Abstract
In the present study, we generated novel cre driver mice for gene manipulation in pancreatic β cells. Using the CRISPR/Cas9 system, stop codon sequences of Ins1 were targeted for insertion of cre, including 2A sequences. A founder of C57BL/6J-Ins1em1 (cre) Utr strain was produced from an oocyte injected with pX330 containing the sequences encoding gRNA and Cas9 and a DNA donor plasmid carrying 2A-cre. (R26GRR x C57BL/6J-Ins1em1 (cre) Utr) F1 mice were histologically characterized for cre-loxP recombination in the embryonic and adult stages; cre-loxP recombination was observed in all pancreatic islets examined in which almost all insulin-positive cells showed tdsRed fluorescence, suggesting β cell-specific recombination. Furthermore, there were no significant differences in results of glucose tolerance test among genotypes (homo/hetero/wild). Taken together, these observations indicated that C57BL/6J-Ins1em1 (cre) Utr is useful for studies of glucose metabolism and the strategy of bicistronic cre knock-in using the CRISPR/Cas9 system could be useful for production of cre driver mice.
Keywords: bicistronic, cre-loxP, CRISPR/Cas9, pancreatic β cells, mouse
Introduction
Although knockout mouse strains are invaluable for investigating gene function in vivo, roughly one third of all genes cause embryonic lethality in homozygous knockout mice (https://www.jax.org/research-and-faculty/tools/knockout-mouse-project/high-throughput-production). It is not possible to utilize the traditional knockout approach to examine the functions of genes that are lethal in the embryonic stage but may be involved in distinct roles during postnatal life. Using traditional knockout mice, it is also difficult to determine tissue-specific functions of genes that are expressed in multiple tissues. Such difficulties can be resolved by production of conditional knockout mice using the cre-loxP system, in which conditional knockouts are produced by crossing floxed mice with cre driver mice. To improve tissue- or cell-restricted expression of the cre enzyme, there are two major approaches to the generation of bacterial artificial chromosome (BAC)-carrying transgenic cre mice through zygotic microinjection and knock-in cre mice through targeted embryonic stem (ES) cells. However, there are also disadvantages to both approaches. As BAC cre constructs are randomly integrated into the mouse genome, there is a danger of disrupting endogenous genes. Although the cre gene is integrated into the targeted site through homologous recombination, the target gene is usually disrupted by cre gene insertion in knock-in mice.
The development of genome engineering will markedly alter the methods used for production of transgenic animals. The CRISPR/Cas9 system is a very convenient method for generating specific mutations in animals. This system is composed of a single guide RNA (sgRNA) and Cas9 protein which cause site-specific DNA double-strand breaks (DSB), leading to indel mutations by non-homologous end joining (NHEJ). In contrast, when a large amount of homologous DNA donor is present in Cas9/sgRNA-mediated DNA break, site-specific knock-in is generated by homologous direct repair (HDR). Indeed, we reported that simple production of albino C57BL/6J mice was possible using C57BL/6J zygote microinjection with the CRISPR/Cas9 system composed of a plasmid carrying a single guide RNA for Tyr and single-stranded DNA (G291T in Tyr) donor [11]. Furthermore, reporter gene knock-in mice can also be generated relatively easily by zygote co-microinjection of CRISPR/Cas9 components and reporter gene DNA, compared with conventional production using ES cells through homologous recombination [15].
Recently, we developed a cre driver mouse strain, C57BL/6N-Tg (Ins1-cre) 25Utr/Rbrc, carrying the cre gene in the BAC of insulin 1 (Ins1) to establish pancreatic β-cell-specific cre-loxP recombination [4]. Although the BAC Ins1 transgene was located in a position far distal to the centromere of chromosome 15, it is unclear whether an endogenous gene is damaged by insertion of the BAC transgene in this strain. In fact, homozygous mutant mice have not yet been obtained from intercrosses of the C57BL/6N-Tg (Ins1-cre) 25Utr/Rbrc strain. To avoid the disadvantages of BAC transgenic and conventional knock-in mice, we generated novel bicistronic Ins1-cre knock-in mice by CRISPR/Cas9-mediated homologous recombination in mouse zygotes without disruption of the endogenous Ins1 gene.
Materials and Methods
Construction of pX330 for Ins1 and donor plasmid
The CRISPR target sequence (5′-CTGGAGAACTACTGCAACTAAGG-3′) was selected for integration of the 2A-cre sequence just before the stop codon of Ins1. The pX330 plasmid, carrying both gRNA and Cas9 expression units, was a gift from Dr. Feng Zhang (Addgene plasmid 42230) [2]. Ins1-CRISPR-F (5′-caccCTGGAGAACTACTGCAACTA-3′) and Ins1-CRISPR-R (5′-aaacTAGTTGCAGTAGTTCTCCAG-3′) were annealed and inserted into the entry site of pX330 as described previously [11]. This plasmid was designated as pX330-Ins1. The cleavage activity of pX330-Ins1 was confirmed by the traffic reporter system using the p2color vector containing the CRISPR target (Supplementary Fig. 1). Transfection to HEK293T cells and fluorescence observations were performed as described [11].
