the neonatal stage would therefore make it possible to store the largest possible number of oocytes.
The common marmoset (Callithrix jacchus) is a New World monkey that has attracted attention as a nonhuman primate model for biological and medical research [4, 22]. Among nonhuman primates, the marmoset has a comparative advantage as a laboratory animal because of its small size (300–450 g) and high reproductive efficiency. However, many monkeys die before or soon after birth in breeding. According to a study of mortality in the common marmoset [24], the frequency of fetal death was 10.5% of total births, and the highest ratios of fetal death occurred in quadruplet (37.5%) and singleton pregnancies (25.0%). In addition, some cases of infanticide occur immediately after birth. Therefore, cryopreservation of the neonatal ovaries may be useful for rescuing female germ cells when the individuals have a particularly important genotype.
The aim of this study was to investigate the effects of cryopreservation by a vitrification method on cryodamage in neonatal marmoset ovaries.
Materials and Methods
Animals
This study was conducted in accordance with protocols approved by the ethics committee for primate research at the National Institute of Neuroscience (NIN), National Center of Neurology and Psychiatry (NCNP), Japan. Female neonatal common marmoset monkeys (Callithrix jacchus, 0–10 days old) produced in the breeding colony of the NIN/NCNP were donated to us after they had been euthanized by intraperitoneal injection of sodium pentobarbital at doses of 100 mg/kg and utilized for neuroscience research. A total of 12 ovaries from 7 neonates were used in this study. Six ovaries were used for histology (3 ovaries were used as fresh controls). The remaining 6 ovaries were used for both a comet assay and analysis of ovarian cell viability (3 ovaries were used as fresh controls).
Cryopreservation and warming
A commercially available vitrification kit (Kitazato BioPharma Co., Ltd., Shizuoka, Japan) was used in these experiments. Neonatal ovaries were transferred into Leibovitz’s L-15 medium supplemented with 10% heat-inactivated fetal bovine serum (L-15 and FBS; Life Technologies Corporation, Carlsbad, CA, USA). After a washing step, they were initially equilibrated in 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) in a basal medium composed of HEPES-buffered TCM199 supplemented with 20% serum substitute supplement (SSS; Irvine Scientific, Santa Ana, CA, USA) for 15 min at room temperature and then in a second equilibration solution composed of 15% EG, 15% DMSO, and 0.5 M sucrose in the basal medium for 30 min at 4°C [9]. The ovaries and a minimum volume of solution were placed on the polypropylene strip of the Cryotop (Cryotop-N, Kitazato BioPharma Co., Ltd., Shizuoka, Japan) and then immediately submerged into liquid nitrogen. For warming, the straw cap from the Cryotop was removed in liquid nitrogen, and the polypropylene strip was immersed directly into the basal medium supplemented with 1 M sucrose for 3 min at 37°C. The ovaries were then transferred into the basal medium with 0.5 M sucrose for 5 min and washed twice in the basal medium without sucrose for 15 min at room temperature.
Histology and evaluation
The ovaries were fixed in 4% formaldehyde in PBS for 24 h, and then embedded in paraffin, sectioned at a thickness of into 1–2 µm at 50 µm intervals, stained with hematoxylin and eosin, and then observed with a light microscope (Axio Imager M1, Carl Zeiss, Germany).
For the analysis, 9 to 10 sections per ovary were randomly selected, and the morphologically normal oocytes in each section were all counted. Oocytes were counted as morphologically normal if they showed no pyknosis of the oocyte nucleus, they showed no large or multiple vacuoles in the oocyte, and they did not have shrunken ooplasm [38].
Ovarian cell viability
To evaluate the pre- and post-vitrification viability of ovarian cells that contain both ovarian somatic cells and primordial oocytes, each ovary was incubated in 5 ml HBSS containing 1.5 mg/ml collagenase (Type IV) for 15 min at 37°C with gentle agitation and was treated with HBSS containing 0.05% trypsin for 10 min. The trypsin was neutralized by adding 10% FBS, and the ovary was dispersed by pipetting. Floating cells were collected by centrifugation at 1,000 rpm for 5 min. The cells (105–106 cells/ml) were washed by centrifugation in PBS and were incubated in PBS supplemented with 2 µM calcein-AM and 4 µM propidium iodide (Double Staining Kit, Dojindo Molecular Technologies Inc., Kumamoto, Japan) for 15 min at 37°C. Cell viability was examined under fluorescence microscopy (BZ-9000, Keyence, Osaka, Japan) after washing the cells. Surviving cells and the nuclei of dead cells were emitted green and red fluorescence, respectively. Lastly, the survival rate was calculated.
