Abstract
Background: Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) is a negative regulator of immune responses that suppresses the activity of effector T cells and contributes to the maintenance of self tolerance. When blocked therapeutically, CTLA-4 leads to an overall activation of T cells that has been exploited for cancer control, a control associated however with a variety of immune-related side effects such as autoimmune thyroiditis. To investigate the mechanism(s) underlying this form of thyroiditis, we used the NOD-H2h4 mouse, a model that develops thyroiditis at very high incidence after addition of iodine to the drinking water.
Methods: NOD-H2h4 mice were started on drinking water supplemented with 0.05% sodium iodide when 8 weeks old and then injected with a hamster monoclonal antibody against mouse CTLA-4, polyclonal hamster immunoglobulins, or phosphate buffered saline when 11 weeks old. One month later (15 weeks of age), mice were sacrificed to assess thyroiditis, general immune responses in blood and spleen, and expression of indoleamine 2, 3-dioxygenase (IDO) in the thyroid and in isolated antigen-presenting cells after stimulation with interferon gamma. The study also analyzed IDO expression in four autopsy cases of metastatic melanoma who had received treatment with a CTLA-4 blocking antibody, and six surgical pathology Hashimoto thyroiditis controls.
Results: CTLA-4 blockade worsened autoimmune thyroiditis, as assessed by a greater incidence, a more aggressive mononuclear cell infiltration in thyroids, and higher thyroglobulin antibody levels when compared to the control groups. CTLA-4 blockade also expanded the proportion of splenic CD4+ effector T cells, as well as the production of interleukin (IL)-2, interferon gamma, IL-10, and IL-13 cytokines. Interestingly, CTLA-4 blockade induced a strong expression of IDO in mouse and human thyroid glands, an expression that could represent a counter-regulatory mechanism to protect against the inflammatory environment.
Conclusions: This study shows that CTLA-4 blockade exacerbates the iodine-accelerated form of thyroiditis typical of the NOD-H2h4 mouse. The study could also have implications for cancer patients who develop thyroiditis as an immune-related adverse event after CTLA-4 blockade.
Introduction
Autoimmune thyroiditis has been modeled in animals since the mid-1950s. For the first four decades, models were mainly based on immunizations with whole thyroid extracts (1) or thyroglobulin (2). Since the early 1990s, autoimmune thyroiditis has also been studied using mice that develop it spontaneously, the so-called NOD-H2h4 model. The NOD-H2h4 mouse was discovered serendipitously by Linda Wicker's laboratory at Merck while studying the influence of the major histocompatibility complex on the NOD model of type 1 diabetes (3). The authors noted that the congenic NOD-H2h4 strain (Kk, Ak, E0, Db) lost the spontaneous development of diabetes typical of the parental NOD strain (Kd, Ag7, E0, Db) but acquired thyroiditis, as assessed by the appearance of mononuclear cell infiltration in the thyroid gland and circulating thyroglobulin antibodies. It is now well established that thyroiditis in NOD-H2h4 mice first emerges at about four months of age and becomes fully prevalent at 12 months (4,5). In contrast to the human counterpart (Hashimoto thyroiditis), in NOD-H2h4 mice thyroperoxidase antibodies develop only later (6), thyroxine remains normal (7), and males are as equally affected as females (4,5). Interestingly, the original authors also noted that addition of sodium iodide to the drinking water accelerated the incidence and severity of thyroiditis in the NOD-H2h4 but not the parental NOD strain (8), an observation later confirmed and expanded by others (9,10). More specifically, once iodine-rich water supplementation is started (typically done at two months of age), thyroiditis ensues within two weeks and becomes fully prevalent at about five months of age (5).
This anticipation and worsening of thyroiditis by iodine has been the subject of numerous studies and hypotheses (4,5). One view is that incorporation of iodine in thyroglobulin renders this autoantigen more immunogenic, and thus more easily recognizable by autoreactive T cells. Another view is that iodine directly affects the thyrocytes by making them more susceptible to apoptosis via dysregulation of oxidative stress control mechanisms or by rendering them a better homing site for circulating effector T cells via upregulation of adhesion molecules (11).
In addition to these thyroid-centered mechanisms, it has also been shown that iodine reduces the number and/or function of regulatory T cells (Tregs), potentially tipping the immunoregulatory balance toward autoimmunity. In fact, iodine feeding decreases the proportion of CD4+ CD25+ Foxp3+ Tregs in the spleen (12,13) and thyroid glands (14) of NOD-H2h4 mice. In addition, NOD-H2h4 mice lacking the T cell costimulatory molecule CD28 develop a more severe form of iodine-accelerated thyroiditis and have fewer Tregs in spleen and cervical lymph nodes (15). Similarly, Tregs depletion by injection of an anti-CD25 antibody for four days prior to the iodine supplementation induced a more severe form of thyroiditis and higher thyroglobulin antibody titers (16). Overall, these studies emphasize the importance of Tregs and costimulatory signals in the pathogenesis of autoimmune thyroiditis, in keeping with the findings reported in other autoimmune conditions (17).
