Abstract
The capacity of slightly acidic hypochlorous acid water (SAHW), in both liquid and spray form, to inactivate bacteria was evaluated as a potential candidate for biosecurity enhancement in poultry production. SAHW (containing 50 or 100 ppm chlorine, pH 6) was able to inactivate Escherichia coli and Salmonella Infantis in liquid to below detectable levels (≤2.6 log10 CFU/ml) within 5 sec of exposure. In addition, SAHW antibacterial capacity was evaluated by spraying it using a nebulizer into a box containing these bacteria, which were present on the surfaces of glass plates and rayon sheets. SAHW was able to inactivate both bacterial species on the glass plates (dry condition) and rayon sheets within 5 min spraying and 5 min contact times, with the exception of 50 ppm SAHW on the rayon sheets. Furthermore, a corrosivity test determined that SAHW does not corrode metallic objects, even at the longest exposure times (83 days). Our findings demonstrate that SAHW is a good candidate for biosecurity enhancement in the poultry industry. Spraying it on the surfaces of objects, eggshells, egg incubators and transport cages could reduce the chances of contamination and disease transmission. These results augment previous findings demonstrating the competence of SAHW as an anti-viral disinfectant.
Keywords: bacteria, biosecurity, SAHW (slightly acidic hypochlorous acid water), spraying, surface disinfection
Bacterial contamination is a significant and recurring problem affecting the poultry industry worldwide. This problem varies from severe acute infections with sudden and high mortality, to mild infections of a chronic nature with low morbidity and mortality rates, but always with injurious impacts on meat and egg production, egg hatchability and public health [19, 20, 22, 29, 34, 46, 49]. A wide variety of bacteria are present in the air, and on surfaces of the equipment and facilities of farms and hatcheries [22, 30, 40]. In addition, upon laying eggs in adequate environments, eggshells are highly contaminated with various kinds of bacteria [22, 32, 33]. Bacteria found on the eggshell can be easily distributed from farms to hatcheries. Given that hatcheries play critical roles in collecting hatching eggs from breeder farms and selling newly hatched chicks to commercial farms, they are a potential source of various infectious disease contaminations across farms. Furthermore, these contaminations cause significant economic losses for the poultry industry [22, 34, 41].
Among the bacterial infections affecting the poultry industry, Salmonella spp. and Escherichia coli are the most common; they are widely distributed in nature and have been isolated from large numbers of animal species and humans [6, 12, 17, 21, 24, 35, 37, 38, 45]. E. coli is a common pathogen of commercial poultry farms, causing colibacillosis worldwide [1, 3, 5, 8, 34, 40]. Salmonellosis is another important bacterial disease of the poultry industry, causing heavy economic losses through chick mortality and reduced meat and egg production [2, 29, 46]. Infected chicks shed these enteric bacteria through their feces and products (meat and eggs), and contaminate environments and nearby objects including air, food water, manure, bedding materials and soil, and the bacteria survive for up to several months [6, 10, 15, 23, 24, 29, 37]. The long-term survival of these bacteria in the environment increases their chance of transmission to sensitive hosts via the ingestion of such contaminated products, food and water, or through their contact with such inanimate objects [6, 10, 17, 37]. Furthermore, farm personnel also play a role in transmission of bacteria within and among the farms through their contact with contaminated hands, clothes and boots [4, 16, 18, 36].
Bacterial survival in food, water, soil, and porous and non-porous surfaces plays a critical role in the transmission of bacterial infections within and between the farms and flocks [17, 21, 28, 44]. Among the bacterial diseases transmissible from poultry to human, salmonellosis is the primary concern for public health, although the risk of colibacillosis cannot be ignored [9, 11, 16, 29, 37, 47]. Thus, inactivation on the surfaces of objects through application of materials with strong and broad-spectrum disinfectant capacity is very important to prevent their infection and colonization in poultry farms, as well as their transmission to humans. To reach these goals, enhancement of biosecurity within the poultry industry is essential. Slightly acidic hypochlorous acid water (SAHW) is a chlorine-based solution that contains a high concentration of hypochlorous acid (HOCl) at pH 6 (ratio in the molecule and the ion is 97.18% and 2.82% at 25°C); overall, the molecule form is the most effective disinfectant of the chlorine species present in solution. Given that HOCl is uncharged and has a relatively low molecular weight, it easily penetrates cell walls and reacts more rapidly than any other chlorine species, in both oxidative and substitution reactions. It has a high capacity for killing pathogens by irreversibly denaturing the critical components of cells, such as nucleic acids (DNA/RNA), mitochondria, enzymes and surface proteins [48]. Previously, we have reported that SAHW has a virucidal efficacy toward avian influenza virus (AIV) on surfaces [13] and Newcastle disease virus (NDV) in the air, along with confirmed safety for chicks [14]. In the present study, we evaluated sprayed SAHW for its efficacy in inactivating Salmonella Infantis and E. coli on rayon sheets and glass plates, as models for porous and non-porous surfaces, respectively, to confirm its applicability to biosecurity enhancement of poultry production.
