Abstract
Chlorine dioxide (ClO2) is a strong oxidant that possesses an antimicrobial activity. We demonstrated here that ClO2 gas is easily generated by mixing 3.35% sodium chlorite solution (Purogene) and 85% phosphoric acid at a 10:1 volume ratio without using an expensive machine. In a test room (87 m3), experiments were carried out using various amounts of sodium chlorite solution (0.25 ml/m3 to 20.0 ml/m3). The gas concentration increased in a sodium chlorite volume-dependent manner and reached peak values of from 0.8 ppm to 40.8 ppm at 2 h–3 h, and then gradually decreased. No differences in gas concentrations were observed between 0.1 and 2.5 m above the floor, indicating that the gas was evenly distributed. Under high-humidity (approximately 80% relative humidity), colony formation of both Staphylococcus aureus and Escherichia coli was completely inhibited by ClO2 gas exposure at 1.0 ml/m3 sodium chlorite solution (mean maximal concentration of 3.0 ppm). Exposure at 4.0 ml/m3 sodium chlorite solution (mean maximal concentration of 10.6 ppm) achieved complete inactivation of Bacillus atrophaeus spores. In contrast, without humidification, the efficacy of ClO2 gas was apparently attenuated, suggesting that the atmospheric moisture is indispensable. Delicate electronic devices (computer, camera, etc.) operated normally, even after being subjected to more than 20 times of fumigation. Considering that our method for gas generation is simple, reproducible, and highly effective at decontaminating microbes, our approach is expected to serve as an inexpensive alternative method for cleaning and disinfecting animal facilities.
Keywords: animal facilities, bacteria, biological indicator, chlorine dioxide gas, fumigant
Introduction
Environmental cleaning efforts in animal research facilities have been shown to play an important role in the control of the spread of infectious microbes, which often influence the outcome of animal experiments. We have routinely employed the manual application of aqueous disinfectant in our animal facilities, but it is time consuming, labor intensive, and prone to error. In addition, aqueous agents are generally not employable for disinfection of various measurement instruments, whereas gaseous disinfectants may be. With the advantages of high efficacy, large disinfection volume, low corrosion, low hazard and simple operation, gaseous disinfection may be the best decontamination method. Traditional fumigants, such as formaldehyde and ethylene oxide, have been used to decontaminate spaces of microbes, but their flammability and carcinogenicity limit their use as decontaminants [5, 17, 21, 22]. Although ozone gas and hydrogen peroxide vapor have been used as alternatives to formaldehyde [11, 14, 18, 26], so far there is no method employed widely to fumigate laboratory animal areas, which has prompted investigations into alternative methods for environmental disinfection in animal facilities.
Chlorine dioxide (ClO2) is a powerful oxidant that has a potent antimicrobial activity against bacteria, fungi and viruses [1, 2, 4]. Aqueous ClO2 is known as an environment-friendly disinfectant and has been successfully employed for the treatment of drinking water [3, 19, 27] because, unlike chlorine, it does not result in the formation of trihalomethanes or react with ammonia to form chloramine in water. Recent studies presented that gaseous ClO2 also has a potent antimicrobial efficacy [6, 8,9,10, 23,24,25]. However, because the generation of ClO2 typically requires employing an expensive machine, the gas so far has not been widely used as a fumigant. We therefore examined a method that is not dependent upon an expensive machine for ClO2 gas generation. We also determined the antibacterial activity of ClO2 gas against bacteria to further validate the utility of this method for ClO2 gas generation.
Materials and Methods
Organisms
Two organisms were selected to test antibacterial activities of ClO2 gas: Staphylococcus aureus ATCC 12600 and Escherichia coli ATCC 11775. Each bacterium was bound to porous beads (MicrobankTM, Pro-Lab Diagnostics, Round Rock, TX, USA) and stored at −80°C. The inoculated beads were streaked onto blood agar plates and incubated at 37°C for 24 h. Each colony was diluted in heart infusion broth (Eiken Chemical Co., Ltd., Tokyo, Japan) and adjusted to about 9 × 108 colony forming units per ml (CFU/ml) by turbidity measurements (DensiCHEK, bioMerieux Inc., Durham, NC, USA). Eighty microliters of each suspension was inoculated onto a 1-cm paper disc (Advantec Co., Ltd., Tokyo, Japan) and then dried inside a biosafety cabinet before use.
Commercially available biological indicator strips preloaded with>106 spores of Bacillus atrophaeus ATCC 9372 (ACE test, Fukuzawa Shoji Co., Ltd., Yokohama, Japan) were also used to validate ClO2 gas decontaminations.
