Abstract
Background: Radiation is a well-described risk factor for differentiated thyroid carcinoma (DTC). Although the natural history of DTC following nuclear disasters and in healthcare workers with chronic radiation exposure (RE) has been described, little is known about DTC following short-term exposure to therapeutic medical radiation for benign disease. This study compares DTC morphology and outcomes in patients with and without a prior history of therapeutic external RE.
Methods: A retrospective review was performed of patients with DTC treated at The University of Chicago between 1951 and 1987, with a median follow-up of 27 years (range 0.3–60 years). Patients were classified as either having (RE+) or not having (RE–) a history of therapeutic RE. Variables examined included sex, age at RE, dose of RE, indication for RE, DTC histology, and outcome. Morphology was determined by blinded retrospective review of all available histologic slides. Outcomes were assessed using Cox proportional hazards model and Kaplan–Meier curves.
Results: Of 257 DTC patients, 165 (64%) were RE– and 92 (36%) were RE+, with males comprising a greater proportion of the RE+ group (43.5% vs. 27.3%; p = 0.01). A total of 94.2% of DTC cases were classic papillary cancers; histology did not differ between RE+ and RE– cohorts (p = 0.73). RE was associated with an increased median overall survival (OS; 43 years vs. 38 years; hazard ratio [HR] = 0.55 [confidence interval (CI) 0.34–0.89]; p = 0.01). Survival for males in the RE– group was significantly worse than it was for RE– females (HR = 1.78 [CI 1.05–3.03]; p = 0.03) or RE+ males (HR = 2.98 [CI 1.39–6.38]; p = 0.01). Recurrence did not differ between the RE+ and RE– groups (HR = 0.85 [CI 0.52–1.41]; p = 0.54), nor did DTC-specific mortality (HR = 0.54 [CI 0.21–1.37]; p = 0.20).
Conclusions: While DTC following RE has historically been considered a more aggressive variant than DTC in the absence of RE, the present data indicate that RE+ DTC is associated with better OS than RE– DTC, especially for males. Additionally, recent reports are confirmed of equivalent rates of thyroid cancer recurrence. These results warrant further investigation into the factors underlying this unexpected finding.
Introduction
Considerable data have demonstrated a causal link between radiation exposure (RE) and the subsequent development of a number of malignancies, including differentiated thyroid carcinoma (DTC) and, in particular, the papillary DTC subtype (1). Since initial observations associating RE with DTC, there have been numerous studies investigating the natural history of post-radiation DTC, most of which have involved cohorts of individuals exposed to radiation either following disasters such as Chernobyl or in the workplace such as with medical X-ray technologists. Results from the Chernobyl cohort suggest rates of recurrence do not differ between individuals with a history of RE and the general population. This has recently led many to consider DTC in patients with a history of RE (RE+) to be clinically equivalent to DTC in patients without a history of RE (RE–) (2,3).
In recent years, the incidence of DTC has skyrocketed, from 4.9:100,000 annually in 1975 to 14.3:100,000 annually in 2014 (4). It has been proposed that this dramatic increase is due in part to the vastly expanded role of radiation in medical care (5). Medical therapeutic radiation most often associated with thyroid cancer is often referred to as “external radiation.” While indications for external radiation have changed over time, rates of utilization continue to increase, with radiation therapy currently utilized by >25% of Medicare enrollees (6,7). Despite the ubiquitous role of radiation in modern medical care, few studies have described the natural history of DTC following external radiation exposure as opposed to DTC in the absence of external radiation exposure.
The pattern of radiation exposure of individuals treated with external radiation is markedly different from that of individuals exposed to radiation either following disasters or in the workplace. In the treatment of benign disease, external radiation exposure typically occurs as multiple seconds-long bursts over a short period of time, and involves doses of <30 Gy. Treatment of malignant diseases typically involves higher doses (>30 Gy) and is less associated with the development of DTC than is external radiation used for benign diseases (8). In contrast, the pattern of RE following disasters such as Chernobyl occurs in two phases. The initial phase is exposure to a high-dose radiation burst of varying length and dose. The second phase is a long-term exposure to radiation secondary to contamination of drinking water and soil and subsequent food-chain contamination (9,10). The pattern of RE in the medical workplace occurs as hundreds of incidental low-dose seconds-long bursts that are not directed to a specific tissue taking place over the course of years or even decades (11).