MATERIALS AND METHODS
SAHW: SAHW containing free chlorine at the rate of 50 ppm was prepared by a Well Clean-TE generator (OSG Co., Ltd., Osaka, Japan) in our laboratory with the normal tap water on the day of use, and SAHW containing 100 ppm chlorine was kindly supplied by OSG Co., Ltd.
Aerosol sprayer and spray box: A nebulizer (NE-C28 Camp A-I-R) with the ability to produce small droplets (<3 µm in diameter) was purchased from Omron Corp. (Kyoto, Japan). SAHW was sprayed at a rate of 200 µl/min. Plastic boxes, measuring W360 × D290 × H112 mm, were purchased from a local market.
Inocula preparation: E. coli strain NBRC106373 was purchased from the National Institute of Technology and Evaluation Biological Resource Center (NBRC) (Chiba, Japan), and S. Infantis was kindly provided by Prof. Hiroshi Fujikawa (Laboratory Public Health, Department of Veterinary Medicine, Tokyo University of Agriculture and Technology, Tokyo, Japan). Both bacterial species were stored in 10% skim milk at −80°C until they were used. For the experiments, both bacteria were sub-cultured by plating on Luria-Bertani (LB) agar (containing 1% Bacto tryptone, 0.5% Bacto yeast extract, 1% sodium chloride and 1.5% Bacto agar, pH 7.4), followed by overnight incubation at 37°C. Colonies were then picked from the overnight culture and cultivated in LB medium (containing 1% Bacto tryptone, 0.5% Bacto yeast extract and 1% sodium chloride, pH 7.4) as previously described [31]. Stationary-phase bacteria were centrifuged at 1,750 ×g for 20 min at 4°C to remove organic materials. Cell pallets were re-suspended twice in phosphate-buffered saline (PBS: 0.14 M NaCl, 2 mM KCl, 3 mM Na2HPO4 and 1.5 m KH2PO4, pH 7.4) and then adjusted to a bacterial concentration of about 2 × 108 colony forming units (CFU)/ml. Both E. coli and S. Infantis were enumerated by surface plating on deoxycholate hydrogen sulfide-lactose agar after serial tenfold dilution in PBS, followed by overnight incubation at 37°C. The number of colonies was then determined and converted to log10 CFU/ml.
Computation of reduction factor: Reduction factor (RF) was calculated using the equation below after conversion of sample titer to log10 CFU/ml:
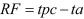 |
In the above equation, tpc is bacterial titer from an untreated sample in log10 CFU/ml, whereas ta is the titer of recovered bacteria from treated samples. The inactivation rate was acceptable when the RF was greater than or equal to 3 [13, 27, 42, 43].
Direct exposure: Fifty microliters of E. coli and S. Infantis were individually inoculated into 225 µl of 50 or 100 ppm SAHW, in a reaction tube and mixed, using a vortex mixer. After different exposure times, 225 µl of fetal bovine serum (FBS) was added to stop SAHW activity and determine the time required for bacterial inactivation. To confirm whether 225 µl of FBS is able to completely stop SAHW activity, a reaction mixture was prepared with the same volumes of SAHW and FBS, and then, 50 µl of E. coli or S. Infantis was inoculated onto the mixture. Given that there was no contact between these bacteria and SAHW before adding FBS, this exposure time was marked as the zero-second contact time. For the positive control, 50 µl of E. coli and S. Infantis was individually inoculated in 450 µl of PBS and indicated as treatments. This experiment was performed in triplicate.
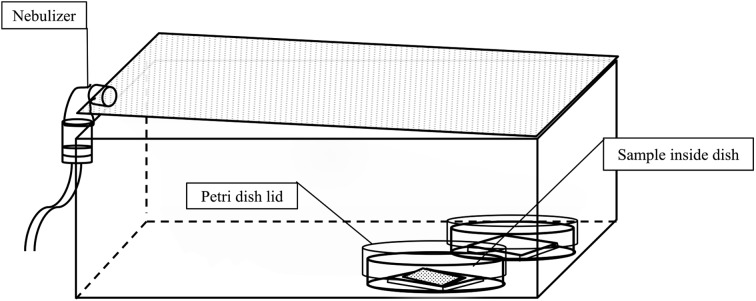
Indirect exposure: A. Dry condition. One hundred microliters of E. coli and S. Infantis were individually inoculated onto a 5 × 5 cm glass plate placed inside a 90-mm diameter petri dish without a lid and incubated for 30 min to facilitate evaporation of PBS and attachment of bacteria on the surface of the glass plate. Then, the petri dishes with lids were transferred to a spraying box and 50 or 100 ppm SAHW was sprayed for 3, 5 or 7 min inside the box, using a nebulizer from one side, and the samples were kept covered on the opposite side of the box (Fig. 1). Reverse osmosis (RO) water was sprayed for the positive control under the same conditions as for SAHW treatments. After stopping aerosol spraying of SAHW or RO water, the lids of the petri dishes were removed and the lid of the box was kept closed for 5 min. The samples were then removed and placed inside a stomacher bag (size 100 × 150 × 0.09 mm and capacity 80 ml; Organo Corp., Tokyo, Japan) containing 900 µl of 20% (v/v) FBS in PBS, to stop the activity of SAHW and to manually harvest the remaining viable bacteria. The supernatant was then transferred into a microfuge-tube for titration of the remaining viable bacteria.