The donor plasmid pIns1/2A-cre contained the P2A sequence and nuclear translocation signal (NLS)-cre. The 2.0-kb 5′-arm (from 1.4 kb upstream of exon 1 to just before the stop codon of Ins1) and the 1.7-kb 3′-arm (from the stop codon to 1.1 kb downstream of exon 2) were cloned into this vector. The strategy of bicistronic cre expression in pancreatic cells is shown in Fig. 1A. The pX330-Ins (RDB13945), pIns1/2A-cre (RDB13946), p2color-Ins1 (RDB13947) and p2color (RDB13948) were deposited in the RIKEN Bio-Resource Center (DNA Bank, Tsukuba, Japan) for distribution to the scientific community.
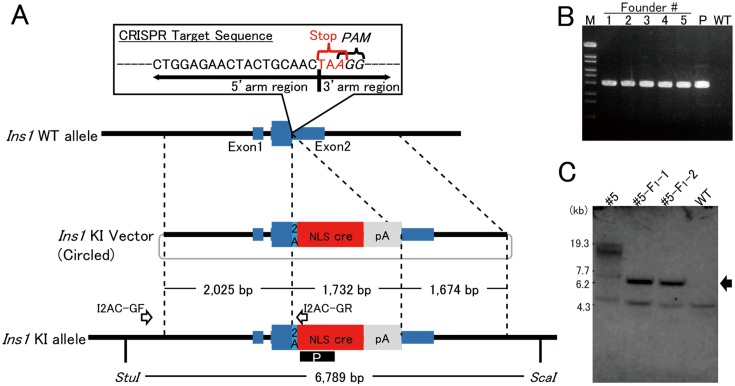
Fig. 1.
Strategy of bicistronic cre expression in pancreatic beta cells. A: To integrate the 2A-cre sequence just before the stop codon of Ins1, the 23-nt sequence (5′-CTGGAGAACTACTGCAACTAAGG-3′) containing both PAM and stop codon was chosen as the CRISPR target. The 3′-end of the region of 5′-homology arm (2.0 kb) is the final coding sequence of Ins1. The 5′-end of the region of 3′-homology arm is the stop codon of Ins1. The arrows labeled I2AC-GF and I2AC-GR indicate the primers for detecting the knock-in allele. The black box including the white letter P indicates the probe used for Southern blotting. B: PCR products, amplified with the founder genome DNA as the template and the primers I2AC-GF and I2AC-GR, of the appropriate size (2,631 bp) were detected. M: Marker 6 (Nippongene). C: Southern blotting analysis with founder #5 and its offspring (#5-F1-1 and #5-F1-2). In #5, the knock-in band (arrow) and longer random integration band were detected. In contrast, the KI band and no random integration band were detected in offspring. Lower weight nonspecific bands were detected in all samples.
Animals
C57BL/6N-Gt (ROSA) 26Sortm1 (CAG-EGFP,tdsRed) Utr/Rbrc, R26GRR [5], were obtained from the RIKEN BioResource Center (Tsukuba, Japan) through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The R26GRR cre reporter mice show bright green fluorescence in non-cre-recombined cells and markedly stronger bright red fluorescence in cre-recombined cells. C57BL/6J were purchased from Charles River Laboratories International, Inc. (Yokohama, Japan). Mice were kept in plastic cages under pathogen-free conditions in a room maintained at 23.5°C ± 2.5°C and 52.5% ± 12.5% relative humidity under a 14-h light:10-h dark cycle. Mice had free access to commercial chow (MF diet; Oriental Yeast Co., Ltd., Tokyo, Japan) and filtered water. All mouse experiments were approved by the University of Tsukuba Animal Experiment Committee.
Microinjection
DNA vectors (pX330-Ins1 and pIns1/2A-cre) for microinjection were isolated with FastGene Gel/PCR Extraction Kit (Nippon genetics, Tokyo, Japan) and diluted to 5 ng/µl by deionized distilled water and mix. The DNA solution was filtrated by MILLEX-GV® 0.22 µm Filter unit (Merk Millipore, Darmstadt, Germany). The DNA vectors were microinjected into the male pronuclei of fertilized oocytes which were harvested from superovulated mated C57BL/6J females. Survived one-cell embryos were transferred into the oviduct of pseudopregnant ICR females.
Genomic DNA analyses
The genomic DNA were purified from the tail with PI-200 (KURABO INDUSTREIS LTD, Osaka, Japan) according to manufacture’s protocol. Genomic PCR was performed with PrimeSTAR GXL DNA Polymerase® (TAKARA Bio, Shiga, Japan) and the primers (Supplementary Table 1). PCR products of off-target candidate were sequenced with BigDye Terminator v3.1 Cycle Sequencing Kit and 3500 genetic analyzer (Thermo Fisher Scientific, Massachusetts, U.S.).
For Southern blotting, 10 µg genomic DNA was double-digested by StuI and ScaI (New England Biolabs, Massachusetts, U.S.) and electrophoresed in 0.8% agarose gel. The DNA in gel was transferred to hybond n+ membrane (GE Healthcare Life Sciences, Chicago, U.S.). The DIG labeling probe for cre was synthesized with PCR DIG Probe Synthesis Kit (Roche, Basel, Switzerland) and primers (Supplementary Table 1), then hybridized to DNA transferred membrane at 45°C. Hybridization and detection steps were performed with DIG DNA Labeling and Detection Kit (Roche, Basel, Switzerland) according to manufacture’s protocol.