Comet assay
The cryodamage of genomic DNA in ovarian tissue after warming was assessed using an alkaline version of the comet assay for single cell gel electrophoresis (#4250–050-K; Trevigen, Inc., Gaithersburg, MD, USA). The tissue preparation and comet assay protocol described by Trevigen was adopted (http://www.trevigen.com/docs/protocol_4250-050-K.pdf). The ovaries were transferred into Ca2+/Mg2+-free PBS with 20 mM EDTA and thereafter were dissected into very small pieces (less than 1 mm3) and allowed to stand for 5 min. Each ovary was incubated in 5 ml Hank’s Balanced Salt Solution (HBSS; Life Technologies Corporation, Carlsbad, CA, USA) containing 1.5 mg/ml collagenase (Type IV, Sigma-Aldrich Corporation, St. Louis, MO, USA) for 15 min at 37°C with gentle agitation, and was treated with HBSS containing 0.05% trypsin for 10 min. The trypsin was neutralized by adding 10% FBS. After gentle pipetting for cell dissociation, the suspension was passed through a 70 µm cell strainer and centrifuged at 1,000 rpm for 5 min at 4°C. After removal of the supernatant, the pellet was resuspended in PBS at a concentration of 1 × 105 cells/ml and maintained at 4°C until the comet assay. These cells contained both ovarian somatic cells and primordial oocytes. Electrophoresis was carried out for 5 min at 25 V. Comet images of single cells were captured with a fluorescence microscope (BZ-9000, Keyence, Japan), converted to gray scale, and scored using the Comet Score software (ver. 1.5, TriTek, Sumerduck, VA, USA). Forty to 80 comets were measured per sample. We compared two different comet parameters. The percent of DNA in the tail was defined as the total acreage of tail intensity / (total acreage of head intensity + total acreage of tail intensity) × 100. The tail moment was defined as the tail length × the percent of DNA in the tail / 100.
Statistical analysis
Each experiment was repeated three times. All data were expressed as means ± SEM. Significance testing was carried out using one-way ANOVA. Percentage data were subjected to arcsine transformation, followed by ANOVA. Values of P<0.05 were considered to be statistically significant.
Results
Morphology and histology
The internal genital organs of a representative female neonatal marmoset are shown in Fig. 1A. In shape, the ovary was a concave ellipsoid (Figs. 1A–1D). The major and minor axes were 2.8 ± 0.1 µm and 1.2 ± 0.1 µm (n=3, mean ± SEM) in length, respectively. Therefore, it was possible to put an ovary on the Cryotop, a vitrification device (Fig. 1B). The vitrified/warmed ovaries had the same appearances as the fresh ovaries (Figs. 1C and 1D). A histological examination demonstrated the presence of primordial follicles (non-growing oocytes) and primary follicles (slightly grown oocytes). Follicles were also found enclosed by round cells and somewhat flattened cells. No preantral follicles were detected (Figs. 1E and 1G). In sections of cryopreserved ovarian tissue, some areas were cryodamaged by the vitrification/warming (Fig. 1F, Arrows), but areas with good preservation were found that showed a similar morphology to the fresh ovaries (Fig. 1H).
Fig. 1.
The morphology and histology of fresh and vitrified/warmed ovaries from female neonatal marmosets. (A) The reproductive organs of a neonate. Arrow: an ovary. (B) An ovary placed on a cryotop. (C) The appearance of a fresh ovary. (D) The appearance of a vitrified/warmed ovary. (E) A histological section of a fresh ovary. (F) A histological section of a vitrified/warmed ovary. Arrows: cryodamaged areas. (G) An enlarged view of an area in E. (H) An enlarged view of the preserved area in F. The scale bars present 500 µm in (C, D), 200 µm in (E, F), or 50 µm (G, H).