Fig. 1.
Indirect spray of SAHW on bacteria. SAHW was sprayed using a nebulizer, from the side opposite to that of the petri dishes, into the box for 3, 5 and 7 min, after which the petri dishes lids were removed and the bacteria present on the glass plate or rayon sheets were exposed to SAHW for 5 min.
B. Wet condition. In this test, samples were prepared, and bacteria were inoculated in the same way as for the dry condition on the glass plate, except that samples were directly transferred to the spraying box after inoculation and exposed to the sprayed SAHW.
C. Inactivation on the rayon sheets. In this study, bacteria were inoculated on to the 3 × 3 cm double-fold rayon sheets, which were placed on the 5 × 5 cm glass plate surface inside a 90-mm diameter petri dish, and then transferred to the spraying box and sprayed with SAHW according to the wet and dry conditions described above. The rest of the procedure was performed identical to that in A and B. All experiments were conducted in triplicate.
Direct exposure of non-stainless metals to SAHW: This exposure was performed to examine the corrosivity of SAHW towards metallic objects. Fifty milliliters of SAHW containing 50 or 100 ppm chlorine was added to 90-mm diameter petri dishes, and then, non-stainless steel screws with or without a flat washer, to act as models for the flat and rough surfaces of metallic objects, were placed inside them and incubated at room temperature (25 ± 2°C) under a desk in the laboratory (dark area). At the same time, 100 and 400 ppm sodium hypochlorite (NaOCl), pH 7.97 and 9.64, respectively, were evaluated to compare their corrosivity. RO water was used as the negative control. These liquids neither changed nor refilled until the end of the experiment. The experiment was performed in duplicate, except for 50 ppm SAHW and 100 ppm NaOCl.
RESULTS
Inactivation efficacy in liquid (direct exposure): Table 1 summarizes the capacity of SAHW to inactivate bacteria in liquid. Both 50 and 100 ppm SAHW inactivated E. coli from 108.35 CFU/ml to below the detection limit (≤102.6 CFU/ml) (RF≥5.75), as well as S. Infantis from 108.76 CFU/ml to below the detectable level (≤102.6 CFU/ml) (RF≥6.16) within 5 sec of exposure time, respectively. In the 0-sec samples treated with 50 ppm SAHW, no reduction was observed in the E. coli and S. Infantis titers (RF=0.00), whereas in the 100-ppm samples, the E. coli titer was reduced from 108.76 CFU/ml to 107.95 CFU/ml (RF=0.39), and that of S. Infantis was reduced from 108.76 CFU/ml to 108.39 CFU/ml (RF=0.37). This experiment demonstrated that the efficacy of SAHW was negated by adding an equal volume of FBS.
Table 1. SAHW bactericidal effects after direct exposure.
| SAHW | Bacteria | Controlb) | RFa) | |
|---|---|---|---|---|
| 0c) sec | 5 sec | |||
| 50 ppm | E. coli | 8.35 ± 0.36d) | 0.00 ± 0.00 | ≥5.75 ± 0.44 |
| S. Infantis | 8.76 ± 0.08 | 0.00 ± 0.00 | ≥6.16 ± 0.08 | |
| 100 ppm | E. coli | 8.35 ± 0.44 | 0.39 ± 0.19 | ≥5.74 ± 0.44 |
| S. Infantis | 8.76 ± 0.08 | 0.37 ± 0.33 | ≥6.16 ± 0.08 | |
a) Reduction factor (RF)=log10 (titer of control/ml) −log10 (titer of treated samples/ml). b) Titer of bacteria in the control (log 10 CFU/ml) . c) Contact times. d) Data represent means ± standard deviation of three different experiments.