Stereomicroscopic findings
After anesthesia with a mixture of medetomidine, midazolam, and butorphanol [8], animals were fixed by perfusion with cold PBS and then with 4% paraformaldehyde (PFA) in cold PBS. EGFP and tdsRed fluorescence in 36 tissues were examined by fluorescence stereomicroscopy (M205FA; Leica, Wetzlar, Germany) provided with internal light sources and appropriate filter sets (excitation and emission: 470 ± 40 nm and 525 ± 50 nm and 545 ± 30 nm and 620 ± 60 nm band-pass filters for EGFP and tdsRed, respectively).
Immunohistochemical findings
For immunohistological demonstration of insulin and glucagon in pancreatic islets of Langerhans tissue, fixed samples were equilibrated in sucrose by placing in 50-ml tubes containing graded concentrations of sucrose (10%, 20%, and 30% in PBS). Samples were embedded in Tissue-Tek OCT (Fisher, Pittsburgh, PA) and frozen in liquid nitrogen. Frozen tissue blocks were brought to −20°C and sections 14 µm thick were cut and mounted on amino silane-coated slides. Tissue sections were incubated with guinea pig anti-insulin antibody (Dako, Carpinteria, CA) or mouse anti-glucagon antibody (Sigma-Aldrich, St. Louis, MO) for 1 h at room temperature. The antigens were visualized using appropriate secondary antibodies conjugated with Alexa 647 and then sections were stained with diamidino-2-phenylinodole (DAPI) to detect nuclei (Invitrogen, Carlsbad, CA). Fluorescence was examined by fluorescence microscopy (BZ-X710; Keyence, Osaka, Japan) with internal light sources and appropriate filter sets (band-pass filter excitation and emission: 360 ± 40 nm and 460 ± 50 nm for DAPI, 470 ± 40 nm and 525 ± 50 nm for EGFP, 560 ± 40 nm and 630 ± 75 nm for tdsRed, and 620 ± 60 nm and 700 ± 75 nm for Alexa 647). Image data were analyzed with a BZ-X analyzer (Keyence).
Glucose tolerance test
Glucose tolerance tests were performed in 16 h fasted mice after intraperitoneal injection of glucose (2 g/kg body weight) [9]. Blood samples were collected from the tail veins. Blood glucose values were measured immediately before and 15, 30, 60, and 120 mins after glucose injection by One Touch Ultra (Johnson and Johnson, U.S.). All results are reported as means ± SD for equivalent groups and compared with the independent t test. In all analyses, P<0.05 was taken to indicate statistical significance.
Results
The pX330-Ins1 vector and donor plasmid pIns1/2A-cre were co-injected into the pronuclei of fertilized oocytes obtained from C57BL/6J mice to obtain integration of the knock-in vector into the Ins1 allele target site. After birth, developed weanlings were genotyped by PCR designed to amplify an integrated knock-in fragment of 2,631 bp in length (Fig. 1B). Five founder mice were obtained from 373 injected oocytes, and the pX330-Ins1 construct was not detected in the tail genome of founders by PCR (data not shown). The efficiency of knock-in mouse production is shown in Supplementary Table 2. The inheritance of the knock-in construct was identified in one (#5) of the five founders, i.e., mouse #5, although Southern blotting analysis indicated that it carried a randomly integrated Ins1/2A-cre construct (s) in addition to the knock-in construct. The extra Ins1/2A-cre construct (s) was segregated from the knock-in construct in the progeny derived by backcrossing with C57BL/6J (Fig. 1C). The heritability was also confirmed by PCR analysis (Supplementary Fig. 2). The knock-in mice were designated as C57BL/6J-Ins1em1 (cre) Utr. Moreover, the ORF of Ins1 was connected to 2A and NLS cre sequence correctly in this strain (Supplementary Fig. 3).
We then investigated the off-target effects in founder #5. Cong et al. [2] reported that single-base mismatch up to 11 bp 5′ of the PAM sequence (5′-NGG-3′) completely abolished genomic cleavage by Streptococcus pyogenes Cas9, which was used in the present study. Therefore, we examined off-target sites that were completely consistent with 12 bases at the 3′ end and PAM (5′-CTACTGCAAACTANGG-3′ or 5′-CCNTAGTTGCAGGTAG-3′). Fifteen off-target candidates were isolated from the whole mouse genome sequence with CRISPRdirect (https://crispr.dbcls.jp/) (Supplementary Table 3), and these 15 off-target candidates were confirmed by direct sequencing analysis. No mutations were found in any of the off-targets, indicating that little or no Cas9-mediated off-target mutation occurred in C57BL/6J-Ins1em1 (cre) Utr. Addition, we obtained 23 offspring from natural mating between #5 and wild-type and the knock-in allele were found in 12 of them (12/23, 52.2%), suggesting that founder #5 carried hemizygous, not mosaical, knock-in allele. However, the other founders (#1, #2, #3 and #4) were mosaics, because there were no their offspring carrying the knock-in allele.