In recent years, T-cell costimulatory signals have become the target of monoclonal antibody therapies in patients with a variety of cancers, firmly establishing immunotherapy as the fourth pillar for cancer treatment (accompanying conventional chemotherapy, radiotherapy, and surgery). For example, ipilimumab, a monoclonal antibody that blocks the T-cell molecule cytotoxic T lymphocyte antigen-4 (CTLA-4), was approved by the U.S. Food and Drug Administration in 2011 for the treatment of advanced melanoma and is now used in several other types of cancer. Immunotherapy is effective and is revolutionizing oncology (18) but induces numerous side effects collectively referred to as immune-related adverse events (19). Thyroiditis/hypothyroidism is seen in about 5% of patients treated with anti-CTLA-4 antibody [reviewed by Corsello et al. (20)], and about 20% of those treated with anti-PD1 antibody [reviewed by Ryder et al. (21)]. The mechanisms through which these immune checkpoint inhibitors cause thyroiditis and other autoimmune side effects remain unknown. In this study we used the iodine-accelerated mouse model of thyroiditis to assess the effect of blocking CTLA-4 on disease incidence and severity, and we begin the investigation of its mechanism of action.
Materials and Methods
NOD-H2h4 mice and iodine administration
The breeding stock used to establish our NOD-H2h4 colony was originally obtained from Dr. Linda Wicker (Merck Laboratories) in 1993 and then maintained in the specific pathogen-free facilities of the Johns Hopkins University. Mice were placed on drinking water supplemented with 0.05% sodium iodide (Sigma) from 8 weeks of age to the termination of the experiments (week 15). A total of 38 mice (24 for the antibody injections, 6 for dendritic cells isolations, and 8 for the macrophage studies) were used, including both males and females since this form of thyroiditis is known to be similar in the two sexes.
Human pathological specimens
We queried the autopsy database of the Johns Hopkins Hospital Department of Pathology for cases that had been administered a CTLA-4 blocking antibody (ipilimumab from Bristol-Meyer-Squibb, or tremelimumab from Astra Zeneca). Of the total of 617 autopsies performed from November 2013 to January 2016, four were cases with metastatic melanoma treated with ipilimumab: all males, aged 32, 60, 66, and 68 years old. As controls, we used six surgical pathology thyroids from patients with Hashimoto thyroiditis who underwent thyroidectomy because of a suspicious cytology (n = 3) or compressive symptoms (n = 3). Human pathological specimens were de-identified and analyzed blindly under institutional review board protocol number 04-07-12-05e.
Injection of CTLA-4 blocking antibody and control antibodies
After three weeks of iodine supplementation (11 weeks of age), mice were separated into three groups: one (n = 9) was injected 10 times during the first two weeks (days 1, 2, 3, 4, and 5 of each week) with a hamster monoclonal antibody directed against the extracellular portion of mouse CTLA-4 (hybridoma clone UC-10-4F10-11, from American Type Culture Collection, Manassas, VA); a second group (n = 8) was injected at the same times with polyclonal hamster immunoglobulins (from Jackson Immunoresearch); and a third group (n = 7) with phosphate buffered saline solution. All mice were sacrificed four weeks after the first injections (at 15 weeks of age). The CTLA-4 antibody was purified from the hybridoma cultures using protein-G columns and dissolved in phosphate buffered saline at a concentration of one milligram per microliter. The hamster control immunoglobulins were also dissolved at the same conditions. Mice were injected intraperitoneally with 100 μL of the antibody solutions (corresponding to 100 μg) or phosphate buffer saline. The protocol was approved by the Animal Care and Use Committee of the Johns Hopkins University.
Assessment of iodine-accelerated thyroiditis
The development of thyroiditis was assessed by measuring serum thyroglobulin antibodies and analyzing thyroid histopathology for evidence of mononuclear cell infiltration. Blood was collected from the retro-orbital plexus before the start of iodine supplementation (week 8), before injection of the antibodies (week 11), and at the end of the experiments (week 15). Serum was separated by centrifugation and used to measure thyroglobulin antibodies by enzyme-linked immunosorbent assay, as described by Kimura et al. (22). Thyroid glands were collected at the time of sacrifice, fixed overnight in 10% buffered formalin, embedded in paraffin, and sectioned at a thickness of five micrometers. Thyroid sections were stained with hematoxylin and eosin and scored for mononuclear cell infiltration using a discrete scale from 0 (normal thyroid, no infiltration) to 5 (complete effacement of the thyroid architecture by the infiltrating mononuclear cells) (23).
Assessment of splenic lymphoid population and cytokine profiles
Spleens were collected at the time of sacrifice, disrupted mechanically, and passed through a metal mesh to prepare single cell suspensions. After lysing the erythrocytes in hypotonic solution (ammonium chloride potassium buffer, Biosource), splenocytes were washed in phosphate buffered saline containing 1% fetal bovine serum and 0.05% sodium azide and incubated for 10 min at 4°C with Fc block [a cocktail of monoclonal antibodies directed against mouse Fcγ receptor II (CD32) and III (CD16)]. Cells were then incubated for 20 min with panels of fluorochrome-labeled antibodies against the following lymphoid markers: CD3-CyChrome, CD4-FITC, CD8-FITC, CD69-PE, CD25-PE, B220-PE, and DX5-PE (DX5 is a monoclonal antibody that recognizes murine CD49b, an alpha-2 integrin expressed by natural killer cells during maturation). Stained cells were collected on a FACScan cytometer (Becton-Dickinson, Immunocytometry Systems) and analyzed using CellQuest software.