Inactivation by sprayed SAHW: Table 2 presents the results of spraying SAHW to inactivate bacteria on the glass plate or rayon sheet surfaces after the indicated spray times and 5 min of exposure. In the dry condition, the sprayed 50 ppm SAHW reduced the titer of E. coli from 106.94 CFU/ml to 104.46 CFU/ml (RF=2.48) at 3 min of spraying, from 106.94 CFU/ml to 102.91 CFU/ml (RF=4.03) at 5 min of spraying and from 106.94 CFU/ml to 102.6 CFU/ml (RF=4.34) at 7 min of spraying. In addition, 50 ppm reduced the titer of S. Infantis from 107.14 CFU/ml to 102.99 CFU/ml (RF=4.15) at 3 min of spraying, from 107.14 CFU/ml to 103.26 CFU/ml (RF=3.88) at 5 min of spraying and from 107.14 CFU/ml below the detectable level (≤102.6 CFU/ml) (RF≥4.54) at 7 min of spraying. In addition, in the wet condition, 50 ppm SAHW could not inactivate bacteria even at the 7-min exposure. In the dry condition, after 5 min of exposure, 100 ppm SAHW reduced the titer of E. coli from 107.04 CFU/ml to 103.28 CFU/ml (RF=3.76) at 3 min of spraying from 107.04 CFU/ml to ≤102.6 CFU/ml (RF≥4.44) at 5 min of spraying and from 107.04 CFU/ml to ≤102.6 CFU/ml (RF≥4.44) at 7 min spraying. In addition, 100 ppm SAHW reduced the titer of S. Infantis from 107.56 CFU/ml to 104.89 CFU/ml (RF=2.67) at 3 min of spraying and from 107.56 CFU/ml to 103.49 CFU/ml (RF=4.07) at 5 min of spraying, and S. Infantis was not tested at 7 min of spraying at dry condition with 100 ppm SAHW. In the wet condition, sprayed 100 ppm SAHW reduced the E. coli titer from 108.65 CFU/ml to 107.92 CFU/ml (RF=0.73) at 3 min of spraying, from 108.65 CFU/ml to 107.57 CFU/ml (RF=1.08) at 5 min of spraying and from 108.65 CFU/ml to 108.12 CFU/ml (RF=0.53) at 7 min of spraying. Furthermore, 100 ppm also reduced the titer of S. Infantis from 108.49 CFU/ml to 106.83 CFU/ml (RF=1.66) at 3 min of spraying, from 108.49 CFU/ml to 106.77 CFU/ml (RF=1.72) at 5 min of spraying and from 108.49 CFU/ml to 106.44 CFU/ml (RF=2.05) at 7 min of spraying.
Table 2. SAHW bactericidal effects on bacteria on glass plates or rayon sheets within 5 min of exposure time.
| SAHW | Bacteria | Conditions | RFa) | ||
|---|---|---|---|---|---|
| 3b) min | 5 min | 7 min | |||
| 50 ppm | E. coli | Wet | Not tested | Not tested | 0.00 ± 0.00 |
| Dry | 2.48 ± 1.43c) | 4.03 ± 0.35 | 4.34 ± 0.15 | ||
| On rayon | Not tested | 1.14 ± 0.51 | 1.16 ± 0.85 | ||
| S. Infantis | Wet | Not tested | Not tested | 0.00 ± 0.00 | |
| Dry | 4.15 ± 0.69 | 3.88 ± 0.57 | 4.54 ± 0.15 | ||
| On rayon | Not tested | 0.40 ± 0.02 | 0.50 ± 0.08 | ||
| 100 ppm | E. coli | Wet | 0.73 ± 0.92 | 1.08 ± 1.25 | 0.53 ± 0.18 |
| Dry | 3.76 ± 0.89 | 4.44 ± 0.59 | 4.44 ± 0.00 | ||
| On rayon | 1.50 ± 0.41 | 3.15 ± 0.90 | 5.89 ± 0.52 | ||
| S. Infantis | Wet | 1.66 ± 1.58 | 1.72 ± 0.50 | 2.05 ± 1.12 | |
| Dry | 2.67 ± 1.14 | 4.07 ± 1.05 | Not tested | ||
| On rayon | 2.05 ± 1.97 | 4.45 ± 1.44 | 5.70 ± 0.00 | ||
SAHW was sprayed in a box for indicated periods (3, 5 or 7 min). Subsequently, the lids of the dishes that contained bacteria on either glass plates or rayon sheets were removed, and the bacteria were exposed to SAHW for 5 min. a) Reduction factor (RF)=log10 (titer of control/ml) −log10 (titer of treated samples/ml). b) Spraying times. c) Data represent means ± standard deviation of three different experiments.
On the rayon sheets (Table 2), sprayed 50 ppm SAHW reduced the E. coli titer from 108.17 CFU/ml to 107.03 CFU/ml (RF=1.14) at 5 min of spraying and from 108.17 CFU /ml to 107.01 CFU/ml (RF=1.16) at 7 min of spraying. In addition, 50 ppm reduced the titer of S. Infantis from 108.25 CFU/ml to 107.85 CFU/ml (RF=0.4) within 5 min of spraying and from 108.25 CFU /ml to 10 7.75 CFU/ml (RF=0.50) at 7 min of spraying. The 100 ppm SAHW reduced the titer of E. coli from 108.49 CFU/ml to 106.99 CFU/ml (RF=1.50) at 3 min of spraying, from 108.49 CFU/ml to 105.34 CFU/ml (RF=3.15) at 5 min of spraying and from 108.49 CFU/ml to below the detection limit (≤102.6 CFU/ml) (RF≥5.89) within 7 min of spraying. In addition, 100 ppm reduced the S. Infantis titer from 108.30 CFU/ml to 106.25 CFU/ml (RF=2.05) at 3 min of spraying, from 108.30 CFU/ml to 103.86 CFU/ml (RF=4.44) at 5 min of spraying and from 108.30 CFU/ml to the detectable limit (RF≥5.70) at 7 min of spraying.