ClO2 gas generation
In this study, we used 3.35% sodium chlorite solution (Purogene, Fuji Techno Service Co., Ltd., Sendai, Japan) and 85% phosphoric acid (Kanto Chemical Co., Inc., Tokyo, Japan) without dilution. ClO2 gas was generated by mixing the sodium chlorite solution and phosphoric acid. The resulting chemical reaction is as follows: 15NaClO2 + 4H3PO4 → 12ClO2 + 6H2O + 3NaCl + 4Na3PO4. The concentration of gas was measured with either of two distinct ClO2 detector tubes (No. 23M: detection range of 0.1 ppm−10 ppm, No. 8H: detection range of 12.5 ppm−250 ppm, Gastec Co., Inc., Ayase, Japan). When using the No. 8H detector tube, the gas concentration was corrected using the conversion factor obtained by following the written instructions.
Test chamber
ClO2 gas was generated in a 14-l chamber (width 38 cm × length 19 cm × height 19.5 cm) at room temperature. The proper amount of 3.35% sodium chlorite solution and 85% phosphoric acid was mixed in a 5-ml glass tube by vortexing, and the requisite amount was dispensed into a small glass tube. The mixtures at volume ratios of 2:1, 10:1 and 50:1 consisted of 4.0 ml/m3 of sodium chlorite solution (actual amount: 56 µl) and phosphoric acid (actual amounts: 28, 5.6 and 1.1 µl, respectively). The gas concentrations dependent upon sodium chlorite solution volume were generated by mixing 3.35% sodium chlorite solution in a range of from 0.5 ml/m3 to 4.0 ml/m3(actual amounts: 7.0 µl to 56 µl) and 85% phosphoric acid at a 10:1 volume ratio. For monitoring gas in the test chamber, air samples were obtained through the line connected to the ClO2 detector. Changes in gas concentrations were monitored for up to 8 h.
Test room
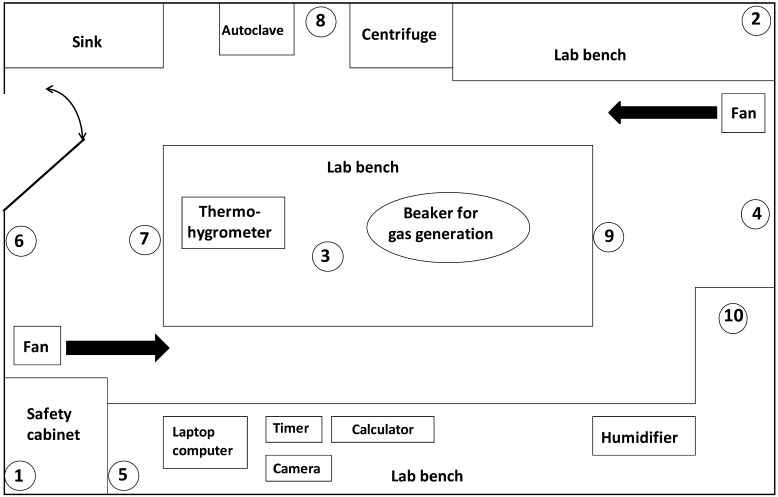
To further evaluate the properties of the ClO2 gas, the experiment was performed in a test room (87 m3) with the dimensions of 5.5 m wide, 6.1 m long, and 2.6 m high (Fig. 1). ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 volume ratio in a glass beaker using a magnetic stirrer. Experiments were carried out using various amounts of sodium chlorite solution ranging from 0.25 ml/m3 to 20.0 ml/m3(actual amounts: 21.8 ml to 1,740 ml). Two air circulators were set up at the corner of the room to promote circulation and mix the gas. Immediately after starting gas generation, the entry door was sealed with duct tape from outside to limit gas leakage into the adjacent room. Two polyethylene tubes (No. 6, Hibiki Co., Tokyo, Japan) entering the test room through the small space between the floor and bottom of the entry door were placed at the positions of 0.1 and 2.5 m above the floor of the test room. The tube lines allowed air sampling of the room to monitor gas concentrations. Air remaining in the tube lines was eliminated by applying suction with a 5-ml syringe, and shortly thereafter, air samples in the test room were obtained. To evaluate whether ClO2 gas damages delicate equipment, a laptop computer, digital camera, timer, and calculator were placed on the laboratory bench for every experiment (Fig. 1). Filter paper discs with each bacterium or biological indicators were placed on the ceiling, walls and floor (Fig. 1). During gas exposure at room temperature, the humidity in the test room was controlled using an electric humidifier to maintain a high-humidity environment. The relative humidity and temperature were recorded by a thermo-hygrometer (Thermo-Hygrograph, Ota Keiki Co., Ltd. Tokyo, Japan) during gas exposure. In addition, filter paper discs without exposure to ClO2 gas were placed under the same conditions of temperature and humidity, and used as a control. All experiments in the test room were performed after stopping the air conditioning.
Fig. 1.