The number of morphologically normal oocytes per square millimeter was evaluated from histological sections (Fig. 2). There was no significant difference between fresh and vitrified/warmed ovaries (504.8 ± 112.6 vs. 394.8 ± 62.1, P=0.4402).
Fig. 2.
The effects of vitrification/warming on the number of morphologically normal oocytes per unit area in the ovaries from female neonatal marmosets.
Ovarian cell viability
The influence of vitrification/warming on the cell viability is shown in Fig. 3. The survival rate of cells from vitrified/warmed ovaries was significantly lower than that of the cells from fresh ovaries (86.0 ± 0.9 vs. 69.1 ± 6.3, P=0.0498).
Fig. 3.
The effects of vitrification/warming on the cell viability in the ovaries from female neonatal marmosets.
Comet assay
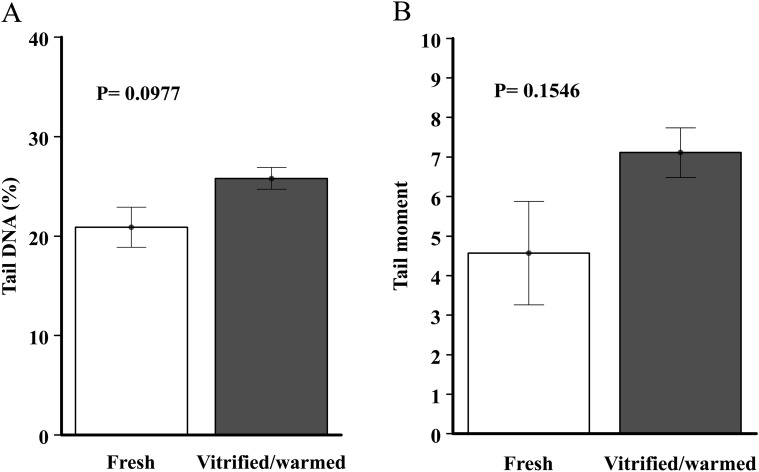
The comet assay was performed to determine the extent of genomic DNA damage. The results of two different comet parameters are shown in Fig. 4. The comet parameter of the DNA, the percentage of DNA in the tail, was not significantly different between fresh and vitrified/warmed ovaries (20.9 ± 3.5 vs. 25.8 ± 1.9, P=0.0977, Fig. 4A). The tail moment parameters calculated from the mean values of each experiment were 4.6 ± 2.3 in the fresh group and 7.1 ± 1.1 in the vitrified/warmed group, which were also not significantly different (P=0.1546, Fig. 4B).
Fig. 4.
The effects of vitrification/warming on the comet assay parameters of ovarian cells. (A) The percentages of tail DNA. (B) The tail moment.
Discussion
To date, ovarian cryopreservation has been applied to mice [28, 39], humans [29] and other animals [25]. Initially, slow-freezing methods were utilized for the cryopreservation of ovarian tissues, and later, a rapid and simple cryopreservation method, vitrification, was tried. The Cryotop method is widely used as an ultra-rapid cooling “vitrification” system for preserving oocytes and embryos [15]. This method has also been applied to ovarian tissue cryopreservation, with modifications of the equilibration procedure. In mice, live pups were obtained through in vivo transplantation or in vitro oocyte growth after ovaries were vitrified/warmed using this method [9, 13]. The ovaries of neonatal marmosets resemble those of prepubertal mice in terms of tissue size and softness, which influence the permeability of the tissues to cryoprotectants. Our results indicate that this vitrification method using the Cryotop method and cryoprotectants (EG, DMSO, and sucrose) may be effective for cryopreservation of ovaries from neonatal marmosets, but carefull optimization of protocols is needed for maximum viability after thawing.
The comet assay, a single cell gel electrophoresis assay, is a simple and sensitive technique for detecting DNA damage at the level of the individual cell. The alkaline version of the comet assay enables detection of single- and double-strand breaks in single cells [30]. This technique has also been used to assess the cryodamage of the genomic DNA of cumulus cells [18], oocytes [2, 20, 27, 31], and sperm [16]. In addition, it is also possible to analyze the damage in cryopreserved somatic tissues [11, 23]. Therefore, the comet assay is useful for evaluating the cryodamage of cells following ovarian cryopreservation.
