Abstract
Accumulating evidence suggests that reactive oxygen species (ROS) generated by endogenous metabolic enzymes are involved in a variety of intracellular mechanisms. In particular, superoxide-generating NADPH oxidase (Nox) 1 is highly expressed in the colon and has been implicated in physiological and pathophysiological states of colon tissues. However, its role in tissue repair following colitis has not been fully elucidated. Our study using experimental colitis in mice showed that repair of the mucosal layer did not occur in Nox1-deficient mice following dextran sulfate sodium-induced colitis. This was accompanied by inhibition of proliferation, cell survival, migration, and terminal differentiation (generation of goblet cells) of crypt progenitor cells, as determined by histochemical analyses. Furthermore, Nox1 expression as well as ROS production in the colon crypt was increased during the repair process, and Nox1 deficiency suppressed these events. The results suggest that Nox1 promotes colon mucosal wound repair by sustaining the bioactivity of crypt progenitor cells and plays a crucial role in the epithelial restitution in the case of damage associated with colitis.
Keywords: colitis, epithelial restitution, NADPH oxidase (Nox) 1, reactive oxygen species (ROS)
Introduction
NADPH oxidase (Nox) is a multi-subunit enzyme composed of transmembrane catalytic parts, Nox and p22phox, associated with several cytosolic regulatory subunits. Nox isoforms constitute a family composed of Nox1~5 and Duox1 and 2 [12]. Noxs catalyze ROS generation in a tightly regulated manner and represents a major cellular source of ROS. In particular, Nox1-derived ROS modulate signaling events involved in growth, differentiation, and apoptosis in a variety of non-phagocytic tissues [11]. Nox1 has also been implicated in the pathogenesis of hypertension [16] and inflammatory pain [5]. As for colon bioactivity, Nox1 is highly expressed in the human and mouse colon epithelium [3, 8], and Nox1-generated ROS have been postulated to play a role in events such as host defense against luminal microorganisms, although its exact physiological role remained enigmatic.
In the colon epithelium, the stem cells self-renew, producing precursor cells in the lower part of crypt, and transit-precursor cells subsequently differentiate into colonocytes and secretory cells forming the surface epithelium [25]. Stem cells not only self-renew throughout life but also contribute to the repair of damaged tissue upon injury. A recent study using Nox1-deficient mice reported that Nox1 controls proliferation and differentiation of progenitor cells in the normal colon that integrate Wnt/β catenin and Notch1 signaling [2]. This finding suggested a novel role of Nox1 in colon homeostasis. However, the study did not provide direct evidence for the role of Nox1 in pathological states associated with epithelial injury and acute/chronic colitis. More recently, new data demonstrated that Nox1 regulates epithelial cell migration, one of the initial responses to colon injury. Nox1 was found to be coupled to the epithelial N-formyl peptide receptor (FPR) system in the colon and to thereby mediate a FPR ligand-stimulated motility of colon cells, promoting mucosal wound repair [14]. Furthermore, combined deletion of Nox1 and IL-10 recapitulated the features of human ulcerative colitis by affecting ER stress in goblet cells [26]. These observations raise the possibility that Nox1-generated ROS play a critical role in maintenance of the colon mucus barrier as well as in rebuilding of the injured colon epithelium.
In the present study, we investigated the role of Nox1 in the repair processes of the colon epithelium following colitis with a special focus on the status of proliferation and differentiation of crypt progenitor cells. No study so far has addressed these aspects of the Nox1-mediated healing process in the inflamed colonic mucosa. Our study using in vivo experimental colitis revealed that Nox1 participates in control of proliferation, anti-apoptotic activity, migration, and terminal differentiation of progenitor cells, thereby contributing to repair from mucosal injury.
Materials and Methods
Animals
Generation and characterization of Nox1-/Y (Nox1KO) mice were previously described [16]. Nox1-/Y mice were backcrossed into the C57BL/6 genetic background for at least 16 generations. Mice were housed under a standard day/night cycle with free access to food and water. Experiments were performed using 5 to 12 mice per group. All experiment procedures were approved by the Experimental Animal Research Committee of the Shinshu University School of Medicine.