Because these patterns of RE are so distinct, it was hypothesized that DTC following therapeutic medical radiation may be biologically and clinically distinct from DTC following radiation disasters or in the medical technologist cohorts. To test this, outcomes were characterized and measured in a unique cohort of 257 patients with DTC, all followed at a single institution for a median of 27 years, of whom 92 had a history of antecedent RE for medical purposes, and 165 who had a history of no antecedent medical RE. Of note, one particular strength of this cohort was that the presence or absence of RE had been defined for each individual, as had the dose and indication for RE for most individuals. Additionally, the median length of follow-up for this cohort was almost 30 years, thereby allowing the natural history of RE+ DTC to be compared to that of RE–DTC in patients followed regularly over the course of decades at a single institution. This is especially significant because DTC patients rarely die of their disease and are frequently young at the time of diagnosis. Consequently, it may be decades before the long-term sequelae of DTC emerge.
Materials and Methods
Patients
After obtaining approval from The University of Chicago Institutional Review Board, a retrospective chart review was performed of medical records of 269 patients with an initial diagnosis of DTC treated at the authors' institution between 1951 and 1987. These patients and their outcomes are summarized in detail by Grogan et al. (12). Patients were classified as either having or not having a history of antecedent exposure to therapeutic medical radiation (RE+ vs. RE–). No patients were included that were exposed solely to diagnostic radiation. All radiation doses recorded and analyzed reflect treatment rather than thyroid specific doses. Of the 269 subjects, eight patients were exposed to high-dose radiation as treatment of a malignant condition. Because these patients were treated with a higher dose of external radiation and any subsequent analysis would be confounded by their primary malignancy and high radiation dose, these patients were excluded from the primary analysis. Demographic data collected included age at diagnosis, sex, race/ethnicity, indication for medical radiation, age at time of radiation exposure, initial histology, history of thyroid disease, family history of thyroid disease, and family or personal history of other cancer diagnoses. Race/ethnicity was not reliably reported for all patients, and was thus not included in the analysis. Follow-up data were obtained through continuing direct patient care, through direct patient contact via systematic telephonic follow-up, or through the University of Chicago Cancer Research Registry (linked to the National Death Index) (12). Thus, based on patient characteristics, there were 94 RE+ cases and 167 RE– cases available for analysis.
Histology
After exclusion of the eight high-dose RE patients, blinded independent histologic review of all available pathology slides and samples from RE+ and RE– patients was conducted by a single pathologist with expertise in thyroid disease (N.C.). Hematoxylin and eosin slides were recut from paraffin blocks when necessary. In total, blocks and/or histologic slides from 104/261 patients were analyzed and assigned a diagnosis using the World Health Organization (WHO) criteria for diagnosis of thyroid tumors (13). This was done to confirm the presence of a malignancy for all possible cases and to study any differences in histology between the RE+ and RE– cohorts. Three instances in which specimens did not meet contemporary pathologic criteria for a thyroid malignancy were excluded (two from the RE+ group and one from the RE– group). A single case of a RE– thyroid cancer reclassified as poorly differentiated thyroid cancer was also excluded. Thus, after histologic review, 92 RE+ cases and 165 RE– cases were available for analysis.
Statistical analysis
Chi-square tests and analysis of variance were performed to compare demographic, clinical, and histologic data between RE+ and RE– cohorts. Overall and DTC-specific mortality and recurrence rates were assessed by Kaplan–Meier survival curves separately for RE+ and RE– DTC patients. Patients for whom RE was documented but not specified were confirmed not to have a history of prior malignancy or other indication for high-dose RE, and were included in the RE+ group for survival and recurrence analyses. They were excluded from analyses of the effect of dose on DTC outcomes.
Recurrence events were defined as any local, nodal, or distant recurrence occurring six months or more after the patient's initial surgery. Cox proportional hazards model was performed to examine the effects of RE on overall survival (OS), as well as DTC-specific mortality and recurrence as primary outcomes. Age at diagnosis was controlled for in all analyses of survival. Differences in OS, DTC-specific mortality, and recurrence were then examined in subgroups of histologically confirmed classic papillary thyroid carcinoma (PTC), and those with Stage I or II disease, in order to confirm these findings were not due to heterogeneity between the RE+ and RE– groups. Finally, multivariate logistic regression was performed to investigate survival and time to recurrence in patients with DTC using the following variables: radiation exposure, indication for radiation exposure, age at time of radiation exposure, multifocality, extrathyroidal extension, age at diagnosis, site of primary treatment, and extent of surgical resection. The assumption of proportional hazards was tested for all models, and competing risks were studied. All statistical analyses were performed using STATA v13 (College Station, TX) (14).
Results
Clinical characteristics
In total, 257 patients were included in the final study cohort. Of these, 92 were RE+ and 165 were RE–. Cohort demographics, histology, age at diagnosis, and stage at diagnosis by RE+ and RE– cohort are summarized in Table 1. Excluded high-dose patients are described in Supplementary Table S1. The only statistically significant difference between the RE+ and RE– groups was the percentage of male patients: 43.5% in the RE+ group versus 27.7% in the RE– group (p = 0.01).