Schematic diagram of a test room. Size of the test room was as follows: volume 87 m3, width 5.5 m×length 6.1 m×height 2.6 m. Paper discs with bacterium were placed on the ceiling (No. 1, 2), walls (No. 4, 5) and floor (No. 7, 8). Biological indicators were placed on the ceiling (No.1, 2, 3), walls (No. 4, 5, 6), floor (No. 7, 8, 9), and in a covered plastic petri dish on the lab bench (No.10). Two tube lines for air sampling were placed at the wall close to the entry door.
Colony forming unit determination
After 24 h of treatment with gas, control (non-exposed) and gas-exposed paper discs were transferred into a 15-ml sterile tube containing 10 ml PBS and vortexed for 1 min. Following extraction, 10-fold dilutions were performed as needed, and 0.1 ml of each dilution was inoculated onto two kinds of selective agar: desoxycholate-hydrogen sulfite-lactose agar (Eiken Chemical Co., Ltd., Tokyo, Japan) for E. coli and X-SA agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) for S. aureus. Following incubation at 37°C for 24 h, the number of colonies was counted, and the viable cell counts on the plates were determined as CFU/disc. Log reduction in viable organisms exposed to ClO2 gas was assessed through comparison with the serial dilution plate counts of the non-exposed control organisms. Samples with 0 CFU on plates were assigned a value of 1 CFU in order to obtain a log value of 0.
Statistics
All data are expressed as mean values ± SD. Statistical analyses were performed by using EXSAS version 7. 6 (Arm, Osaka, Japan) which is based on SAS release 9.1.3 (SAS Institute, Tokyo, Japan). The antibacterial effects of ClO2 gas were analyzed by Student’s t tests. Differences yielding a P value less than 0.05 were considered statistically significant.
Results
Generation of ClO2 gas in a test chamber
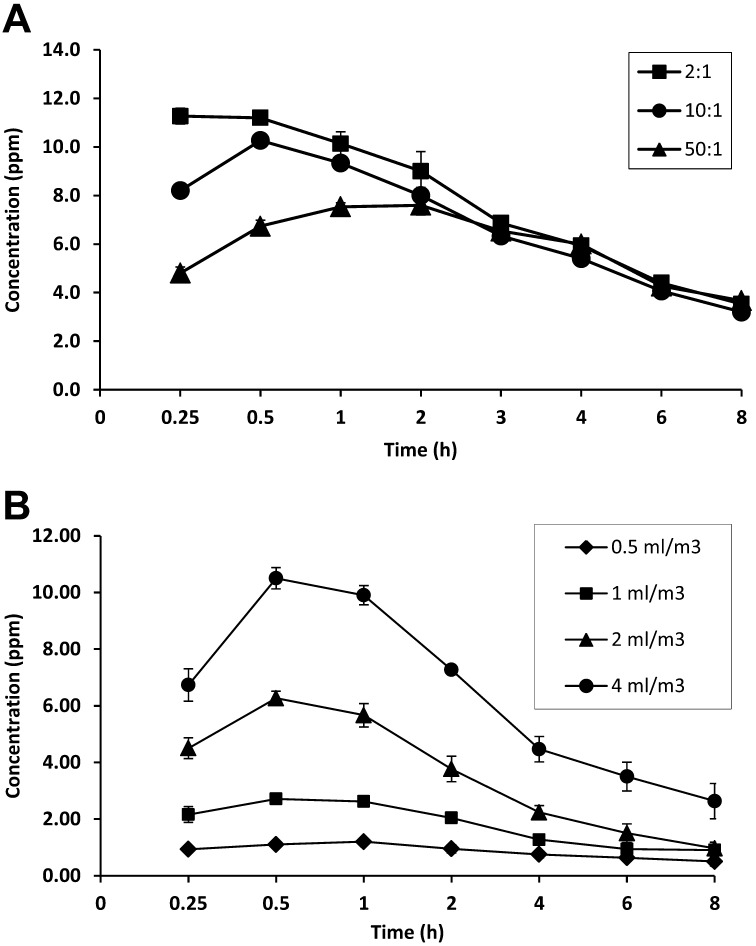
We first evaluated the changes in the gas concentrations generated by different ratios of 3.35% sodium chlorite solution (4.0 ml/m3) to 85% phosphoric acid. When mixed at a 2:1 volume ratio, ClO2 gas levels increased rapidly and reached maximum levels within 15 min, while a mixture at a 50:1 volume ratio exhibited a slow increase in gas levels (Fig. 2A). In the case of a 10:1 volume ratio, gas levels peaked at 30 min after gas generation, and then decreased thereafter (Fig. 2A). The ratio of 10:1 was chosen for further evaluation because it was found to be a ratio neither too fast nor too slow for gas generation.
Fig. 2.