Antibodies and reagents
Dextran sulfate sodium salt (DSS: molecular mass, 36–50 kDa) was purchased from MP Biochemicals (Santa Ana, CA, USA), DPI was purchased from Calbiochem (San Diego, CA, USA), Hydro-CY3 (commercial name: ROS 550) was purchased from LI-COR Biosciences (Lincoln, NB, USA), and BrdU was purchased from Sigma-Aldrich (St. Louis, MO, USA). A TUNEL assay kit was purchased from Roche Applied Science (Manheim, Germany). The following antibodies were used: rabbit anti-Cox-2 from Cayman Chemicals (Ann Arbor, MI, USA), rabbit anti-HSP70 from Enzo Life Sciences (Villeurbanne, France), mouse anti-BrdU from Sigma-Aldrich, rabbit anti-Mucin 2 from Santa Cruz Biotechnology (Dallas, TX, USA), mouse anti-Ki-67 (BD Biosciences, San Jose, CA, USA), and mouse anti-IκBα and, rabbit anti-phospho Erk Tyr-204/Thr-202 from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-Nox1 antibodies were provided by Dr. D. Lambeth, who produced the antibodies through collaboration with diaDexus (South San Francisco, CA, USA).
Induction of colitis
Mice deficient in Nox1-/Y and wild type (WT) littermates received 2% (wt/vol) DSS in drinking water for 4 days, and the DSS was withdrawn to allow recovery from colitis for an additional 5 days. In DPI treatment, Nox1-/Y and WT mice received both 2% DSS in drinking water for 4 days and a daily intraperitoneal injection of DPI (0.08 mg/kg/day). The control group received DMSO. Mice were then allowed recovery as described above. Mice were sacrificed on day 9, and colons were removed and processed for histological and biochemical analyses. DSS-administered animals were monitored clinically for blood in stool and diarrhea.
Histological analysis
Colon tissue samples were fixed in 10% formalin, embedded in paraffin, deparaffinized, and retrieved as described previously [4]. The colon sections were immunostained with various antibodies by using second antibodies conjugated with horseradish peroxidase (Nichirei Biosciences Inc., Tokyo, Japan). Peroxidase activity was visualized using 3,3′-diaminobenzidine tetrahydrochloride (Nacalai Tesque, Inc., Kyoto, Japan). Counterstaining was performed with hematoxylin and eosin (H & E). Alcian Blue (pH2.5)/periodic acid-Schiff base (AB-PAS) staining was used for detection of sugar chains attached to glycoproteins. Histochemical and biochemical analyses were performed in at least three separate experiments. A group of five WT mice and a group of five Nox1-/Y mice were used for each experiment. At least three colon sections were analyzed, with at least three images examined for each. Twenty crypts/colon were counted in analyses of histochemical damage, goblet cell damage, and BrdU/Ki-67 staining. Colon crypt damage was evaluated by the presence of leukocyte recruitment/infiltration, thickening of the colon wall, and loss or immaturity of goblet cells, as described previously [21].
Measurement of ROS generation
Colons were dissected, washed three times with Hanks’ balanced salt solution (HBSS), and labeled with 25 µM Hydro-CY3 in HBSS at 37°C for 30 min. Labeled colon tissues were fixed in 3.7% paraformaldehyde, sectioned, and mounted with 1 mg/ml p-phenylenediamine (Nacalai Tesque, Inc., Kyoto, Japan) in PBS/glycerol. Fluorescence was analyzed by confocal microscopy with the excitation source at 514 nm and an emission wavelength of 543 nm. The histogram was analyzed as described previously [19].
Immunoblotting
Colon tissues were homogenized in RIPA buffer by using a Teflon homogenizer, and lysates were subjected to immunoblotting analysis after clarification by centrifugation as described previously [10].
Statistics
Data represent the mean ± SD of at least three separate experiments. Differences or correlations between two groups were assessed by Student’s t test. Differences with values of P<0.05 were considered to be statistically significant.