During the fetal stage in mammals, mitotically primordial germ cells develop into oocytes that enter the meiotic cell cycle and then are arrested at prophase I. Near the time of birth in rodents, and during mid-gestation in humans, the oocytes are enclosed by a single layer of flattened epithelial cells (progenitors of follicular granulosa cells). These oocytes and their surrounding follicles are called primordial oocytes and primordial follicles, respectively [21]. In the neonatal marmoset, numerous primordial oocytes (non-growing oocytes) are stored as primordial follicles or naked oocytes in the ovaries. Furthermore, the existence of primitive oogonia in the ovarian surface epithelium can be revealed by detecting markers of pluripotency and proliferation [6].In breeding, many marmosets die before or soon after birth. Ovarian tissue cryopreservation is useful for rescuing female germ cells. One of the methods for recovery of animals is xenograft into immunodeficient mice. If oocytes are fully grown, it is possible to produce the offspring using in vitro maturation (IVM), in vitro fertilization (IVF) and embryo transfer (ET) [13].
In conclusion, cryopreservation of neonatal marmoset ovaries using vitrification will likely represent a useful storage system for whole ovaries. The results of this study are considered to provide useful information on the preservation of reproductive (genetic) resources for nonhuman primates.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) (KAKENHI 24700443) from the Japan Society for the Promotion of Science (JSPS), and in part by a Research Grant (20–10) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare, Japan, and an Intramural Research Grant (23–9) for Neurological and Psychiatric Disorders from the NCNP.
References
- 1.Amorim C.A., Jacobs S., Devireddy R.V., Van Langendonckt A., Vanacker J., Jaeger J., Luyckx V., Donnez J., Dolmans M.M.2013. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum. Reprod. 28: 2146–2156. doi: 10.1093/humrep/det103 [DOI] [PubMed] [Google Scholar]
- 2.Aye M., Di Giorgio C., De Mo M., Botta A., Perrin J., Courbiere B.2010. Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: dimethyl sulfoxide, ethylene glycol and propylene glycol. Food Chem. Toxicol. 48: 1905–1912. doi: 10.1016/j.fct.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 3.Candy C.J., Wood M.J., Whittingham D.G.1995. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum. Reprod. 10: 2334–2338. doi: 10.1093/oxfordjournals.humrep.a136295 [DOI] [PubMed] [Google Scholar]
- 4.Carrion R., Jr, Patterson J.L.2012. An animal model that reflects human disease: the common marmoset (Callithrix jacchus). Curr. Opin. Virol. 2: 357–362. doi: 10.1016/j.coviro.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edgar D.H., Gook D.A.2012. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum. Reprod. Update 18: 536–554. doi: 10.1093/humupd/dms016 [DOI] [PubMed] [Google Scholar]
- 6.Fereydouni B., Drummer C., Aeckerle N., Schlatt S., Behr R.2014. The neonatal marmoset monkey ovary is very primitive exhibiting many oogonia. Reproduction 148: 237–247. doi: 10.1530/REP-14-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forabosco A., Sforza C., De Pol A., Vizzotto L., Marzona L., Ferrario V.F.1991. Morphometric study of the human neonatal ovary. Anat. Rec. 231: 201–208. doi: 10.1002/ar.1092310208 [DOI] [PubMed] [Google Scholar]
- 8.Gougeon A., Ecochard R., Thalabard J.C.1994. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol. Reprod. 50: 653–663. doi: 10.1095/biolreprod50.3.653 [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa A., Mochida N., Ogasawara T., Koyama K.2006. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil. Steril. 86: (Suppl): 1182–1192. doi: 10.1016/j.fertnstert.2005.12.082 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto S., Suzuki N., Yamanaka M., Hosoi Y., Ishizuka B., Morimoto Y.2010. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod. Biomed. Online 21: 501–509. doi: 10.1016/j.rbmo.2010.04.029 [DOI] [PubMed] [Google Scholar]