Generation of ClO2 gas in a 14-l chamber. ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid. (A) Gas generation at three different volume ratios. The mixtures at 2:1, 10:1 and 50:1 volume ratios were prepared by mixing 4 ml/m3(actual amount: 56 µl) of sodium chlorite solution and phosphoric acid (actual amounts were 28, 5.6 and 1.1 µl, respectively). (B) The dependency of gas concentrations on sodium chlorite solution volume. Sodium chlorite solution and phosphoric acid were mixed at a 10:1 ratio, and the mixture was used at various volumes of sodium chlorite ranged from 0.5 ml/m3 to 4.0 ml/m3(actual amounts: 7.0 µl to 56 µl). Three independent experiments were performed, and data represent mean ± SD (n=3).
We then evaluated whether ClO2 gas levels are dependent on the volume of the sodium chlorite-phosphoric acid mixture. In the volumes of sodium chlorite solution ranged from 0.5 ml/m3 to 4.0 ml/m3, gas levels volume-dependently increased, peaking at 1.2 ppm to 10.5 ppm (Fig. 2B). In our preliminary tests, when we generated ClO2 gas at 50.0 ml/m3 of sodium chlorite solution, maximal concentrations of gas reached approximately 100 ppm.
Generation of ClO2 gas in a test room
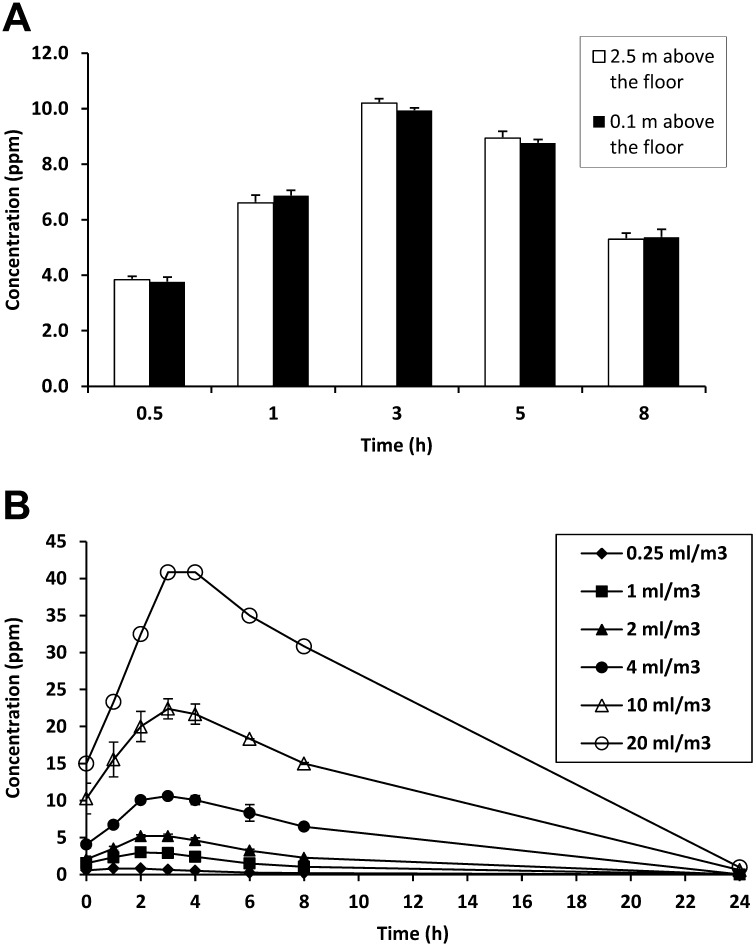
The generation of ClO2 gas was performed under high-humidity conditions using a humidifier. Ambient conditions in a test room were observed in the range of 75%–85% relative humidity and 20°C–25°C during exposure to ClO2 gas. To confirm the distribution of ClO2 gas in the test room, we measured the gas levels at both 0.1 and 2.5 m from the floor. When gas was generated by mixing 4.0 ml/m3 of 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 volume ratio, there were no differences in gas concentrations between lower and upper positions during exposure to the ClO2 gas (Fig. 3A), indicating that gas was equally distributed over the entire test room. Therefore, subsequent measurements of gas levels were performed at 2.5 m above the floor, unless stated otherwise in the text.
Fig. 3.
Generation of ClO2 gas in a test room (87 m3). Under high-humidity (75% to 85% relative humidity), ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 ratio. (A) Distribution of ClO2 gas in the test room. The mixture of sodium chlorite solution (4.0 ml/m3) and phosphoric acid was as follows: actual amounts were 348 ml and 34.8 ml, respectively. ClO2 gas concentrations were measured at both 0.1 m and 2.5 m from the floor. (B) Time course of changes in ClO2 gas concentrations. Experiments were carried out using various amounts of sodium chlorite solution ranged from 0.25 ml/m3 to 20.0 ml/m3 (actual amounts: 21.8 ml to 1,740 ml). Three independent experiments were performed, and data represent mean ± SD (n=3) except 20 ml/m3 (n=2).