Splenocytes from individual mice were also added to 24-well tissue culture plates at a density of 5 × 106 cells/mL in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen), supplemented with 1% l-glutamine, 100 U/mL of each penicillin and streptomycin, 1% nonessential amino acids, 1% sodium pyruvate and 10% fetal bovine serum. Following a 48-hour incubation without stimulants, culture supernatants were collected to measure interleukin (IL)-2, IL-4, IL-10, IL-12, IL-13, tumor necrosis factor alpha, and interferon (IFN)-γ by Quantikine murine cytokine ELISA kits (R&D Systems).
Isolation of splenic dendritic cells
Splenic mononuclear cells were re-suspended in phosphate-buffered saline containing 2 mM EDTA and incubated for 20 minutes at 4°C with an antibody against dendritic cells CD11c (clone N418) coupled to magnetic microbeads (Miltenyi Biotech). Cells were then washed and isolated by positive selection using the magnetic MACS separator (Miltenyi Biotech). After allowing unbound CD11c-negative cells to pass through, the column was unloaded from the magnetic field and cells and flushed with 3 mL of phosphate-buffered saline, 2 mM EDTA, 0.5% bovine serum albumin. Cells positive for CD11c were then cultured for 48 hours in RPMI medium containing recombinant mouse IFN-γ (200 U/mL) or anti-IFN-γ (both from BD PharMingen) to assess the expression of indoleamine 2, 3-dioxygenase (IDO) by immunohistochemistry.
Isolation of peritoneal macrophages
At week 11, two days after the first injection of the CTLA-4 antibody or controls, cells from peritoneal lavage were cultured for 3 hours in complete RPMI-1640 medium (Invitrogen) in multiwell slides (Nalge Nunc) and allowed to adhere. Nonadherent cells were then removed by flushing with excess medium and adherent macrophages cultured in RPMI-1640 supplemented with 1% l-glutamine, 100 U/mL of each penicillin and streptomycin, 1% nonessential amino acids, 1% sodium pyruvate, and 10% fetal bovine serum. To assess IDO expression, macrophages were cultured with IFN-γ, as described above for splenic dendritic cells.
Immunohistochemistry to detect IDO expression in mouse thyroid glands, mouse cultured dendritic cells and macrophages, and human thyroid glands
Four-micron sections were cut from the formalin-fixed, paraffin-embedded thyroid blocks, and deparaffinized using standard protocols. Slides containing the thyroid sections or cultured cells were first digested with proteinase K for eight minutes and then incubated in 3% hydrogen peroxide to block endogenous peroxidase. After attenuation of nonspecific binding with the Dual block enzyme system (Dako), mouse sections were incubated with a rabbit polyclonal antibody directed against IDO (Chemicon International Inc.). A biotinylated secondary antibody directed against rabbit immunoglobulin G (IgG) was then used, along with streptavidin conjugated to alkaline phosphatase (LSAB®2 Dako code numbers K1017 and K1018 respectively). Antibody binding was visualized by Fast Red chromogen (K0597 Dako). Human thyroid sections were similarly analyzed on an autostainer (Bond III, Leica Microsystems) using a mouse monoclonal antibody (clone 10.1 EMD Millipore). Staining was developed using the Bond™ Polymer Refine Red Detection system (DS9390, Leica Microsystems), as per manufacturer's instructions. Briefly, after incubation with the mouse primary antibody, a rabbit anti-mouse IgG linker was added to localize the mouse antibodies, followed by a polymeric alkaline phosphatase conjugate anti-rabbit IgG to localize the rabbit antibodies. The substrate chromogen Fast Red was then added to visualize the antigen–antibody complexes with a red precipitate, followed by Mayer's hematoxylin counterstain to visualize (in blue) the cell nuclei.
Immunohistochemistry to detect CD68, a macrophage marker, in human thyroid glands
Human thyroid sections were also stained for CD68 using a mouse monoclonal antibody (clone KP-1, Ventana Medical Systems Inc.) on the Ultra Benchmark autostainer (Ventana Medical Systems Inc.). Following the heat-induced epitope retrieval, deparaffinized and rehydrated sections were incubated (eight minutes at room temperature) with the primary antibody, and the binding then visualized using the iVIEW DAB detection kit (Ventana-Roche Medical Systems) per manufacturer's instructions.
Statistical analyses
Median thyroiditis severity and thyroglobulin levels among the three experimental groups were compared by Mann–Whitney U-test. Mean cytokine levels in splenic supernatants and splenic lymphoid populations were compared by one-way analysis of variance; p values less than 0.05 were considered significant. Analyses were performed using Stata (software release 14, from Stata Corporation).
