Abstract
Previously, we have identified a calcium-binding protein that is specifically expressed in spermatids and localized to the flagella of the mature sperm in mouse, so-called mCABS1. However, the physiological roles of CABS1 in the male reproductive system have not been fully elucidated yet. In the current study, we aimed to localize and clarify the role of CABS1 in porcine (pCABS1). We determined for the first time the full nucleotides sequence of pCABS1 mRNA. pCABS1 protein was detected on SDS-PAGE gel as two bands at 75 kDa and 70 kDa in adult porcine testis, whereas one band at 70 kDa in epididymal sperm. pCABS1 immunoreactivity in seminiferous tubules was detected in the elongated spermatids, and that in the epididymal sperm was found in the acrosome as well as flagellum. The immunoreactivity of pCABS1 in the acrosomai region disappeared during acrosome reaction. We also identified that pCABS1 has a transmembrane domain using computational prediction of the amino acids sequence. The treatment of porcine capacitated sperm with anti-pCABS1 antiserum significantly decreased acrosome reactions. These results suggest that pCABS1 plays an important role in controlling calcium ion signaling during the acrosome reaction.
Keywords: porcine, calcium-binding protein, CABS1, transmembrane, testis, acrosome, epididymal sperm
Introduction
In mammalian testis, spermatozoa are immotile and incapable of fertilizing the oocytes. Spermatozoa are sequentially exposed to different environments and successfully modified to acquire the ability of fertilization during passing through male and female reproductive tracts until their arrival to the oocytes, at the ampulla of fallopian tube where oocyte exists. Fertilization ability of sperm depends on successful completion of capacitation, hyperactivation, and the acrosome reaction, where extracellular Ca2+ are involved in each of these steps [4, 13, 24, 25]. Although the exact mechanisms regulating Ca2+ signaling in the sperm remain to be elucidated, a number of studies have shown that Ca2+ channel and pump proteins on the plasma membrane and Ca2+ stores are likely responsible for regulation of the intracellular Ca2+ levels in sperm [8, 15, 18, 23]. Calcium-binding proteins have also been reported to participate in Ca2+ signaling [3, 14, 20, 29]. Thus, identifying the function of such proteins is important to understand the Ca2+ signaling pathways as well as the male fertility.
Calcium binding protein and spermatid specific-1 (CABS1) is one of the calcium-binding proteins identified by us in mouse testis, which is also named as Casein-Like PHosphoprotein (CLPH) in rat [5, 17]. The Cabs1 gene is located in a chromosomal region of the mammalian cluster of secretory calcium-binding phosphoprotein genes, and its protein interacts with Ca2+ since CABS1 has many acidic amino acids. Moreover, rat CLPH (rCABS1) was classified as an intrinsically disordered protein owing to its unique amino acid contents and sequences [5].
In testis, previous results showed that both mouse CABS1 (mCABS1) and rat CABS1 (rCABS1) are expressed in the round and elongated spermatids. In the epididymis, however, mCABS1 was localized in the sperm flagellum, but rCABS1 was not [5, 17]. McClintock et al., reported that Cilia-flagellum-related mouse genes include Cabs1, indicating that mCABS1 would be localized in the flagellum/Cilia [19]. These data suggested that CABS1 might be involved in Ca2+ signaling during spermiogenesis and/or sperm maturation.
In the present study, we found that similar to mouse, porcine CABS1 (pCABS1) is expressed during the late stages of spermatogenesis in the elongated spermatids. However, in mature sperm, we found that pCABS1 localizes to the acrosome in addition to the tail where mCABS1 only localizes, suggesting that pCABS1 is involved in the acrosome reaction.
Materials and Methods
Animals
All animal experiments were approved by the Animal Experiment Committee of the University of Tsukuba. Fresh testes and epididymis were obtained from mature porcine (Sus scrofa domestica) at the local slaughterhouse. The mature sperm was collected by retrograde flush of air through epididymal ducts and then washed twice by centrifugation at 2,500 rpm for 10 min at room temperature. The precipitated spermatozoa were suspended in an appropnate buffer.
Sequence analysis of pCABS1 mRNA by the RACE methods
Total RNA was isolated from porcine testis using ISOGEN reagent (Nippon Gene, Tokyo, Japan) and subjected to RT-PCR (QuantiTect Reverse Transcription kit, Qiagen, Hilden, Germany). The nucleotide sequence of pCABS1 mRNA was determined by the RACE method using the 3′-Full RACE core set (Takara, Shiga, Japan). For 3′-RACE, first-strand cDNAs were synthesized by reverse transcription reaction of the porcine testis RNA using the Oligo dT-3 sites adaptor primer contained in the 3′-Full RACE core set. Polymerase chain reaction was performed with a Cabs1 specific primer 5′-TAGATGTGCATGGTGCCACT-3′, according to the NCBI database (dbEST ID=26461132 & GenBank gi=84125897), which corresponds to the 5′-terminal sequence of mouse Cabs1 mRNA and the 3 sites adaptor primer. The product was then cloned into the pGEM-T vector (Promega, Madison, WI, USA) and sequenced.
The identity among CABS1 from different species; Sus, Bos (accession no. NM_001040539, XM_597308), Homo sapiens (accession no. BC046111), Mus (accession no. NM_027631, XM_132142), and Rattus (accession no. NM_022263, XM_341196) were calculated by clustalw tool (www.genome.jp/tools/clustalw).
Preparation of pCABS1 recombinant protein
The recombinant protein was prepared for antigen production and analysis of calcium-binding activity as follows. pCABS1 cDNA fragments were synthesized by RT-PCR from testis total RNAs as a template using the primer set 5′-ATGGCTGAAGATGGATCCCAGAA-3′ and 5′-TCAGGAACTCCCCGGGTTCTTCTTTCAG-3′. The product was ligated into the BamHI and SmaI sites of the bacterial expression vector pGEX-6P-2 (GE Healthcare; Piscataway, NJ, USA), which was transformed into Escherichia coli DH5α. An overnight culture of the transformant in LB medium was diluted and shaken at 37°C until the OD 600 reached 0.4–0.6. After addition of 0.2 mM isopropyl-β-D-thiogalactopyranoside (Sigma, Saint Louis, MO, USA), the culture was shaken at 25°C for 5 h. The bacterial cells were collected by centrifugation, washed with PBS, and suspended in 20 mM Tris-HCl (pH 7.4) containing 200 mM NaCl, 1 mM EDTA, 1 mM DTT, and 1/1,000 volume of protease inhibitor cocktail (Sigma). The suspended solutions were sonicated and Triton-X 100 was added to a final concentration of 0.01%. The suspension was incubated for 30 min at 4°C. The lysates were centrifuged at 16,000 ×g for 30 min, and the supernatant was bound to Glutathione Sepharose 4B beads (GE Healthcare). pCABS1 protein fragments were removed from GST by PreScission Protease (GE Healthcare). The beads were centrifuged at 12,000 ×g for 10 min, and the supernatant fraction was obtained as purified recombinant pCABS1.
pCABS1 antiserum
Purified recombinant pCABS1 was used as an antigen to produce rabbit antiserum. Subcutaneous injection of 1 mg of purified antigen with Freund’s complete adjuvant (Sigma) was followed by three additional booster injections of 300 µg of purified antigen with Freund’s incomplete adjuvant (Sigma) at two-weeks intervals.