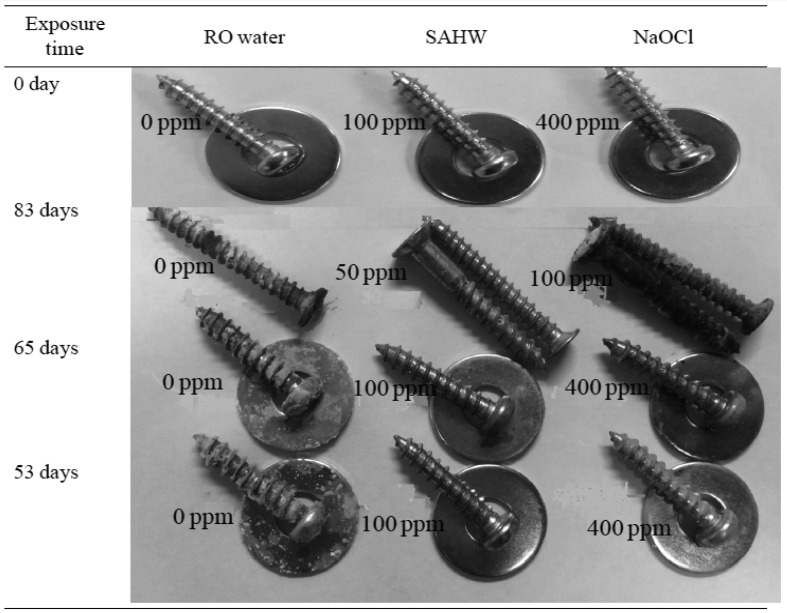
Corrosivity of SAHW towards metallic objects: Figure. 2 illustrates the results of corrosivity tests of SAHW as well as NaOCl towards metals. Within 83 days of exposure, 50 ppm SAHW did not corrode the non-stainless metals, as there was no change observed in their color, in comparison to 100 ppm NaOCl, which clearly changed the normal color of metallic objects to oxidized iron pigments. Such changes were slightly observed in the RO water-exposed metals, which was used as a negative control. Furthermore, within 65 days of non-stainless metal exposure to 100 ppm SAHW, no corrosion was observed in comparison to 400 ppm NaOCl or RO water.
Fig. 2.
SAHW effects on non-stainless metallic objects and its comparison with NaOCl and RO water.
DISCUSSION
Bacterial contamination is always a significant concern for poultry producers, not only in terms of morbidity and mortality of the chicks, but also as the main cause of poor hatchability and chick performance of the hatcheries, and as a potential risk to public health. Understanding the mechanisms underlying effective bacterial inactivation under different conditions, and selection of an appropriate disinfectant with a capacity for fast and strong disinfection efficacy against a broad range of pathogens in the air, on surfaces and in liquids, is vital for designing proper infection control strategies, enhancement of biosecurity and prevention of zoonotic infections. To minimize contamination of hatcheries, disinfection of eggs and hatcheries is necessary. There are several methods for disinfection of eggs, such as wiping, spraying, dipping into disinfectant and most importantly, fumigation of the hatching eggs, which can be performed during incubation (during or just after transfer to the hatchery), but most commonly prior to incubation. The most common disinfectant used as fumigant is formaldehyde, which is an excellent anti-microbial agent, but in comparison to SAHW, requires higher concentration and longer exposure time [25, 26, 50]. Furthermore, it is highly toxic and causes serious damage to the embryos, if fumigation is not properly carried out [2, 7, 30, 39]. Such damage mostly occurs on the outermost organic layer and cuticle, which constitute an important barrier to microbial invasion; hence, such damage may cause serious problems during incubation [2].