To evaluate the site of cre expression in the fetal stage after expressing endogenous Ins1, heterozygous C57BL/6J-Ins1em1 (cre) Utr males were crossed with homozygous R26GRR females. In (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice (n=3) on embryonic day (E) 16.5, red fluorescence was observed in the tissues surrounded by a rectangular frame and in the intestinal cavity as indicated with an arrow in Fig. 2A. Immunohistochemistry using anti-insulin antibody clearly demonstrated the co-localization of red fluorescence with insulin (Alexa647) signals, indicating that the staining-positive tissue in the abdomen was the pancreas. However, the red signals in the intestinal cavity appeared to be nonspecific because there was not staining with DAPI or for insulin (data not shown). Examination of whole embryos indicated no red signals in other tissues. These results suggested that cre in C57BL/6J-Ins1em1 (cre) Utr was expressed dominantly in the fetal pancreas at E16.5, but not in other tissues.
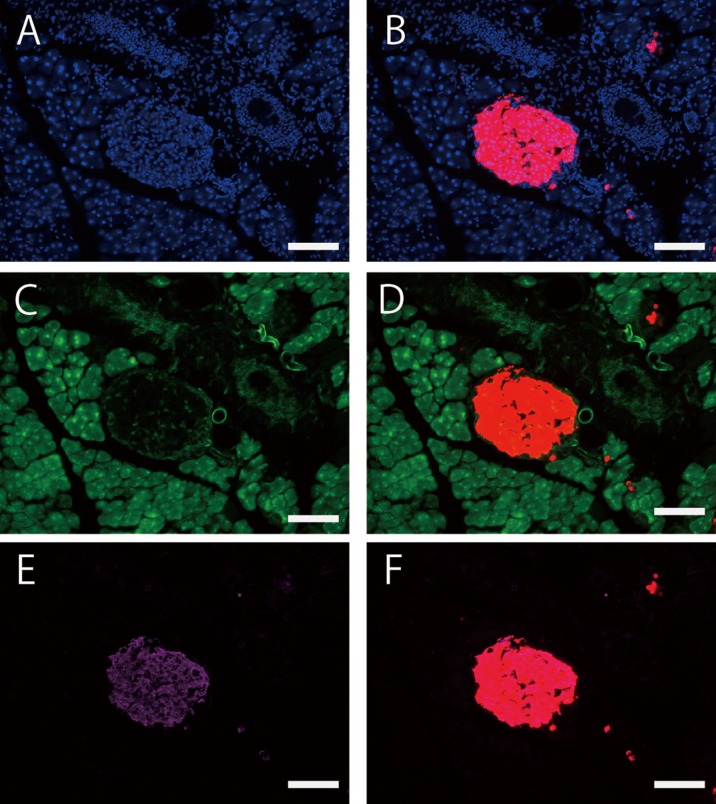
Fig.2.
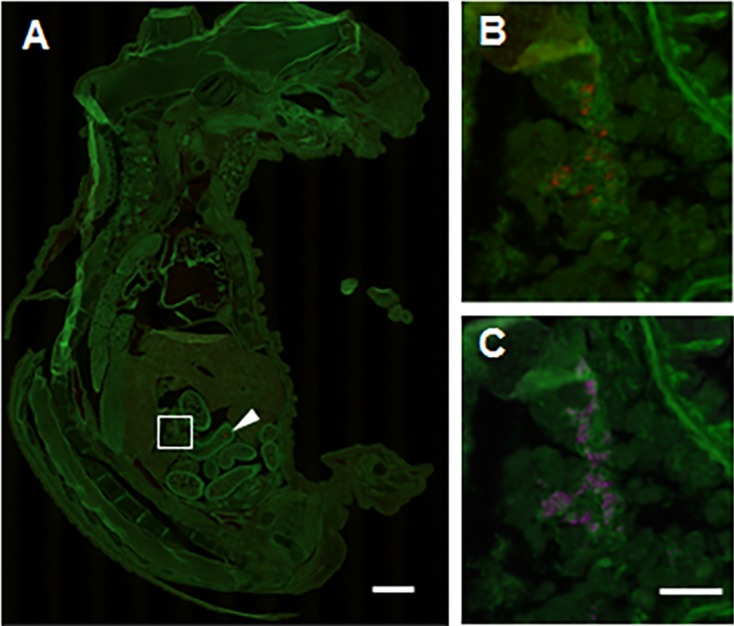
Histological analysis of cre-loxP recombination in the fetal stage of (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice. A: tdsRed signals (cre-loxP recombination) in the whole body of a fetus at E16.5. Specific recombination signals were detected in the fetal pancreas (surrounded by a square) (B and C). The arrowhead indicates nonspecific signal in the intestine. B: tdsRed signals in the pancreas of a fetus at E16.5. C: Insulin signals in the pancreas of a fetus at E16.5. Each experiment (n=4). Scale bar: 1 mm (low-power field) and 100 µm (high-power field).