Analysis of calcium-binding activity
The properties of the calcium binding activity were analyzed by Stains-All staining (Sigma) or ruthenium red (Sigma) using recombinant pCABS1 protein. For Stains-All staining, the protein was resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The gels were stained with Coomassie Brilliant Blue (CBB) kit (Nacalai Tesque, Kyoto, Japan). Alternatively, the gels were washed overnight with 30% isopropyl alcohol, then stained with the Stains-All dye for 48 h, and destained with 25% isopropyl alcohol until a clear background was achieved. For ruthenium red staining, recombinant pCABS1 protein was transferred to PVDF membrane and stained with Ponceau S or ruthenium red (25 mg/l of ruthenium red in 60 mM KCl, 5 mM MgCl2, 10 mM Tris-HCl, pH 7.5) for 48 h [28].
Protein extraction from testis and cauda epididymal sperm
Porcine testis was homogenized using Polytron (Kinematica AG, Lucerne, Switzerland) in Tris-buffered saline (TBS) containing a 1% protease inhibitor cocktail and 1% SDS. The homogenates were centrifuged at 100,000 ×g for 60 min at 4°C and the supernatant was stored at −80°C until use. Cauda epididymal sperm were washed twice by centrifugation at 500 ×g for 5 min at 4°C in modified Krebs-Ringer bicarbonate medium composed of 4.8 mM KCl, 1.2 mM KH2PO4, 95 mM NaCl, 5.56 mM glucose, 25 mM NaHCO3, 2 mM CaCl2, and 1 mM pyruvate as described previously [27]. The sperm pellets were resuspended in homogenizing buffer (20 mm Tris-HCl, pH 7.4, containing 2.5% protease inhibitor cocktail and 0.25% phosphatase inhibitor cocktail), sonicated three times on ice for 30 s, and then centrifuged at 50,000 rpm for 30 min at 4°C. The supernatant was collected and protein concentrations were determined using a protein assay reagent (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Proteins extracted from porcine testis and sperm were separated by 12.5% SDS-PAGE and electroblotted to PVDF membrane (GE Healthcare) under semidry conditions (Atto, Tokyo, Japan). The membranes were blocked with 3% skim milk and 1% bovine serum albumin (BSA) in PBS overnight at 4°C and then incubated with anti-pCABS1 antiserum (1:500) in PBS for 2 h at 37°C. After three times washing with PBS containing 0.1% Tween 20 (PBS-T), the membranes were then incubated with peroxidase-conjugated anti-rabbit IgG secondary antibody (Sigma, 1:2,000) in blocking buffer for 1 h at room temperature. After three times washing with PBS-T, signals were detected with Western Blotting Luminol Reagents (Nacalai). The protein marker used was WIDE-VIEW prestained protein size marker III (Wako, Osaka, Japan). BSA was used as the negative control.
Immunohistochemical analysis of porcine testis
Porcine testes were fixed by soaking in Bouin fixing fluid at room temperature for 24 h, and they then were dehydrated and embedded in paraffin. The block was sectioned at a thickness of 4 µm and mounted on slides. After deparaffinization, the sections were soaked in 0.1 M sodium citrate buffer at 37°C and washed with distilled water. The sections were incubated with anti-pCABS1 antiserum (1:500) at 37°C for 1 h. Signals were visualized by an avidin-biotin complex method using a Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA) as previously reported [21]. Specificity control for the immunohistochemical reaction was carried out on adjacent sections, which were incubated with the neutralized antisera instead of the anti-pCABS1 antiserum. Counterstaining of the sections was done using hematoxylin.
Immunofluorescence analysis of porcine testis
Porcine testes were dissected and immediately transferred to 4% paraformaldehyde (PFA) in PBS (pH 7.4). The tissues were excised and postfixed in the same fixative overnight, and embedded in 4% carboxymethylcellulose compound (Fintec, Tokyo, Japan). Testis sections (5 µm) were cut (8–9 serial sections for each sample), and then mounted on polylysine-precoated slides (Dako, Tokyo, Japan). Testis sections were boiled in 20 mM citrate buffer (pH 6.0) for 30 min and cooled down to room temperature. After being washed in PBS, sections were permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. The sections were blocked with 3% skim milk in PBS at 4°C overnight and then with 10% goat serum in PBS at 37°C for 1 h. The sections were incubated with anti-pCABS1 antiserum (1:500) at room temperature for 2 h. They were then washed three times with PBS-T and incubated with Alexa Fluor 594 goat anti-rabbit IgG (1:1,000) (Invitrogen, Carlsbad, USA) and 20 µg/ml FITC-conjugated peanut agglutinin (FITC-PNA) (Sigma) dissolved in blocking buffer (10% goat serum in PBS) at room temperature for 1 h. Sections were counterstained with hoechst and subjected to observation with an immunofluorescence microscope (BX50/BX-FLA; Olympus, Tokyo, Japan).
Immunocytochemical analysis of porcine sperm
The washed cauda epididymal sperm were suspended to a final concentration of 4 × 106 cells/ml in PBS. The sperm suspension was placed on the polylysine-precoated slides (Dako, Glostrup, Denmark) and dried at 50°C for 30 min. After fixation with 4% PFA in PBS for 5 min at room temperature, sperm were washed with PBS three times and permeabilized with 0.1% Triton X-100 for 10 min. The samples were washed with PBS and then blocked with 5% skim milk in PBS (Blocking Buffer) for 1 h. The samples were then incubated with anti-pCABS1 antiserum (1:300) diluted in blocking buffer at 37°C for 1 h, followed by washing three times with PBS. Immunodetection was done as the immunofluorescence analysis except that Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) was used as the secondary antibody.