SAHW is also an excellent anti microbial agent with a fast and strong capacity for inactivating pathogens. We previously confirmed its efficacy towards NDV in the air and AIV on surfaces, as well as its safety for chicks [13, 14], and the present study documented its high performance for inactivation of bacteria in liquid and on porous and non-porous surfaces. Moreover, inactivation of bacteria in the dry condition was very easy, as even 50 ppm SAHW was able to inactivate them to an acceptable level. However, in the wet condition, it was not demonstrated to be effective for inactivation of bacteria even at the higher concentration, due to limited contact with sprayed SAHW, caused by PBS. Furthermore, inactivation of bacteria on a porous surface required a higher concentration of SAHW (Table 2). These data also suggest limited contact of SAHW with the bacteria, especially covered with water that may dilute SAHW. Aerosol spraying (indirect exposure) of SAHW caused great reduction of the bacterial titer on the surfaces of glass plates and rayon sheets, and is equivalent to the fumigation method. It is therefore a good candidate for disinfection of eggshells by spraying, and most importantly, fumigation of the egg incubators and hatcheries by aerosol spraying. Along with its excellent capacity for inactivating pathogens, SAHW is also harmless for metallic objects, even less corrosive than RO water, and safe for chicks; hence, it can be applied without hesitation at farms and hatcheries.
In conclusion, this study demonstrated SAHW’s fast and strong capacity against bacteria in liquid and on surfaces, and confirmed its non-corrosivity towards metallic objects. Confirmation of its non-corrosivity towards metallic objects and its safety for chickens increases its applicability to poultry production. Despite its safety and non-corrosivity, SAHW is also less expensive, readily available and applicable, inactivates a broad range of pathogens, and requires shorter exposure time. These characteristics of SAHW would encourage farmers to use these materials as ideal disinfectants on their farms and in other poultry facilities. From the results we obtained with S. Infantis and E. coli, its effectiveness towards other bacterial pathogens may well be inferred. Given that a study was conducted at the laboratory level, further investigation may be required to evaluate its remarkable properties and capacity to inactivate pathogens in poultry production units.
Acknowledgments
This study was supported in part by Regulatory Science, Ministry of Agriculture, Forestry and Fisheries, Japan (MAFF) 2015.
REFERENCES
- 1.Agunos A., Carson C., Léger D.2013. Antimicrobial therapy of selected diseases in turkeys, laying hens, and minor poultry species in Canada. Can. Vet. J. 54: 1041–1052. [PMC free article] [PubMed] [Google Scholar]
- 2.Ball R. F., Logan V., Hill J. F.1975. Factors affecting the cuticle of the egg as measured by intensity of staining. Poult. Sci. 54: 1479–1484. doi: 10.3382/ps.0541479 [DOI] [Google Scholar]
- 3.Barnes H. J., Nolan L. K., Vaillancourt J. F.1997. Colibacillosis. pp. 691–732. In: Diseases of Poultry, 12th ed. (Saif, M. Y., Fadly M. A., Glisson, R. J., McDougald, R. L., Nolan, K. L and Swayne, E. D. eds.) Blackwell Publishing, Ames. [Google Scholar]
- 4.Barua H., Biswas P. K., Olsen K. E. P., Christensen J. P.2012. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS ONE 7: e35914. doi: 10.1371/journal.pone.0035914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas P. K., Uddin G. M., Barua H., Roy K., Biswas D., Ahad A., Debnath N. C.2006. Causes of loss of Sonali chickens on smallholder households in Bangladesh. Prev. Vet. Med. 76: 185–195. doi: 10.1016/j.prevetmed.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Branham L. A., Carr M. A., Scott C. B., Callaway T. R.2005. E. coli O157 and Salmonella spp. in white-tailed deer and livestock. Curr. Issues Intest. Microbiol. 6: 25–29. [PubMed] [Google Scholar]
- 7.Cadirci S.2009. Disinfection of hatching eggs by formaldehyde fumigation. Arch. Geflugelkd. 73: 116–123. [Google Scholar]
- 8.Diarrassouba F., Diarra M. S., Bach S., Delaquis P., Pritchard J., Topp E., Skura B. J.2007. Antibiotic resistance and virulence genes in commensal Escherichia coli and Salmonella isolates from commercial broiler chicken farms. J. Food Prot. 70: 1316–1327. [DOI] [PubMed] [Google Scholar]
- 9.European Food Safety Authority2007. Report of the task force on zoonoses data collection on the analysis of the baseline study on the prevalence of salmonella in holdings of laying hen flocks of gallus gallus. Eur. Food Saf. Authority J 97: 1–84. [Google Scholar]