Table 1.
Demographic and Tumor Characteristics of University of Chicago Thyroid Cancer Cohort (n = 257)
| Radiation exposure (n = 92) | No radiation exposure (n = 165) | p | |
|---|---|---|---|
| Age at first thyroid surgery, years | 34.4 ± 13.6 | 36.7 ± 16.3 | 0.25 |
| Sex | 0.01 | ||
| Female | 52 (56.5%) | 120 (72.7%) | |
| Male | 40 (43.5%) | 45 (27.3%) | |
| Treatment | 0.88 | ||
| Total or near total | 60 (65.2%) | 106 (63.9%) | |
| Subtotal or lobectomy | 32 (34.8%) | 59 (36.1%) | |
| Radioactive iodine | 0.74 | ||
| Received | 46 (50%) | 86 (52.1%) | |
| Did not receive | 46 (50%) | 79 (47.9%) | |
| Histology (n = 104) | 0.83 | ||
| Classic papillary | 40 (93.1%) | 56 (91.8%) | |
| Follicular variant | 2 (4.6%) | 3 (4.8%) | |
| Columnar | 0 (0%) | 1 (1.6%) | |
| Follicular | 1 (2.3%) | 1 (1.6%) | |
| Multifocality | 0.89 | ||
| Unifocal | 51 (55.4%) | 93 (56.4%) | |
| Multifocal | 41 (44.6%) | 72 (43.6%) | |
| Lymph node involvement | 0.72 | ||
| 0 | 56 (60.9%) | 99 (60.0%) | |
| 1–5 | 20 (21.7%) | 42 (25.5%) | |
| >5 | 16 (17.4%) | 24 (14.5%) | |
| Extrathyroidal invasion | 36 (39.1%) | 56 (33.7%) | 0.39 |
| Stage | 0.10 | ||
| 1 | 83 (90.2%) | 127 (77.0%) | |
| 2 | 2 (2.2%) | 8 (4.8%) | |
| 3 | 1 (1.1%) | 7 (4.2%) | |
| 4a | 4 (4.3%) | 13 (7.9%) | |
| 4b | 2 (2.2%) | 10 (6.1%) | |
| Decade of diagnosis | 0.15 | ||
| Before 1960 | 10 (6.5%) | 12 (13.5%) | |
| 1960–1969 | 12 (7.8%) | 10 (11.2%) | |
| 1970–1979 | 74 (48.1%) | 42 (47.2%) | |
| After 1980 | 58 (37.7%) | 25 (28.1%) | |
| Primary treatment site | 0.12 | ||
| University of Chicago | 60 (65.2%) | 91 (55.2%) | |
| Other | 74 (44.9%) | 32 (34.8%) |
Four patients in the RE+ group (4.3%) and three patients (1.8%) in the RE– group had a history of hyperthyroidism (p = 0.18). A single patient (1.1%) in the RE+ group and none in the RE– group had a history of untreated hypothyroidism (p = 0.18). One patient (1.1%) in the RE+ group and two patients (1.2%) in the RE– group had a first-degree family member with thyroid cancer (p = 0.92). Five patients in the RE+ group (5.2%) and six in the RE– group (3.1%) had a first-degree relative with a documented malignancy (p = 0.39). None of the patterns of documented malignancies was suggestive of a known familial cancer syndrome such as Cowden syndrome, and these patients did not differ in any clinical characteristic from other members of the RE+ or RE– groups.
Within the RE+ group, sites of medical radiation varied and reflect the differing non-malignant indications for radiation therapy. Of the 92 RE+ patients, all had RE to the head and neck for the following indications: 25 received radiation to their tonsils (24.5%); 15 received radiation to their face for acne (14.7%); 12 received thymic radiation (11.8%); two received both tonsilar and thymic radiation in separate treatments (2.0%); one received tonsilar radiation and radiation to their face for acne in separate treatments (1.0%); and 40 did not have the indication for radiation reported (39.2%). Overall, the median age of RE was 9.6 ± 11.1 years. Only two patients received radiation exposure at age >21 years. When stratified by indication, the mean age of RE was 1.5 ± 1.6 years for thymic radiation; 4.1 ± 2.7 years for tonsilar radiation; 18.2 ± 4.6 years for acne radiation; and 16.7 ± 17.9 years for uncategorized radiation. The radiation dose to which each patient was exposed was not always recorded, but was assumed to be the standard dose for each indication. External dose estimates for the treated area are: 2 Gy for thymic RE, 5 Gy for tonsilar RE, and 15 Gy for facial acne (8). The mean interval between RE and diagnosis of DTC was 24.3 ± 12.6 years. This interval between RE and time to DTC diagnosis did not vary by indication for radiation (p = 0.45). Interestingly, it also did not vary by age at RE (p = 0.12), suggesting that medical radiation-induced DTC carcinogenesis may occur over a fixed time period.