Induction of the acrosome reaction
To examine the expression of pCABS1 protein before and after the acrosome reaction, cauda epididymal sperm were capacitated as described by Katoh et al. with few modifications [16]. Briefly, sperm were adjusted to 2 × 106/ml in modified Krebs-Ringer bicarbonate medium containing 0.4% BSA (Sigma) and then cultured at 39°C in 5% CO2 for 120 min. They were then incubated for an additional 15 min with or without calcium ionophore A23187 at a final concentration of 2.5 µM. Samples were then subjected to imunocytochemical analysis as described above to evaluate pCABS1 expression status. Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen) was used as a secondary antibody together with 20 µg/ml FITC-conjugated peanut agglutinin (FITC-PNA) as an acrosomal marker.
Effect of anti-pCABS1 antiserum on acrosome reaction
The effect of anti-pCABS1 antiserum on the acrosome reaction was examined as following; capacitated sperm (2 × 106/ml) were further incubated at 39°C for 30 min in the capacitation medium with anti-pCABS1 antiserum (1:200 dilution and 1:50). Anti-pCABS1 was neutralized by incubation with purified pCABS1 recombinant antigen (5 mg/ 80 µl) overnight at 4°C and used as the control. Ca2+ ionophore A23187 (final concentration 2.5 µmol/l; Sigma) was added simultaneously to induce the acrosome reaction. The percentage of acrosome reactions of living spermatozoa was evaluated using FITC-PNA in conjunction with DNA-specific fluorochrome propidium iodide (PI) as a viability test [10]. As described by Siciliano et al. for the evaluation of acrosome reaction of live spermatozoa, the sperm suspension was placed in a 96-well plate and exposed to FITC-PNA (10 µg/ml) and PI (12 µmol/l) for 10 min at 39°C then fixed by adding 4% (w/v) paraformaldehyde [26]. The samples were then examined with a fluorescence microscope with a multiple fluorescence filters (BX50/BX-FLA; Olympus, Tokyo, Japan). Sperm were scored as acrosome-reacted when a bright green stain was observed on the acrosome, or as acrosome-intact when either green fluorescent staining was restricted to the equatorial segment or no labeling was observed. The acrosomal status was determined for 200 live sperm (PI-negative sperm) per well. Triplicate wells were analyzed, total 600 sperm for each treatment. The results were reported as means ± SD. Differences of the means were analyzed by the unpaired t test, with P<0.05 considered significantly different.
Results
Determination of the full nucleotides sequence of the pCABS1 cDNA
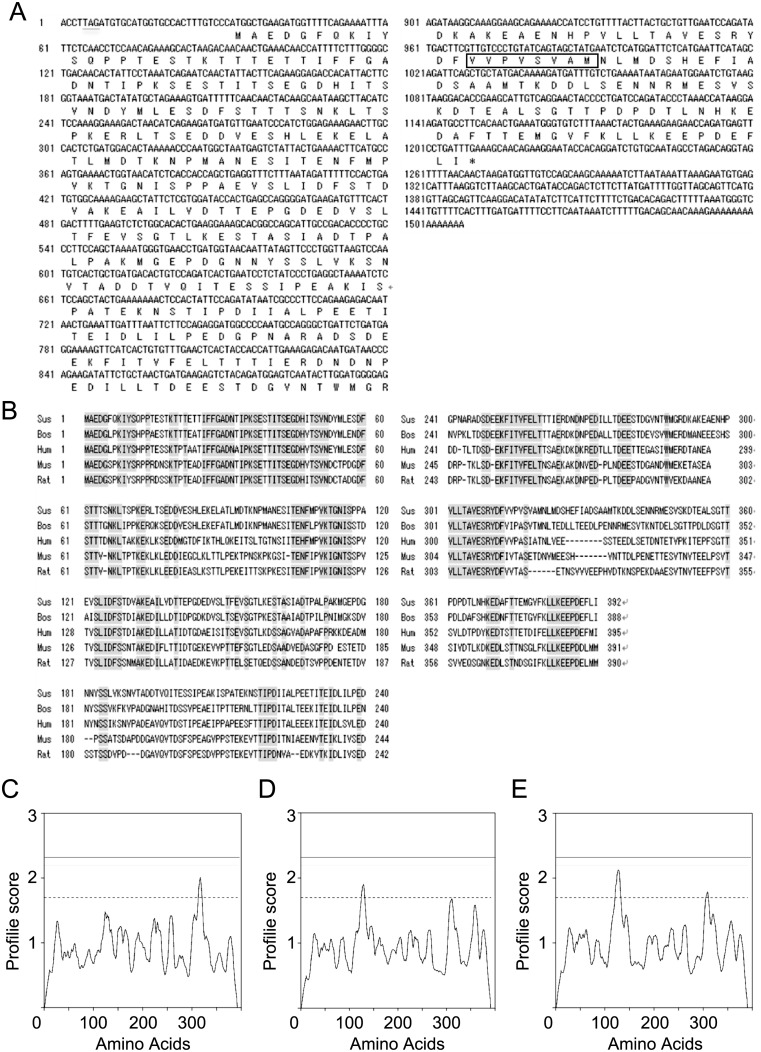
According to the dbEST from the national center for biotechnology information (NCBI) there was a porcine sequence (dbEST ID=26461132 & GenBank gi=84125897) that corresponds to the 5′-terminal sequence of mouse Cabs1 mRNA. We identified full nucleotides sequence of the porcine sequence by doing 3′-RACE, which has 1,507 bp (Fig. 1A). After the in-frame stop codon TAG in 5′ UTR region, there were three possible sites of the initiation codon ATG at base 8, 14, and 32 in the same frame of the matured mRNA sequence. The third one corresponded to the predicted initiation site in mouse and rat. An open reading frame consisted of 1,176 bp coding 392 amino acid residues, which was followed by a long 3′-untranslated region.
Fig. 1.
Nucleotide sequence of pCABS1 cDNA and a comparison of its predicted amino acid sequence with that of other species. (A) Nucleotide sequence of pCABS1 cDNA and deduced amino acid sequence. The predicted transmembrane domain is boxed. (B) Comparison of deduced amino acid sequences of CABS1 homologs among swine, cattle, humans, mice, and rats. Identical residues throughout the five species are shown with a shadow. Transmembrane region predicted for (C) porcine CABS1 (D) mouse CABS1 (E) rat CABS1 using the DAS server (http://www.sbc.su.se/~miklos/DAS/). The solid and dot lines represent strict (2.2) and loose (1.7) cutoff values for prediction of the transmembrane domain, respectively. The peak above 1.7 indicates the transmembrane region.