Results
Nox1 deficiency affects mucosal restitution following colitis
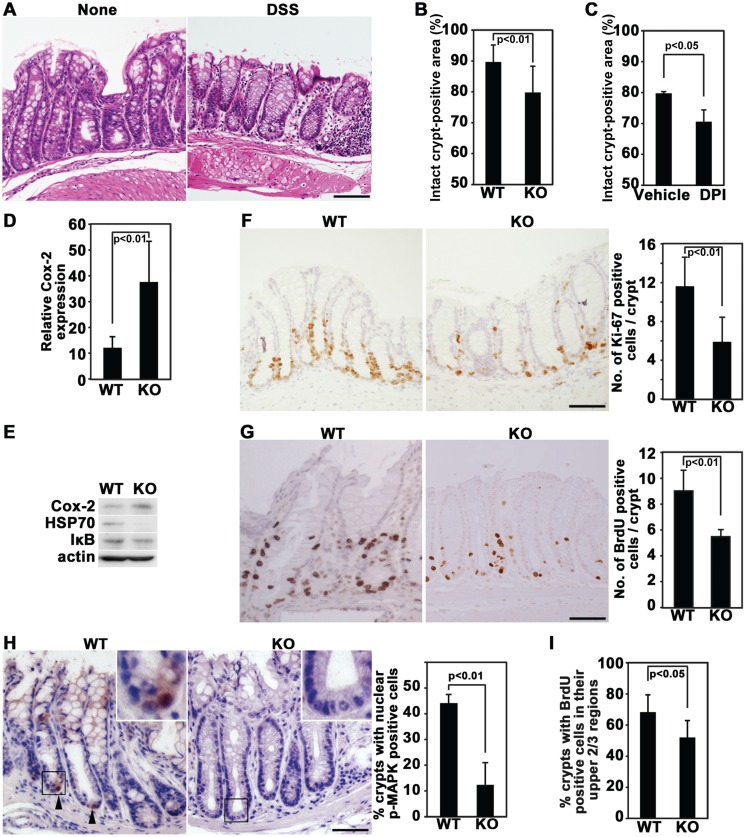
To address the functional role of Nox1 in colon homeostasis, we used a mouse model of colon injury and inflammation induced by oral administration of DSS, which is known to be toxic to the colon epithelium [22]. The original assay protocol recommends that the 3–10% DSS phase is 6–10 days long, followed by 6–10 days recovery [22]. In our experiments, however, this condition seemed to be too harsh, and more than half of the mice died. We therefore modified the protocol so that DSS treatment could be tolerated by the majority of the mice. Thus, WT mice were given 2% DSS in drinking water for 4 days and recovered with normal drinking water for 5 days. Mice displayed clinical signs such as colonic bleeding and diarrhea at day 4 after administration of DSS. This was stopped by subsequent water intake, but histological analyses showed some remains of epithelial injury (Fig. 1A), confirming partial recovery from DSS-induced colitis as described previously [14]. Then, WT and Nox1-/Y were treated with DSS under the same conditions as described above. Histological analyses revealed more severe epithelial injury in Nox1-/Y mice compared with the WT controls: the number of intact crypts in Nox1-/Y mice was significantly smaller than that in WT mice (Fig. 1B). Injection of DPI, a general inhibitor of Nox isozymes, similarly decreased the number of restored crypts compared with DPI-untreated mice (Fig. 1C). Taken together, these data are consistent with a role of Nox1 in mediating the process of crypt restoration.
Fig. 1.
Inhibition of Nox1 suppresses recovery from DSS-induced colitis, which is accompanied by decreased growth and migration of colonic cells. (A) Representative pictures of colon tissues on day 9 in WT mice with and without administration of DSS, followed by recovery from colitis. Colon sections were stained with H & E. The lengths of the analyzed colons were 6 cm and 4.5 cm for DSS-treated and DSS-untreated mice, respectively. (B and C) WT control and Nox1-/Y mice were given DSS (B). Alternatively, WT mice were given DSS together with DPI or DMSO (C). Then, the animals were allowed to recover from colitis. Histological damage was quantified by estimating the percentage of intact crypts found within an entire section of the colon (4.5 to 6 cm length). Histograms represent mean ± SD values [n=12 for WT and Nox1-/Y mice (B) and n=5 for DMSO and DPI-treated mice (C)]. (D) Immunohistochemical analysis of Cox-2 expression on day 9 in WT and Nox1-/Y mice treated with DSS, followed by recovery from colitis in the colon. Histograms represent the percentages of Cox-2-positive cells found within 0.1 mm2 sections of colons (mean ± SD, n=5 for WT and Nox1-/Y mice). (E) Immunoblotting analysis of Cox-2, HSP70, and IκB expression in the colon. Colonic extracts were prepared from WT and Nox1 KO mice at 5 days after termination of DSS administration. β-actin was used as a loading control. (F) WT and Nox1-/Y mice were treated with 2% DSS and allowed to recover from colitis. Colon sections were stained with anti-Ki-67 antibodies. Histograms represent the number of Ki-67-positive cells per crypt (mean ± SD, n=5 for WT and Nox1-/Y mice). (G) WT and Nox1-/Y mice were treated with DSS and allowed to recover from colitis. Colon sections were prepared following administration of BrdU 2 h prior to sacrifice and stained with anti-BrdU antibodies. Histograms represent the number of BrdU-positive cells per crypt (mean ± SD, n=5 for WT and Nox1-/Y mice). (H) WT and Nox1-/Y mice were treated with DSS and allowed to recover as in Fig. 1A. Colon sections were stained with anti-phospho-MAPK (Erk) Thr-202/Tyr-204 antibodies. (Insert): magnification x3.5. Histograms represent the percentage of crypts with nuclear phospho-MAPK-positive cells (arrowheads) per 30 crypts (mean ± SD, n=5 for WT and Nox1-/Y mice). (I) WT and Nox1-/Y mice were treated with DSS, allowed to recover from colitis, administered BrdU, 2 h before sacrifice, and stained with anti-BrdU antibodies. Histograms represent the percentage of crypts with BrdU-positive cells in their upper 2/3 regions per 20 crypts (mean ± SD, n=5 for WT and Nox1-/Y mice. Scale bar=50 µm.