Results
CTLA-4 blockade increases thyroiditis incidence and severity in the NOD-H2h4 strain
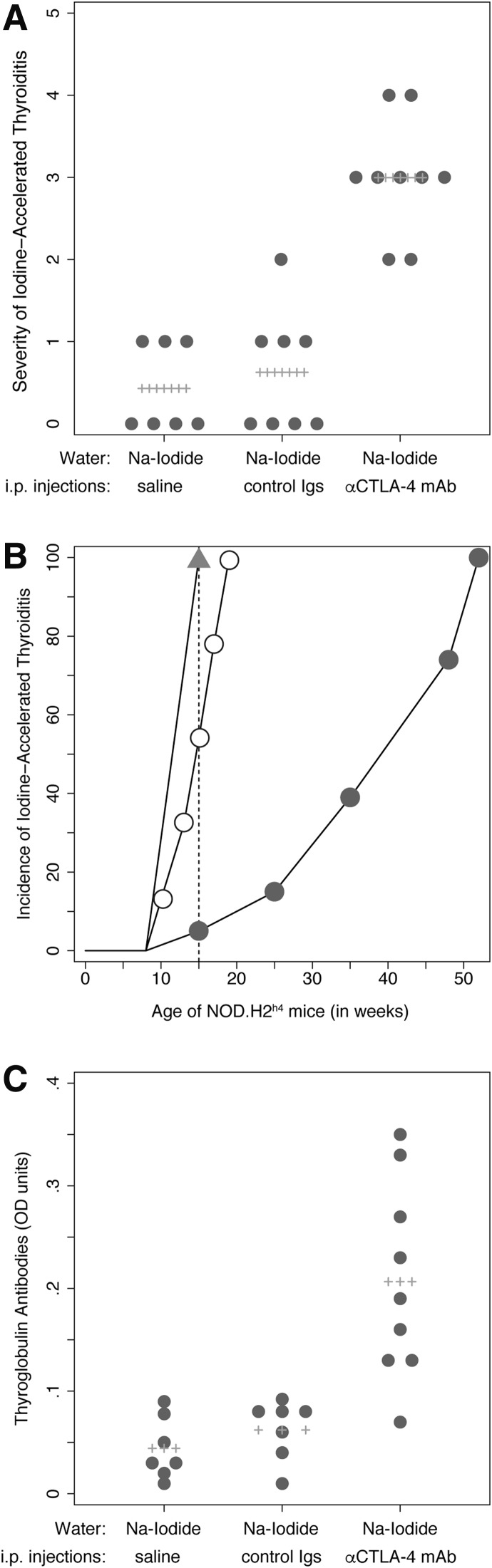
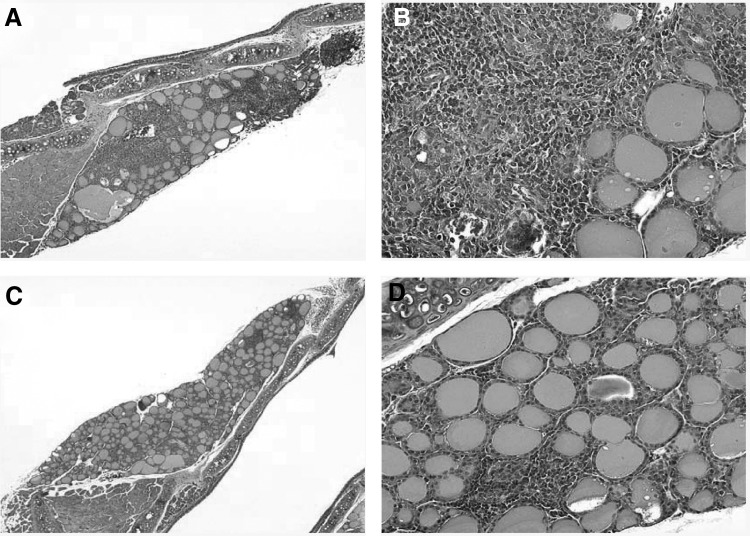
The severity of thyroiditis was significantly greater in mice injected with the CTLA-4 blocking antibody than in those injected with the control antibody or saline solution (Fig. 1A). The mean thyroiditis score was 3 in the CTLA-4 blockade group versus 0.62 in the control antibody (p = 0.0006) or 0.43 in the saline (p = 0.0006; Fig. 1) groups. There was no difference in thyroiditis severity between the iodine-fed mice injected with the control hamster immunoglobulin and those injected with saline (p = 0.648, Fig. 1). The mononuclear cell infiltrate involved large areas of the gland, effacing the normal follicular architecture in the CTLA-4 group, (Fig. 2A, B), whereas it was more focal and minimally destructive in the two control groups (Fig. 2C, D).
FIG. 1.
Iodine-accelerated thyroiditis: scoring of thyroid mononuclear infiltration and thyroglobulin antibodies. (A) Severity of thyroiditis: NOD-H2h4 mice treated with the cytotoxic T lymphocyte antigen-4 (CTLA-4) blocking antibody and drinking sodium iodide (NaI)–supplemented water developed a more severe autoimmune thyroiditis than controls. (B) Incidence of thyroiditis: At 15 weeks of age, all mice treated with the CTLA-4 blocking antibody and drinking NaI-supplemented water (triangles) developed thyroiditis, as opposed to 50% of the mice drinking only NaI-supplemented water (open circles) or 5% of those drinking regular water (closed circles). (C) Thyroglobulin antibodies: Levels were significantly higher in the CTLA-4 group than in controls. Igs, immunoglobulins; i.p., intraperitoneal.
FIG. 2.
Histopathological appearance of the thyroid gland in the in NOD-H2h4 mouse model. CTLA-4 blockade induced a more severe form of autoimmune thyroiditis (A, B) than the administration of control immunoglobulins (C, D). Panels A and C are at 40 × magnification, and panels B and D are 64 × .