The amino acid sequence showed a high degree of identity with CABS1 proteins from different species, suggesting that the sequence we determined is porcine CABS1 (Fig. 1B). The molecular mass and the isoelectric point (pI) of the predicted pCABS1 protein were calculated to be 42,863 Daltons and 4.07, respectively. Porcine CABS1 shares the highest identity with bovine (72.4%), followed by human (58.4%), mouse (47.6%), and the lowest identity with rat (46.4%). The identity among the five species is 39.4%. A transmembrane region at a cutoff value of 1.7 was predicted for pCABS1 spanning from 313–320, mCABS1 spanning from 125–131, and rCABS1 spanning from 121–132 (Figs. 1C–1E).
Calcium-binding activity of pCABS1
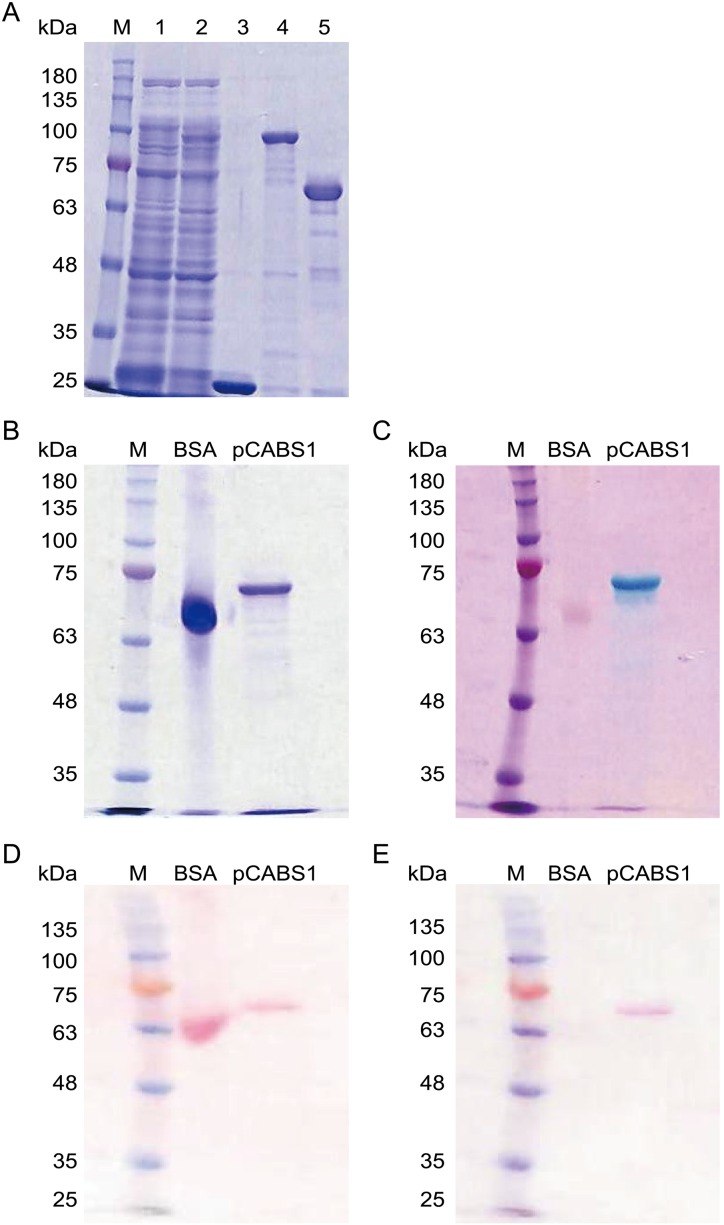
Recombinant pCABS1 protein was successfully purified and used for calcium-binding analyses (Fig. 2A). The calcium-binding activity of recombinant pCABS1 protein was determined by the colorimetric assays using Stains-All (Figs. 2B and 2C) or ruthenium red (Figs. 2D and 2E). In Stains-All staining, proteins that are very rich in acidic amino acids has the ability for the calcium binding and stained in blue or purple on gels, whereas other proteins stained in red or pink [6]. Control BSA and pCABS1 were separated on SDS-PAGE gel and stained with CBB (Fig. 2B) or Stains-All (Fig. 2C). The Stains-All stained pCABS1 in blue, while BSA in pink. Alternatively, ruthenium red is known to interact with calcium binding proteins and show red [7]. After SDS-PAGE, proteins were transferred electrically into a PVDF membrane and stained with Ponceau S (Fig. 2D) or ruthenium red (Fig. 2E). The ruthenium red stained pCABS1 in red, not BSA. Both results of Stains-All and ruthenium red suggested that pCABS1 protein has a calcium-binding activity.
Fig. 2.
Calcium-binding activity of the recombinant pCABS1 protein. (A) Purification of recombinant pCABS1 protein. Lane 1: Crude extract of GST as a negative control; lane 2: Crude extract of GST- pCABS1; lane 3: Purified GST; lane 4: Purified GST-pCABS1; and lane 5: Purified pCABS1. (B, C) 5 µg of bovine serum albumin (BSA) and the recombinant pCABS1 protein were separated on 10% SDS-PAGE, and stained with Coomassie Brilliant Blue (B) or Stains-All (C). (D, E) PVDF transfer of BSA and recombinant pCABS1 protein was stained with Ponceau S (D) or ruthenium red (E). M: Molecular marker (kilodalton; kDa).