RE and DTC histology
Because there have been significant changes over time in the histological classification of DTC, a pathologic review was performed using WHO classification of all cases with tissue available (n = 106; 42 [39.6%] RE+ and 64 [60.4%] RE–). The clinical characteristics of this subset of patients did not differ significantly from those of the overall RE+ and RE– groups (Table 1). By WHO classification, the vast majority of patients were found to have classic PTC: 93.1% in the RE+ group and 91.8% in the RE– group. This is in contrast to the original pathologic diagnosis of follicular variant PTC in 52.7% of the RE+ group and 57.8% of the RE– group (12). Using the revised classification, no difference in histology were observed between the RE+ and RE– groups (p = 0.72; Table 1).
Extrathyroidal extension was noted in the initial pathology reports for 36 (39.1%) of the RE+ patients and 56 (33.7%) of the RE– patients (p = 0.39). Multifocality was observed in 72 (43.6%) of the RE– and 41 (44.6%) of the RE+ patients (p = 0.89). As expected, multifocality correlated with extent of resection and did not differ between the RE+ and RE– groups. Total thyroidectomy and near total thyroidectomy specimens showed multifocality more often than subtotal thyroidectomy and lobectomy specimens, irrespective of the presence or absence of RE (odds ratio = 1.94; p = 0.01).
RE, recurrence, and survival after DTC
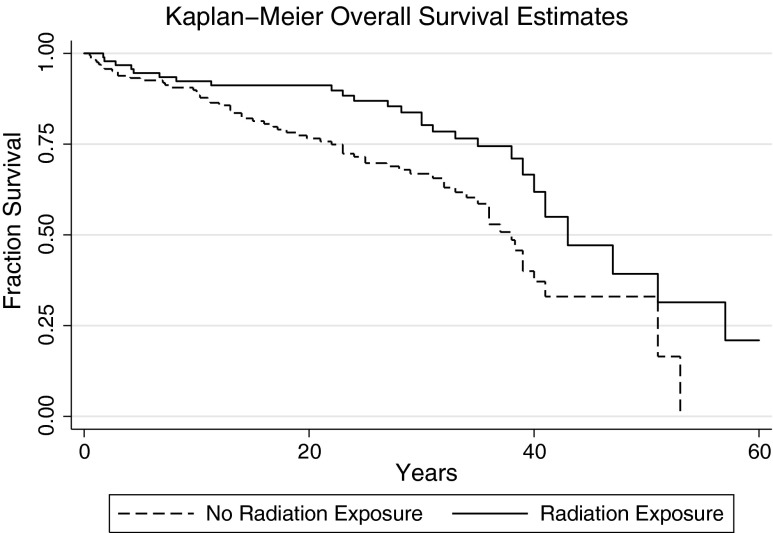
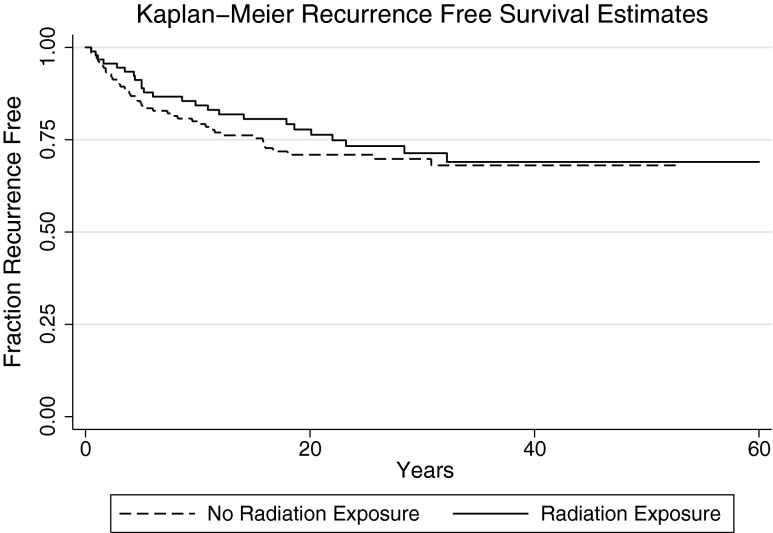
To determine whether outcomes following RE+ DTC differed from RE– DTC, OS, recurrence, and DTC-specific mortality were contrasted between these groups. Unexpectedly, a significant increase in OS was observed after controlling for age at diagnosis in the RE+ group compared with the RE– group (median OS: 43 years vs. 38 years; Cox hazard ratio [HR] = 0.55 [confidence interval (CI) 0.34–0.89]; p = 0.01; Fig. 1). By univariate analysis, risk of recurrence was not significantly different between the RE+ and RE– groups (Cox HR = 0.85 [CI 0.52–1.41]; p = 0.54; Fig. 2), nor was DTC-specific mortality (Cox HR = 0.54 [CI 0.21–1.37]; p = 0.19; Fig. 3). The assumption of proportional hazards was fulfilled for overall (p = 0.86) and DTC-specific (p = 0.85) mortality, as well as for recurrence (p = 0.16). The dose of RE was not associated with OS (p = 0.17), recurrence (p = 0.69), or DTC-specific mortality (p = 0.20). Indication for radiation exposure did not affect OS (p = 0.87–0.96), recurrence (p = 0.31–0.98), or DTC-specific mortality (p = 0.49–1.00). Controlling for decade of treatment initiation similarly did not significantly affect associations between outcomes (OS, DTC-specific mortality, and recurrence) and RE. Controlling for the bias introduced by thyroid cancer recurrence on thyroid cancer–specific mortality, there is no change in significance (p = 0.40). Outcomes for the eight patients with high-dose exposure compared with the RE– group are shown in Supplementary Figures S1–S3 (Supplementary Data are available online at www.liebertpub.com/thy).