The time course of changes in concentration of ClO2 gas was investigated at various volumes of 3.35% sodium chlorite solution in the range of 0.25 ml/m3 to 20.0 ml/m3. Respectively, the gas concentrations volume-dependently increased, peaking at 2 h–3 h, ranging from 0.8 ppm to 40.8 ppm, and then gradually decreased thereafter (Fig. 3B). In the volumes of sodium chlorite solution ranged from 0.25 ml/m3 to 4.0 ml/m3, gas levels were nearly undetectable (<0.05 ppm) after 24 h of gas generation. When ClO2 gas was generated at sodium chlorite solution volumes of 10.0 ml/m3 and 20.0 ml/m3, gas concentrations of 0.6 ppm–1 ppm were detected after 24 h of gas generation, but became undetectable after 30 min of aeration. Although study staff stayed in an adjacent room to monitor gas levels, leakage from the treated room was undetectable (<0.05 ppm) throughout the time of the gas exposure.
Delicate electronic devices, including a laptop computer, digital camera, timer and calculator, were placed on the laboratory bench for every trial (Fig. 1). There were no signs of functional damage even after these devices were exposed to ClO2 gas more than 20 times.
Antibacterial effects of ClO2 gas
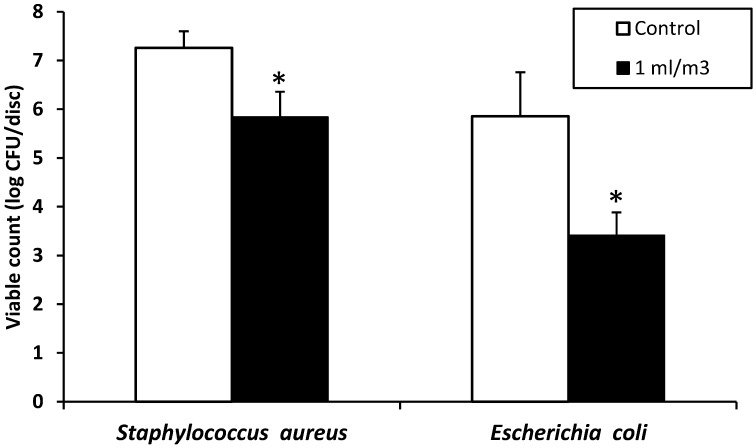
As model microbes, we selected both Gram-positive S. aureus and Gram-negative E. coli. Bacteria dried onto paper disc surfaces were placed at 6 sites (Fig. 1) and exposed for 24 h to ClO2 gas at the sodium chlorite solution volumes of 0.25, 1.0 and 4.0 ml/m3 under high-humidity conditions (Table 1). The colony formation of S. aureus and E. coli was completely inhibited by exposure to ClO2 gas generated at 4.0 ml/m3(mean maximal concentration of 10.6 ppm). Similarly, gas exposure at 1.0 ml/m3(mean maximal concentration of 3.0 ppm) also achieved complete inactivation of both bacteria. Although exposure to lower levels of gas (0.25 ml/m3, mean maximal concentration of 0.8 ppm) did not result in complete inactivation, its effects were more than 3 log10 CFU reductions in both bacteria. By contrast, without a humidifier (relative humidity of 30% to 40%), the efficacy at 1.0 ml/m3 apparently declined to less than a 2 log10 CFU reduction (Fig. 4).
Table 1. Antibacterial effects of ClO2 gas on S. aureus and E. coli.
| Bacteria | Group | Sodium chlorite (ml/m3) | ||
|---|---|---|---|---|
| 0.25 | 1.0 | 4.0 | ||
| (Log10 CFU/disc) | ||||
| S. aureus | Control | 7.1 ± 0.22 | 7.4 ± 0.33 | 7.2 ± 0.36 |
| ClO2 gas | 2.1 ± 1.49** | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| E. coli | Control | 3.5 ± 0.91 | 4.8 ± 0.45 | 4.8 ± 0.61 |
| ClO2 gas | 0.28 ± 0.20** | 0.0 ± 0.00 | 0.0 ± 0.00 | |
Three independent experiments were performed under high-humidity conditions (75% to 85%) using a humidifier. Paper discs with each bacterium were placed at 6 locations in the test room as shown in Fig. 1. ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 ratio. Experiments were carried out at 0.25, 1.0 and 4.0 ml/m3 of 3.35% sodium chlorite solution. After 24 h of ClO2 gas exposure, the viable cell counts were determined as log10 CFU per disc. Data represent mean ± SD (n=3). **P<0.01, significantly different from control paper discs treated without ClO2 gas (Student’s t-test).
Fig. 4.
Antibacterial effects of ClO2 gas on S. aureus and E. coli without using an electric humidifier. The relative humidity in the test room was 30% to 40%. ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 volume ratio. Sodium chlorite solution was used at a volume of 1.0 ml/m3(actual amount: 87 ml). After 24 h of gas generation, the viable cell counts were determined as log10 CFU per disc. Three independent experiments were performed, and data represent mean ± SD (n=3). * P<0.05, significantly different from control discs treated without ClO2 gas (Student’s t-test).