Western blot analysis of pCABS1
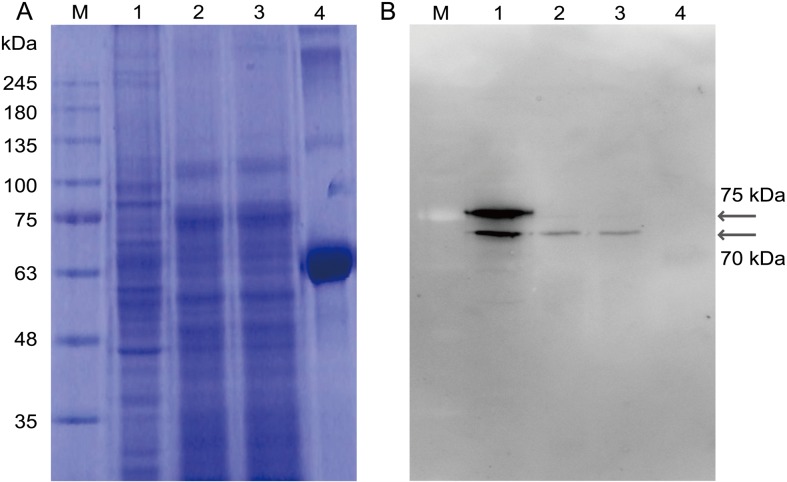
Western blot analysis of the proteins extracted from porcine testis and epididymal sperm was performed. Two bands at 75 kDa and 70 kDa were detected for the proteins extracted from testis, and one band at 70 kDa was detected for the proteins extracted from capacitated and non-capacitated sperm as indicated in Fig. 3 (Figs. 3A and 3B).
Fig. 3.
Western blot analysis of pCABS1 from testis and epididymis. 20 µg proteins extracted from testis, noncapacitated sperm, or capacitated sperm were separated on 10% SDS-PAGE. (A) Coomassie Brilliant Blue-staining. (B) Western blot analysis with anti-pCABS1 antiserum. Lane 1: Testis; lane 2: Noncapacitated sperm; lane 3: Capacitated sperm; and lane 4: bovine serum albumin (BSA) as a negative control. M: Molecular marker (kilodalton; kDa).
Localization of pCABS1 in the seminiferous epithelium
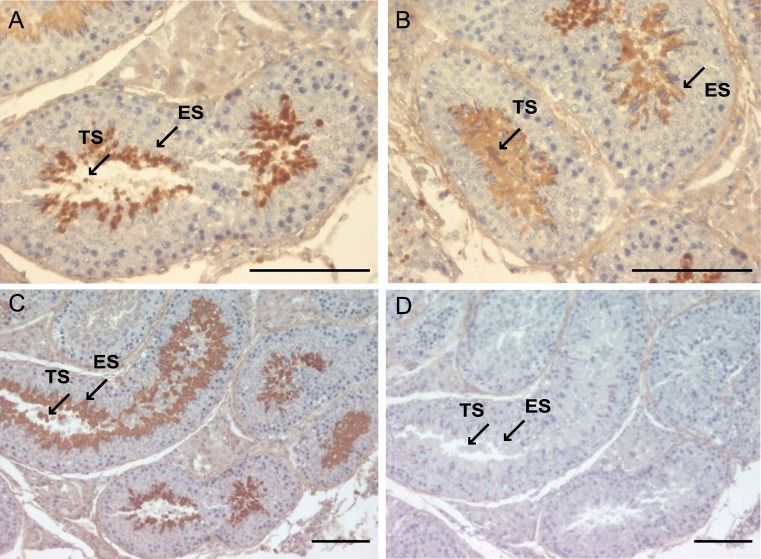
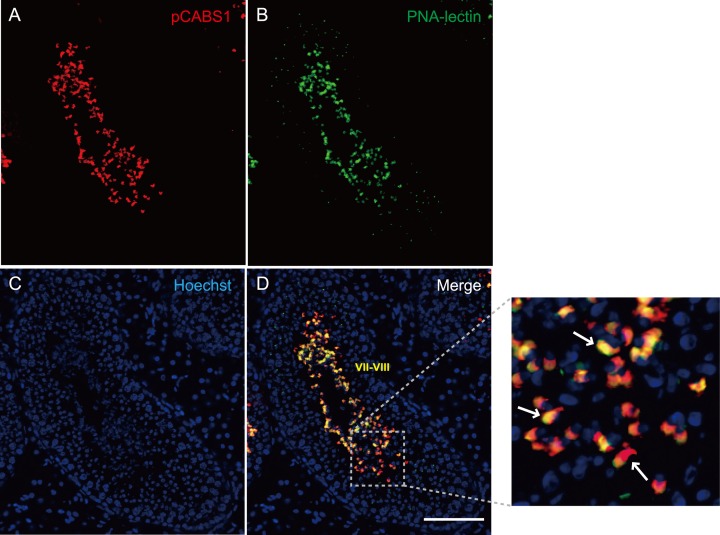
Immunohistochemical as well as immunofluorescent staining of the frozen sections from porcine testis with anti-pCABS1 antiserum revealed that the pCABS1 protein is localized in the elongated spermatids (ES) and testicular sperm (TS) within the lumen of the seminiferous tubules (Figs. 4A-4D). No staining was detected in the spermatogonia, spermatocytes, round spermatids, or somatic cells. We examined pCABS1 localization in relationship to acrosomal development (Figs. 5A–5D). Fig. 5 shows double staining of the acrosome-specific marker PNA-lectin together with pCABS1. The pCABS1 was strongly detected in the acrosomal regions, where the staining by PNA and pCABS1 antiserum was overlapped. pCABS1 was also expressed in the cytoplasm of the elongated spermatid, most of which then moved into the residual body of sperm.
Fig. 4.
Immunohistochemical analysis of pCABS1 in porcine testis. Localization of pCABS1 protein in the porcine testis was analyzed with anti-pCABS1 antiserum (A–C). pCABS1 antiserum was localized in the elongated spermatids (ES) as well as in the testicular sperm (TS) in the lumen, as indicated by the arrows. Neutralized pCABS1 antiserum gave no specific staining (D). Scale bars: 100 µm.
Fig. 5.
Localization of pCABS1 in the acrosomal region of elongated spermatids and luminal sperm. Immunofluorescence labeling of frozen sections of porcine testis with (A) pCABS1 (B) acrosomal marker PNA-lectin (C) nuclear staining with Hoechst. (D) merged images for the localization of pCABS1 on the acrosome of testicular sperm, and acrosome and cytoplasm of the elongated spermatid as indicated by arrows in the high magnification image. Scale bar: 100 µm.