FIG. 1.
Kaplan–Meier survival estimates for overall survival. History of therapeutic radiation exposure (RE+) Cox hazard ratio (HR) = 0.55 [confidence interval (CI) 0.34–0.89]; p = 0.01.
FIG. 2.
Kaplan–Meier estimates for thyroid cancer recurrence. RE+ Cox HR = 0.85 [CI 0.52–1.41]; p = 0.54.
FIG. 3.
Kaplan–Meier survival estimates for thyroid cancer–specific mortality. RE+ Cox HR = 0.54 [CI 0.21–1.37]; p = 0.19.
Because there were more advanced-stage DTC patients in the RE– cohort than in the RE+ cohort (18.2% vs. 7.6% patients with Stage III–IV DTC; p = 0.10), a separate analysis of patients with Stage I–II disease (85 RE+, 135 RE–) was performed in order to ensure that this difference in stage did not explain the observed difference in OS. Again, longer OS was found in the RE+ group compared with the RE– group, with a median OS of 47 versus 39 years (Cox HR = 0.52 [CI 0.30–0.90]; p = 0.02). There was no difference in DTC-specific mortality (Cox HR = 0.89 [CI 0.20–4.03]; p = 0.88) or risk of recurrence (Cox HR = 0.95 [CI 0.54–1.67]; p = 0.79). The assumption of proportional hazards was again fulfilled for overall (p = 0.89) and DTC-specific (p = 0.49) survival, as well as for recurrence (p = 0.34).
To ensure that histologic reclassification did not alter the results of the outcome analysis, outcomes were investigated in the subset of patients with pathologically confirmed classic PTC (RE+: 40 patients; RE–: 56 patients). Again, OS was found to be longer in the RE+ group than in the RE– group, with a median survival of 51 versus 36 years (Cox HR = 0.36 [CI 0.16–0.82]; p = 0.02). There was no difference in either PTC-specific mortality (Cox HR = 0.65 [CI 0.12–3.57]; p = 0.62) or risk of recurrence (Cox HR = 0.89 [CI 0.36–2.21]; p = 0.84). The assumption of proportional hazards was fulfilled for overall (p = 0.65) and DTC-specific (p = 0.97) survival, as well as for recurrence (p = 0.13). By multivariate analysis, neither histology nor lymph node involvement were associated with DTC-specific mortality (p = 0.73 and 0.42, respectively), or OS (p = 0.27 and 0.12, respectively).