We next examined the expression status of inflammation-related proteins. The level of a pro-inflammatory protein, Cox-2, expressed in mostly infiltrating leukocytes was higher in the colon from Nox1-/Y mice than its WT counterpart (Figs. 1D and 1E). The NFκB pathway targeting genes encoding pro-inflammatory cytokines and chemokines also remained more active in Nox1-/Y mice compared with WT mice, as indicated by a decrease in the level of IκB (Fig. 1E). The expression of a heat-shock protein HSP72, a cytoprotective factor, was also compromised in the colon epithelium of Nox1-/Y mice (Fig. 1E). These data implicate that DSS-induced inflammatory responses remained more persistently in Nox1-/Y mice compared with WT mice. Thus, we conclude that Nox1 deficiency leads to impaired recovery from colon epithelial injury following withdrawal of DSS.
Nox1 promotes both proliferation and migration of crypt progenitor cells
Because our results indicated that dysfunction of Nox1 leads to impaired restoration of the injured colon, we investigated whether Nox1 deficiency affects proliferation of crypt progenitor cells during recovery from colitis. To this end, the proliferation of colonic crypt cells was examined by analyzing the expression of Ki-67, a cell proliferation-associated marker, after 5 days of recovery following DSS treatment. Ki-67-positive proliferating cells was reduced in Nox1-/Y mice compared with WT controls (Fig. 1F), suggesting that Nox1 is required for proliferation of progenitor cells. To further confirm this, the frequency of BrdU-labeled growing cells was examined. Similar to the result obtained by Ki-67 staining, the colonic crypt of Nox1-/Y mice showed a markedly decreased number of BrdU-labeled proliferating cells (Fig. 1G). As Nox1 drives the growth factor receptor-coupled MEK/Erk signaling pathway in growing colon cancer cells [10], we therefore analyzed whether this signaling cascade is regulated by Nox1 in the recovering intestinal epithelium. Phosphorylation of Erk at Tyr-204/Thr-202 residues was dramatically suppressed by Nox1 deletion, indicating that Nox1-controlled proliferation of progeny cells possibly involves Erk (Fig. 1H). Notably, Ki-67-stained cells were present even in the middle regions of crypts in WT mice, in contrast to Nox1-/Y mice (Fig. 1F). The number of crypts with BrdU-labeled cells present in their upper 2/3 regions was lower in Nox1-/Y mice than WT controls (Fig. 1I). Since these crypt regions are remote from the stem cells and considered to be differentiated and nonproliferating [23], the data suggest that BrdU-incorporated cells rapidly migrated from the stem cell area and that Nox1 deficiency suppressed their migration. Thus, Nox1 deletion inhibited both proliferation and migration of progenitor cells along the crypt axis. Consistently, we previously demonstrated the mediating role of Nox1 in both proliferation and migration of colon cancer cells [6, 24].