B. atrophaeus spore strip biological indicators were the standard for validation of ClO2 gas decontamination. As shown in Fig.1, biological indicators were placed at 10 sites, including a biological indicator in a covered petri dish, in addition to the ceiling, walls, and floor. Gas exposure at 10.0 ml/m3 and 20.0 ml/m3 of sodium chlorite solution (mean maximal concentration of 22.4 ppm and 40.8 ppm, respectively) achieved complete inactivation of B. atrophaeus at all 10 placement sites (Table 2). Generation of ClO2 gas at 4.0 ml/m3 also completely inactivated spore strip indicators at 9 sites, but failed to inactivate the biological indicator at placement site 10, which was located in the covered petri dish (Table 2). However, a second trial at the same volume resulted in complete inactivation of biological indicators at all placement sites (Table 2).
Table 2. Effect of ClO2 gas on B. atrophaeus biological indicator.
| Sodium chlorite (ml/m3) |
B. atrophaeus spores | |
|---|---|---|
| No. of sites with growth |
No. of sites without growth |
|
| 4.0 | 1 | 9 |
| 0 | 10 | |
| 10.0 | 0 | 10 |
| 0 | 10 | |
| 20.0 | 0 | 10 |
| 0 | 10 | |
Two independent experiments were performed under high-humidity conditions (75% to 85%) using a humidifier. Biological indicators were placed at 10 locations in the test room as shown in Fig. 1. ClO2 gas was generated by mixing 3.35% sodium chlorite solution and 85% phosphoric acid at a 10:1 ratio. Experiments were carried out at 4.0, 10.0 and 20.0 ml/m3 of 3.35% sodium chlorite solution. After 24 h of ClO2 gas exposure, indicator strips were incubated at 37°C for 48 h.
Discussion
Microbial decontamination is one of the practical issues of maintaining animal facilities for research. Here, we describe an alternative method for ClO2 gas generation that can be applied to cleaning and disinfecting animal facilities.
For our study, ClO2 gas was easily generated by mixing 3.35% sodium chlorite solution (Purogene) and 85% phosphoric acid without using an expensive gas generator. In a test room, the mixture volume-dependently produced ClO2 gas, reaching maximum levels at 2 h to 3 h, followed by a decline, and becoming nearly undetectable or up to 30-fold less than peak levels after 24 h of gas generation. The mean peak values in the test room were similar to those found in a 14-l test chamber, suggesting that our approach for gas generation is highly reproducible and flexibly applicable in various sizes of spaces.
According to safety guidelines, ClO2 has a permissive exposure limit of 0.1 ppm and a short term exposure limit of 0.3 ppm set by the American Conference of Governmental Industrial Hygienists (ACGIH). Since we adjusted the seal around the entry door to the test room, leakage of gas into the adjacent room was undetectable (<0.05 ppm) at any volumes of the mixture.
Aqueous disinfectants are generally not employable for disinfection of animal rooms, particularly where various measurement instruments and expensive equipment are placed, whereas gaseous agents may be. However, it remains unknown whether vaporous peracetic acid, hydrogen peroxide and ozone gas are applicable to delicate equipment without causing functional damage. A laptop computer, digital camera, timer and calculator were placed in the test room for every experiment. We confirmed that there were no signs of functional damage even after more than 20 times of fumigation. We cannot, however, rule out the possibility that ClO2 gas reduces the durability of such devices.
Although gaseous ClO2 inactivates bacteria, viruses and fungi [6, 8,9,10, 23,24,25], its antimicrobial effect is known to be affected by the atmospheric moisture [14, 16, 17]. Therefore, under conditions of high-humidity (75%–85% relative humidity), we evaluated the antibacterial effect of ClO2 gas against S. aureus, E. coli and B. atrophaeus spores. ClO2 gas generated at 1.0 ml/m3 of sodium chlorite solution (mean peak concentration of 3.0 ppm) completely inhibited the colony formation of S. aureus and E. coli. In addition, nearly complete inactivation was also observed for spore strip indicators exposed to ClO2 gas (4.0 ml/m3 of sodium chlorite, mean peak concentration of 10.6 ppm). In previous reports, sporicidal activities have been evaluated at high levels of ClO2 gas (170 ppm to 3,000 ppm) [7, 8, 13, 20]. Of note, we found that under our experimental conditions, the sporicidal activity of ClO2 gas was achieved with comparatively low levels of gas. On the other hand, when performing gas exposure without a humidifier (relative humidity of 30% to 40%), the efficacy of ClO2 gas was apparently attenuated, which is consistent with previous reports indicating that the atmospheric moisture is indispensable for ClO2 gas to inactivate microbes [13, 15, 16]. Thus, it is possible that humidification may result in a higher probability of the ClO2 gas making contact with bacteria and potentially increasing the efficacy of the ClO2 gas.