In addition to severity, CTLA-4 blockade also increased the incidence of iodine-accelerated thyroiditis. In our historic cohort of NOD-H2h4, thyroiditis begins to appear at about 15 weeks of age and becomes 100% penetrant at about 52 weeks of age without iodine supplementation (Fig. 1B, closed circles). When iodine is added to the drinking water starting at 8 weeks of age, thyroiditis develops at week 10 and becomes fully penetrant at week 19 (Fig. 1B, open circles). In the present cohort, all mice receiving the CTLA-4 blocking antibody developed thyroiditis at 15 weeks of age (Fig. 1B, closed triangles). Therefore, at 15 weeks of age, the incidence of thyroiditis is about 5% in the non-iodine-fed mice, 50% in the iodine-fed mice, and 100% in the iodine-fed, CTLA-4 antibody treated mice (Fig. 1B, dotted line; p < 0.0001).
Thyroglobulin antibodies followed a pattern similar to that described for the thyroidal infiltrate: they were significantly more prevalent and at higher levels in mice treated with the CTLA-4 blocking antibody than in controls (Fig. 1C). Overall these results indicate that inhibition of the CLTA-4 inhibitory pathway synergizes with iodine to induce a more aggressive, early-onset form of thyroiditis.
CTLA-4 blockade expands the number of splenic CD4 effector T cells and the production of splenic cytokines
To assess the immunological effects of blocking the inhibitory CTLA-4 pathway in the NOD-H2h4 model of thyroiditis, we examined the distribution of the major lymphoid populations in the spleen and their cytokine production capacity. CTLA-4 blockade treatment given in an iodine-rich environment significantly increased the number of CD4+ T cells, despite the total spleen cellularity remaining the same (Table 1). The increase specifically involved the T effector population, as assessed by the expression of the activation markers CD69 (Table 1) and CD25 (Table 1; Fig. 3A, B) and the broad (i.e., not T-cell subset restricted) cytokine signature (Fig. 3C, D), and it was also consistent with previous reports in animals (24) and humans (25).
Table 1.
Percentage of Splenic Lymphoid Populations in the Three Treatment Groups of NOD-H2h4 Mice, According to the Major Lymphoid Subpopulations
| Phosphate buffered saline (group C) | Control hamster immunoglobulins (group B) | Hamster anti-mouse CTLA-4 (group A) | p-Value comparing group A vs. B | |
|---|---|---|---|---|
| Total splenocytes | 5.34 ± 0.75 | 4.45 ± 0.73 | 6.01 ± 1.04 | 0.1675 |
| CD3+ T cells | 42.68 ± 5.81 | 43.45 ± 4.99 | 47.44 ± 3.36 | 0.082 |
| CD4+ T cells | 23.16 ± 4.31 | 24.4 ± 1.23 | 27.6 ± 1.15 | <0.001 |
| CD8+ T cells | 19.48 ± 2.65 | 19.1 ± 2.43 | 19.9 ± 1.77 | 0.464 |
| CD4/CD8 ratio | 1.20 ± 0.24 | 1.29 ± 0.11 | 1.3 ± 0.17 | 0.890 |
| CD4+CD69+ T cells | 2.37 ± 0.16 | 2.62 ± 0.20 | 3.24 ± 0.22 | <0.001 |
| CD4+CD25+ T cells | 2.78 ± 0.50 | 3.55 ± 0.33 | 5.16 ± 0.46 | <0.001 |
| DX5+ NK cells | 0.91 ± 0.20 | 1.19 ± 0.09 | 0.95 ± 0.09 | 0.001 |
| B220+ B cells | 57.2 ± 2.68 | 59.5 ± 3.11 | 55.8 ± 4 | 0.058 |
For total spleen cells, numbers are expressed as absolute values.
FIG. 3.
Expansion of splenic effector T cells and increased cytokine production after CTLA-4 blockade in the in NOD-H2h4 mouse model. CTLA-4 blockade induced a two-fold increase in the number of splenic effector T cells (CD4/CD25 double positives) as compared with the administration of a control antibody or saline solution. A representative mouse for the control and CTLA-4 groups is shown in panels A and B, respectively. CTLA-4 blockade induced a significant increase of splenic interleukin (IL)-2, interferon gamma, and tumor necrosis factor alpha cytokines (C), as well as IL-13 and IL-10 (D).
There was also a significant decrease in the number of DX5+ natural killer cells (Table 1), in keeping with what has been previously reported in cancer patients treated with a CTLA-4 blocking antibody (26). The numbers of CD8+ T cells and B220+ B cells, however, were not affected by CTLA-4 blockade (Table 1).
Splenocytes produced significantly greater levels of pro-inflammatory T helper 1 cytokines such as IL-2, IFNγ, and tumor necrosis factor alpha (Fig. 3C), as well anti-inflammatory cytokines such as IL-10 and IL-13 (Fig. 3D) in mice exposed in vivo to CTLA-4 blockade than in controls. Overall, these findings are in keeping with the notion that CTLA-4 blockade induces a strong stimulation of effector T cells (24,25), thus potentiating the thyroiditis-inducing effect of iodine. They also suggest that this pro-inflammatory state induces a compensatory increase in counter-regulatory mechanisms, such as the production of IL-10.