Sex as a modifying factor for DTC outcomes
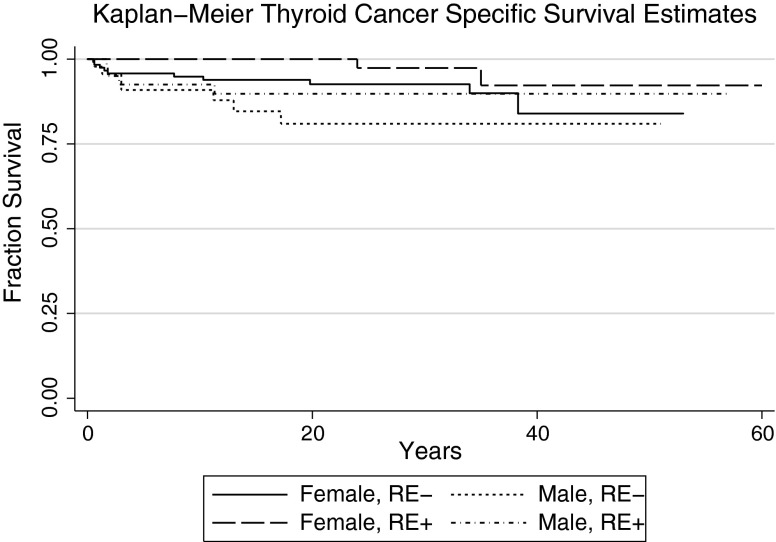
As noted, there were significantly more males in the RE+ DTC group than in the RE– DTC group. Sex was not significantly associated with OS (p = 0.17), DTC-specific mortality (p = 0.09), or risk of recurrence (p = 0.49) by univariate analysis in the combined RE+/RE– cohort. Multivariate analysis, however, revealed a significant effect of sex when controlling for RE. Within the RE+ group, OS did not differ between males and females (Cox HR = 1.36 [CI 0.88–2.11]; p = 0.84). In the RE– group, however, it was found that males had significantly shorter OS than females did (Cox HR = 1.95 [CI 1.13–3.36]; p = 0.02; Fig. 4). Using a multivariate model including RE and sex, it was observed that male sex was associated with a significantly worse OS (Cox HR = 1.56 [CI 1.00–2.45]; p = 0.05) and DTC-specific mortality (Cox HR = 2.32 [CI 1.01–5.35]; p = 0.05) in RE– males (Figs. 4 and 5). Conversely, when controlling for sex, OS was significantly better for RE+ as opposed to RE– males (Cox HR = 0.50 [CI 0.31–0.82]; p = 0.005), although there was no difference in DTC-specific mortality (Cox HR = 0.46 [CI 0.018–1.18]; p = 0.11), possibly due to power. Taken together, these data indicate that sex significantly influences outcomes in the absence of RE.
FIG. 4.
Kaplan–Meier survival estimates for overall survival by radiation exposure and sex. There were 120 patients in the female RE– group (median survival 38.3 years), 52 patients in the female RE+ group (median survival 43 years), 45 patients in the male RE– group (median survival 32 years), and 40 patients in the male RE+ group (median survival 57 years).
FIG. 5.
Kaplan–Meier survival estimates for thyroid cancer–specific mortality by radiation exposure and sex. There were 120 patients in the female RE– group, 52 patients in the female RE+ group, 45 patients in the male RE– group, and 40 patients in the male RE+ group.
Discussion
Over the past 20 years, the incidence of DTC has skyrocketed. Paralleling this rise has been an enormous increase in the use of medical radiation (6,7). As radiation is a major risk factor for DTC, these observations have led some investigators to suggest that increased use of medical radiation has contributed significantly to the burgeoning incidence of DTC (5). Little is known, however, about the effect of medical radiation on outcomes for DTC.
While there are considerable data describing outcomes for RE-induced DTC following disasters such as Chernobyl and in medical technologists, both the mechanism and dose of RE in these cohorts is different from that of patients who develop DTC following medical RE (8–11). Additionally, although other studies have investigated DTC outcomes following therapeutic medical radiation, the length of follow-up has been short, and all have used DTC in the general population as the comparator group rather than individuals with RE– DTC. Given the high prevalence of RE in the general population, this is likely to obscure clinical features unique to either RE+ or RE– DTC (10,13,14–17). Here, outcomes and histology were directly contrast in 92 RE+ DTC patients and 165 RE– DTC patients, all treated and followed at the same institution over the same time period for a median length of 27 years. In light of initial historic reports suggesting thyroid cancer after RE was more aggressive and, more recently, equivalent to thyroid cancer in the absence of RE, it was a surprise to observe in this cohort that OS for RE+ DTC patients is significantly better than that for RE– DTC patients (18). There was no difference between the groups for either DTC-specific mortality or risk of recurrence, similar to findings reported for the Chernobyl cohort (2,3).
Another surprising finding was that there was a significantly higher proportion of male patients in the RE+ group compared with the RE– group. Intriguingly, this sex skewing has been observed in other external medical radiation cohorts, but not in cohorts of individuals exposed to ionizing radiation after Chernobyl or the atomic bomb (1–3,10,11,15–17), but its cause remains unknown. Although more males were observed in the RE+ than in the RE–group, it was found that males in the RE– group had worse OS and DTC-specific mortality. This was not noted in earlier descriptions of this cohort, as radiation exposure for benign versus malignant disease was not controlled for (12). While the etiology of these sex-specific differences is unclear, the results suggest that sex is a powerful modifier of outcomes based on the presence or absence of RE. Furthermore, because these differences in outcome are unique to comparisons between medical RE+ DTC patients and RE– DTC patients, they may suggest that medical RE-induced DTC is biologically and medically distinct from DTC induced by radiation disasters or occupational exposure. These results underscore the importance of both long-term follow-up and accounting for RE in studies of outcomes in DTC patients.