- 11.Hu M.L., Chuang C.H., Sio H.M., Yeh S.L.2002. Simple cryoprotection and cell dissociation techniques for application of the comet assay to fresh and frozen rat tissues. Free Radic. Res. 36: 203–209. doi: 10.1080/10715760290006420 [DOI] [PubMed] [Google Scholar]
- 12.Kagabu S., Umezu M.2000. Transplantation of cryopreserved mouse, Chinese hamster, rabbit, Japanese monkey and rat ovaries into rat recipients. Exp. Anim. 49: 17–21. doi: 10.1538/expanim.49.17 [DOI] [PubMed] [Google Scholar]
- 13.Kagawa N., Kuwayama M., Nakata K., Vajta G., Silber S., Manabe N., Kato O.2007. Production of the first offspring from oocytes derived from fresh and cryopreserved pre-antral follicles of adult mice. Reprod. Biomed. Online 14: 693–699. doi: 10.1016/S1472-6483(10)60670-0 [DOI] [PubMed] [Google Scholar]
- 14.Kardak A., Leibo S.P., Devireddy R.2007. Membrane transport properties of equine and macaque ovarian tissues frozen in mixtures of dimethylsulfoxide and ethylene glycol. J. Biomech. Eng. 129: 688–694. doi: 10.1115/1.2768107 [DOI] [PubMed] [Google Scholar]
- 15.Kuwayama M.2007. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 67: 73–80. doi: 10.1016/j.theriogenology.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 16.Lewis S.E., Agbaje I.M.2008. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis 23: 163–170. doi: 10.1093/mutage/gem052 [DOI] [PubMed] [Google Scholar]
- 17.Li G., Thirumala S., Leibo S.P., Devireddy R.V.2006. Subzero water transport characteristics and optimal rates of freezing rhesus monkey (Macaca mulatta) ovarian tissue. Mol. Reprod. Dev. 73: 1600–1611. doi: 10.1002/mrd.20541 [DOI] [PubMed] [Google Scholar]
- 18.Lindley E.M., Jacobson J.D., Corselli J., King A., Chan P.J.2001. Cryopreservation of human cumulus cells for co-cultures and assessment of DNA damage after thawing using the comet assay. J. Assist. Reprod. Genet. 18: 534–538. doi: 10.1023/A:1011991806423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda F., Inoue N., Manabe N., Ohkura S.2012. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J. Reprod. Dev. 58: 44–50. doi: 10.1262/jrd.2011-012 [DOI] [PubMed] [Google Scholar]
- 20.Men H., Monson R.L., Parrish J.J., Rutledge J.J.2003. Detection of DNA damage in bovine metaphase II oocytes resulting from cryopreservation. Mol. Reprod. Dev. 64: 245–250. doi: 10.1002/mrd.10249 [DOI] [PubMed] [Google Scholar]
- 21.Moniruzzaman M., Miyano T.2010. Growth of primordial oocytes in neonatal and adult mammals. J. Reprod. Dev. 56: 559–566. doi: 10.1262/jrd.10-071H [DOI] [PubMed] [Google Scholar]
- 22.Okano H., Hikishima K., Iriki A., Sasaki E.2012. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal Neonatal Med. 17: 336–340. doi: 10.1016/j.siny.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Recio L., Kissling G.E., Hobbs C.A., Witt K.L.2012. Comparison of Comet assay dose-response for ethyl methanesulfonate using freshly prepared versus cryopreserved tissues. Environ. Mol. Mutagen. 53: 101–113. doi: 10.1002/em.20694 [DOI] [PubMed] [Google Scholar]
- 24.Rothe H., Darms K., Koenig A.1992. Sex ratio and mortality in a laboratory colony of the common marmoset (Callithrix jacchus). Lab. Anim. 26: 88–99. doi: 10.1258/002367792780745922 [DOI] [PubMed] [Google Scholar]
- 25.Santos R.R., Amorim C., Cecconi S., Fassbender M., Imhof M., Lornage J., Paris M., Schoenfeldt V., Martinez-Madrid B.2010. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim. Reprod. Sci. 122: 151–163. doi: 10.1016/j.anireprosci.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 26.Schnorr J., Oehninger S., Toner J., Hsiu J., Lanzendorf S., Williams R., Hodgen G.2002. Functional studies of subcutaneous ovarian transplants in non-human primates: steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum. Reprod. 17: 612–619. doi: 10.1093/humrep/17.3.612 [DOI] [PubMed] [Google Scholar]