Effects of Nox1 deletion on goblet cell differentiation and colonal cell viability
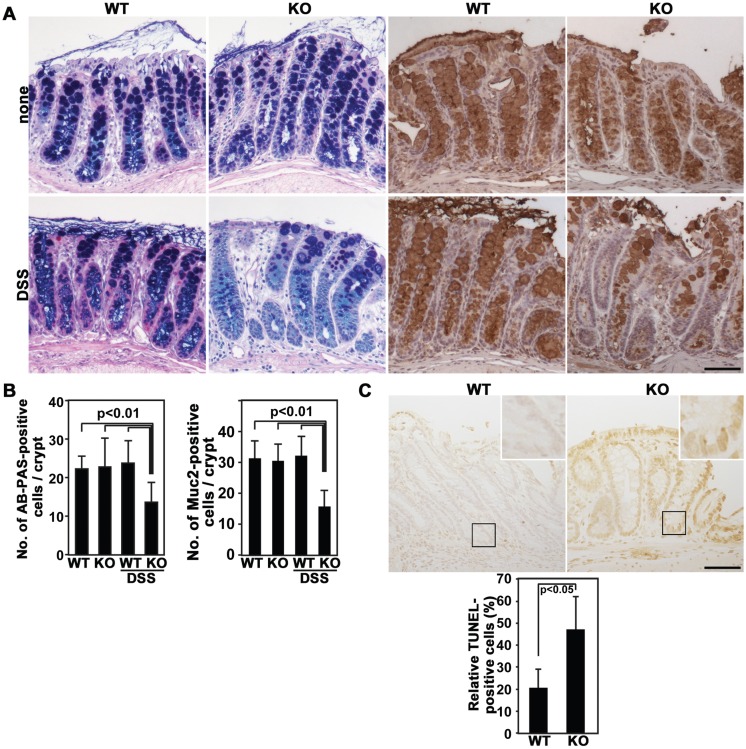
To further investigate the involvement of Nox1 in colon mucosal healing, differentiation of goblet cells was evaluated with the expression of acidic, neutral, and Muc2 mucins by using Alcian Blue (AB), Periodic acid-Schiff (PAS), and anti-Muc2 antibodies, respectively. Without DSS treatment, neither AB-PAS nor Muc2 staining differed between WT and Nox1-/Y mice (Figs. 2A and 2B). After DSS withdrawal, strikingly, the colon of DSS-treated Nox1-/Y mice exhibited a reduction in both mucin staining and goblet cell number compared with those in DSS-treated control littermates (Figs. 2A and 2B), suggesting that Nox1 deficiency causes a lack of maturation in goblet cells. In addition, the effect of Nox1 deletion on the survival activity of colon epithelial cells was examined by TUNEL assay, because Nox isozymes including Nox4 have previously been shown to protect cells from apoptosis in various biological systems [7]. The number of TUNEL-positive cells was higher in Nox1-/Y crypts compared with that in WT controls after 5 days of recovery from colitis (Fig. 2C), suggesting that Nox1 sustains cell viability in the colon epithelium.
Fig. 2.
Goblet cell differentiation and survival of colonic cells during recovery from colitis. (A and B) Colon sections prepared from WT and Nox1-/Y mice at day 5 after termination of DSS administration were stained with AB-PAS (dark blue) or anti-Mucin 2 (brown) antibodies (A). Histograms represent the number of AB-PAS-stained cells or Mucin 2-positive per crypt (mean ± SD, n=5 for WT and Nox1-/Y) (B). (C) TUNEL staining of colon tissue from WT and Nox1-/Y mice 5 days after termination of DSS administration. (Insert): magnification x3.5. Histograms represent the ratios of TUNEL-positive cells (arrowhead) within 0.1 mm2 sections of crypts (mean ± SD, n=5 for WT and Nox1-/Y). Scale bar=50 µm.