On pathology review, no differences in histology, multifocality, extrathyroidal extension, stage, or lymph node involvement were observed to explain the difference in outcomes between the RE+ and RE– groups. Interestingly, the majority (54.7%) of patients in both groups were initially classified as having follicular variant of PTC. On re-review using contemporary WHO classifications of DTC, almost all patients for whom samples were available were reclassified as having classic PTC (92.3%). This change in diagnosis exemplifies the updated understanding of PTC and its follicular variant. Previous descriptions of this cohort noted a change in rates of recurrence based on the original histologic classification of these malignancies that did not hold true in the updated analysis and contemporary histologic classification (12).
In the initial patient cohort, there were eight patients who developed DTC following medical radiation of >30 Gy for treatment of a prior malignancy. Compared with RE– DTC patients, OS (p = 0.001) and risk of recurrence (p = 0.05) were worse for these patients (Supplementary Table S1), although results must be interpreted with caution in light of the small sample size. These data are intriguing because they again suggest that not all RE are equivalent, and that dose and schedule may influence the natural history of RE-induced DTC. As a caveat, it must be kept in mind that all patients in this group had an antecedent history, not only of high-dose RE but also of malignancy. Therefore, these patient outcomes may not reflect exclusively the effect of high-dose RE on DTC outcomes, but may instead reflect other factors such as the consequences of their original RE-requiring disease, innate genetic predispositions, exposure to other genotoxic therapies, and the fact that as survivors, they are subject to increased screening compared with healthy individuals. Nonetheless, the findings are in contrast to previous reports that DTC after radiation for antecedent malignancies do not behave more aggressively than sporadic DTC (3).
This study is limited by its retrospective nature. Patients were treated for DTC based upon the standard of care at their time of diagnosis, which varied over the course of this study. Thus, it is possible that different therapeutic modalities were associated with different outcomes, independent of the innate biology of DTC. Increased awareness of the risks associated with RE over time may have led to increased surveillance of individuals with a history of RE and a lead-time bias for detection of DTC in these individuals, which may account for their improved survival compared with those without a history of RE. Arguing against this, treatment and stage at diagnosis did not differ significantly between the RE+ and RE– groups, suggesting that RE+ DTC patients were not diagnosed significantly earlier than RE– DTC patients. Multiple regression analyses were performed, increasing the possibility of a type I error due to multiple testing. Finally, information regarding socioeconomic background and ethnicity were not available for this analysis. During the time period in which these patients were exposed to medical radiation, these treatments were considered cutting edge and oftentimes sought out by well-informed and high socioeconomic status (SES) medical consumers (8). As higher SES has been associated with improved thyroid cancer–specific outcomes, even in countries with universal healthcare, it is possible that RE+ DTC patients were of higher SES than DTC– patients (19,20). As males of low SES often have less access to healthcare and seek out healthcare less frequently than other groups of individuals, it may be that the finding that RE– males with DTC have worse outcomes than other groups is a reflection of disparities in healthcare rather than of biology (20).
In summary, PTC following therapeutic medical radiation has improved OS compared with classic non-radiation-induced PTC. These findings may suggest that RE+ and RE– DTC are distinct entities with distinct outcomes. Furthermore, the observation that DTC following RE for benign indications and medical RE for malignant conditions have different outcomes, both of which are different from outcomes following RE from nuclear catastrophes, implies that different forms of RE are associated with distinct DTC outcomes and even possibly distinct etiological subtypes.
Recent changes in the American Thyroid Association guidelines for the treatment of DTC have decreased the indications for total thyroidectomy in favor of thyroid lobectomy in some cases (21). These changes, however, do not extend to patients with a history of prior head and neck radiation therapy or environmental radiation exposure. The present findings demonstrate that RE+ DTC-specific outcomes do not differ from those of RE– DTC, while OS is improved. As rates of multifocality and extrathyroidal extension are also equivalent between the RE+ and RE– groups, this may suggest that RE+ patients would also benefit from the expanded indications for thyroid lobectomy, as excision of the contralateral lobe may only be of prophylactic benefit. Further work will be required to confirm the finding that DTC following therapeutic medical RE for benign indications is associated with a better prognosis than DTC in the absence of RE, and to determine whether the observed clinical differences between the RE+ and RE– DTC patients is the result of a unique biology or due to other as yet unknown environmental or SES factors.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (HD0433871, CA129045, and CA40046 to K.O.; K12CA139160 to R.H.G.); the American Cancer Society—Illinois Division (K.O.); and the Cancer Research Foundation (K.O.).
Author Disclosure Statement
The authors have no relevant financial disclosures.