The exacerbation of iodine-accelerated thyroiditis caused by CTLA-4 blockade is associated with increased IDO expression by thyroidal and peripheral antigen-presenting cells
To gain insights into the mechanisms through which CTLA-4 blockade exacerbates thyroiditis in NOD-H2h4 mice exposed to iodine, we analyzed by immunohistochemistry the presence of IDO in mouse thyroid glands collected at 15 weeks of age. IDO was expressed above baseline in thyroid glands of thyroiditis-prone mice treated with the control antibody or saline (Fig. 4A), but more strongly so in the CTLA-4 treated group (Fig. 4B, arrows). In all groups, IDO expression was found in the cytoplasm of mononuclear cells infiltrating the interstitial space in-between the thyroid follicles (more clearly seen in Fig. 4A). Similar findings were observed in human specimens. IDO was found in scattered interstitial macrophages in Hashimoto thyroiditis controls (Fig. 4C), and in interstitial, intracolloidal, and intrametastatic CD68-positive macrophages in the autopsy thyroids from CTLA-4 blocked melanoma cases (Fig. 4D, Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy).
FIG. 4.
Indoleamine 2, 3-dioxygenase (IDO) expression in murine and human thyroid glands, as well as in cultured peritoneal macrophages and splenic dendritic cells. Thyroidal IDO expression, detectable above normal levels in thyroiditis-prone NOD-H2h4 mice (A; 64 × magnification), was increased even further after CTLA-4 blockade (B, black arrows; 64 × ). Similarly, thyroidal IDO expression, detectable in patients affected by Hashimoto thyroiditis (C, black arrows; 100 × ) was seen more strongly in cancer patients who had been treated with a CTLA-4 blocking antibody (D; 100 × ). IDO expression was mainly found in the cytoplasm of infiltrating macrophages (C, inset, black arrow; 160 × ) as well as in the nests of melanoma cells that metastasize to the thyroid gland (D, inset, black arrow indicates the macrophages and blue arrow the melanoma cells; 100 × ). Cultured mouse peritoneal Mac1+ macrophages (E) and splenic CD11c+ dendritic cells (F) strongly expressed IDO upon stimulation with interferon gamma.
To determine whether the CTLA-4 effect on IDO was mediated by IFN-γ, we isolated peritoneal macrophages and splenic dendritic cells from mice that had been supplemented with iodine for 21 days and then injected intraperitoneally with the CTLA-4 blocking or control antibodies two days before the collection. Following a one-day stimulation with mouse recombinant IFN-γ, macrophages (Fig. 4E) and dendritic cells (Fig. 4F) displayed strong cytosolic expression of IDO, which was instead not present in cells derived from the control antibody group (data not shown). Co-incubation with an antibody directed against IFN-γ completely blocked the increased expression of IDO.
Discussion
This study demonstrates that treatment with a CTLA-4 blocking antibody exacerbates disease in the NOD-H2h4 mouse model of iodine-accelerated thyroiditis. Thyroiditis, in fact, was more severe, more prevalent, and occurred earlier in mice receiving the CTLA-4 blocking antibody than in those injected with the control antibody or saline. The study also reveals that CTLA-4 blockade is associated with increased IDO expression, in both murine and human thyroid specimens.
IDO is the first and rate-limiting enzyme in the pathway that degrades the essential amino acid tryptophan to kynurenine (27). The function of IDO has been best characterized in tumor immunology. Here, IDO is expressed in the cytoplasm of tumor cells and antigen-presenting cells [such as dendritic cells, macrophages, and B cells (28)], where it degrades tryptophan, ultimately decreasing its concentration in the extracellular milieu. Lower tryptophan levels induce two main downstream effects: firstly, they attenuate the activity of effector and helper T cells, thus decreasing their ability to eliminate tumor cells; secondly, they activate Foxp3-lineage CD4+ regulatory T cells, which in turn further increase IDO. Thus, increased IDO expression in the tumor microenvironment lead to an immunosuppressive phenotype that impairs the antitumor capacity of effector T cells (29). This lymphosuppressive function of IDO has also been reported in patients with papillary thyroid cancer (30,31), a neoplasia long known to be associated with a prominent lymphocytic infiltration (32). Several drugs are therefore being developed to decrease the levels of IDO (and thus increase the extracellular concentration of tryptophan) as a way to boost the activity of tumor-specific effector T cells (33).
IDO function in organs targeted by autoimmunity has been more difficult to interpret (34). Here, one would predict that increased IDO expression is beneficial to the host because it suppresses a key pathogenic cell type in autoimmunity: the antigen-specific effector T cell (35). Moreover, a beneficial effect has been shown after administration of a subset of myeloid cells that increase IDO synthesis, leading to expansion of regulatory T cells and correction of hyperglycemia in a mouse model of type 1 diabetes (36). Similarly, NOD-H2h4 mice developed an attenuated form of thyroiditis when injected with an adenovirus expressing IDO directly into the thyroid gland one day before and four weeks after the beginning of iodine supplementation in the drinking water (37). But contrasting results have been obtained in other diseases. For example, pharmacological inhibition of IDO with 1-methyl-tryptophan attenuates joint inflammation in the K/BxN mouse model of rheumatoid arthritis (38). In humans, IDO mRNA levels in peripheral blood mononuclear cells are significantly increased during the acute phase of relapsing–remitting multiple sclerosis (39). Similarly, sera from patients with active systemic lupus erythematosus showed increased IDO activity, as determined by a higher kynurenine/tryptophan liquid chromatography ratio (40), and in patients with active Crohn's disease, abundant IDO positive dendritic cells are found lining the ulcerative fissures that are scattered throughout the intestinal mucosa (41). By contrast, diminished numbers of IDO positive plasmacytoid dendritic cells have been reported in patients with autoimmune thyroid disease (42). Therefore, both decreased and increased IDO function has been associated with autoimmune diseases. In this study we found that IDO is expressed in thyroids of mice and patients treated with a CTLA-4 blocking antibody. We interpret this IDO increase as a counter-regulatory mechanism: in particular, the pro-inflammatory milieu induced by the CTLA-4 blockade would lead to an increased IDO expression to protect against an overwhelming inflammatory response. The different IDO levels reported in autoimmunity could also be attributed to different disease stage and severity. For example, in patients with long-standing Hashimoto thyroiditis, where these counter-regulatory mechanisms are likely exhausted, the expression of IDO is significantly diminished in plasmacytoid dendritic cells (42).