Upregulation of Nox1 and ROS production during restitution following colitis
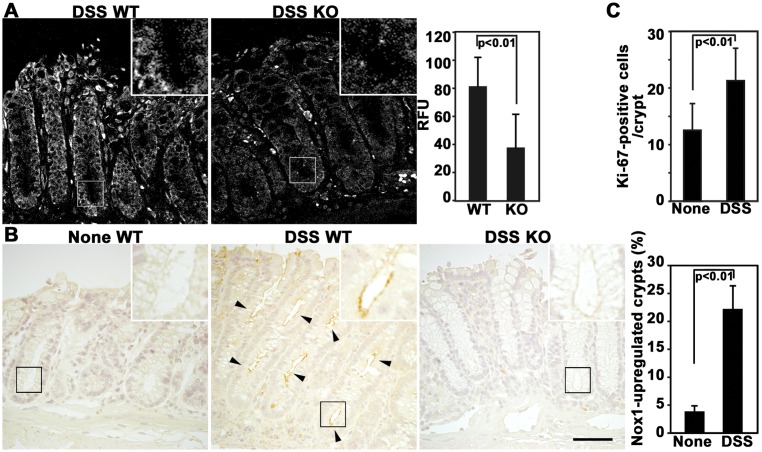
To further assess the association of Nox1 with the repair process, we investigated the expression of Nox1 in colon tissues during recovery from colitis. The effect of Nox1 deletion on superoxide production was first examined by using the fluorescent probe Hydro-Cy3, which is a hydrocyanine derivative and highly sensitive to ROS [9]. A substantial level of ROS production was observed in WT crypts following colitis, whereas it was diminished in their Nox1-/Y counterparts (Fig. 3A). To determine whether ROS production correlates with the expression level of Nox1, the Nox1 expression was examined by immunostaining with a monoclonal antibody to Nox1, which has been shown to specifically recognize Nox1 proteins [1, 27]. Increased Nox1 was detected in the apical membrane of colon epithelial cells in WT mice, but it was undetectable in the same areas in Nox1-/Y mice (Fig. 3B). The results suggest that Nox1 is indeed responsible for ROS generation in the colon epithelial cells and exerts its biological role through ROS signaling. Furthermore, both the Nox1 level (Fig. 3B) and Ki-67-positive cells (Fig. 3C) significantly increased in DSS-treated WT mice compared with untreated controls during the recovery period, confirming the upregulation of Nox1 and concomitant increased cell growth during epithelial restitution. These results are consistent with the notion that Nox1 facilitates mucosal repair by sustaining the bioactivity of progenitor cells.
Fig. 3.
Both Nox1 and ROS levels are increased during restitution from colitis. (A) Representative confocal fluorescent images of colon sections. Colons obtained from WT and Nox1-/Y mice at day 5 after termination of DSS administration were labeled with 25 µM Hydro-CY3. (Insert): magnification ×3.5. Histograms represent the relative intensity of fluorescence per crypt (mean ± SD, n=20 for WT and Nox1-/Y mice). (B) WT mice were treated with or without DSS, and Nox1-/Y mice were treated with DSS, followed by recovery from colitis. Colon sections were stained with anti-Nox1 antibodies. Arrowheads indicate the Nox1 expression in colon epithelial cells. (Insert): magnification ×3.5. Histograms represent the percentage of Nox1-upregulated crypts found in 20 crypts in WT mice (mean ± SD, n=8 for WT mice). (C) WT mice were treated with or without DSS and allowed to recover from colitis. Colon sections were stained with anti-Ki-67 antibodies. Histograms represent the number of Ki-67-positive cells per crypt (mean ± SD, n=8 for WT mice). Scale bar=50 µm.
Discussion
Colon epithelial homeostasis is dysregulated in inflammatory bowel diseases, including ulcerative colitis, and renewal of the damaged colon epithelium is tightly regulated by cellular processes, such as proliferation, differentiation, migration, and cell death of the epithelial cells. On the other hand, little is known about the exact physiological role of Nox1 in the colon, although it has been speculated that Nox1 provides an oxidative host defense barrier against intestinal pathogens [3]. The results presented here suggest a required role of Nox1 in epithelial restitution from colitis. Our study using genetically engineered Nox1-/Y mice showed that Nox1 deficiency significantly delayed recovery from experimental mucosal injury. This aberrant repair process was accompanied by suppression of both proliferation and migration of transit progenitor cells derived from colon stem cells. Nox1 deletion also attenuated differentiation of progenitor cells into mucus-secreting goblet cells and the survival activity of colonocytes. Thus, we propose that Nox1 plays a critical mediating role in restitution from colon epithelial injury, contributing to restoration of intestinal homeostasis.