Localization of pCABS1 in the epididymal sperm
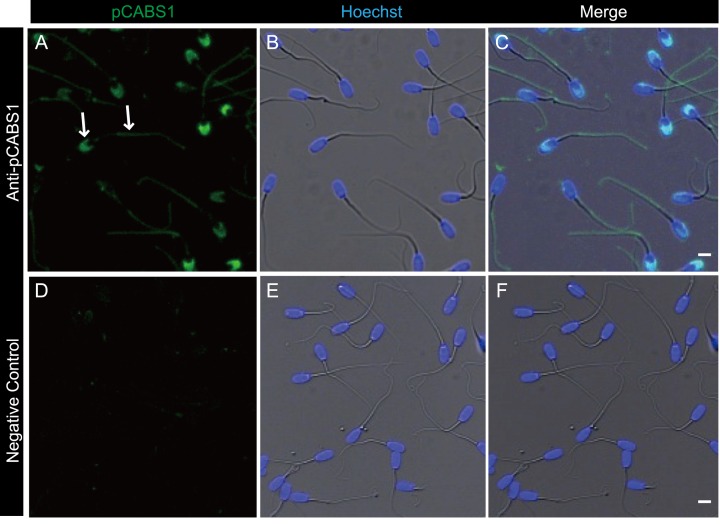
Immunofluorescent staining of cauda epididymal sperm shown in Fig. 6 indicated that pCABS1 is localized to the principal and end piece of the flagellum as with mCABS1. Moreover, pCABS1 was further localized in the acrosome of porcine sperm (Figs. 6A–6F).
Fig. 6.
Immunofluorescence detection of pCABS1 protein in cauda epididymal sperm. (A–C) The localization of pCABS1 in the cauda epididymal sperm was detected in the sperm tail and acrosome as indicated by arrows. (D–F) negative control obtained with neutralized anti-pCABS1 antiserum. Nuclear stainings by Hoechst are shown as blue (B and E). Scale bars: 5 µm.
Loss of pCABS1 through the acrosome reaction
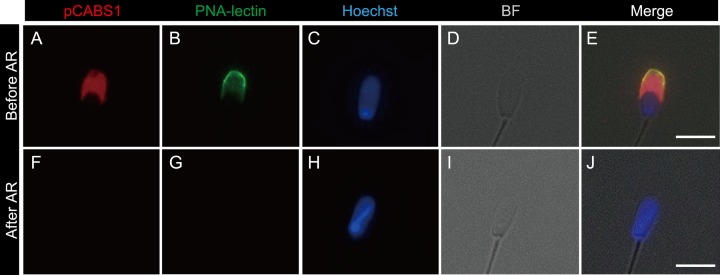
In order to further study the specific localization of pCABS1, we investigated its localization during the acrosome reaction. After capacitation, the sperm cells were incubated in the presence of the calcium ionophore A23187, fixed by 4%PFA, and then double staining was performed using FITC-PNA to assess the status of the acrosome and using anti-pCABS1 antiserum to localize pCABS1 (Figs. 7A–7J). Acrosome-reacted sperm were evidenced by the loss of an acrosome marker PNA-lectin of fixed sperm (Fig. 7G). Fig. 7 clearly shows that pCABS1 was lost during the acrosome reaction since pCABS1 immunostaining was no longer detectable in the acrosome reacted-spermatozoa.
Fig. 7.
Loss of pCABS1 protein during the acrosome reaction (AR) of the cauda epididymal sperm. Double-staining by anti-pCABS1 antiserum with FITC-conjugated peanut agglutinin (PNA)-lectin. (A–E) before the acrosome reaction. (F–J) after the acrosome reaction. pCABS1 (A and F), PNA-lectin (B and G), nuclear staining (C and H), bright field (D and I). merged photos (E and J). Acrosome-reacted sperm lost the pCABS1 staining (F). The AR is evidenced by the absence of PNA-lectin binding of fixed sperm. Scale bars: 5 µm.
Effect of anti-pCABS1 antiserum on the acrosome reaction
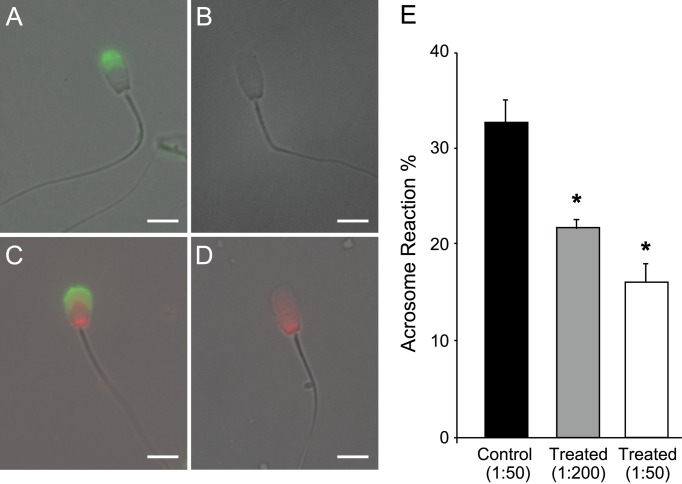
To analyze the involvement of pCABS1 in the acrosome reaction, capacitated sperm were incubated with anti-pCABS1 antisera (1:200 and 1:50 dilution) or pCABS1-neutralized antisera (1:50 dilution) as a control. Because no abnormal sperm motility was observed, sperm acrosome reaction was then artificially induced by the addition of the calcium ionophore A23187. Evaluations were done by staining with FITC-PNA and propidium iodide (PI) as a viability test. Four fluorescence patterns were observed (Figs. 8A–8D); acrosome-reacted alive sperm (PNA+PI−), acrosome-intact alive sperm (PNA−PI−), acrosome-reacted dead sperm (PNA+PI+), acrosome-intact dead sperm (PNA−PI+). Acrosomal integrity was expressed as the percentage of live acrosome-reacted spermatozoa over total live spermatozoa for each treatment. The live/dead ratios were not affected by the addition of anti-pCABS1 antiserum (control 75.2% ± 9.6, anti-pCABS1 1:200 dilution 71.5% ± 8.8, and anti-pCABS1 1:50 dilution 77.7% ± 10.9), but the treatment resulted in a decrease of acrosome-reacted sperm (Fig. 8E). The percentage of living acrosome-reacted sperm was 21.9% ± 1.4 and 16.2% ± 2.9 in the experimental group when sperm were treated with anti-pCABS1 antiserum at 1:200 and 1:50 dilutions, respectively, although that in the control group was 32.7% ± 4.0 (P<0.05).
Fig. 8.