In summary, this study shows that administration of iodine in combination with CTLA-4 blockade induces an aggressive form of thyroiditis in the NOD-H2h4 mouse model. These findings have implications for cancer patients who develop thyroiditis as an adverse event after administration of CTLA-4 blocking antibodies.
Supplementary Material
Acknowledgments
The work was supported in part by National Institutes of Health grant R01 CA194042 to P.C. The authors are grateful to Drs. Noel Rose and Lynne Burek for their contributions and guidance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rose NR, Witebsky E. 1956. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol 76:417–421 [PubMed] [Google Scholar]
- 2.Jones HE, Roitt IM. 1961. Experimental auto-immune thyroiditis in the rat. Brit J Exp Pathol 42:546–557 [PMC free article] [PubMed] [Google Scholar]
- 3.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. 1993. I-E+ nonobese diabetic mice develop insulitis and diabetes. Exp Med 178:793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braley-Mullen H, Yu S. 2015. NOD.H-2h4 mice: an important and underutilized animal model of autoimmune thyroiditis and Sjogren's syndrome. Adv Immunol 126:1–43 [DOI] [PubMed] [Google Scholar]
- 5.Kolypetri P, King J, Larijani M, Carayanniotis G. 2015. Genes and environment as predisposing factors in autoimmunity: acceleration of spontaneous thyroiditis by dietary iodide in NOD.H2 mice. Int Rev Immunol 34:542–56 [DOI] [PubMed] [Google Scholar]
- 6.Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. 2010. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology 151:4583–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R. 2009. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med 9:51–59 [DOI] [PubMed] [Google Scholar]
- 8.Wicker LS, Todd JA, Peterson LB. 1995. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol 13:179–200 [DOI] [PubMed] [Google Scholar]
- 9.Rasooly L, Burek CL, Rose NR. 1996. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol 81:287–292 [DOI] [PubMed] [Google Scholar]
- 10.Braley-Mullen H, Sharp GC, Medling B, Tang H. 1999. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun 12:157–165 [DOI] [PubMed] [Google Scholar]
- 11.Sharma RB, Alegria JD, Talor MV, Rose NR, Caturegli P, Burek CL. 2005. Iodine and IFN-gamma synergistically enhance intercellular adhesion molecule 1 expression on NOD.H2h4 mouse thyrocytes. J Immunol 174:7740–7745 [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Bi M, Yang R, Zhou J, Zhao S, Fan C, Shan Z, Li Y, Teng W. 2014. Defective expression of regulatory B cells in iodine-induced autoimmune thyroiditis in non-obese diabetic H-2(h4) mice. J Endocrinol Invest 37:43–50 [DOI] [PubMed] [Google Scholar]
- 13.Xue H, Wang W, Shan Z, Li Y, Li Y, Teng X, Gao Y, Fan C, Teng W. 2011. Dynamic changes of CD4+CD25 + regulatory T cells in NOD.H-2h4 mice with iodine-induced autoimmune thyroiditis. Biol Trace Elem Res 143:292–301 [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Gao T, Shi R, Zhou X, Qu J, Xu J, Shan Z, Teng W. 2014. Effect of iodine excess on Th1, Th2, Th17, and Treg cell subpopulations in the thyroid of NOD.H-2h4 mice. Biol Trace Elem Res 159:288–296 [DOI] [PubMed] [Google Scholar]
- 15.Ellis JS, Hong SH, Zaghouani H, Braley-Mullen H. 2013. Reduced effectiveness of CD4+Foxp3+ regulatory T cells in CD28-deficient NOD.H-2h4 mice leads to increased severity of spontaneous autoimmune thyroiditis. J Immunol 191:4940–4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. 2007. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun 29:195–202 [DOI] [PubMed] [Google Scholar]
- 17.Bour-Jordan H, Bluestone JA. 2009. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev 229:41–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couzin-Frankel J. 2013. Breakthrough of the year 2013. Cancer immunotherapy. Science 342:1432–1433 [DOI] [PubMed] [Google Scholar]
- 19.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, Garbe C, Gutzmer R, Grabbe S, Hauschild A, Hein R, Hundorfean G, Justich A, Keller U, Klein C, Mateus C, Mohr P, Paetzold S, Satzger I, Schadendorf D, Schlaeppi M, Schuler G, Schuler-Thurner B, Trefzer U, Ulrich J, Vaubel J, von Moos R, Weder P, Wilhelm T, Goppner D, Dummer R, Heinzerling LM. 2013. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PloS One 8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. 2013. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98:1361–1375 [DOI] [PubMed] [Google Scholar]
- 21.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. 2014. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 21:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura H, Kimura M, Tzou SC, Chen YC, Suzuki K, Rose NR, Caturegli P. 2005. Expression of class II major histocompatibility complex molecules on thyrocytes does not cause spontaneous thyroiditis but mildly increases its severity after immunization. Endocrinology 146:1154–1162 [DOI] [PubMed] [Google Scholar]