ROS are involved in the regulation of proliferation and differentiation of embryonic stem cells and are also considered to act as a second messenger in several self-renewing tissue cells including hematopoietic stem cells, neural stem cells, and spermatogonial stem cells [18]. Our finding that ROS generated by the epithelial Nox1 maintain the bioactivity of committed progenitor cells during repair of colonic epithelial injury is consistent with this viewpoint, emphasizing the importance of Nox1-derived ROS in self-renewal of the colonic epithelium. In the present study, the effect of Nox1 deletion on differentiation status was examined with goblet cells but not other colon epithelial constituents such as M-cells and Paneth cells. Since all colon epithelial cell lineages develop from epithelial stem cells within the crypt [15], it is most likely the case that Nox1 regulates the differentiation of these cells, as in the case of goblet cells. On the other hand, in Nox1 deficiency, mice have a normal colonic mucosa with functional goblet cells as in WT mice (Fig. 2A), and the healing of damage induced by DSS treatment is not inhibited completely. These findings suggest that several signaling pathways, including the Nox1 pathway, contribute to the differentiation.
Of note, upregulation of Nox1 proteins was induced in colon cryptic cells from DSS-treated mice (Fig. 3B), resulting in elevated ROS production. The underlying mechanism for this event is currently unknown. One possibility is that upon disruption of the epithelial barrier by DSS, cytokines or growth factors are released from infiltrating immune cells and thereby activate the gene expression of Nox1. In fact, Nox1 expression has been reported to be induced by TNFα [20], PDGF [13], and EGF [17].
Previous studies suggested a critical role for Nox1 as a signal transducer controlling cell proliferation and fate in the colon epithelium: Nox1 contributes to maintenance of basal intestinal homeostasis [2], and its deficiency, combined with the lack of IL-10, caused ulcerative colitis in mice [26]. However, the studies did not address the Nox1 function in repair following epithelial injury and colitis. Thus, our discovery has a significant implication in the sense that it revealed for the first time the Nox1-based redox regulation of the proliferative phase of tissue remodeling following colonic inflammation. The involvement of Nox1 in mucosal wound repair has recently been reported in other biological systems as well: Annexin A1, the endogenous FPR ligand secreted in the mucosal layer, promoted migration of intestinal epithelial cells through activation of FPR- and Nox1-dependent redox signaling, leading to wound closure in the intestinal epithelium [14]. This observation together with our observations suggests that Nox1 oxidase-derived ROS are critically involved in the redox balance responsible for the regulation of tissue remodeling.
In conclusion, we showed the role of Nox1 expression in mucosal repair following colitis and identified Nox1 as an integral component of the machinery required for maintenance of colon physiology. Further study will be of great interest in determining which downstream signaling pathway (s) Nox1-generated ROS target and how they modulate the signaling events.
Acknowledgments
Financial support for this work was provided by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (22300328, to T.K.) and a Grant for Scientific Research from Nagano Society for the Promotion of Science (to M.K.).
Reference
- 1.Chen W., Shang W.H., Adachi Y., Hirose K., Ferrari D.M., Kamata T.2008. A possible biochemical link between NADPH oxidase (Nox) 1 redox-signalling and ERp72. Biochem. J. 416: 55–63. doi: 10.1042/BJ20071259 [DOI] [PubMed] [Google Scholar]
- 2.Coant N., Ben Mkaddem S., Pedruzzi E., Guichard C., Tréton X., Ducroc R., Freund J.N., Cazals-Hatem D., Bouhnik Y., Woerther P.L., Skurnik D., Grodet A., Fay M., Biard D., Lesuffleur T., Deffert C., Moreau R., Groyer A., Krause K.H., Daniel F., Ogier-Denis E.2010. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol. Cell. Biol. 30: 2636–2650. doi: 10.1128/MCB.01194-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiszt M., Lekstrom K., Brenner S., Hewitt S.M., Dana R., Malech H.L., Leto T.L.2003. NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J. Immunol. 171: 299–306. doi: 10.4049/jimmunol.171.1.299 [DOI] [PubMed] [Google Scholar]
- 4.Harada O., Suga T., Suzuki T., Nakamoto K., Kobayashi M., Nomiyama T., Nadano D., Ohyama C., Fukuda M.N., Nakayama J.2007. The role of trophinin, an adhesion molecule unique to human trophoblasts, in progression of colorectal cancer. Int. J. Cancer 121: 1072–1078. doi: 10.1002/ijc.22821 [DOI] [PubMed] [Google Scholar]