To determine the recombination site of cre-loxP in the adult stage, we dissected adult (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 males (n=3) and females (n=3) after perfusion with 4% PFA and divided into 36 kinds of tissues. Each tissue was cut into coronal, transverse, or sagittal sections, which were then examined by fluorescence stereomicroscopy. As shown in Supplementary Fig. 4, red fluorescence indicating the cre-loxP recombination signal was observed in the pancreas, but not in other tissues. These data were consistent with the results in fetuses. In mice, insulin is synthesized within the β-cells of the islets of Langerhans in the pancreas. As the localization and efficiency of cre-loxP recombination are the most important characteristics in cre driver mice, fluorescence-microscopy was performed using cryosections of the pancreas from adult (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice. More than 40 islets in each mouse (n=3) were examined by Alexa647 fluorescence for insulin and tdsRed fluorescence for cre-loxP recombination. All individuals showed complete recombination in the insulin-positive islets examined (Fig. 3 and Supplementary Table 4).
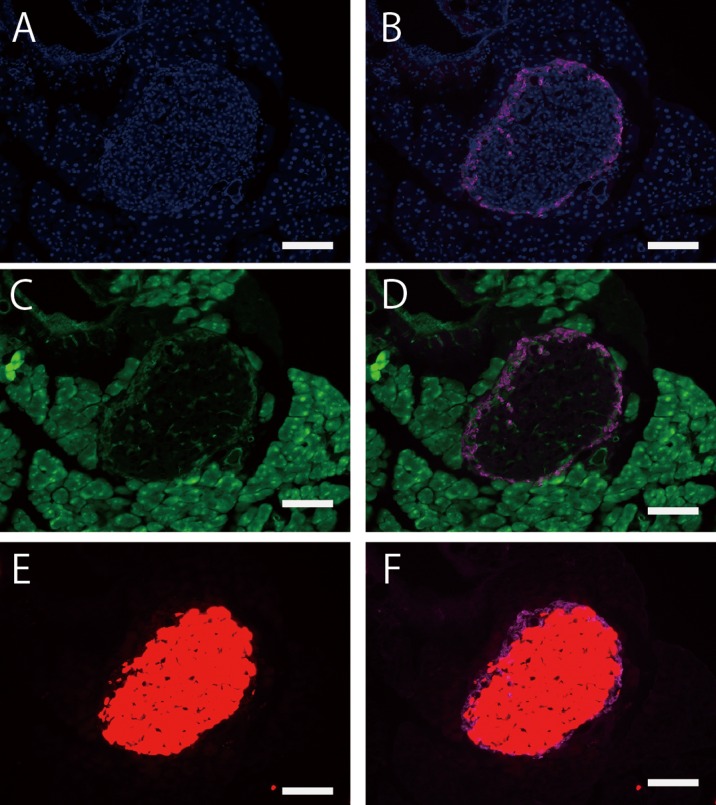
Fig.3.
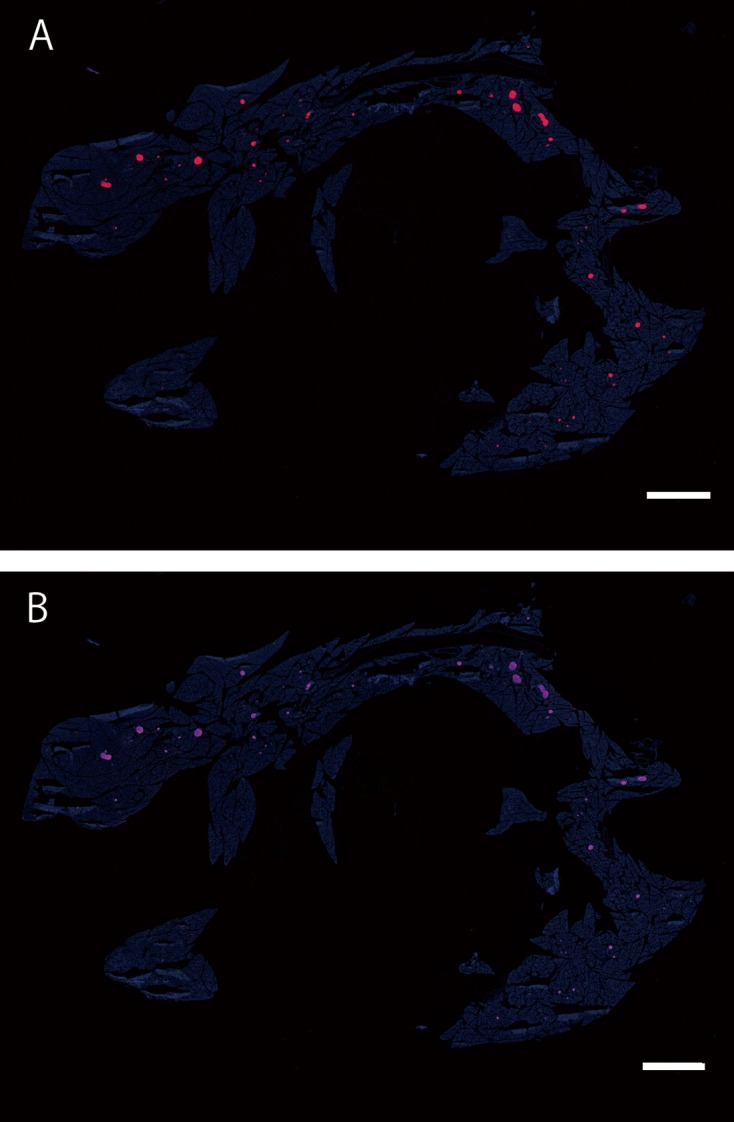
cre-loxP recombination of islets in a wide range of regions in the adult pancreas of (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice. A: tdsRed signals (Cre-loxP recombination), B: Insulin signals (using anti-insulin + 2nd antibody conjugated with Alexa647). All tdsRed-positive islets were insulin-positive. Each experiment (n=3). Scale bar: 1.5 mm
Next, we examined whether cre-loxP recombination was limited to the β-cells of the pancreatic islets of Langerhans. On higher magnification observation, tdsRed-positive cells were well overlaid with insulin-positive cells in the pancreatic islets, but not with glucagon-positive cells (Figs. 4 and 5). Quantitative analysis (n=3) indicated that cre-loxP recombination had occurred in approximately 98% of insulin-positive cells (Supplementary Table 5), suggesting that cre gene expression is spatially regulated in exactly the same way as the endogenous Ins1 gene in C57BL/6J-Ins1em1 (cre) Utr mice.