- 23.Caturegli P, Rose NR, Kimura M, Kimura H, Tzou SC. 2003. Studies on murine thyroiditis: new insights from organ flow cytometry. Thyroid 13:419–426 [DOI] [PubMed] [Google Scholar]
- 24.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. 2013. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210:1389–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, Old LJ, Allison JP, Jungbluth A, Wolchok JD. 2011. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother 60:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohlhapp FJ, Broucek JR, Hughes T, Huelsmann EJ, Lusciks J, Zayas JP, Dolubizno H, Fleetwood VA, Grin A, Hill GE, Poshepny JL, Nabatiyan A, Ruby CE, Snook JD, Rudra JS, Schenkel JM, Masopust D, Zloza A, Kaufman HL. 2015. NK cells and CD8+ T cells cooperate to improve therapeutic responses in melanoma treated with interleukin-2 (IL-2) and CTLA-4 blockade. J Immunother Cancer 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. 2015. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines 3:703–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nouel A, Pochard P, Simon Q, Segalen I, Le Meur Y, Pers JO, Hillion S. 2015. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. J Autoimmun 59:53–60 [DOI] [PubMed] [Google Scholar]
- 29.Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. 2014. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 5:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti S, Menicali E, Voce P, Morelli S, Cantarelli S, Sponziello M, Colella R, Fallarino F, Orabona C, Alunno A, de Biase D, Bini V, Mameli MG, Filetti S, Gerli R, Macchiarulo A, Melillo RM, Tallini G, Santoro M, Puccetti P, Avenia N, Puxeddu E. 2014. Indoleamine 2,3-dioxygenase 1 (IDO1) is up-regulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J Clin Endocrinol Metab 99:E832–840 [DOI] [PubMed] [Google Scholar]
- 31.Ryu HS, Park YS, Park HJ, Chung YR, Yom CK, Ahn SH, Park YJ, Park SH, Park SY. 2014. Expression of indoleamine 2,3-dioxygenase and infiltration of FOXP3+ regulatory T cells are associated with aggressive features of papillary thyroid microcarcinoma. Thyroid 24:1232–1240 [DOI] [PubMed] [Google Scholar]
- 32.Dailey ME, Lindsay S, Skahen R. 1955. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 70:291–297 [DOI] [PubMed] [Google Scholar]
- 33.Sheridan C. 2015. IDO inhibitors move center stage in immuno-oncology. Nature Biotechnol 33:321–322 [DOI] [PubMed] [Google Scholar]
- 34.Opitz CA, Wick W, Steinman L, Platten M. 2007. Tryptophan degradation in autoimmune diseases. Cellular Mol Life Sci 64:2542–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff L, Alvarez S, Dai DL, Soukhatcheva G, Orban PC, Verchere CB. 2015. Cellular mechanisms of CCL22-mediated attenuation of autoimmune diabetes. J Immunol 194:3054–3064 [DOI] [PubMed] [Google Scholar]
- 36.Zoso A, Mazza EM, Bicciato S, Mandruzzato S, Bronte V, Serafini P, Inverardi L. 2014. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur J Immunol 44:3307–3319 [DOI] [PubMed] [Google Scholar]
- 37.Nakahara M, Nagayama Y, Saitoh O, Sogawa R, Tone S, Abiru N. 2009. Expression of immunoregulatory molecules by thyrocytes protects nonobese diabetic-H2h4 mice from developing autoimmune thyroiditis. Endocrinology 150:1545–1551 [DOI] [PubMed] [Google Scholar]
- 38.Pigott E, DuHadaway JB, Muller AJ, Gilmour S, Prendergast GC, Mandik-Nayak L. 2014. 1-Methyl-tryptophan synergizes with methotrexate to alleviate arthritis in a mouse model of arthritis. Autoimmunity 47:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancuso R, Hernis A, Agostini S, Rovaris M, Caputo D, Fuchs D, Clerici M. 2015. Indoleamine 2,3 Dioxygenase (IDO) Expression and Activity in Relapsing-Remitting Multiple Sclerosis. PloS One 10:e0130715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lood C, Tyden H, Gullstrand B, Klint C, Wenglen C, Nielsen CT, Heegaard NH, Jonsen A, Kahn R, Bengtsson AA. 2015. Type I interferon-mediated skewing of the serotonin synthesis is associated with severe disease in systemic lupus erythematosus. PloS One 10:e0125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K, Rutgeerts P, Tilg H. 2004. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol 113:47–55 [DOI] [PubMed] [Google Scholar]
- 42.Leskela S, Rodriguez-Munoz A, de la Fuente H, Figueroa-Vega N, Bonay P, Martin P, Serrano A, Sanchez-Madrid F, Gonzalez-Amaro R, Marazuela M. 2013. Plasmacytoid dendritic cells in patients with autoimmune thyroid disease. JClin Endocrinol Metab 98:2822–2833 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.