- 5.Ibi M., Matsuno K., Shiba D., Katsuyama M., Iwata K., Kakehi T., Nakagawa T., Sango K., Shirai Y., Yokoyama T., Kaneko S., Saito N., Yabe-Nishimura C.2008. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J. Neurosci. 28: 9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajla S., Mondol A.S., Nagasawa A., Zhang Y., Kato M., Matsuno K., Yabe-Nishimura C., Kamata T.2012. A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J. 26: 2049–2059. doi: 10.1096/fj.11-196360 [DOI] [PubMed] [Google Scholar]
- 7.Kamata T.2009. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 100: 1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi H., Hikage M., Miyashita H., Fukumoto M.2000. NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene 254: 237–243. doi: 10.1016/S0378-1119(00)00258-4 [DOI] [PubMed] [Google Scholar]
- 9.Kim J.Y., Choi W.I., Kim Y.H., Tae G.2011. Highly selective in-vivo imaging of tumor as an inflammation site by ROS detection using hydrocyanine-conjugated, functional nano-carriers. J. Control. Release 156: 398–405. doi: 10.1016/j.jconrel.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 10.Komatsu D., Kato M., Nakayama J., Miyagawa S., Kamata T.2008. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene 27: 4724–4732. doi: 10.1038/onc.2008.102 [DOI] [PubMed] [Google Scholar]
- 11.Lambeth J.D.2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4: 181–189. doi: 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 12.Lambeth J.D.2007. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 43: 332–347. doi: 10.1016/j.freeradbiomed.2007.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassègue B., Sorescu D., Szöcs K., Yin Q., Akers M., Zhang Y., Grant S.L., Lambeth J.D., Griendling K.K.2001. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 88: 888–894. doi: 10.1161/hh0901.090299 [DOI] [PubMed] [Google Scholar]
- 14.Leoni G., Alam A., Neumann P.A., Lambeth J.D., Cheng G., McCoy J., Hilgarth R.S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Neish A.S., Nusrat A.2013. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123: 443–454. doi: 10.1172/JCI65831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A.2013. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6: 666–677. doi: 10.1038/mi.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., Yabe-Nishimura C.2005. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112: 2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709 [DOI] [PubMed] [Google Scholar]
- 17.Mitsushita J., Lambeth J.D., Kamata T.2004. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 64: 3580–3585. doi: 10.1158/0008-5472.CAN-03-3909 [DOI] [PubMed] [Google Scholar]
- 18.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A.2010. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7: 150–161. doi: 10.1016/j.stem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki T., Furuta S., Mitsushita J., Shang W.H., Ito M., Yokoo Y., Yamaura M., Ishizone S., Nakayama J., Konagai A., Hirose K., Kiyosawa K., Kamata T.2006. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25: 3699–3707. doi: 10.1038/sj.onc.1209406 [DOI] [PubMed] [Google Scholar]
- 20.Mouzaoui S., Djerdjouri B., Makhezer N., Kroviarski Y., El-Benna J., Dang P.M.2014. Tumor necrosis factor-α-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediators Inflamm. 2014: 312484. doi: 10.1155/2014/312484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurath M.F., Fuss I., Kelsall B.L., Stüber E., Strober W.1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182: 1281–1290. doi: 10.1084/jem.182.5.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R.1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694–702. [DOI] [PubMed] [Google Scholar]
- 23.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R.2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241. doi: 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 24.Shinohara M., Adachi Y., Mitsushita J., Kuwabara M., Nagasawa A., Harada S., Furuta S., Zhang Y., Seheli K., Miyazaki H., Kamata T.2010. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 285: 4481–4488. doi: 10.1074/jbc.M109.071779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons B.D., Clevers H.2011. Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 317: 2719–2724. doi: 10.1016/j.yexcr.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 26.Tréton X., Pedruzzi E., Guichard C., Ladeiro Y., Sedghi S., Vallée M., Fernandez N., Bruyère E., Woerther P.L., Ducroc R., Montcuquet N., Freund J.N., Van Seuningen I., Barreau F., Marah A., Hugot J.P., Cazals-Hatem D., Bouhnik Y., Daniel F., Ogier-Denis E.2014. Combined NADPH oxidase 1 and interleukin 10 deficiency induces chronic endoplasmic reticulum stress and causes ulcerative colitis-like disease in mice. PLoS ONE 9: e101669. doi: 10.1371/journal.pone.0101669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Nakayama J., Kamata T.2015. Nox4-generated ROS regulate TGF-β1-induced motility of colon cancer cells through the low molecular weight protein tyrosine phosphatase-Rho signaling pathway. Shinshu Med. J. 63: 281–293. [Google Scholar]