Effect of anti-pCABS1 antiserum on the acrosome reaction of porcine sperm. (A–D) Representative photos of four fluorescence patterns stained with FITC-conjugated peanut agglutinin (PNA)-lectin and propidium iodide (PI) for the assessment of acrosome status and sperm viability. (A, B) Live sperm without PI staining. (C, D) Dead sperm showing nuclear red PI fluorescence. (A) Acrosome-reacted sperm with acrosomal cap and uniform green fluorescence FITC-PNA. (B) Acrosome-unreacted sperm with no staining of the acrosomal cap. Scale bars: 5 µm. (E) Percentage of acrosome-reacted live sperm after incubation with anti-pCABS1 diluted 1:200 and 1:50 (treated) vs. incubation with the neutralized anti-pCABS1 antiserum diluted 1:50 (control). The bar chart demonstrates mean percentage ± SEM (n=200). *Significantly different from control sperm (P<0.05).
Discussion
Calcium ions are involved in multiple signaling processes needed for fertilization of both invertebrate and vertebrate spermatozoa such as maturation, motility, and the acrosome reaction [9]. Calcium-binding proteins contribute to the control of Ca2+-concentration in the cytosol and participate in numerous cellular functions by acting as Ca2+-transporters across cell membranes or as Ca2+-modulated sensors [29]. Previously, we identified a calcium-binding protein expressed specifically in mouse spermatids, mCABS1, which was also reported in rat spermatids by another group [5, 17]. Here, we report for the first time that CABS1 is also expressed in porcine, which was named pCABS1, and its full nucleotide sequence was determined. As long as tested, pCABS1 amino acid sequence shares the highest identity with cow (72.4%), followed by human (58.4%), and has the lowest homology with rat and mouse (46.4%) and (47.6%), respectively.
pCABS1 consists of 392 amino acids and has a predicted molecular weight of 42,863. The molecular mass of recombinant pCABS1 protein, which contained whole predicted amino acids, was observed at 70 kDa on the SDS-PAGE. This difference between the predicted and observed molecular mass could be due to the fact that some highly acidic proteins show anomalous behavior on SDS-PAGE, migrating to the position with higher molecular mass than expected [1, 11]. pCABS1 is likely to behave in the same way owing to its highly acidic nature, which was also observed in mCABS1 [17].
Furthermore, the native pCABS1 protein was detected by Western blot analysis as two bands at 75 kDa and 70 kDa in the testis and one band at 70 kDa for the proteins extracted from the mature sperm. It is interesting that mCABS1 also showed two kinds of molecular form dependent on the calcium binding. Namely, mCABS1 shifted the electrophoretic migration from 66 kDa in the testis to 58 kDa in the mature sperm by binding calcium during the transit through epididymis.
It was found that pCABS1 was specifically expressed in the elongated spermatids similarly to mCABS1. But the localization of pCABS1 in the cauda epididymal sperm was different from that in mouse. pCABS1 was found in the acrosome in addition to the principal piece of the flagellum, where mCABS1 was present. Acrosome is essential for fertilization process. It has been reported that the uptake of external Ca2+ into the acrosome is enhanced while sperm approach and interact with the egg, which is required for acrosomal exocytosis to be completed [2, 12, 22]. pCABS1 is very rich in acidic amino acids and stained by Stains-All and ruthenium red, suggesting its ability for the calcium binding as same as mCABS1. Both the calcium-binding activity of pCABS1 and its localization in the acrosome suggest its participation in the acrosome reaction. In order to examine this hypothesis, we investigated whether anti-pCABS1 antiserum affects the acrosome reaction. The results showed that anti-pCABS1 antiserum could react to its epitope on the sperm surface and that the percentage of acrosome reactions was significantly decreased. The inhibitory effects of antiserum are not due to the artificial effect of bivalent antibody, since the neutralized antiserum do not have an inhibitory effect on acrosome reaction. However, there is a possibility that the effect of pCABS1 might be indirect on acrosomal exocytosis. Furthermore, pCABS1 was not detected in the acrosome-reacted sperm. These results suggest that pCABS1 is a membrane protein involved in the fusion of plasma membrane and outer acrosomal membrane through its calcium-binding activity. In this connection, it is interesting that pCABS1 has a transmembrane domain spanning from 313 to 320 in the C-terminal region. Both mouse and rat have a transmembrane domain in CABS1 but spanning at different regions (125–131 in mCABS1, and 121–132 in rCABS1) from that of pCABS1, which might make the difference in their subcellular localization. It is interesting that a very weak but significant signal of mCABS1 could be also detected in acrosome [17]. In order to determine the exact physiological function of mCABS1, it would be necessary to generate and analyze the phenotypes of CABS1 knockout mice.
It is indicated that the CABS1 proteins have different physiological functions in mature sperm among species. In mouse, mCABS1 is principally present in flagellum of mature sperm, suggesting its involvement in the regulation of flagellar movement. On the other hand, it has been reported that in rat CABS1 is also expressed in the spermatogenic cells but absent from mature sperm, suggesting that rCABS1 has some roles in the spermatogenesis [5]. In porcine sperm, pCABS1 localizes in the acrosomal region in addition to flagellum and participates in the acrosome reaction. The exact molecular mechanisms of pCABS1 involved in the acrosome reaction must be elucidated in near future.
Acknowledgments
The authors express their deep gratefulness to Dr. Naomichi Okamura for valuable discussions and proofreading of the manuscript. We also would like to thank Flaminia Miyamasu at the Medical English Communications Center of the University of Tsukuba for grammatical revision of the manuscript.