It is generally assumed that gaseous disinfectants can diffuse broadly into rooms by using an air circulator. Indeed, there were no differences in ClO2 gas concentrations between upper and lower positions in the test room, indicating that the gas was evenly distributed in the test room. Supporting this result, the antibacterial effects of ClO2 gas were observed on the ceiling, walls and floor. Furthermore, ClO2 gas was capable of completely inactivating bacteria in covered petri dishes. These results indicate that gaseous ClO2 is highly diffusible and permeable, thereby gaining access to the small spaces that are hard to disinfect with aqueous agents.
Regarding the efficacy of ClO2, parameters other than humidity are also necessary to consider. Previous reporting suggests that the activity of ClO2 gas is different between inoculated porous and nonporous materials, such as cotton cloth and glass [12]. In our preliminary studies, ClO2 gas generated at 1.0 ml/m3 of sodium chlorite solution showed decontamination efficacy against bacteria dried on glass surfaces. For organic contamination, recent reports have shown that the inactivation of microbes by ClO2 gas decreases with the increase of organic substances in a bacteria suspension [15, 16]. In our study, bacteria were suspended with heart infusion broth media, which contains organic matter derived from porcine heart. Further investigation is needed to identify the effects of ClO2 gas on various inoculated test materials and organic contamination.
Overall, our method for ClO2 gas generation was simple, reproducible, and did not require an expensive machine for gas generation. Furthermore, in addition to the high efficacy of ClO2 gas in decontaminating microbes, functional damage to delicate equipment and potential health risks for staff members were negligible. Thus, our approach is expected to serve as an alternative method to formaldehyde fumigation for cleaning and disinfecting animal facilities, and is also expected to be particularly useful in situations where various measurement instruments are present.
Acknowledgments
For their helpful discussion and advice, the authors are grateful to both Dr. Gen Kudou from our Department, and Dr. Yoshihiro Noda of the Laboratory Animal Facility at the Tokyo Metropolitan Institute of Gerontology.
References
- 1.Bang J., Hong A., Kim H., Beuchat L.R., Rhee M.S., Kim Y., Ryu J.H.2014. Inactivation of Escherichia coli O157:H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. Int. J. Food Microbiol. 191: 129–134. doi: 10.1016/j.ijfoodmicro.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 2.Benarde M.A., Israel B.M., Olivieri V.P., Granstrom M.L.1965. Efficiency of chlorine dioxide as a bactericide. Appl. Microbiol. 13: 776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann H., Koparal S.2005. The formation of chlorine dioxide in the electrochemical treatment of drinking water for disinfection. Electrochim. Acta 50: 5218–5228. doi: 10.1016/j.electacta.2005.01.061 [DOI] [Google Scholar]
- 4.Chatuev B.M., Peterson J.W.2010. Analysis of the sporicidal activity of chlorine dioxide disinfectant against Bacillus anthracis (Sterne strain). J. Hosp. Infect. 74: 178–183. doi: 10.1016/j.jhin.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheney J.E., Collins C.H.1995. Formaldehyde disinfection in laboratories: limitations and hazards. Br. J. Biomed. Sci. 52: 195–201. [PubMed] [Google Scholar]
- 6.Czarra J.A., Adams J.K., Carter C.L., Hill W.A., Coan P.N.2014. Exposure to chlorine dioxide gas for 4 hours renders Syphacia ova nonviable. J. Am. Assoc. Lab. Anim. Sci. 53: 364–367. [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbs S.G., Lowe J.J., Smith P.W., Hewlett A.L.2012. Gaseous chlorine dioxide as an alternative for bedbug control. Infect. Control Hosp. Epidemiol. 33: 495–499. doi: 10.1086/665320 [DOI] [PubMed] [Google Scholar]
- 8.Han Y., Applegate B., Linton R.H., Nelson P.E.2003. Decontamination of Bacillus thuringiensis spores on selected surfaces by chlorine dioxide gas. J. Environ. Health 66: 16–21. [PubMed] [Google Scholar]
- 9.Hsu C.S., Huang D.J.2013. Disinfection efficiency of chlorine dioxide gas in student cafeterias in Taiwan. J. Air Waste Manag. Assoc. 63: 796–805. doi: 10.1080/10962247.2012.735212 [DOI] [PubMed] [Google Scholar]