Fig. 4.
Co-localization of insulin-positive islets with tdsRed-positive islets in the adult pancreas of (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice. A: DAPI, B: DAPI/tdsRed (cre-loxP recombination), C: EGFP (non cre-loxP recombination), D: EGFP/tdsRed, E: Insulin (using anti-insulin + 2nd antibody conjugated with Alexa647), F: Insulin/tdsRed. Each experiment (n=3). Scale bar: 100 µm
Fig. 5.
De-localization of glucagon-positive signals with tdsRed-positive signal in adult pancreatic islets of (R26GRR × C57BL/6J-Ins1em1 (cre) Utr) F1 mice. A: DAPI, B: DAPI/Glucagon (using anti-glucagon + 2nd antibody conjugated with Alexa647), C: EGFP (no cre-loxP recombination), D: EGFP/Glucagon, E: tdsRed (cre-loxP recombination), F: Glucagon/tdsRed. Each experiment (n=3). Scale bar: 100 µm
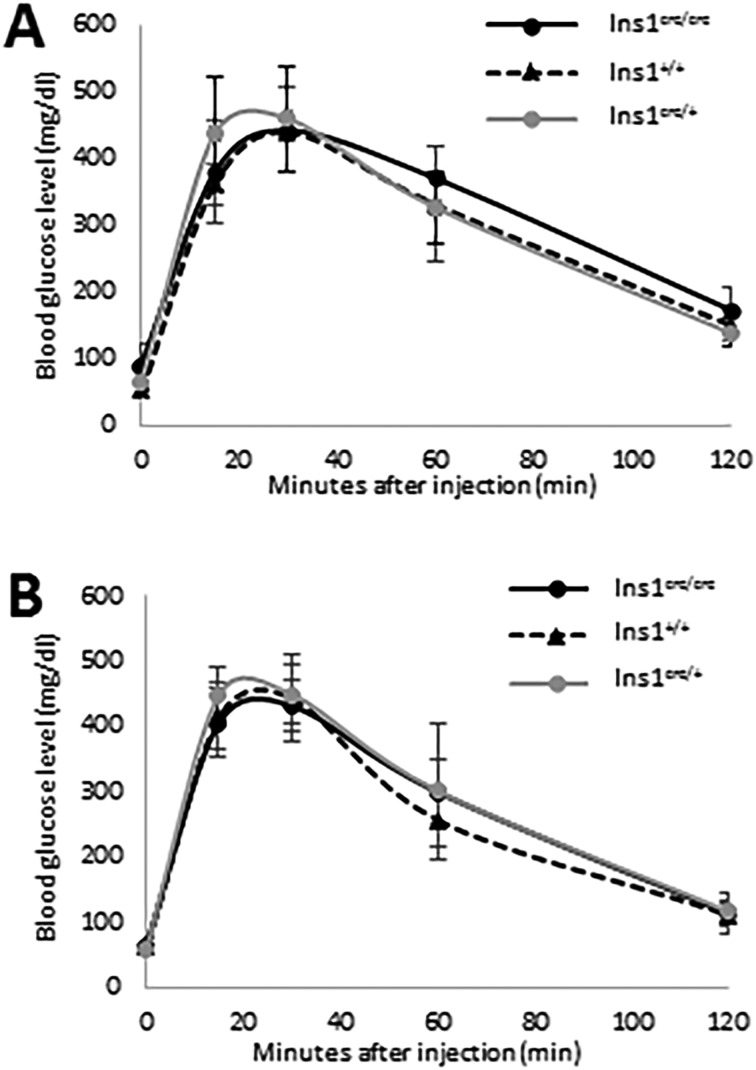
To examine whether Ins1 transcript fused with 2A-cre affects glucose metabolism, we performed glucose tolerance tests in fasted 8-week-old heterozygous C57BL/6J-Ins1em1 (cre) Utr mice. Compared with wild-type males and females, C57BL/6J-Ins1em1 (cre) Utr males and females exhibited no significant differences in glucose clearance at any time point examined after glucose injection, respectively. Finally, we investigated whether homozygous mice were generated from intercrosses of heterozygous mutants in the C57BL/6J-Ins1em1 (cre) Utr strain. As expected, homozygous males and females were obtained, and they showed no significant differences between genotypes of each sex in the glucose tolerance test (Fig. 6). C57BL/6J-Ins1em1 (cre) Utr is a highly useful cre driver mouse strain with induction of β-cell-specific cre-loxP recombination for studying glucose metabolism.