- 27.Sharma G.T., Dubey P.K., Chandra V.2010. Morphological changes, DNA damage and developmental competence of in vitro matured, vitrified-thawed buffalo (Bubalus bubalis) oocytes: A comparative study of two cryoprotectants and two cryodevices. Cryobiology 60: 315–321. doi: 10.1016/j.cryobiol.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 28.Shaw J.M., Trounson A.O.2002. Ovarian tissue transplantation and cryopreservation. Application to maintenance and recovery of transgenic and inbred mouse lines. Methods Mol. Biol. 180: 229–251. [DOI] [PubMed] [Google Scholar]
- 29.Silber S.J.2012. Ovary cryopreservation and transplantation for fertility preservation. Mol. Hum. Reprod. 18: 59–67. doi: 10.1093/molehr/gar082 [DOI] [PubMed] [Google Scholar]
- 30.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L.1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175: 184–191. doi: 10.1016/0014-4827(88)90265-0 [DOI] [PubMed] [Google Scholar]
- 31.Stachowiak E.M., Papis K., Kruszewski M., Iwaneńko T., Bartłomiejczyk T., Modliński J.A.2009. Comparison of the level(s) of DNA damage using Comet assay in bovine oocytes subjected to selected vitrification methods. Reprod. Domest. Anim. 44: 653–658. doi: 10.1111/j.1439-0531.2007.01042.x [DOI] [PubMed] [Google Scholar]
- 32.Suzuki N., Hashimoto S., Igarashi S., Takae S., Yamanaka M., Yamochi T., Takenoshita M., Hosoi Y., Morimoto Y., Ishizuka B.2012. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum. Reprod. 27: 2420–2429. doi: 10.1093/humrep/des178 [DOI] [PubMed] [Google Scholar]
- 33.Ting A.Y., Yeoman R.R., Campos J.R., Lawson M.S., Mullen S.F., Fahy G.M., Zelinski M.B.2013. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum. Reprod. 28: 1267–1279. doi: 10.1093/humrep/det032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting A.Y., Yeoman R.R., Lawson M.S., Zelinski M.B.2011. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum. Reprod. 26: 2461–2472. doi: 10.1093/humrep/der196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting A.Y., Yeoman R.R., Lawson M.S., Zelinski M.B.2012. Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles. Cryobiology 65: 1–11. doi: 10.1016/j.cryobiol.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang W.H., Chow K.L.2010. Cryopreservation of mammalian embryos: Advancement of putting life on hold. Birth Defects Res. C Embryo Today 90: 163–175. doi: 10.1002/bdrc.20186 [DOI] [PubMed] [Google Scholar]
- 37.von Schönfeldt V., Chandolia R., Kiesel L., Nieschlag E., Schlatt S., Sonntag B.2011. Advanced follicle development in xenografted prepubertal ovarian tissue: the common marmoset as a nonhuman primate model for ovarian tissue transplantation. Fertil. Steril. 95: 1428–1434. doi: 10.1016/j.fertnstert.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 38.von Schönfeldt V., Chandolia R., Kiesel L., Nieschlag E., Schlatt S., Sonntag B.2011. Assessment of follicular development in cryopreserved primate ovarian tissue by xenografting: prepubertal tissues are less sensitive to the choice of cryoprotectant. Reproduction 141: 481–490. doi: 10.1530/REP-10-0454 [DOI] [PubMed] [Google Scholar]
- 39.Woods E.J., Benson J.D., Agca Y., Critser J.K.2004. Fundamental cryobiology of reproductive cells and tissues. Cryobiology 48: 146–156. doi: 10.1016/j.cryobiol.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 40.Yeoman R.R., Wolf D.P., Lee D.M.2005. Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil. Steril. 83: (Suppl 1): 1248–1254. doi: 10.1016/j.fertnstert.2004.11.036 [DOI] [PubMed] [Google Scholar]