- 10.Ferens W. A., Hovde C. J.2011. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog. Dis. 8: 465–487. doi: 10.1089/fpd.2010.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley S. L., Nayak R., Hanning I. B., Johnson T. J., Han J., Ricke S. C.2011. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 77: 4273–4279. doi: 10.1128/AEM.00598-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas Neto O.d., Penha Filho R., Barrow P., Berchieri Junior A.2010. Sources of human non-typhoid salmonellosis: A review. Rev. Bras. Ciênc. Avíc. 12: 1–11. [Google Scholar]
- 13.Hakim H., Thammakarn C., Suguro A., Ishida Y., Kawamura A., Tamura M., Satoh K., Tsujimura M., Hasegawa T., Takehara K.2015. Evaluation of sprayed hypochlorous acid solutions for their virucidal activity against avian influenza virus through in vitro experiments. J. Vet. Med. Sci. 77: 211–215. doi: 10.1292/jvms.14-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakim H., Thammakarn C., Suguro A., Ishida Y., Nakajima K., Kitazawa M., Takehara K.2015. Aerosol disinfection capacity of slightly acidic hypochlorous acid water towards Newcastle disease virus in the air: An in vivo experiment. Avian Dis. 59: 486–491. doi: 10.1637/11107-042115-Reg.1 [DOI] [PubMed] [Google Scholar]
- 15.Hao X. X., Li B. M., Wang C. Y., Zhang Q., Cao W.2013. Application of slightly acidic electrolyzed water for inactivating microbes in a layer breeding house. Poult. Sci. 92: 2560–2566. doi: 10.3382/ps.2013-03117 [DOI] [PubMed] [Google Scholar]
- 16.Hoelzer K., Moreno Switt A. I., Wiedmann M.2011. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 42: 34. doi: 10.1186/1297-9716-42-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holley R., Walkty J., Blank G., Tenuta M., Ominski K., Krause D., Ng L. K.2008. Examination of Salmonella and Escherichia coli translocation from hog manure to forage, soil, and cattle grazed on the hog manure-treated pasture. J. Environ. Qual. 37: 2083–2092. doi: 10.2134/jeq2007.0304 [DOI] [PubMed] [Google Scholar]
- 18.Huang G. K., Stewardson A. J., Grayson M. L.2014. Back to basics: hand hygiene and isolation. Curr. Opin. Infect. Dis. 27: 379–389. doi: 10.1097/QCO.0000000000000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim M., Emeash H., Ghoneim N. H., Abdel-Halim M.2013. Seroepidemiological studies on poultry Salmonellosis and its public health importance. J. World’s Poult. Res 3: 18–21. [Google Scholar]
- 20.Ibrahim W. A., Abd El-Ghany W. A., Nasef S. A., Hatem M. E.2014. A comparative study on the use of real time polymerase chain reaction (rt-pcr) and standard isolation techniques for the detection of Salmonella in broiler chicks. Int. J. Vet. Sci. Med. 2: 67–71. doi: 10.1016/j.ijvsm.2013.11.001 [DOI] [Google Scholar]
- 21.Jay M. T., Cooley M., Carychao D., Wiscomb G. W., Sweitzer R. A., Crawford-Miksza L., Farrar J. A., Lau D. K., O’Connell J., Millington A., Asmundson R. V., Atwill E. R., Mandrell R. E.2007. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 13: 1908–1911. doi: 10.3201/eid1312.070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J. H., Kim K. S.2010. Hatchery hygiene evaluation by microbiological examination of hatchery samples. Poult. Sci. 89: 1389–1398. doi: 10.3382/ps.2010-00661 [DOI] [PubMed] [Google Scholar]
- 23.Kramer A., Schwebke I., Kampf G.2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6: 130. doi: 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krawiec M., Kuczkowski M., Kruszewicz A. G., Wieliczko A.2015. Prevalence and genetic characteristics of Salmonella in free-living birds in Poland. BMC Vet. Res. 11: 15. doi: 10.1186/s12917-015-0332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster J. E., Crabb W. E.1953. a. Studies on disinfection of eggs and incubators: The survival of Salmonella pullorum, thompson and typhi-murium on the surface of the hen’s egg and on incubator debris. Br. Vet. J. 109: 139–148. [Google Scholar]
- 26.Lancaster J. E., Crabb W. E.1953. b. Studies on disinfection of eggs and incubators: The value of formaldehyde gas with particular reference to the concentration resulting from the addition of formalin to potassium permanganate. Br. Vet. J. 109: 390–397. [Google Scholar]
- 27.Lombardi M. E., Ladman B. S., Alphin R. L., Benson E. R.2008. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 52: 118–123. doi: 10.1637/8055-070907-Reg [DOI] [PubMed] [Google Scholar]