Fig. 6.
Glucose tolerance test of C57BL/6J-Ins1em1 (cre) Utr mice. Males (A) and females (B) were tested at 8 weeks old. Ins1+/+ (male: n=3, female: n=9), Ins1cre/+ (male: n=7, female: n=7), and Ins1cre/cre (male: n=7, female: n=6) indicate wild-type, heterozygous, and homozygous C57BL/6J-Ins1em1 (cre) Utr, respectively.
Discussion
Several cre driver mouse strains have been developed for investigating gene functions in pancreatic β-cells. Transgenic mice carrying the rat insulin promoter fused to cre (RIP-cre) have been widely used for cre-loxP recombination in pancreatic β-cells. However, cre was also shown to be expressed in the brain in addition to the pancreas in three different RIP-cre mouse strains, i.e., Tg (Ins2-cre) 25Mgn [12], Tg (Ins2-cre) 1Herr [6], and Tg (Ins2-cre/Esr) 1Dam [3]. To obtain exclusive cre expression in pancreatic β-cells, Wicksteed et al. [14] and Hasegawa et al. [4] developed Tg (Ins1-cre/ERT) 1Lphi and C57BL/6N-Tg (Ins1-cre) 25Utr/Rbrc mouse strains, respectively, which lacked cre-loxP recombination in the brain. Similar to both of these transgenic lines carrying cre fused to the Ins1 promoter, the results of the present study showed that C57BL/6J-Ins1em1 (cre) Utr mice have highly specific cre recombinase activity in pancreatic β-cells and lack cre recombinase activity in the brain. The most important difference between C57BL/6J-Ins1em1 (cre) Utr and other β-cell-specific cre driver mice is clarity of the integration site of cre into the genome. Although Thorens et al. [13] reported the generation of Ins1cre knock-in mice through targeted ES cells with conventional homologous recombination techniques, exon 2 encoding the whole of proinsulin was replaced with an exogenous construct encoding cre recombinase, which resulted in loss of Ins1function. In contrast, cre transcripts are expressed in a bicistronic manner of Ins1 in C57BL/6J-Ins1em1 (cre) Utr mice, suggesting no loss of function of Ins1. This is the first report of the development of bicistronic Ins1 cre driver mice.
The bicistronic knock-in strategy is an effective method for generating endogenous gene-dependent cre recombinase activity without losing one copy of the target allele. The internal ribosomal entry site and self-cleaving 2A peptides have been frequently used for multicistronic gene expression. Four different 2A peptides derived from foot-and-mouth disease virus (F2A), equine rhinitis A virus (E2A), porcine teschovirus-1 (P2A), and Thosea asigna virus (T2A) in addition to an internal ribosomal entry site (IRES) were widely used in biomedical research increasing the cleavage efficiency and maintaining the expression of more than one gene. In the present study, a 2A peptide derived from porcine teschovirus-1 was chosen for bicistronic cre expression, because the viral 2A peptide maintains more reliable expression levels of the appended downstream cistron than IRES [1]. Kim et al. [7] reported that P2A has high cleavage efficiency in human cell lines, as well as in zebrafish and mice. C57BL/6J-Ins1em1 (cre) Utr mice showed cre recombinase activity in almost all cells of the pancreatic islets synthesizing insulin. Therefore, our results suggest that P2A peptide is functional in bicistronic constructs containing cre in the pancreatic β-cells of living mice.
From the results of the present study, it is unclear whether insulin protein is translated from the Ins1 knock-in allele, because C57BL/6J-Ins1em1 (cre) Utr mice also possess an Ins2 allele. Further, we should investigate the synthesis and secretion of insulin derived from the Ins1 knock-in allele in Ins2-deficient C57BL/6J-Ins1 em1 (cre) Utr mice.
Bicistronic knock-in mice have been generated from chimeras with genetic contributions from both the recipient embryo and the ES cells targeted with the knock-in vector. Although reliable, this method is laborious, costly, and time consuming. Recently, we [10, 11] and many other groups have adopted the CRISPR/Cas9 technique for gene modification of mouse because of its high degree of flexibility and accuracy in cutting and pasting DNA. As the founder of the C57BL/6J-Ins1em1 (cre) Utr line carried randomly integrated transgene (s) in addition to the knock-in allele, Southern blotting analysis using a restriction enzyme with a recognition site outside of the knock-in construct is necessary for genotyping of CRISPR/Cas9-mediated knock-in mice, similar to conventional knock-in mice. However, the transgene could be easily segregated from the knock-in Ins1 allele by backcrossing. To our knowledge, C57BL/6J-Ins1em1 (cre) Utr is the first bicistronic knock-in cre driver mouse strain generated by the CRISPR/Cas9 system.