- 10.Lee S.Y., Costello M., Kang D.H.2004. Efficacy of chlorine dioxide gas as a sanitizer of lettuce leaves. J. Food Prot. 67: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 11.Lemmen S., Scheithauer S., Häfner H., Yezli S., Mohr M., Otter J.A.2015. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am. J. Infect. Control 43: 82–85. doi: 10.1016/j.ajic.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Li Y.J., Zhu N., Jia H.Q., Wu J.H., Yi Y., Qi J.C.2012. Decontamination of Bacillus subtilis var. niger spores on selected surfaces by chlorine dioxide gas. J. Zhejiang Univ. Sci. B 13: 254–260. doi: 10.1631/jzus.B1100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe J.J., Gibbs S.G., Iwen P.C., Smith P.W., Hewlett A.L.2013. Impact of chlorine dioxide gas sterilization on nosocomial organism viability in a hospital room. Int. J. Environ. Res. Public Health 10: 2596–2605. doi: 10.3390/ijerph10062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moat J., Cargill J., Shone J., Upton M.2009. Application of a novel decontamination process using gaseous ozone. Can. J. Microbiol. 55: 928–933. doi: 10.1139/W09-046 [DOI] [PubMed] [Google Scholar]
- 15.Morino H., Fukuda T., Miura T., Lee C., Shibata T., Sanekata T.2009. Inactivation of feline calicivirus, a norovirus surrogate, by chlorine dioxide gas. Biocontrol Sci. 14: 147–153. doi: 10.4265/bio.14.147 [DOI] [PubMed] [Google Scholar]
- 16.Morino H., Fukuda T., Miura T., Shibata T.2011. Effect of low-concentration chlorine dioxide gas against bacteria and viruses on a glass surface in wet environments. Lett. Appl. Microbiol. 53: 628–634. doi: 10.1111/j.1472-765X.2011.03156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan A.T., Preston R.J., Dellarco V., Ehrenberg L., Generoso W., Lewis S., Tates A.D.1995. Ethylene oxide: evaluation of genotoxicity data and an exploratory assessment of genetic risk. Mutat. Res. 330: 55–70. doi: 10.1016/0027-5107(95)00036-I [DOI] [PubMed] [Google Scholar]
- 18.Passaretti C.L., Otter J.A., Reich N.G., Myers J., Shepard J., Ross T., Carroll K.C., Lipsett P., Perl T.M.2013. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin. Infect. Dis. 56: 27–35. doi: 10.1093/cid/cis839 [DOI] [PubMed] [Google Scholar]
- 19.Radziminski C., Ballantyne L., Hodson J., Creason R., Andrews R.C., Chauret C.2002. Disinfection of Bacillus subtilis spores with chlorine dioxide: a bench-scale and pilot-scale study. Water Res. 36: 1629–1639. doi: 10.1016/S0043-1354(01)00355-4 [DOI] [PubMed] [Google Scholar]
- 20.Rastogi V.K., Ryan S.P., Wallace L., Smith L.S., Shah S.S., Martin G.B.2010. Systematic evaluation of the efficacy of chlorine dioxide in decontamination of building interior surfaces contaminated with anthrax spores. Appl. Environ. Microbiol. 76: 3343–3351. doi: 10.1128/AEM.02668-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers J.V., Choi Y.W., Richter W.R., Rudnicki D.C., Joseph D.W., Sabourin C.L., Taylor M.L., Chang J.C.2007. Formaldehyde gas inactivation of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surface materials. J. Appl. Microbiol. 103: 1104–1112. doi: 10.1111/j.1365-2672.2007.03332.x [DOI] [PubMed] [Google Scholar]
- 22.Sander J.E., Steffens W.L.1997. Transmission electron microscopic demonstration of abnormalities on the tracheal cilia of chicks exposed to formaldehyde during hatching. Avian Dis. 41: 977–980. doi: 10.2307/1592356 [DOI] [PubMed] [Google Scholar]
- 23.Sun X., Bai J., Ference C., Wang Z., Zhang Y., Narciso J., Zhou K.2014. Antimicrobial activity of controlled-release chlorine dioxide gas on fresh blueberries. J. Food Prot. 77: 1127–1132. doi: 10.4315/0362-028X.JFP-13-554 [DOI] [PubMed] [Google Scholar]
- 24.Sy K.V., McWatters K.H., Beuchat L.R.2005. Efficacy of gaseous chlorine dioxide as a sanitizer for killing Salmonella, yeasts, and molds on blueberries, strawberries, and raspberries. J. Food Prot. 68: 1165–1175. [DOI] [PubMed] [Google Scholar]
- 25.Wilson S.C., Wu C., Andriychuk L.A., Martin J.M., Brasel T.L., Jumper C.A., Straus D.C.2005. Effect of chlorine dioxide gas on fungi and mycotoxins associated with sick building syndrome. Appl. Environ. Microbiol. 71: 5399–5403. doi: 10.1128/AEM.71.9.5399-5403.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoutman D., Shannon M., Mandel A.2011. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am. J. Infect. Control 39: 873–879. doi: 10.1016/j.ajic.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 27.Zuo J.L., Cui F.Y., Lin T.2006. Study of removal effect on Mesocyclops leukarti with oxidants. J. Zhejiang Univ. Sci. B 7: 171–179. doi: 10.1631/jzus.2006.B0171 [DOI] [PMC free article] [PubMed] [Google Scholar]