Reference
- 1.Alves V.S., Pimenta D.C., Sattlegger E., Castilho B.A.2004. Biophysical characterization of Gir2, a highly acidic protein of Saccharomyces cerevisiae with anomalous electrophoretic behavior. Biochem. Biophys. Res. Commun. 314: 229–234. doi: 10.1016/j.bbrc.2003.12.086 [DOI] [PubMed] [Google Scholar]
- 2.Bleil J.D., Wassarman P.M.1983. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 95: 317–324. doi: 10.1016/0012-1606(83)90032-5 [DOI] [PubMed] [Google Scholar]
- 3.Braunewell K.H.2005. The darker side of Ca2+ signaling by neuronal Ca2+-sensor proteins: from Alzheimer’s disease to cancer. Trends Pharmacol. Sci. 26: 345–351. doi: 10.1016/j.tips.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Breitbart H.2002. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol. Cell. Endocrinol. 187: 139–144. doi: 10.1016/S0303-7207(01)00704-3 [DOI] [PubMed] [Google Scholar]
- 5.Calvel P., Kervarrec C., Lavigne R., Vallet-Erdtmann V., Guerrois M., Rolland A.D., Chalmel F., Jégou B., Pineau C.2009. CLPH, a novel casein kinase 2-phosphorylated disordered protein, is specifically associated with postmeiotic germ cells in rat spermatogenesis. J. Proteome Res. 8: 2953–2965. doi: 10.1021/pr900082m [DOI] [PubMed] [Google Scholar]
- 6.Campbell K.P., MacLennan D.H., Jorgensen A.O.1983. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye “Stains-all”. J. Biol. Chem. 258: 11267–11273. [PubMed] [Google Scholar]
- 7.Charuk J.H., Pirraglia C.A., Reithmeier R.A.1990. Interaction of ruthenium red with Ca2(+)-binding proteins. Anal. Biochem. 188: 123–131. doi: 10.1016/0003-2697(90)90539-L [DOI] [PubMed] [Google Scholar]
- 8.Costello S., Michelangeli F., Nash K., Lefievre L., Morris J., Machado-Oliveira G., Barratt C., Kirkman-Brown J., Publicover S.2009. Ca2+-stores in sperm: their identities and functions. Reproduction 138: 425–437. doi: 10.1530/REP-09-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darszon A., Nishigaki T., Beltran C., Treviño C.L.2011. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 91: 1305–1355. doi: 10.1152/physrev.00028.2010 [DOI] [PubMed] [Google Scholar]
- 10.Funahashi H.2002. Induction of capacitation and the acrosome reaction of boar spermatozoa by L-arginine and nitric oxide synthesis associated with the anion transport system. Reproduction 124: 857–864. doi: 10.1530/rep.0.1240857 [DOI] [PubMed] [Google Scholar]
- 11.Graceffa P., Jancsó A., Mabuchi K.1992. Modification of acidic residues normalizes sodium dodecyl sulfate-polyacrylamide gel electrophoresis of caldesmon and other proteins that migrate anomalously. Arch. Biochem. Biophys. 297: 46–51. doi: 10.1016/0003-9861(92)90639-E [DOI] [PubMed] [Google Scholar]
- 12.Gupta S.K., Bhandari B., Shrestha A., Biswal B.K., Palaniappan C., Malhotra S.S., Gupta N.2012. Mammalian zona pellucida glycoproteins: structure and function during fertilization. Cell Tissue Res. 349: 665–678. doi: 10.1007/s00441-011-1319-y [DOI] [PubMed] [Google Scholar]
- 13.Ho H.C., Granish K.A., Suarez S.S.2002. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 250: 208–217. doi: 10.1006/dbio.2002.0797 [DOI] [PubMed] [Google Scholar]
- 14.Hofer A.M.2005. Another dimension to calcium signaling: a look at extracellular calcium. J. Cell Sci. 118: 855–862. doi: 10.1242/jcs.01705 [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Gonzalez C., Michelangeli F., Harper C.V., Barratt C.L., Publicover S.J.2006. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum. Reprod. Update 12: 253–267. doi: 10.1093/humupd/dmi050 [DOI] [PubMed] [Google Scholar]
- 16.Katoh Y., Takebayashi K., Kikuchi A., Iki A., Kikuchi K., Tamba M., Kawashima A., Matsuda M., Okamura N.2014. Porcine sperm capacitation involves tyrosine phosphorylation and activation of aldose reductase. Reproduction 148: 389–401. doi: 10.1530/REP-14-0199 [DOI] [PubMed] [Google Scholar]
- 17.Kawashima A., Osman B.A., Takashima M., Kikuchi A., Kohchi S., Satoh E., Tamba M., Matsuda M., Okamura N.2009. CABS1 is a novel calcium-binding protein specifically expressed in elongate spermatids of mice. Biol. Reprod. 80: 1293–1304. doi: 10.1095/biolreprod.108.073866 [DOI] [PubMed] [Google Scholar]
- 18.Marquez B., Ignotz G., Suarez S.S.2007. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 303: 214–221. doi: 10.1016/j.ydbio.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClintock T.S., Glasser C.E., Bose S.C., Bergman D.A.2008. Tissue expression patterns identify mouse cilia genes. Physiol. Genomics 32: 198–206. doi: 10.1152/physiolgenomics.00128.2007 [DOI] [PubMed] [Google Scholar]
- 20.Newton A.C.2010. Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 298: E395–E402. doi: 10.1152/ajpendo.00477.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi J., Hikono H., Sato S., Watanabe G., Taya K., Sasamoto S., Hasegawa Y.1997. Ontogeny of inhibin secretion in the rat testis: secretion of inhibin-related proteins from fetal Leydig cells and of bioactive inhibin from Sertoli cells. J. Endocrinol. 155: 27–34. doi: 10.1677/joe.0.1550027 [DOI] [PubMed] [Google Scholar]
- 22.O’Toole C.M., Arnoult C., Darszon A., Steinhardt R.A., Florman H.M.2000. Ca(2+) entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Mol. Biol. Cell 11: 1571–1584. doi: 10.1091/mbc.11.5.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson S.D., Fauci L.J., Suarez S.S.2011. Mathematical modeling of calcium signaling during sperm hyperactivation. Mol. Hum. Reprod. 17: 500–510. doi: 10.1093/molehr/gar040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Publicover S., Harper C.V., Barratt C.2007. [Ca2+]i signalling in sperm—making the most of what you’ve got. Nat. Cell Biol. 9: 235–242. doi: 10.1038/ncb0307-235 [DOI] [PubMed] [Google Scholar]
- 25.Rahman M.S., Kwon W.S., Pang M.G.2014. Calcium influx and male fertility in the context of the sperm proteome: an update. Biomed. Res. Int. 2014: 841615. doi: 10.1155/2014/841615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siciliano L., Marcianò V., Carpino A.2008. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 6: 5. doi: 10.1186/1477-7827-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tardif S., Dubé C., Bailey J.L.2003. Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol. Reprod. 68: 207–213. doi: 10.1095/biolreprod.102.005082 [DOI] [PubMed] [Google Scholar]
- 28.Will T., Tjallingii W.F., Thönnessen A., van Bel A.J.2007. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 104: 10536–10541. doi: 10.1073/pnas.0703535104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yáñez M., Gil-Longo J., Campos-Toimil M.2012. Calcium binding proteins. Adv. Exp. Med. Biol. 740: 461–482. doi: 10.1007/978-94-007-2888-2_19 [DOI] [PubMed] [Google Scholar]