- 28.Lopez G. U., Gerba C. P., Tamimi A. H., Kitajima M., Maxwell S. L., Rose J. B.2013. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 79: 5728–5734. doi: 10.1128/AEM.01030-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutful Kabir S. M.2010. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 7: 89–114. doi: 10.3390/ijerph7010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magwood S. E.1964. Studies in hatchery sanitation: 3. The effect of air-borne bacterial populations on contamination of egg and embryo surfaces. Poult. Sci. 43: 1567–1572. doi: 10.3382/ps.0431567 [DOI] [Google Scholar]
- 31.Maniatis T., Fritsch E. F., Sambrook J.1982. Molecular cloning: A laboratory manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. [Google Scholar]
- 32.Mauldin J. M.1999. Reducing contamination of hatching eggs. Poult. Dig. 57: 38–44. [Google Scholar]
- 33.North M. O., Bell D. D.1990: Maintaining hatching egg quality. pp. 87–102. In: Commercial Chicken Production Manual, 4th ed. Chapman and Hall, One Penn Plaza, New York. [Google Scholar]
- 34.Reid W. M., Maag T. A., Boyd F. M., Kleckner A. L., Schmittle S. C.1961. Embryo and baby chick mortality and morbidity induced by a strain of Escherichia coli. Poult. Sci. 40: 1497–1502. doi: 10.3382/ps.0401497 [DOI] [Google Scholar]
- 35.Rice D. H., Hancock D. D., Besser T. E.2003. Faecal culture of wild animals for Escherichia coli O157:H7. Vet. Rec. 152: 82–83. doi: 10.1136/vr.152.3.82 [DOI] [PubMed] [Google Scholar]
- 36.Rusin P., Maxwell S., Gerba C.2002. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 93: 585–592. doi: 10.1046/j.1365-2672.2002.01734.x [DOI] [PubMed] [Google Scholar]
- 37.Samiullah S.2013. Salmonella Infantis, a potential human pathogen has an association with table eggs. Int. J. Poult. Sci. 12: 185–191. doi: 10.3923/ijps.2013.185.191 [DOI] [Google Scholar]
- 38.Scherer C. A., Miller S. I.2001. Principles of bacterial pathogenesis. Academic Press, San Diego. [Google Scholar]
- 39.Sibel H., Dürdane K.2008. Investigation of the effects of pre-incubation formaldehyde fumigation on the tracheal epithelium of chicken embryos and chicks. Turk. J. Vet. Anim. Sci. 32: 263–267. [Google Scholar]
- 40.Stebbins M. E., Berkhoff H. A., Corbett W. T.1992. Epidemiological studies of congo red Escherichia coli in broiler chickens. Can. J. Vet. Res. 56: 220–225. [PMC free article] [PubMed] [Google Scholar]
- 41.Swai E. S., Sanka P. N., Daborn C. J.2013. Hatchery hygiene evaluation by questionnaire and microbiological screening of hatchery samples. Livest. Res. Rural Dev. 25: 7. [Google Scholar]
- 42.Takehara K., Chinen O., Jahangir A., Miyoshi Y., Ueno Y., Ueda S., Takada Y., Ruenphet S., Mutoh K., Okamura M., Nakamura M.2009. Ceramic powder made from chicken feces: anti-viral effects against avian influenza viruses. Avian Dis. 53: 34–38. doi: 10.1637/8382-062008-Reg.1 [DOI] [PubMed] [Google Scholar]
- 43.Thammakarn C., Tsujimura M., Satoh K., Hasegawa T., Tamura M., Kawamura A., Ishida Y., Suguro A., Hakim H., Ruenphet S., Takehara K.2015. Efficacy of scallop shell powders and slaked lime for inactivating avian influenza virus under harsh conditions. Arch. Virol. 160: 2577–2581. doi: 10.1007/s00705-015-2517-9 [DOI] [PubMed] [Google Scholar]
- 44.Traoré O., Springthorpe V. S., Sattar S. A.2002. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J. Appl. Microbiol. 92: 549–555. doi: 10.1046/j.1365-2672.2002.01560.x [DOI] [PubMed] [Google Scholar]
- 45.Tseng M., Fratamico P. M., Manning S. D., Funk J. A.2014. Shiga toxin-producing Escherichia coli in swine: the public health perspective. Anim. Health Res. Rev. 15: 63–75. doi: 10.1017/S1466252313000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uddin M. Z., Samad M. A., Kabir S. M. L.2011. Mortality and diseases status in hy-line and isa-brown strians of layer chickens beared in cage system in bangladesh. Bangl. J. Vet. Med. 9: 1–16. [Google Scholar]
- 47.Whiley H., Ross K.2015. Salmonella and eggs: from production to plate. Int. J. Environ. Res. Public Health 12: 2543–2556. doi: 10.3390/ijerph120302543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White G. C.2010. Chemistry of aqueous chlorine. pp. 69–173. In: White’s Handbook of Chlorination and Alternative Disinfectants, 5th ed. (Black and Veatch), John Waley and Sons, Inc., Hoboken. [Google Scholar]
- 49.Xavier J., Pascal D., Crespo E., Schell H. L., Trinidad J. A., Bueno D. J.2011. Seroprevalence of Salmonella and Mycoplasma infection in backyard chickens in the state of Entre Rios in Argentina. Poult. Sci. 90: 746–751. doi: 10.3382/ps.2010-01036 [DOI] [PubMed] [Google Scholar]
- 50.Zeweil H. S., Rizk R. E., Bekhet G. M., Ahmed M. R.2015. Comparing the effectiveness of egg disinfectants against bacteria and mitotic indices of developing chick embryos. J. Basic Appl. Zool. 70: 1–15. doi: 10.1016/j.jobaz.2014.12.005 [DOI] [Google Scholar]