In conclusion, we generated a novel CRISPR/Cas9-mediated bicistronic knock-in cre mouse strain, C57BL/6J-Ins1em1 (cre) Utr. The cre activity was observed in insulin-synthesized cells in the fetal pancreas at E16.5. In adults, cre activity was detected in almost all insulin-synthesizing cells in the pancreatic islets. However, there was no cre activity in other tissues. Moreover, the knock-in cre mice showed no abnormalities in the glucose tolerance test. These results suggest that C57BL/6J-Ins1em1 (cre) Utr is a useful cre driver mouse strain for gene modification in pancreatic β-cells. Utilization of the CRISPR/Cas9 system could be useful for generating bicistronic knock-in cre driver mice. The C57BL/6J-Ins1em1 (cre) Utr mouse strain (RBRC09525) is available from the RIKEN BioResource Center.
Disclosure/Conflict of Interest Statement
The authors declare there are no conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Challenging Exploratory Research (15K14359: to F.S.), Scientific Research (S) (26221004: to S.T., S.M., F.S), and NBRP Fundamental Technologies Upgrading Program (to F.S.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. We thank the members of the Yagami Laboratory for helpful discussions and encouragement.
References
- 1.Chan H.Y., V S., Xing X., Kraus P., Yap S.P., Ng P., Lim S.L., Lufkin T.2011. Comparison of IRES and F2A-based locus-specific multicistronic expression in stable mouse lines. PLoS ONE 6: e28885. doi: 10.1371/journal.pone.0028885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F.2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. doi: 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dor Y., Brown J., Martinez O.I., Melton D.A.2004. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46. doi: 10.1038/nature02520 [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa Y., Daitoku Y., Mizuno S., Tanimoto Y., Mizuno-Iijima S., Matsuo M., Kajiwara N., Ema M., Oishi H., Miwa Y., Mekada K., Yoshiki A., Takahashi S., Sugiyama F., Yagami K.2014. Generation and characterization of Ins1-cre-driver C57BL/6N for exclusive pancreatic beta cell-specific Cre-loxP recombination. Exp. Anim. 63: 183–191. doi: 10.1538/expanim.63.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa Y., Daitoku Y., Sekiguchi K., Tanimoto Y., Mizuno-Iijima S., Mizuno S., Kajiwara N., Ema M., Miwa Y., Mekada K., Yoshiki A., Takahashi S., Sugiyama F., Yagami K.2013. Novel ROSA26 Cre-reporter knock-in C57BL/6N mice exhibiting green emission before and red emission after Cre-mediated recombination. Exp. Anim. 62: 295–304. doi: 10.1538/expanim.62.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera P.L., Orci L., Vassalli J.D.1998. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol. Cell. Endocrinol. 140: 45–50. doi: 10.1016/S0303-7207(98)00028-8 [DOI] [PubMed] [Google Scholar]
- 7.Kim J.H., Lee S.R., Li L.H., Park H.J., Park J.H., Lee K.Y., Kim M.K., Shin B.A., Choi S.Y.2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 6: e18556. doi: 10.1371/journal.pone.0018556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni R.N., Brüning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R.1999. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96: 329–339. doi: 10.1016/S0092-8674(00)80546-2 [DOI] [PubMed] [Google Scholar]
- 10.Mizuno S., Takami K., Daitoku Y., Tanimoto Y., Dinh T.T., Mizuno-Iijima S., Hasegawa Y., Takahashi S., Sugiyama F., Yagami K.2015. Peri-implantation lethality in mice carrying megabase-scale deletion on 5qc3.3 is caused by Exoc1 null mutation. Sci. Rep. 5: 13632. doi: 10.1038/srep13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno S., Dinh T.T., Kato K., Mizuno-Iijima S., Tanimoto Y., Daitoku Y., Hoshino Y., Ikawa M., Takahashi S., Sugiyama F., Yagami K.2014. Simple generation of albino C57BL/6J mice with G291T mutation in the tyrosinase gene by the CRISPR/Cas9 system. Mamm. Genome 25: 327–334. doi: 10.1007/s00335-014-9524-0 [DOI] [PubMed] [Google Scholar]
- 12.Postic C., Shiota M., Niswender K.D., Jetton T.L., Chen Y., Moates J.M., Shelton K.D., Lindner J., Cherrington A.D., Magnuson M.A.1999. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274: 305–315. doi: 10.1074/jbc.274.1.305 [DOI] [PubMed] [Google Scholar]
- 13.Thorens B., Tarussio D., Maestro M.A., Rovira M., Heikkilä E., Ferrer J.2015. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia 58: 558–565. doi: 10.1007/s00125-014-3468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicksteed B., Brissova M., Yan W., Opland D.M., Plank J.L., Reinert R.B., Dickson L.M., Tamarina N.A., Philipson L.H., Shostak A., Bernal-Mizrachi E., Elghazi L., Roe M.W., Labosky P.A., Myers M.G., Jr, Gannon M., Powers A.C., Dempsey P.J.2010. Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59: 3090–3098. doi: 10.2337/db10-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H., Wang H., Shivalila C.S., Cheng A.W., Shi L., Jaenisch R.2013. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154: 1370–1379. doi: 10.1016/j.cell.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.