References
- 1.Schneider AB, Recant W, Pinsky SM, Ryo UY, Bekerman C, Shore-Freedman E. 1986. Radiation-induced thyroid carcinoma. Clinical course and results of therapy in 296 patients. Ann Intern Med 105:405–412 [DOI] [PubMed] [Google Scholar]
- 2.Naing S, Collins BJ, Schneider AB. 2009. Clinical behavior of radiation-induced thyroid cancer: factors related to recurrence. Thyroid 19:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rumyantsev PO, Saenko VA, Ilyin AA, Stepanenko VF, Rumyantseva UV, Abrosimov AY, Lushnikov EF, Rogounovitch TI, Shibata Y, Mitsutake N, Tsyb AF, Yamashita S. 2011. Radiation exposure does not significantly contribute to the risk of recurrence of Chernobyl thyroid cancer. J Clin Endocrinol Metab 96:385–393 [DOI] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG. 2014. Current thyroid cancer trends in the United States. JAMA 140:317–322 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Chen Y, Huang H, Sandler J, Dai M, Ma S, Udelsman R. 2015. Diagnostic radiography exposure increases the risk for thyroid microcarcinoma: a population-based case-control study. Eur J Cancer Prev 24:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargavan M, Sunshine JH. 2005. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology 234:824–832 [DOI] [PubMed] [Google Scholar]
- 7.Warren J, Yabroff K, Meekins A, Topor M, Lamont E, Brown M. 2008. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 100:888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Mhoon D, Kaplan SP, Angelos P. 2009. Radiation-induced thyroid cancer: the Chicago experience. Surgery 146:979–985 [DOI] [PubMed] [Google Scholar]
- 9.Zvonova I, Krajewski P, Berkovsky V, Ammann M, Duffa C, Filistovic V, Homma T, Kanyar B, Nedveckaite T, Simon SL, Vlasov O, Webbe-Wood D. 2010. Validation of 131I ecological transfer models and thyroid dose assessments using Chernobyl fallout data from the Plavsk district, Russia. J Environ Radioact 101:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridman M, Savva N, Krasko O, Mankovskaya S, Branovan DI, Schmid KW, Demidchik Y. 2014. Initial presentation and late results of treatment of post-Chernobyl papillary thyroid carcinoma in children and adolescents of Belarus. J Clin Endocrinol Metab 99:2932–2941 [DOI] [PubMed] [Google Scholar]
- 11.Sigurdson AJ, Doody MM, Rao RS, Freedman DM, Alexander BH, Hauptmann M, Mohan AK, Yoshinaga S, Hill DA, Tarone R, Mabuchi K, Ron E, Linet MS. 2003. Cancer incidence in the US radiologic technologists health study, 1983–1998. Cancer 97:3080–3089 [DOI] [PubMed] [Google Scholar]
- 12.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB. 2013. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154:1436–1446; discussion 1446–1437 [DOI] [PubMed] [Google Scholar]
- 13.Delellis RA LR, Heitz PU, Eng C. 2004. Pathology and Genetics: Tumours of Endocrine Organs. IARC Press, Lyon, France [Google Scholar]
- 14.StataCorp 2013. Stata Statistical Software: Release 13. StataCorp LP, College Station, TX [Google Scholar]
- 15.Cardis E, Hatch M. 2011. The Chernobyl accident—an epidemiological perspective. Clin Oncol (R Coll Radiol) 23:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, Sugiyama H, Soda M, Ozasa K, Mabuchi K. 2013. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer 132:1222–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharya S, Sarafoglou K, LaQuaglia M, Lindsley S, Gerald W, Wollner N, Tan C, Sklar C. 2003. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer 97:2397–2403 [DOI] [PubMed] [Google Scholar]
- 18.Zablotska L, Nadyrov E, Rozhko A, Gong Z, Polyanskaya O, McConnell R, O'Kane P, Brenner A, Little M, Ostroumova E, Bouville A, Drozdovitch V, Minenko V, Demidchik Y, Nerovnya A, Yauseyenka V, Savasteeva I, Nikonovich S, Mabuchi K, Hatch M. 2014. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997–2008) of a cohort of children and adolescents from Belarus exposed to radioiodines after the Chernobyl accident. Cancer 121:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siu S, McDonald JT, Rajaraman M, Franklin J, Paul T, Rachinsky I, Morrison D, Imran SA, Burrell S, Hart R, Driedger A, Badreddine M, Yoo J, Corsten M, Van Uum S. 2014. Is lower socioeconomic status associated with more advanced thyroid cancer stage at presentation? A study in two Canadian centers. Thyroid 24:545–551 [DOI] [PubMed] [Google Scholar]
- 20.Keegan TH, Grogan RH, Parsons HM, Tao L, White MG, Onel K, Horn-Ross PL. 2015. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid 25:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen BR, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.